Abstract
The micronization of ampicillin via supercritical gas antisolvent (GAS) process was studied. The particle size distribution was significantly controlled with effective GAS variables such as initial solute concentration, temperature, pressure, and antisolvent addition rate. The effect of each variable in three levels was investigated. The precipitated particles were analyzed with scanning electron microscopy (SEM) and Zetasizer Nano ZS. The results indicated that decreasing the temperature and initial solute concentration while increasing the antisolvent rate and pressure led to a decrease in ampicillin particle size. The mean particle size of ampicillin was obtained in the range of 220–430 nm by varying the GAS effective variables. The purity of GAS-synthesized ampicillin nanoparticles was analyzed in contrast to unprocessed ampicillin by FTIR and HPLC. The results indicated that the structure of the ampicillin nanoparticles remained unchanged during the GAS process.
KEY WORDS: ampicillin, nanoparticles, precipitation, supercritical gas antisolvent
INTRODUCTION
The dissolution rate and bioavailability of many pharmaceuticals is limited by particle size. Reduction of particle size increases the contact surface with the solvent in the body and dissolution rate increases. The conventional methods of micronization have some problems such as mechanical stress, residual solvent, high thermal and wide particle size distribution. Alternative methods such as the precipitation process, which involves the supercritical fluids (SCFs), have been proposed. The most commonly used SCF is carbon dioxide because it possesses special properties such as being nontoxic, non-reactive, non-flammable, and inexpensive (1).
The rapid expansion of supercritical solutions (RESS), aerosol solvent extraction system (ASES), gas antisolvent (GAS), supercritical antisolvent (SAS), solution-enhanced dispersion by supercritical fluids (SEDS), and particles from gas-saturated solution (PGSS) are some methods for particle precipitation with SCFs. When the pharmaceutical has a high solubility in the supercritical fluids the RESS process has been suggested (2–9). The micronization of pharmaceutical compounds, with low solubility in supercritical fluids, was investigated via antisolvent techniques (10–28).
In the GAS processes, a solute is dissolved in solvent and loaded into a crystallizer. The solution is then expanded by injecting carbon dioxide into the crystallizer. The sharp reduction of solute solubility in liquid phase is observed and subsequently particle precipitation occurs. The antisolvent techniques for drugs with low solubility in the supercritical fluid are used. The mean particle size and particle size distribution are controlled by GAS variables. These variables are pressure, temperature, antisolvent addition rate, and initial solute concentration.
Various drugs are micronized by GAS process. Micro particle production of carbamazepine via GAS process was studied (29). Cocero and Ferrero (30) studied the effect of temperature and solvent type in the GAS process of β-carotene. The GAS method was used to control the cholesterol particle size and particle size distribution (31). The effect of crystallization variables such as temperature and type of solvent was studied on caffeine particle size (32). The results of Bakhbakhi et al. (33) analysis indicated that the mean particle size of phenanthrene was decreased by increasing the antisolvent rate and decreasing temperature.
Fusaro et al. (34) investigated the paracetamol precipitation by GAS process. Solvent and antisolvent were acetone and carbon dioxide, respectively. The experimental results indicated that particle size decreased with increasing the antisolvent rate and decreasing the temperature. The paracetamol particle size was independent of the initial concentration.
The effect of process variables (pressure, temperature, flow rate, and concentration) on the size and shape of Ginkgo particle was studied in the GAS process. Their experiments showed that particle size was reduced by increasing flow rate and decreasing the concentration and temperature (35). The experiments for production of belcomethasone-17,21-dipropionate with GAS process was investigated by Bakhbakhi et al. (36). The effect of variables such as antisolvent rate (1, 50, 75, and 100 mL/min), temperature (25°C, 32.5°C, 40°C, and 52.5°C), solute concentration (5%, 25%, 70%, and 100%) and stirrer speed (500, 1000, 2000, and 3000 rpm) was investigated (36). The micronization of 5-fluorouracil via GAS process was investigated (37).
Ampicillin is used to treat different types of infections caused by bacteria. Reverchon (38) proposed the supercritical assisted atomization method to improve size distribution of ampicillin. Micronized particles of ampicillin were generated with SAS process (39). Tenorio et al. (40) investigated the experimental precipitation of ampicillin via SAS technique. They studied the effect of solvents and pressure on particle size. Homogeneous spherical ampicillin nanoparticles were obtained by the SAS process with 1-methylene-2-pyrrolidone as solvent (41). Montes et al. (42) studied the ampicillin precipitation via SAS process. The N-methylpyrrolidone was used as solvent in their work. The thermodynamic and Kinetic modeling of ampicillin nanoparticles synthesis via supercritical gas antisolvent process was investigated (43).
