Abstract
In this work, heat stable dry powders of oxytocin (OT) suitable for delivery by oral inhalation were prepared. The OT dry powders were prepared by spray drying using excipients chosen to promote OT stability including trehalose, isoleucine, polyvinylpyrrolidone, citrate (sodium citrate and citric acid), and zinc salts (zinc chloride and zinc citrate). Characterization by laser diffraction indicated that the OT dry powders had a median particle size of 2 μm, making them suitable for delivery by inhalation. Aerodynamic performance upon discharge from proprietary dry powder inhalers was evaluated by Andersen cascade impaction (ACI) and in an anatomically correct airway (ACA) model, and confirmed that the powders had excellent aerodynamic performance, with respirable fractions up to 77% (ACI, 30 L/min). Physicochemical characterization demonstrated that the powders were amorphous (X-ray diffraction) with high glass transition temperature (modulated differential scanning calorimetry, MDSC), suggesting the potential for stabilization of the OT in a glassy amorphous matrix. OT assay and impurity profile were conducted by reverse phase HPLC and liquid chromatography-mass spectrometry (LC-MS) after storage up to 32 weeks at 40°C/75%RH. Analysis demonstrated that OT dry powders containing a mixture of citrate and zinc salts retained more than 90% of initial assay after 32 weeks storage and showed significant reduction in dimers and trisulfide formation (up to threefold reduction compared to control).
Electronic supplementary material
The online version of this article (doi:10.1208/s12249-015-0314-0) contains supplementary material, which is available to authorized users.
KEY WORDS: dry powder, oral inhalation, oxytocin, peptide delivery, postpartum hemorrhage
INTRODUCTION
Oxytocin (OT) is a cyclic nonapeptide hormone (Cys-Tyr-Ile-Gln-Asn-Cys-Pro-Leu-Gly-NH2) secreted by the posterior pituitary gland that causes uterine contraction by binding to oxytonergic receptors. Clinically, OT is the first choice uterotonic agent for the prevention of uterine bleeding (1) and is available as an aqueous solution administered by injection (Pitocin, oxytocin injection USP, JHP Pharmaceuticals) or as a nasal spray formulation, though the latter is not widely used for therapeutic purposes (2). Chemically, OT contains a free amino group and two cysteine residues linked together by a disulfide bond. It undergoes rapid degradation to inactive products when exposed to elevated temperature and therefore requires storage below 25°C (3,4). Due to interest in reducing maternal death related to postpartum hemorrhage in developing countries, numerous efforts have been recently reported in the literature to develop stable OT formulations (5–7).
Oxytocin delivery by oral inhalation as a dry powder has the potential to address the refrigeration challenge and parenteral limitations (such as the requirements for sterilization and injection-trained staff) of current OT formulations. Drug delivery by oral inhalation has long been the standard of care for pulmonary diseases like asthma. Recently, however, a drug delivered by oral inhalation was approved by the FDA and made available in the USA for treating a systemic disease, diabetes (www.afrezza.com). The lungs provide a large surface area for drug absorption (80–100 m2/adult), avoid first pass metabolism, and can allow a rapid onset of therapeutic action. In particular, an inhaled dry powder OT formulation was shown to provide a more rapid onset of action than an intramuscular injection (5).
The challenges associated with preparing powders suitable for inhalation include engineering particles within a suitable size range while accommodating the physicochemical properties of the protein/peptide being delivered (8–11). A low-density powder with particle size between 1 and 5 μm is optimal for delivery to the deep lung (12). Methods used to engineer protein/peptide-containing particles within this size range include freeze-drying and spray-drying (13,14). Spray-drying has gained popularity because it offers ready control over particle size and density. In addition, stabilization of proteins or peptides in spray-dried powders can be achieved by engineering amorphous matrices using excipients with high glass transition temperatures (Tg) (13). The peptide or protein is entrapped in a glassy matrix, limiting its mobility and reactivity. Excipients with high Tg include polyols and saccharides such as lactose and trehalose (13). Saccharides have been shown to provide additional benefit because they contain hydrogen bond donors, which can displace water during processing, and lead to increased protein/peptide protection during drying and storage (15).
Multiple degradation pathways lead to OT instability (6,7,16). Degradation is mainly the result of chemical instability at the disulfide bond leading to dimerization and formation of sulfur-related impurities such as trisulfide, tetrasulfide, and trimers (16–18). Instability at the glutamine and asparagine groups leads to deamidation products. OT stability in solution can be improved by using stabilizing agents, in particular buffers and divalent cations (7,17,18). Recently, Avanti et al. demonstrated the beneficial effect of combining divalent zinc and a citrate buffer that dramatically reduced the formation of dimers and other sulfur-related impurities in solution (17–19). OT’s high binding affinity to divalent cations, especially Zn2+, is believed to play an important role in binding to its receptor (19,20).
