Abstract
A color reaction of aspirin with ferrous gluconate was studied by UV-Vis spectrophotometry and HPLC-MS. It was found that the UV-Vis spectra of the two drugs were different before and after they were mixed in water at about 0.3 M (diluted by >20 times for analysis), indicating that a complexation reaction took place. The drug-iron complex dissociated when the reacting solution was diluted by 400 times. The by-products of the reaction identified by HPLC-MS were salicylic acid, acetylated gluconic acid, salicylate-gluconic acid conjugate, and an oxidized product of salicylic acid that was complexed with iron with a molecular weight of 212. This reaction may be used as an important consideration to optimize the dosing regime of the two drugs and to help explain some pharmacological reactions between aspirin and biomolecules.
KEY WORDS: aspirin, ferrous gluconate, reaction
INTRODUCTION
Aspiring is a common over-the-counter drug to reduce fever and relieve pain. It is also prescribed at low dose to prevent blood clogs and reduce the risk of stroke and heart attack. Aspirin is a cyclooxygenase (COX) inhibitor (1,2). It selectively acetylates a serine residue (Ser 530) of the enzyme (3,4). At high concentrations, aspirin will also non-specifically acetylate a variety of proteins and nucleic acids (3). Recently, it was discovered that aspirin acetylates the tumor suppressor proteins, which may contribute to its anticancer effects (5). On the other hand, majority of the therapeutic dose of aspirin is metabolized in the liver to salicylic acid, which is conjugated with glycine and glucuronic acid and excreted in the urine (1,2,6). However, the mechanism of these pharmacological reactions is unclear because they do not happen in vitro without any enzymatic catalysis.
Iron is an essential element in the human body and is critical for many biological functions such as breathing, metabolism, and signal transduction (7–10). Ferrous compounds are used as iron supplements to treat anemia; they are found to interact with various drugs taken concurrently (11). Because the ferrous ion itself is not quite stable against oxidation, it is often synthesized as salts of organic acids such as gluconate and fumarate. The organic salts of ferrous ion are also absorbed better than inorganic salts in patients (12). It was reported that the hydrolysis product of aspirin, salicylic acid can have a color reaction with ferric ion through a chelation mechanism, and this property is used for the analysis of this compound (13–15). Aspirin, however, is not known to react with iron because firstly, the phenolic oxygen on this molecule is esterized and can not complex with ferric ion, and secondly, the rate of hydrolysis of aspirin in water is slow at room temperature (rate constant = 0.0436 day−1) although the hydrolysis in blood is much faster due to the presence of esterase (6).
This brief communication reports a new color reaction between aspirin and ferrous gluconate in water (the structures of the two drugs and salicylic acid are shown in Scheme 1). This observation will serve as an important consideration if the two drugs should be dosed together. Additionally, as the majority of iron exists as Fe(II) in vivo and complexes with biomolecules such as glucose derivatives and proteins, it will be logical to use a complexed Fe(II) compound as a model if one wants to simulate the drug-iron-biomolecules interaction. It is hypothesized that the pharmacological reactions of aspirin mentioned above is catalyzed by this drug-iron interaction.
Scheme 1.

Aspirin salicylic acid ferrous gluconate
METHODS
Aspirin and ferrous gluconate were dissolved in water and mixed for 2 h at room temperature. The concentration of aspirin and ferrous gluconate in the mixture solution were 0.27 and 0.31 M, respectively. The solution was diluted 20, 100, and 400 times and UV-Vis spectra were acquired for the aliquots of these samples in comparison to pure (unmixed) aspirin and ferrous gluconate solutions. The drug solutions were subsequently analyzed by mass spectrometry (MS) following separation by high-performance liquid chromatography (HPLC).