In this study, the GAS technique was employed to generate ampicillin nanoparticles. The effect of the GAS process variables such as temperature (34°C, 40°C, and 46°C), concentration (20, 60, and 100 mg/mL), pressure (9, 12, and 15 MPa), and antisolvent flow rate (1.6, 2, and 2.4 mL/min) on the particle size and size distribution of ampicillin particles was investigated. The final product was characterized with HPLC and FTIR analysis.
EXPERIMENTAL
Materials
Ampicillin was obtained from Sigma-Aldrich Co. Dimethyl sulfoxide (DMSO) (≥99%), n-hexane (≥95%), and all of the chemicals used for HPLC analysis were purchased from Merck Co. (≥99%). Industrial-grade carbon dioxide (≥99%; Zamzam Co.) was used as the supercritical fluid.
Experimental Set-Up
A scheme of GAS apparatus is shown in Fig. 1. Carbon dioxide was stored in cylinder (1). It was filtered with a column of molecular sieve beads (2). CO2 was liquefied in a cooler (3), and subsequently pumped by high pressure pump (4). Carbon dioxide was heated before entering the precipitation vessel (7) by spiral preheater (6) in an oven (9). The pressure was adjusted by back pressure regulator (10). Particles were precipitated on a sinter filter (8) at the bottom of precipitation vessel.
Fig. 1.
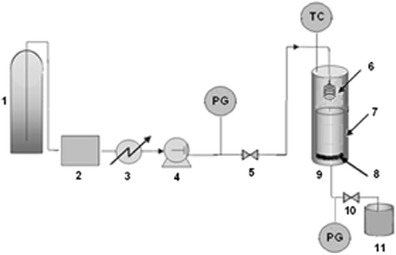
Experimental apparatus for the recrystallization of ampicillin by GAS process: 1 CO2 cylinder, 2 molecular sieve column, 3 cooler, 4 HPLC pump, 5 valve, 6 spiral heat exchanger, 7 precipitation vessel, 8 sinter metal filter, 9 oven, 10 back pressure valve, 11 organic solvent collection vessel
Characterization
The generated ampicillin particles were characterized by SEM and Zetasizer Nano ZS. Moreover, HPLC (using UV-visible detector and column C18, 5 μm, 25 cm × 4.6 mm; Jasco) analysis was used to investigate the purity of the final experimental product. The 50 mM sodium acetate solution:acetonitrile (75:25) with a flow rate of 1 mL/min was used as mobile phase (44). FTIR spectroscopy (FTIR; Tensor 27, Bruker) was performed to analyze the purity and structure of ampicillin nanoparticles.
Experimental Procedure
Batches of ampicillin solutions dissolved in DMSO were prepared with various concentrations. One milliliter of this solution was added into the precipitation vessel. The carbon dioxide injection into the precipitation vessel was started and continued until the desired pressure was reached. Subsequently, the carbon dioxide injection was stopped and the system was allowed 30 min to reach equilibrium. Then, the outlet valve was opened and the system was flushed with pure CO2 at the final pressure which is the same as initial pressure. The solvent was separated from the ampicillin particles with a constant CO2 flow rate. Then the particles were dried. The particles were precipitated to the bottom of the vessel. Finally, the particles were collected and analyzed by SEM, Zetasizer Nano ZS, HPLC, and FTIR.
RESULTS AND DISCUSSIONS
The solubility and sustained release of drugs in the body is usually controlled by size and size distribution of particles. Therefore, investigation of the effective GAS variables on the size and size distribution of medicines is considered to be a vital research step in the pharmaceutical industry. In this regard, GAS process variables such as initial solute concentration, temperature, pressure, and antisolvent addition rate change the particle size. Thus, GAS experiments were conducted to study the influence of each variable on the ampicillin particle size distribution. This investigation was carried out with the optimization method of one-at-a-time technique in which the effect of one variable was studied, while the other variables were kept constant at a selected value.
Effect of the Initial Solute Concentration
The particle size distribution was studied by varying the initial solute concentration (20, 60, and 100 mg/mL) at fixed temperature, pressure, and antisolvent addition rate of 40°C, 12 MPa, and 2 mL/min, respectively. Figure 2 shows the produced volume percent distribution for three sets of experiments at different initial solute concentration. Figure 3 shows the mean particle size as a function of initial solute concentration. When the solute concentration was increased, a larger mean particle diameter was obtained.
Fig. 2.