Herein, we report the preparation of OT powders suitable for oral inhalation and containing excipients to promote OT stability at accelerated storage conditions. Powders were prepared by spray-drying solutions containing stabilizing and bulking excipients, including trehalose, isoleucine, polyvinylpyrrolidone (PVP), citrate (sodium citrate and citric acid), and zinc salts (zinc chloride and zinc citrate).
MATERIALS AND METHODS
Materials
Oxytocin was obtained from American Peptide (Sunnyvale, CA). Trehalose, trisodium citrate, and isoleucine were obtained from Alfa Aesar (Ward Hill, MA). Citric acid was obtained from EMD Chemicals (Darmstadt, Germany). Zinc chloride and zinc citrate were obtained from Sigma Aldrich (St Louis, MO). PVP K30 was obtained from Spectrum Chemicals (New Brunswick, NJ). Unless otherwise stated all materials were analytical or pharmaceutical grade.
Solution and Powder Preparation
The final powders contained 1% OT by weight and were prepared by spray drying feed solutions containing 5% solids by weight. The powders were prepared on a Büchi B290 spray dryer using nitrogen at 60 psi as the atomizing gas and the aspirator set at 80%. The solution feed rate was 1 g/min with the pump set at 5% of its maximum capacity. The inlet temperature of the drying gas (air) was controlled at 130°C, and the outlet temperature was measured at 60°C. Powders containing citrate salts were prepared from solutions containing 75 mM citrate buffer at either pH 4.5 (powders A-C, E-F) or pH 6.5 (powder D). Feed solutions were prepared with molar ratios of citrate to OT up to 100:1 and Zn to OT ratios up to 50:1 (Table I), and the final solution/suspension pH was measured before spray drying. Powder E was spray-dried from a suspension due to the low solubility of zinc citrate. Particles were collected using a high efficiency cyclone.
Table I.
Ratio OT-Zn2+
| Ratio to OT (molar equivalent) | |||||||
|---|---|---|---|---|---|---|---|
| ZnCl2 | Zn citrate | ||||||
| 0 | 0 | 10 | 25 | 50 | 50 | 50 | |
| Sodium citrate | |||||||
| 0 | G | – | – | – | – | – | – |
| 20 | – | – | C | – | – | – | – |
| 50 | – | – | – | B | – | – | – |
| 100 | – | F | – | – | A | D | E |
| Initial buffer pH | – | pH 4.5 | pH 6.5 | ||||
Moisture Content
Residual water content was analyzed using a thermogravimetric analyzer (Q500, TA Instruments, New Castle, DE). Samples (∼10 mg) were placed in platinum pans and heated under a nitrogen purge (30 L/min) at a rate of 20°C/min up to 110°C then held at temperature for 30 min. Moisture content was reported as loss on drying (LOD), expressed as percent by weight.
Phase Transition Temperature
Phase transition temperatures were measured by modulated differential scanning calorimetry (MDSC) using a DSC Q2000 (TA Instruments, New Castle, DE). Samples (∼5 mg) were filled in aluminum TzeroTM pans and sealed using a sample encapsulation press. Prior to analysis, the pan cover was punched with a small hole. The analysis was conducted with temperature amplitude of ±0.64°C every 60 s superimposed on a 5-min initial isotherm and 2°C/min ramp to 200°C under a 50 mL/min nitrogen purge.
Particle Morphology
Particle morphology was determined by ultra-high resolution scanning electron microscopy (SEM) (Micron Inc. Wilmington, DE). Samples were analyzed using a Hitachi SEM s-5500. Samples were prepared by aerosol dispersion onto a double-sided sticky carbon tape affixed onto an aluminum block. The mounted powders were then sputter-coated with platinum.
Amorphous Content by X-Ray Powder Diffraction
The amorphous content of the dry powders was assessed by X-ray diffraction (Micron Inc., Wilmington, DE). Samples were analyzed using a Shimadzu, LabX, XRD-6000 from 4 to 45 degrees two-theta at a continuous scan of 2.0° per minute using Cu radiation (40.0 kV, 35 mA). Data points were collected every 0.02° two-theta.
Powder Density
Density was measured with a tapped density analyzer (Autotap, Quantachrome Instruments, Boynton Beach, FL). Bulk density was determined by filling a 5-mL graduated cylinder with approximately 2 mL of powder. Tapped density was determined by measuring the powder volume after 3000 taps.