RESULTS
Figure 1a shows the UV-Vis spectra of the pure and mixture solutions of aspirin and ferrous gluconate. The maximal absorption wavelength of aspirin and ferrous gluconate occurs at 274 and 324 nm, respectively. When the two drugs were mixed, the UV-Vis spectrum of the solution changed. The maximal absorption wavelength of aspirin shifted to 265 nm (as a shoulder peak) and the absorbance of ferrous gluconate at 324 nm decreased by 46%. At shorter wavelengths such as 237 nm, the absorbance of the mixture solution was greater than the sum of the two pure drugs. These indicate that there was an interaction between aspirin and Fe(II) and that the chemical bond of ferrous gluconate was altered. The color of the mixture solution appeared brownish, which is consistent with a higher molar absorptivity of the mixture solution than the unmixed drugs at wavelengths above 400 nm; and the color of the mixture solution continued to darken slowly. Figure 1b reveals that the UV spectrum of the mixture solution that is diluted by 20 times was almost the same as the one that was diluted by 100 times, while the mixture solution that was diluted by 400 times had a lower molar extinction coefficient than the former two solutions. This suggests that the drug-iron complex was intact at 100-time dilution, but dissociated upon dilution by 400 times. The UV spectrum of the dissociated samples also indicates that there was no large amount of free salicylic acid present which should give an absorption maximum at 303 nm (13,16). The absorption peak of the salicylate-(Fe3+) complex at 530 nm reported in the literature (14) was also absent in the spectrum of the sample solutions.
Fig. 1.

a UV-vis spectra of the pure and mixture solutions of aspirin and ferrous gluconate (diluted by 20 times). b UV-vis spectra of the mixture solutions of aspirin and ferrous gluconate (diluted by 20, 100 and 400 times)
High-performance liquid chromatography separated the mixture solution into six different components, gluconic acid, aspirin, salicylic acid, and three other new impurities. The mass spectra of these substances are displayed in Fig. 2. The molecular weight of gluconic acid, aspirin, and salicylic acid are 196, 180, and 138, respectively, which corresponds to the molecular ion peaks of their spectra (Fig. 2a–c). The peak of m/z 237 in the spectrum of Impurity 1 (Fig. 2d) was the molecular ion of acetylated gluconic acid, and the peak of m/z 318 in the spectrum of Impurity 2 (Fig. 2e) was the molecular ion of salicylate-gluconic acid conjugate. Based on the ion peak of m/z 211 (Fig. 2f), Impurity 3 may be an oxidized product of salicylic acid that is complexed with iron. The presence of the ion peaks of salicylic acid (m/z 137) and ferrous gluconate (m/z 445) in the same spectrum partly supported this deduce. The amount of salicylic acid presented in this sample solution (about 33% of initial aspirin concentration) was significantly greater than the one in pure aspirin solution, which suggests that ferrous gluconate catalyzes the production of salicylic acid. The rate of this reaction is expected to be lower in more dilute solutions.
Fig. 2.

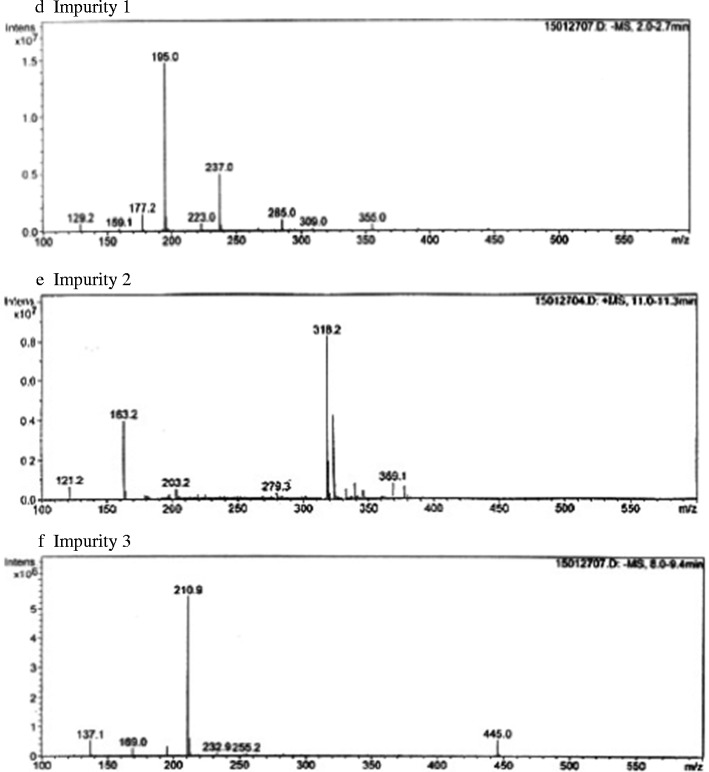
Mass spectra of gluconic acid, aspirin, and salicylic acid and three new impurities in the mixture solution separated by HPLC
DISCUSSION
The volume of gastric fluid in human is about 50–100 mL (average 75 mL). The concentration of the mixture solution that is diluted by 100 times in this experiment is 0.003 M. Using these parameters, it is calculated that the average doses of aspirin and ferrous gluconate at which the two drugs can react are 40.5 and 108.5 mg, respectively. These doses are smaller than the ones that are normally prescribed for human use. The pH of the reaction mixture is from 3 to 5. As we know, the pH of gastric fluid ranges from 1 to 5 depending on if food is ingested, and the gastric emptying time is about 2 h or longer with food intake. Therefore, the two drugs can potentially react in the upper GI tract during the absorption phase and they may not be taken together with little water.