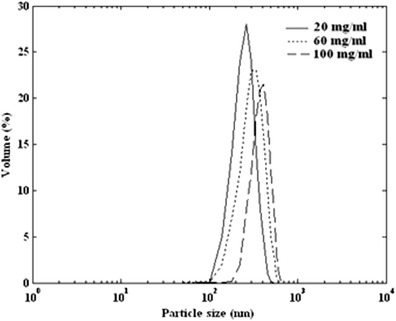
Particle size distribution of produced ampicillin by GAS process (34°C, 12 MPa, and 2 mL/min) at various initial solute concentration of 20, 60, and 100 mg/mL
Fig. 3.
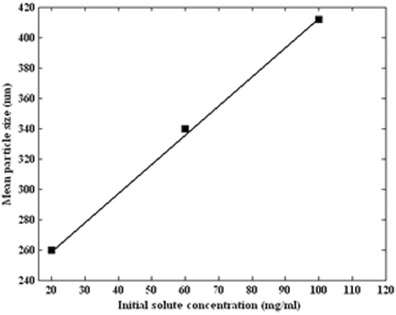
Mean particle size of produced ampicillin by GAS process (34°C, 12 MPa, and 2 mL/min) as a function of initial solute concentration (20, 60, and 100 mg/mL)
The observed results in our study can be explained in conjunction with the plot of solute concentration versus volume expansion. There are three distinguished zones (45) in this plot, namely, stable, metastable, and nucleation. When initial solute concentration increased, the nucleation was obtained at lower volume expansion. Therefore, the nucleuses were allowed to grow in a longer time. Chen et al. (35) obtained similar results for the precipitation of Ginkgo ginkgolides. The effect of BECD concentration on mean diameter particles was investigated by Bakhbakhi et al. (36). Their report indicated that mean particle diameter increased with increasing concentration.
Effect of Temperature
A set of experiments was carried out at temperature of 34°C, 40°C, and 46°C, at pressure, initial solute concentration, and carbon dioxide addition rate of 12 MPa, 60 mg/mL, and 2 mL/min, respectively. The particle size and particle size distribution of ampicillin were analyzed.
Figure 4 shows the produced volume percent distribution for three sets of experiments at different temperature. For the lowest temperature, i.e., 34°C, mean particle diameter is 340 nm. When temperature was increased, larger mean particle diameter was indicated. The mean particle diameter at three levels of temperature is shown in Fig. 5. As illustrated in Fig. 5, an increase in the temperature for the precipitation process increased the ampicillin mean particle size.
Fig. 4.
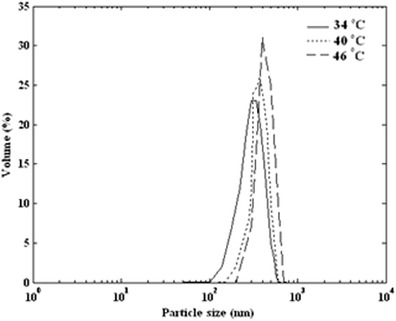
Particle size distribution of produced ampicillin by GAS process (12 MPa, 2 mL/min, and 60 mg/mL) at various temperatures of 34, 40, and 46°C
Fig. 5.
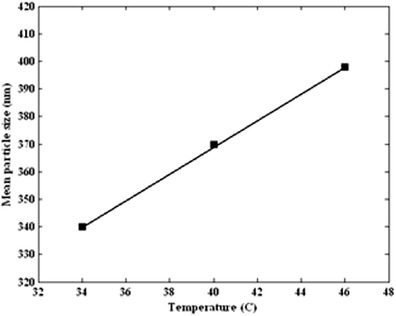
Mean particle size of obtained ampicillin by GAS process (34°C, 2 mL/min, and 60 mg/mL) as a function of temperature (34, 40, and 46°C)
Particle precipitation via GAS process occurs by increasing the volume expansion and decreasing the solubility. Temperature is an important effect in this process because it can change the solubility, supersaturation, and nucleation. While the temperature increases, the solubility of many drugs increases in organic solvent. Volumetric expansion decreases with increasing temperature in GAS process. Then, saturated and critical supersaturation lines are shifted upwards. The profile of the GAS moves to saturation line. The prevailing mechanism is growth. Therefore, larger mean particle sizes are obtained at the higher temperature. The effect of temperature on GAS process for different materials was also studied by Muller et al. (45), Bakhbakhi et al. (33), Chen et al. (35), Bakhbakhi et al. (36), and Gonzalez et al. (46). Similar results and trends were obtained and reported by the previous researchers.
Effect of Pressure
The effect of the pressure on particle size and particle size distribution was investigated using the pressures of 9, 12, and 15 MPa at constant temperature, CO2 flow rate and initial ampicillin concentration of 34°C, 2 mL/min, and 60 mg/mL, respectively. The SEM photomicrographs of ampicillin particles at pressure 12 and 15 MPa are shown in Figs. 6 and 7. The comparison of particles in these figures indicated that particle size was decreased when the pressure was increased. The nucleation mechanism prevailed when the pressure was increased. Thus, smaller particles were observed.