Aerodynamic Testing by Andersen Cascade Impaction
Aerodynamic particle size distributions were determined at ambient conditions (22°C/35%RH) using an 8-stage Andersen cascade impactor (ACI) and a flow controller (Copley Scientific, Nottingham, UK) at a flow rate of 30 L/min. A reusable dry powder inhaler (Dreamboat, MannKind Corporation) was initially selected to conduct the ACI screening test (21). The inhaler was connected to the induction port using a molded silicone adapter. Inhaler cartridges were filled with 5 mg of powder. Cartridges were discharged over 8 s at 30 L/min (4 L air flow volume); five cartridges were discharged per determination. The respirable fraction on fill (% RF/fill) was determined by dividing the mass of powder recovered from stages 2 to 7 of the impactor by the total mass of powder filled into the cartridges. Cartridge emptying was determined by weighing the cartridges before and after discharge.
Particle Size by Laser Diffraction
Particle size was determined using two different methodologies. Primary particle size of the bulk powders was determined by laser diffraction using a Sympatec HELOS unit equipped with a VIBRI vibratory feeder and a RODOS/M dry powder dispersing unit (Sympatec GmbH, Clausthal-Zellerfeld, Germany). An R1 lens with a measuring range of 0.1–35 μm was used. Particle size analysis was performed by WINDOX 5.6 software. Particle size distributions of dry powder plumes emitted from the inhaler were determined by laser diffraction using a Sympatec HELOS BR with an R3 lens equipped with an inhaler module (MannKind Corporation) (22). OT formulations were tested in a reusable dry powder inhaler (Dreamboat, MannKind Corporation) in which the air flow path was modified to improve cartridge emptying for amorphous powders (23). For each measurement, 5 mg of the OT formulation was filled into a new cartridge. A pressure profile lasting 4 s with a peak inspiratory pressure (PIP) of 4 kPa was used to discharge the inhalers and aerosolize the powder. A total of five samples were tested for each powder/inhaler combination. Cartridge emptying was determined by weighing the cartridges before and after discharge.
Patient Simulation by Anatomically Correct Airway Model
Powder performance was assessed in an anatomically correct airway (ACA) model, which includes a three dimensional stereolithograph made from MRI scans of a human male oropharyngeal tract (24). For each determination, 5 mg of powder was pre-filled into a reusable dry powder inhaler (Dreamboat, MannKind Corporation). A pressure profile lasting 4 s with a PIP of 4 kPa was used to discharge the inhaler and aerosolize the powder. The powder collected on a filter at the base of the ACA represented the mass of drug presented to the lung. The mass on the filter was determined relative to the initial filled mass of the device (%Mass to Filter/Fill, %MtF/F). ACA data points represent the average of three determinations.
HPLC and LC/MS of Oxytocin and its Degradation Products
RP-HPLC was performed using a 150 × 3.0 mm Kinetex C18 column with 2.6 μm particle size (Phenomenex, Torrance, CA) on an Alliance 2695 HPLC equipped with a 2487 dual wavelength detector (Waters, Milford MA). Samples (20 μL) were injected, and the separation was carried out at a flow rate of 0.5 mL/min using UV detection at 214 nm. Gradient elution with 0.1% TFA/H2O as solvent A and 0.1% TFA/acetonitrile (ACN) as solvent B was used. The ACN increased linearly from 10 to 35% over 30 min, returned to 10% at 35 min, and equilibrated for 5 min prior to the next injection.
All MS experiments were conducted in positive ion mode on an Agilent LC/MS-TOF 6210 mass spectrometer equipped with a 1200 series HPLC (Agilent Technologies, Wilmington, DE) using the HPLC parameters described above. The gas temperature was 350°C, gas flow was 10.0 L/min, and the nebulizer pressure was 60 psi. The Vcap was 4500 V, fragmentor 220 V, and skimmer 60.0 V. Mass spectra were acquired by scanning a mass-to-charge ratio (m/z) range from 300 to 2500 with a scan rate of 1.00 spectra.
Stability Studies
Powders (∼75 mg) were weighed into scintillation vials with fluoropolymer resin lined screw caps and placed in heat-sealed aluminum pouches as a first-pass model to probe the powder’s sensitivity to moisture and heat. Pouches were stored at 40°C and 75% relative humidity (RH) for up to 32 weeks.
RESULTS
Spray-Drying Yield, Residual Water Content, and MDSC
Spray-dried powders were prepared from aqueous feed solutions containing bulking agents (trehalose, isoleucine and PVP) with or without additional stabilizing agents, including citrate buffers and zinc salts (Table II). Powders A to F were prepared from feed solutions containing citrate at pH 3.8–6.0 and were compared to a control powder (G) that was prepared from bulking agents alone. Average yields were ∼75% using a high efficiency cyclone. Residual water in the powders (LOD) was 4.8–6.5%. All powders exhibited high glass transition temperatures (Tg > 100°C) (supplemental information).