Fig. 6.
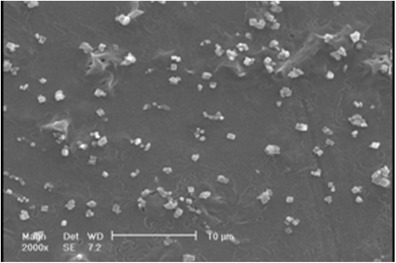
SEM photomicrographs of obtained ampicillin by GAS process at temperature of 34°C, CO2 flow rate of 2 mL/min, initial solute concentration of 60 mg/mL, and pressure of 12 MPa
Fig. 7.
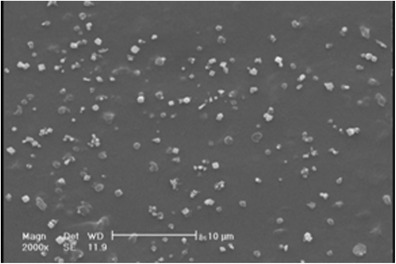
SEM photomicrographs of obtained ampicillin by GAS at temperature of 34°C, CO2 flow rate of 2 mL/min, initial solute concentration of 60 mg/mL, and pressure of 15 MPa
Figure 8 shows the produced volume percent distribution for three set of experiments at different pressure that were measured by Zetasizer Nano ZS. The mean particle diameter is 220 nm at pressure 15 MPa. As indicated in Fig. 9, it is clear that the average particle size decreases with increasing pressure in a linear trend. Similar results were obtained for gas antisolvent precipitation of pharmaceutical compound (G. ginkgolides) with supercritical CO2 by Chen et al. (35).
Fig. 8.
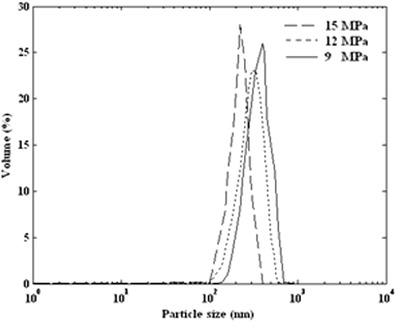
Particle size distribution of produced ampicillin by GAS process (34°C, 2 mL/min, and 60 mg/mL) at various pressures of 9, 12, and 15 MPa
Fig. 9.
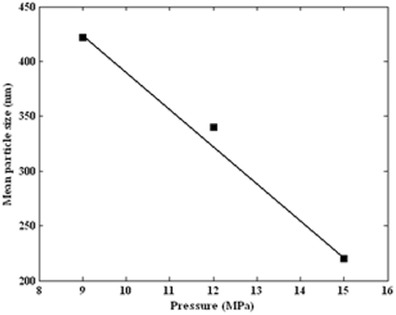
Mean particle size of obtained ampicillin by GAS process (34°C, 2 mL/min and 60 mg/mL) as a function of pressure (9, 12, and 15 MPa)
Effect of Antisolvent Addition Rate
The particle size was investigated by varying the carbon dioxide addition rate (1.6, 2, and 2.4 mL/min). Fixed variables were temperature (40°C), pressure (12 MPa), and initial ampicillin concentration (60 mg/mL). Figure 10 shows the volume percent distributions at three experimental flow rates. When the antisolvent addition rate was increased, a smaller mean particle diameter was obtained. The mean particle diameter versus carbon dioxide addition rate is shown in Fig. 11.
Fig. 10.
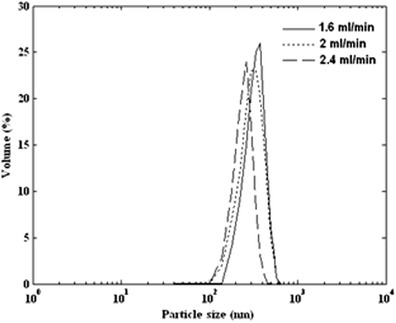
Particle size distribution of produced ampicillin by GAS process (34°C, 12 MPa, and 60 mg/mL) at various antisolvent flow rates of 1.6, 2, and 2.4 mL/min
Fig. 11.