Table II.
SD Powders: OT Content by wt%, Yield, LOD, Feed Solution pH, Tg
| Formulation | OT Assay (wt %) | Weight percent on dry basis (wt%) | Yield (%) | LOD (%) | pH feed solution | Tg (°C) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Trehalose | IsoLeu | PVP | Citrate salt | Zn salt | ||||||
| ZnCl2 | ||||||||||
| A | 0.98 | 59.94 | 6.89 | 1.38 | 24.03 | 6.76 | 76.0 | 5.8 | 3.8 | 102 |
| B | 0.99 | 73.47 | 8.45 | 1.69 | 12.01 | 3.38 | 78.8 | 6.5 | 3.9 | 125 |
| C | 0.93 | 81.59 | 9.38 | 1.88 | 4.81 | 1.35 | 74.6 | 5.9 | 4.0 | 106 |
| D | 0.92 | 56.69 | 6.52 | 1.30 | 27.73 | 6.76 | 76.9 | 6.2 | 6.0 | 130 |
| Zn citrate | ||||||||||
| E | 1.13 | 39.26 | 4.51 | 0.90 | 24.03 | 30.30 | 67.5 | 5.4 | 4.6 | 132 |
| F | 0.96 | 65.89 | 7.57 | 1.51 | 24.03 | – | 76.0 | 4.8 | 4.7 | 120 |
| G | 1.06 | 87.00 | 10.00 | 2.00 | – | – | 75.7 | 6.2 | 5.7 | 111 |
Particle Morphology and Homogeneity
The control formulation (G) containing trehalose, PVP, and isoleucine was observed to have a corrugated appearance similar to leucine-containing powders (Fig. 1a) (13,25). The corrugated morphology was maintained when zinc and citrate salts were added to the feed solutions (Fig. 1b–d). X-ray diffraction confirmed a uniform amorphous phase of the spray-dried powders formulated with high Tg excipients (supplemental information).
Fig. 1.
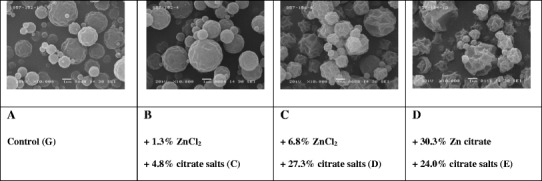
Representative SEM images of OT spray-dried powders
Powder Density
Bulk and tap density measurements are shown in Table III. Spray-drying produced particles with bulk density below 0.48 g/cm3 and tapped density below 0.65 g/cm3, regardless of salt content.
Table III.
Summary of Aerodynamic Performance, Particle Size, and Density
| Formulation | Aerodynamic performance (ACI) | Particle size (Laser diffraction) ×50 average (μm) | Density (g/cm3) | ||||
|---|---|---|---|---|---|---|---|
| Rf (%) | Rf/fill (%) | CE (%) | 0.5 bar | 3 bar | Bulk | Tap | |
| A | 76.5 | 59.1 | 77.2 | 1.99 | 1.68 | 0.472 | 0.594 |
| B | 77.5 | 60.3 | 77.8 | 1.87 | 1.63 | 0.408 | 0.582 |
| C | 71.4 | 55.4 | 77.3 | 1.97 | 1.85 | 0.456 | 0.623 |
| D | 69.4 | 59.8 | 86.2 | 1.89 | 1.96 | 0.389 | 0.620 |
| E | 70.8 | 53.2 | 75.1 | 2.06 | 1.81 | 0.397 | 0.580 |
| F | 74.3 | 55.9 | 75.3 | 1.96 | 1.65 | 0.454 | 0.553 |
| G | 58.4 | 40.9 | 70.0 | 1.87 | 1.57 | 0.411 | 0.649 |
Aerodynamic Performance by ACI
Powder performance, measured as respirable fraction on fill (%RF/fill) and cartridge emptying (%CE) from a reusable dry powder inhaler by Andersen cascade impaction (ACI), is shown in Table III. Both %RF/fill and %CE were improved relative to the control powder (G) when the stabilizing agents citrate and/or divalent zinc were added (Powders A–F).
Geometric Particle Size by Laser Diffraction
The volumetric median geometric diameter (VMGD) for all powders was below 2.0 μm by laser diffraction, making them suitable for pulmonary delivery (9,12).
The VMGD (×50) of powder discharged from a reusable dry powder inhaler was consistently below 2.4 μm with standard deviations below 0.12 μm (Table IV). Excellent cartridge emptying (CE) was obtained. CE for formulations A, B, and C was 89% or greater. The improvement in CE was realized with discharges from an inhaler using a modified flow path designed especially for compatibility with amorphous powders.