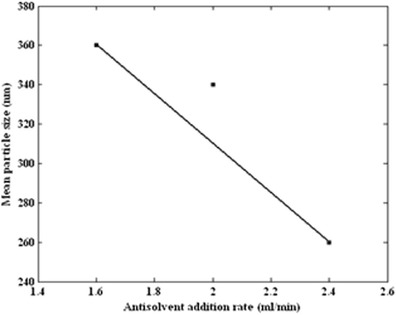
Mean particle size of obtained ampicillin by GAS process (34°C, 12 MPa, and 60 mg/mL) as a function of antisolvent flow rate (1.6, 2, and 2.4 mL/min)
The volume expansion and then supersaturation occurs when enough antisolvent is added to the precipitator. Then, the primary nucleuses of particle are created. A higher number of nucleus forms with primary nucleation at higher antisolvent addition rate. Therefore, solute concentration in liquid phase and supersaturation reduced quickly. At this time, a large number of particles with high surface area are formed. Thus, secondary nucleation occurs and a large amount of supersaturation is discharges. The solute concentration in liquid phase reduces when supersaturation drains. The driving force of particle growth is the solute concentration difference between the solution and particle surface. Therefore, the nucleation mechanism succeeds and growth rate is decreased. The same results were observed by Bakhbakhi et al. (36), Muller et al. (45), and Park and Yeo (32).
The influence of antisolvent addition rate on particle size was illustrated by Muller et al. (45). As indicated in their investigation, the system shifts into the nucleation zone and then the solute concentration reduces at higher antisolvent addition rate. Therefore, the system moves to metastable zone, where particles grow on this zone. The large amount of supersaturation is discharged in nucleation zone rather than in metastable zone, thus the smaller mean particle size is obtained at a higher antisolvent addition rate. Similarly, Bakhbakhi et al. (33) studied the effect of carbon dioxide addition rate on the phenanthrene concentration versus volume expansion. Their result showed the same trend.
Ampicillin Nanoparticles Analysis
The HPLC chromatograms of the GAS produced and unprocessed ampicillins are shown in Fig. 12. A very similar retention time (4 min) was observed in the chromatographic characteristics of ampicillin before and after the micronization by GAS process.
Fig. 12.
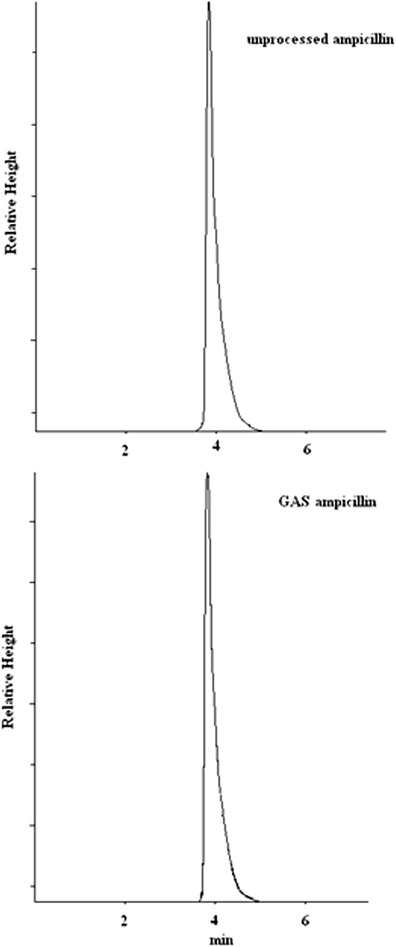
HPLC analysis of virgin and GAS precipitated ampicillin
Figure 13 shows the FTIR analysis of the ampicillin before and after the micronization. The comparison of FTIR spectra of GAS and virgin ampicillin indicated that the nature of ampicillin did not change.
Fig. 13.
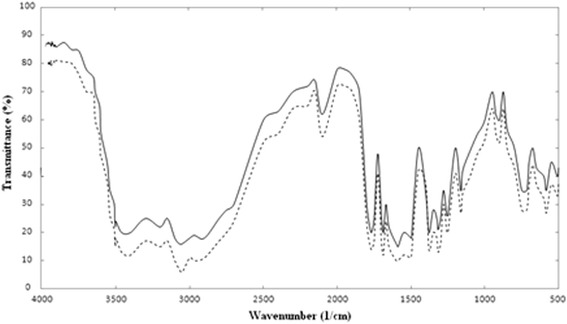
Comparison of FTIR spectra of unprocessed and GAS precipitated ampicillin
This analysis indicated that the GAS process was a suitable method for micronization of ampicillin component. The ampicillin nanoparticles without any change in nature and structure were produced.
CONCLUSIONS
In summary, the GAS and its effective variables such as initial solute concentration, temperature, pressure, and antisolvent addition rate for production of ampicillin nanoparticles were investigated. Ampicillin, carbon dioxide, and DMSO were used as solute, antisolvent, and solvent, respectively. The influence of each variable was investigated at three levels. Experimental results indicated that the mean particle size and particle size distribution were controlled with effective GAS process variables. These results illustrated that increasing the antisolvent flow rate from 1.6 to 2.4 mL/min led to a decrease in ampicillin particle size from 359 to 260 nm. The mean particle size was 425 nm for the lowest pressure (9 MPa). When the pressure was increased, a smaller mean particle size (220 nm) was obtained. The smaller mean particle size was observed in the lower temperature and initial solute concentration.