Table IV.
Particle Size Distribution Discharged from Reusable Dry Powder Inhaler
| Formulation | Pressure (kPa) | CE (%) | Particle size distribution (μm) | ||
|---|---|---|---|---|---|
| ×16 average | ×50 average | ×84 average | |||
| A | 4 | 94.6 | 1.21 | 2.28 ± 0.12 | 3.93 ± 0.31 |
| B | 4 | 89.0 | 1.15 | 2.19 ± 0.02 | 3.71 ± 0.05 |
| C | 4 | 90.6 | 1.16 | 2.19 ± 0.01 | 3.68 ± 0.01 |
| D | 4 | 85.6 | 1.26 | 2.32 ± 0.03 | 3.89 ± 0.06 |
| E | 4 | 80.5 | 1.17 | 2.17 ± 0.01 | 3.63 ± 0.03 |
Patient Simulation Using an Anatomically Correct Airway Model (ACA)
Powder performance was assessed in an anatomically correct airway (ACA) model to simulate patient usage, estimate the amount of powder deliverable to the lungs, and simulate oropharyngeal deposition patterns. Using a simulated inhalation profile with a PIP of 4 kPa, more than 88% of Powder A was discharged from a reusable dry powder inhaler and more than 63% of the dose reached the lower part of the airway model (%Mass to Filter/Fill, %MtF/F) simulating the expected delivered dose to the lungs. Reducing the content of zinc and citrate salts from 30% (Powder A) to about 6% (Powder C) improved the device emptying to more than 94% and the dose reaching the lower part of the airway model to more than 73%.
Powder Stability
Powders were prepared with a target drug content of 1%. Assay by HPLC confirmed that the OT content was between 0.92 and 1.13% for all powders (Table II). After 32 weeks at 40°C/75% relative humidity (RH), three of the seven powders maintained more than 89% of their initial assay (Fig. 2). The control formulation prepared without zinc or citrate (Powder G) retained only 51.6% assay after 32 weeks. The most stable powder (Powder D) was prepared from a pH 6.5 citrate buffer with zinc and retained 98% of its initial assay after 32 weeks.
Fig. 2.
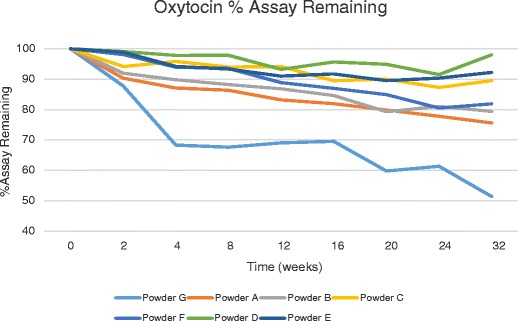
Stability results for oxytocin powders at 40°C/75%RH
Overall, powders prepared in the presence of both citrate and zinc salts exhibited the highest stability (Powders A to E). The stabilizing effect of this combination was achieved using either zinc chloride or zinc citrate. OT has been shown to coordinate strongly with Zn+2 in an octahedral complex formed with the peptide backbone carbonyl oxygen of Tyr2, Ile3, Gln4, Cys6, Leu8, and Gly9 (19,20). The formation of this OT-zinc complex induces a conformational change in OT to a form which facilitates binding to the OT receptor. It is possible that this conformational change may be contributing to the OT stability observed in this study and will be investigated in future work.
Decreasing the zinc/citrate concentration increased oxytocin stability for formulations prepared at pH ≤ 4 as demonstrated in Powders A, B, and C. Powder C maintained more than 89% assay after 32 weeks at 40°C/75% RH, while Powders A and B maintained less than 89% assay after 4 and 8 weeks, respectively. The control powder without zinc/citrate (Powder G) maintained only 87% assay after 2 weeks at 40°C/75%RH.
OT stability was also affected by feed solution pH. Powders A and D were prepared using the same components but using different feed solution pH (3.8 and 6.0, respectively; Table II). The increase in formulation pH improved the duration of >90% stability from 4 weeks (Powder A) to more than 32 weeks (Powder D).
Impurity Profile
All powders were evaluated after 32 weeks at 40°C/75% RH to compare impurity profiles. Degradation products included deamidated species, OT dimers, and trisulfide OT (Figs. 3 and 4). These species are the result of well-documented routes for OT degradation (7,17). All powders also contained an OT-glucose Amadori adduct (see Supplemental Information) formed by reaction with glucose, a contaminant in the trehalose used, which is not considered a normal OT degradation route (26,27). The OT dimers and the OT-glucose adduct were confirmed by synthesis of authentic samples with molecular weight determination by LC/MS (supplemental information). The presence of citrate salts in the solid state did not lead to the formation of citrate adducts as described previously for solutions (28).