Acknowledgments
The financial support provided for this project by Isfahan University of Technology (IUT) is gratefully acknowledged.
References
- 1.Alibouri M, Ghoreishi SM, Aghabozorg HR. Effect of supercritical deposition synthesis on dibenzothiophene hydrodesulfurization over NiMo/Al2O3 nanocatalyst. AIChE J. 2009;55:2665–74. doi: 10.1002/aic.11867. [DOI] [Google Scholar]
- 2.Byrappa K, Ohara S, Adschiri T. Nanoparticles synthesis using supercritical fluid technology—towards biomedical applications. Adv Drug Deliv Rev. 2008;60:299–327. doi: 10.1016/j.addr.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Turk M, Lietzow R. Formation and stabilization of submicron particles via rapid expansion processes. J Supercrit Fluids. 2008;45:346–55. doi: 10.1016/j.supflu.2008.01.019. [DOI] [Google Scholar]
- 4.Atila C, Yildiz N, Cahmh A. Particle size design of digitoxin in supercritical fluids. J Supercrit Fluids. 2010;51:404–14. doi: 10.1016/j.supflu.2009.10.006. [DOI] [Google Scholar]
- 5.Chiou AH-J, Yeh M-K, Chen C-Y, Wang D-P. Micronization of meloxicam using a supercritical fluids process. J Supercrit Fluids. 2007;42:120–8. doi: 10.1016/j.supflu.2006.12.024. [DOI] [Google Scholar]
- 6.Varshosaz JF, Hassanzade M, Mahmoudzadeh M. Preparation of cefuroxime axetil nanoparticles by rapid expansion of supercritical fluid technology. Powder Technol. 2009;189:97–102. doi: 10.1016/j.powtec.2008.06.009. [DOI] [Google Scholar]
- 7.Yildiz N, Tuna S, Doker O, Calimi A. Micronization of salicylic acid and taxol (paclitaxel) by rapid expansion of supercritical fluids (RESS) J Supercrit Fluids. 2007;41:440–51. doi: 10.1016/j.supflu.2006.12.012. [DOI] [Google Scholar]
- 8.Kayrak D, Akman U, Hortacsu O. Micronization of ibuprofen by RESS. J Supercrit Fluids. 2003;26:17–31. doi: 10.1016/S0896-8446(02)00248-6. [DOI] [Google Scholar]
- 9.Charpentier PA, Ming J, Rahima AL. Study of the RESS process for producing beclomethasone-17,21-dipropionate particles suitable for pulmonary delivery. Pharm Sci Technol. 2008;9:39–46. doi: 10.1208/s12249-007-9004-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reverchon E. Supercritical antisolvent precipitation of micro and nanoparticles. J Supercrit Fluids. 1999;15:1–21. doi: 10.1016/S0896-8446(98)00129-6. [DOI] [Google Scholar]
- 11.Vezzu K, Bertucco A, Lucien FP. Solid-liquid equilibria of multicomponent lipid mixtures under CO2 pressure: measurement and thermodynamic modeling. AIChE J. 2008;54:2487–94. doi: 10.1002/aic.11543. [DOI] [Google Scholar]
- 12.Miguel F, Martin A, Mattea F, Cocero MJ. Precipitation of lutein and co-precipitation of lutein and poly lactic acid with the supercritical anti-solvent process. Chem Eng Process. 2008;47:1594–1602. doi: 10.1016/j.cep.2007.07.008. [DOI] [Google Scholar]
- 13.Miguel F, Martin A, Gamse T, Cocero MJ. Supercritical antisolvent precipitation of lycopene effect of the operating parameters. J Supercrit Fluids. 2006;36:225–35. doi: 10.1016/j.supflu.2005.06.009. [DOI] [Google Scholar]
- 14.Mattea F, Martin A, Matias-Gago A, Cocero MJ. Supercritical antisolvent precipitation from an emulsion: β-carotene nanoparticle formation. J Supercrit Fluids. 2009;51:238–47. doi: 10.1016/j.supflu.2009.08.013. [DOI] [Google Scholar]
- 15.Jin H, Xia F, Jiang C, Zhao Y, He L. Nanoencapsulation of lutein with hydroxypropylmethyl cellulose phthalate (HPMCP) by supercritical antisolvent. Chin J Chem Eng. 2009;4(17):672–7. doi: 10.1016/S1004-9541(08)60262-1. [DOI] [Google Scholar]