Fig. 3.
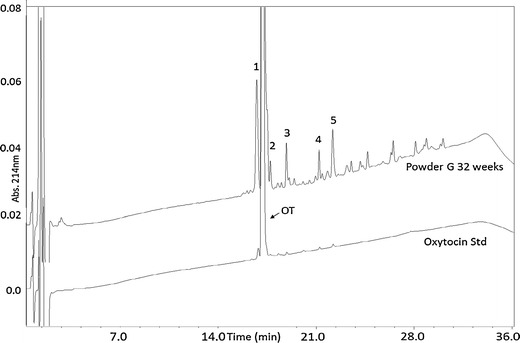
HPLC chromatograms of an oxytocin standard solution overlaid with Powder G after 32 weeks at 40°/75% RH. Peak labels: OT oxytocin, 1 glucose adduct, 2 deamidation product, 3 trisulfide, 4 dimer, 5 dimer
Fig. 4.
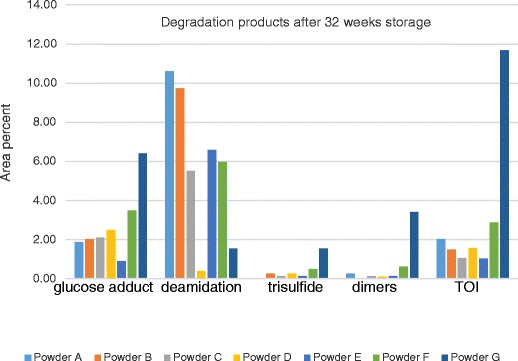
Degradation products (area%) in OT formulations after 32 weeks at 40°C/75% RH as determined by HPLC
The zinc/citrate combination influenced the amount of total impurities and the nature of the impurities formed on stability. Powder G, formulated without zinc or citrate, formed the highest amount of dimer and trisulfide. In addition, almost 12% of uncharacterized impurities referred as total other impurities (TOI) formed in Powder G after 32 weeks at 40°C/75% RH (Fig. 4). TOI remained less than 2% after 32 weeks at 40°C/75% RH in formulations containing zinc and citrate (Fig. 4). Taken together, this indicates that the zinc/citrate combination suppresses several routes of oxytocin degradation. Degradation via deamidation was influenced by the feed solution pH. Deamidation was significantly reduced at the higher pH (Fig. 4), likely because the buffering properties of citrate salts controlled the acid-catalyzed reactions at the asparagine, glutamine, and the amidated C-terminal glycine residues (18–20).
DISCUSSION
This study demonstrated that dry powder formulations of oxytocin suitable for pulmonary delivery can be prepared and are stable after extended storage under tropical and subtropical conditions. The spray-drying process produced amorphous powders well suited to pulmonary delivery: homogeneous median particle size distribution less than 2 μm and low powder density (13).
Powders were delivered from breath activated reusable dry powder inhalers (21,23) (http://www.mannkindtechnologies.com). The additives used during formulation, zinc chloride, sodium citrate, and zinc citrate, influenced the aerodynamic properties of the powders to produce high respirable fraction and high cartridge emptying. In a patient simulation model (24), device emptying was greater than 94% following a single discharge, and 73.5% of the dose (3.7 mg of the 5 mg cartridge fill) reached the lower part of the airway model, simulating the dose expected to reach the lungs. After accounting for the OT content of the powder (1%), and the minimum USP recommended OT activity (400U/mg), this correlates to a dose of approximately 15 U of oxytocin, which is in line with a clinically relevant dose (10 U) for controlling postpartum uterine bleeding (2).
Chemical stability of the OT powders was also influenced by the additives used during formulation (zinc and citrate salts). The control formulation (G) lost more than 10% of its activity (89% assay remaining) after only 2 weeks at 40°C/75% RH, and lost almost half the initial content (51% assay remaining) after 32 weeks of storage. Powders containing the combination of citrate and zinc salts in molar ratio to OT as low as 20:1 and 10:1 retained at least 89% of initial assay after 32 weeks of storage at 40°C/75%RH. Improvements in stability for such powders were attributed to the stabilization of the cysteine residue by the combination of citrate and zinc (17). Compared to control powders without citrate and zinc, these powders exhibited up to a threefold reduction of dimers and trisulfide, which are known inactive degradation products that arise from disulfide scrambling due to oxidation and sulfur exchange (29). Stability was improved with as little as 1.3% by weight of zinc and as little as 4.8% by weight of sodium citrate. Reduction in the formation of deamidation products was associated with high feed solution pH.