- 16.Moribe K, Fukino M, Tozuka Y, Higashi K, Yamamoto K. Prednisolone multicomponent nanoparticles preparation by aerosol solvent extraction system. Int J Pharm. 2009;380:201–5. doi: 10.1016/j.ijpharm.2009.06.030. [DOI] [PubMed] [Google Scholar]
- 17.Steckel H, Thies J, Muller BW. Micronization of steroids for pulmonary delivery by supercritical carbon dioxide. Int J Pharm. 1997;152:99–110. doi: 10.1016/S0378-5173(97)00071-9. [DOI] [Google Scholar]
- 18.Bustami RT, Chan H-K, Dehghani F, Foster NR. Generation of microparticles of proteins for aerosol delivery using high- pressure modified carbon dioxide. Pharm Res. 2000;17:1360–6. doi: 10.1023/A:1007551006782. [DOI] [PubMed] [Google Scholar]
- 19.Moshashaee S, Bisrat M, Fobes RT, Nyquist H, York P. Supercritical fluid processing of proteins I, lysozyme precipitation from organic solution. Eur J Pharm Sci. 2000;11:239–45. doi: 10.1016/S0928-0987(00)00108-1. [DOI] [PubMed] [Google Scholar]
- 20.Jeong H-H, Yoo K-P, Lim JS. Preparation of polystyrene submicron particles using ASES process in supercritical carbon dioxide. J Ind Eng Chem. 2008;14:77–83. doi: 10.1016/j.jiec.2007.08.002. [DOI] [Google Scholar]
- 21.Hong HL, Suo QL, Han LM, Li CP. Study on precipitation of astaxanthin in supercritical fluid. Powder Technol. 2009;191:294–8. doi: 10.1016/j.powtec.2008.10.022. [DOI] [Google Scholar]
- 22.Priamo WL, de Cezaro AM, Ferrwira SRS, Oliveira JV. Precipitation and encapsulation of β-carotene in PHBV using carbon dioxide as antisolvent. J Supercrit Fluids. 2010;54:103–9. doi: 10.1016/j.supflu.2010.02.013. [DOI] [Google Scholar]
- 23.Rodrigues M, Peirico N, Matos H, Gomes de Azevedo E, Lobato MR, Almeida AJ. Microcomposites theophylline/hydrogenated palm oil from a PGSS process for controlled drug delivery systems. J Supercrit Fluids. 2004;29:175–84. doi: 10.1016/S0896-8446(03)00034-2. [DOI] [Google Scholar]
- 24.Mandžuka Z, Knez Z. Influence of temperature and pressure during PGSS micronization and storage time on degree of crystallinity and crystal forms of monostearate and tristearate. J Supercrit Fluids. 2008;45:102–11. doi: 10.1016/j.supflu.2007.11.006. [DOI] [Google Scholar]
- 25.Sencar-Bozic P, Srcic S, Knez Z, Kerc J. Improvement of nifedipine dissolution characteristics using supercritical CO2. Int J Pharm. 1997;148:123–30. doi: 10.1016/S0378-5173(96)04838-7. [DOI] [Google Scholar]
- 26.Li J, Matos HA, Gomes de Azevedo E. Two-phase homogeneous model for particle formation from gas-saturated solution processes. J Supercrit Fluids. 2004;32:275–86. doi: 10.1016/j.supflu.2004.01.004. [DOI] [Google Scholar]
- 27.Sierra-Pallares J, Marchisio DL, Parra-Santos MT, Garcia-Serna J, Castro F, Cocero MJ. A computational fluid dynamics study of supercritical antisolvent precipitation: mixing effects on particle size. AIChE J. 2012;58:385–98. doi: 10.1002/aic.12594. [DOI] [Google Scholar]
- 28.Martin A, Pham HM, Kilzer A, Kareth S, Weidner E. Micronization of polyethylene glycol by PGSS (particles from gas saturated solution)-drying of aqueous solutions. Chem Eng Process. 2010;49:1259–66. doi: 10.1016/j.cep.2010.09.014. [DOI] [Google Scholar]
- 29.Moneghini M, Kikic I, Voinovich D, Perissutti B, Filipovic-Grcic J. Processing of carbamazepine-PEG 4000 solid dispersions with supercritical carbon dioxide: preparation, characterization and in vitro dissolution. Int J Pharm. 2001;222:129–38. doi: 10.1016/S0378-5173(01)00711-6. [DOI] [PubMed] [Google Scholar]
- 30.Cocero MJ, Ferrero S. Crystallization of β-carotene by a GAS process in batch effect of operating conditions. J Supercrit Fluids. 2002;22:237–45. doi: 10.1016/S0896-8446(01)00125-5. [DOI] [Google Scholar]