Future work includes evaluating the in vivo activity of OT powders for oral inhalation using a suitable animal model along with confirmation of the powder stability and performance after storage in field-suitable containers. In addition, evaluation of off-target effects, such as the potential for OT to stimulate contraction of the airways, would also be conducted. Encouragingly, a recent study published by Prankerd et al. (5) showed that pulmonary administration of oxytocin as a dry powder to postpartum sheep lead to authentic response of uterine tissue and no contractile responses to oxytocin in isolated airway tissue.
CONCLUSION
In this study, dry powder formulations of oxytocin were prepared by spray drying. Physicochemical characterization of the powders demonstrated that they had suitable median particle size (2 μm) for inhalation. Aerodynamic testing results indicated that a clinically relevant OT dose was achievable after discharging the powders from a dry powder inhaler. The use of divalent zinc salt and sodium citrate gave excellent stability under conditions of elevated temperature and humidity (40°C/75%RH), without negatively impacting aerodynamic performance of the powders. Both the buffering capacity of citrate salts and OT binding affinity for divalent zinc are known to stabilize OT in solution and likely played roles in the reduction of sulfur-related impurities and deamidation products in the dried state. Based on this study, orally inhaled dry powder OT formulations discharged from breath activated inhalers have demonstrated desirable product attributes while overcoming challenges presented by cold-chain storage.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
(DOCX 294 kb)
Abbreviations
- OT
Oxytocin
- Tg
Glass transition temperature
- PVP
Polyvinylpyrrolidone
- MDSC
Modulated differential scanning calorimetry
- ACI
Andersen cascade impactor
- PIP
Peak inspiratory pressure
- ACA
Anatomically correct airway
References
- 1.Dept. of Reproductive Health and Research, WHO WHO recommendations for the prevention and treatment of postpartum hemorrhage; 2012.
- 2.Guastella AJ, Hickie IB, McGuinness MM, et al. Recommendations for the standardisation of oxytocin nasal administration and guidelines for its reporting in human research. Psychoneuroendocrinology. 2013;38(5):612–25. doi: 10.1016/j.psyneuen.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 3.Hogerzeil HV, Walker GJA, de Goeje MJ. Stability of injectable oxytocics in tropical climates. WHOreportWHO/DAP/93.6;1993.
- 4.Groot ANJA, Vree TB, Hogerzeil HV, Walker GJA. Stability of oral oxytocics in tropical climates: Results of simulation studies on oral ergometrine, oral ethylergometrine, buccal oxytocin and buccal desamino-oxytocin. Geneva: World Health Organization; 1997. [Google Scholar]
- 5.Prankerd RJ, Nguyen T-H, Ibrahim JP, et al. Pulmonary delivery of an ultra-fine oxytocin dry powder formulation: potential for treatment of postpartum hemorrhage in developing countries. PLoS ONE. 2013;8(12):e82965. doi: 10.1371/journal.pone.0082965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hawe A, Poole R, Romeijn S, Kasper P, Jiskoot W. Towards heat-stable oxytocin formulations: analysis of degradation kinetics and identification of degradation products. Pharm Res. 2009;26(7):1679–88. doi: 10.1007/s11095-009-9878-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Avanti C, Amorij JP, Setyaningsih D, Hawe A. A new strategy to stabilize oxytocin in aqueous solutions: I. The effects of divalent metal ions and citrate buffer. The AAPS Journal. 2011;13(2):284–90. doi: 10.1208/s12248-011-9268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patton J, Byron P. Inhaling medicines: delivering drugs to the body through the lungs. Nat Rev Drug Discov. 2007;6:67–74. doi: 10.1038/nrd2153. [DOI] [PubMed] [Google Scholar]
- 9.Depreter F, Pilcer G, Amighi K. Inhaled proteins: challenges and perspectives. Int J Pharm. 2013;447(1–2):251–80. doi: 10.1016/j.ijpharm.2013.02.031. [DOI] [PubMed] [Google Scholar]
- 10.Manning MC, Chou D, Murphy B, Payne R, Katayama D. Stability of protein pharmaceuticals: an update. Pharm Res. 2010;27(4):544–75. doi: 10.1007/s11095-009-0045-6. [DOI] [PubMed] [Google Scholar]
- 11.Chang L, Pikal M. Mechanisms of protein stabilization in the solid state. J Pharm Sci. 2009;98:2886–908. doi: 10.1002/jps.21825. [DOI] [PubMed] [Google Scholar]
- 12.Carvalho T, Peters J, Williams R. Influence of particle size on regional lung deposition–what evidence is there? Int J Pharm. 2011;406(1–2):1–10. doi: 10.1016/j.ijpharm.2010.12.040. [DOI] [PubMed] [Google Scholar]
- 13.Reinhard V. Pharmaceutical particle engineering via spray drying. Pharm Res. 2008;25(5):999–1022. doi: 10.1007/s11095-007-9475-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elversson J, Millqvist-Fureby A, Alderborn G, Elofsson U. Droplet and particle size relationship and shell thickness of inhalable lactose particles during spray drying. J Pharm Sci. 2003;92:900–10. doi: 10.1002/jps.10352. [DOI] [PubMed] [Google Scholar]
- 15.Liao YH, Brown M, Nazir T, Quader A, Martin G. Effects of sucrose and trehalose on the preservation of the native structure of spray-dried lysozyme. Pharm. Res. 2002;19(12):1847–53. doi: 10.1023/A:1021445608807. [DOI] [PubMed] [Google Scholar]
- 16.Mozziconacci O, Schöneich C. Photodegradation of oxytocin and thermal stability of photoproducts. J Pharm Sci. 2012;101(9):3331–46. doi: 10.1002/jps.23204. [DOI] [PubMed] [Google Scholar]
- 17.Avanti C, Permentier HP, van Dam A, et al. A new strategy to stabilize oxytocin in aqueous solutions: II. Suppression of cysteine-mediated intermolecular reactions by a combination of divalent metal ions and citrate. Mol Pharm. 2012;9(3):554–62. doi: 10.1021/mp200622z. [DOI] [PubMed] [Google Scholar]
- 18.Christina Avanti C, Hinrichs W, Casini A, et al. The formation of oxytocin dimers is suppressed by the zinc–aspartate–oxytocin complex. J Pharm Sci. 2013;102(6):1734–41. doi: 10.1002/jps.23546. [DOI] [PubMed] [Google Scholar]
- 19.Liu D, Seuthe AB, Ehrler OT, et al. Oxytocin-receptor binding: why divalent metals are essential. J Am Chem Soc. 2005;127(7):2024–5. doi: 10.1021/ja046042v. [DOI] [PubMed] [Google Scholar]
- 20.Wyttenbach T, Liu D, Bowers MT. Interactions of the hormone oxytocin with divalent metal ions. J Am Chem Soc. 2008;130(18):5993–6000. doi: 10.1021/ja8002342. [DOI] [PubMed] [Google Scholar]
- 21.Smutney C, Kinsey PS, Sahi C, Adamo B, Polidoro J, McLean S, et al. Dry powder inhaler and system for drug delivery, MannKind Corporation. USA Patent 8,636,001 B2.
- 22.Adamo B, Shah S, Smutney C. Inhaler adaptor for a laser diffraction apparatus and method for measuring particle size distribution, MannKind Corporation. USA Patent 8,508,732 B2.
- 23.Smutney C, Adamo B, Laurenzi B, Kinsey PS. Dry powder drug delivery system and methods, MannKind Corporation. USA Patent Appln. US2014/001106 A1.
- 24.Adamo B, Polidoro J, Overfield D, Sahi C, Laurenzi B, Smutney C, Kinsey PS. Apparatus and method for simulating inhalation efforts, MannKind Corporation. USA Patent Appln. US 2012/0247235 A1.
- 25.Lechuga-Ballesteros D, Charan C, Stults C, Stevenson C, Miller D, Vehring R, et al. Trileucine improves aerosol performance and stability of spray-dried powders for inhalation. J Pharm Sci. 2008;97(1):287–302. doi: 10.1002/jps.21078. [DOI] [PubMed] [Google Scholar]
- 26.Tarelli E, Corran PH, Bingham BR, Mollison H, Wait R. Lysine vasopressin undergoes rapid glycation in the presence of reducing sugars. J Pharm Biomed Anal. 1994;12(11):1355–61. doi: 10.1016/0731-7085(94)00098-0. [DOI] [PubMed] [Google Scholar]
- 27.Wolfenden R, Yuan Y. Rates of spontaneous cleavage of glucose, fructose, sucrose, and trehalose in water, and the catalytic proficiencies of invertase and trehalas. J Am Chem Soc. 2008;130(24):7548–9. doi: 10.1021/ja802206s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poole R, Kasper P, Jiskoot W. Formation of amide- and imide-linked degradation products between the peptide drug oxytocin and citrate in citrate-buffered formulations. J Pharm Sci. 2011;100:3018–22. doi: 10.1002/jps.22495. [DOI] [PubMed] [Google Scholar]
- 29.Yamashiro D, Hope D, du Vigneaud V. Isomeric dimers of oxytocin. J Am Chem Soc. 1968;90(14):3857–60. doi: 10.1021/ja01016a048. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 294 kb)