- 31.Liu Z, Wang J, Song L, Yang G, Han B. Study on the phase behavior of cholesterol-acetone-CO2 system and crystallization of cholesterol by antisolvent CO2. J Supercrit Fluids. 2002;24:1–6. doi: 10.1016/S0896-8446(02)00007-4. [DOI] [Google Scholar]
- 32.Park S, Yeo S. Recrystallization of caffeine using gas antisolvent process. J Supercrit Fluids. 2008;47:85–92. doi: 10.1016/j.supflu.2008.05.010. [DOI] [Google Scholar]
- 33.Bakhbakhi Y, Rohani S, Charpentier PA. Micronization of phenanthrene using the gas antisolvent process. 1. Experimental study and Use of FTIR. Ind Eng Chem Res. 2005;44:7337–7344. doi: 10.1021/ie050206e. [DOI] [Google Scholar]
- 34.Fusaro F, Mazzotti M, Muhrer G. Gas antisolvent recrystallization of paracetamol from acetone using compressed carbon dioxide as antisolvent. Cryst Growth Des. 2004;4:881–9. doi: 10.1021/cg034172u. [DOI] [Google Scholar]
- 35.Chen K, Zhang X, Pan J, Zhang W, Yin G. Gas antisolvent precipitation of ginkgo ginkgolides with supercritical CO2. Powder Technol. 2005;152:127–32. doi: 10.1016/j.powtec.2005.01.009. [DOI] [Google Scholar]
- 36.Bakhbakhi Y, Charpentier PA, Rohani S. Experimental study of the GAS process for producing microparticles of belcomethasone-17,21-di propionate suitable for pulmonary delivery. Int J Pharm. 2006;309:71–80. doi: 10.1016/j.ijpharm.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 37.Esfandiari N, Ghoreishi SM. Synthesis of 5-fluourouracil nanoparticles via supercritical gas antisolvent process. J Supercrit Fluids. 2013;84:205–10. doi: 10.1016/j.supflu.2013.10.008. [DOI] [Google Scholar]
- 38.Reverchon E. Supercritical-assisted atomization to produce micro-and/or nanoparticles of controlled size and distribution. Ind Eng Res. 2002;41:2405–11. doi: 10.1021/ie010943k. [DOI] [Google Scholar]
- 39.Reverchon E, Porta GD. Micronization of antibiotics by supercritical assisted atomization. J Supercrit Fluids. 2003;26:243–52. doi: 10.1016/S0896-8446(02)00162-6. [DOI] [Google Scholar]
- 40.Tenorio A, Gordilio MD, Pereyra CM, Martinez de la Ossa EJ. Controlled submicro particle formation of ampicillin by supercritical antisolvent precipitation. J Supercrit Fluids. 2007;40:308–16. doi: 10.1016/j.supflu.2006.07.003. [DOI] [Google Scholar]
- 41.Tenorio A, Gordilio MD, Pereyra CM, Martinez de la Ossa EJ. Supercritical antisolvent process applied to the pharmaceutical industry. Part Sci Technol. 2010;28:262–6. doi: 10.1080/02726351.2010.481589. [DOI] [Google Scholar]
- 42.Montes A, Tenorio A, Gordillo MD, Pereyra CM, Martines de la Ossa EJ. Supercritical antisolvent precipitation of ampicillin in complete miscibility conditions. Ind Eng Chem Res. 2011;50:2343–7. doi: 10.1021/ie101334v. [DOI] [Google Scholar]
- 43.Esfandiari N, Ghoreishi SM. Kinetics modeling of ampicillin nanoparticles synthesis via supercritical gas antisolvent process. J Supercrit Fluids. 2013;81:119–27. doi: 10.1016/j.supflu.2013.05.018. [DOI] [Google Scholar]
- 44.Kurian T, Kurien J, William H. Simultaneous multicomponent analysis of ampicillin and probenecid in pharmaceutical formation by reverse phase high performance liquid chromatography. J Drug Med. 2012;4:65–9. [Google Scholar]
- 45.Muller M, Meier U, Kessler A, Mazzotti M. Experimental study of the effect of process parameters in the recrystallization of an organic compounds using compressed carbon as antisolvent. Ind Eng Chem Res. 2000;39:2260–8. doi: 10.1021/ie990828y. [DOI] [Google Scholar]
- 46.Gonzalez DA, Mabe G, Zabaloy M, Brignole EA. Gas antisolvent crystallization of organic salts from aqueous solution. J Supercrit Fluids. 2000;17:249–58. doi: 10.1016/S0896-8446(99)00056-X. [DOI] [Google Scholar]