It is apparent from this study that the acetylation and conjugation of gluconic acid by aspirin was catalyzed by iron through the formation of a drug-Fe(II)-gluconate complex. It is possible the same reaction happens in the liver where iron and glycogen are abundant. While ferrous gluconate dissociates at dilute concentration, iron-protein complexes do not because the structure of proteins are rigid. The iron concentrations in blood and in liver are 0.0045 and 0.0018 M, respectively, and the metal remain complexed with hemoglobin in blood and metabolic proteins such as cytochromes in liver. Therefore, it’s possible that aspirin will bind to these proteins along with iron at low concentration. It was reported that aspirin was a CYP 2C9 substrate and inducer (2). A recent study utilized computer simulation to investigate dextromethorphan oxidation by cytochrome P450 at the atomic level (17). Dextromethorphan was found to be oxidized by the heme group of the active site of CYP 2D6. There was another study that employed ferrous gluconate to analyze the affinity of some weakly alkaline and aromatic drugs for Fe(II) (18). This study showed that acetaminophen, dextromethrophan, guaifenesin, caffeine, or phenylephrine could bind to ferrous gluconate and these complexed mixtures reduce oxygen and hydrogen peroxide to water as in a battery reaction. Since the carboxyl, amino, imidazole, hydroxyl, and thiol groups on various amino acids can be potentially complexed with iron, it is possible that the binding of aspirin to proteins involves the formations of coordination bonds among the drug, Fe(II), and the proteins.
Future studies should focus on whether this in vitro reaction will have any biological effect in the body such as bioavailability of the two drugs, toxicity of the by-products and the interaction of aspirin with other biomolecules of interest.
CONCLUSION
Aspirin reacts with ferrous gluconate in water at 0.003–0.3 M, forming salicylic acid, acetylated gluconic acid, salicylate-gluconic acid conjugate, and an oxidized product of salicylic acid that is complexed with iron with a molecular weight of 212. This reaction provides useful information on the optimal dosing regime of the two drugs and preliminary assessment of the interaction of aspirin-Fe(II)-biomolecules.
Acknowledgments
The technical assistance from Ms. K.Y. Cui and Ms. F. Huang is greatly appreciated.
References
- 1.Wikipedia on aspirin (http://en.wikipedia.org/wiki/Aspirin).
- 2.Drug Bank (http://www.drugbank.ca).
- 3.Vane JR, Botting RM. The mechanism of action of aspirin. Thromb Res. 2003;110:255–8. doi: 10.1016/S0049-3848(03)00379-7. [DOI] [PubMed] [Google Scholar]
- 4.Roth GJ, Majerus PW. The mechanism of the effect of Aspirin on human platelets: 1 Acetylation of a particulate fraction protein. J Clin Invest. 1975;56:624–32. doi: 10.1172/JCI108132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alfonso LF, Srivenugopal KS, Arumugam TV, Abbruscato TJ, Weidanz JA, Bhat GJ G. Aspirin inhibits camptothecin-induced p21CIP1 levels and potentiates apoptosis in human breast cancer cells. Int J Oncol. 2009;34:597–608. doi: 10.3892/ijo_00000185. [DOI] [PubMed] [Google Scholar]
- 6.Hartwigotto H. Pharmacokinetic considerations of common analgesics and antipyretics. Am J Med. 1983;75(5):30–7. doi: 10.1016/0002-9343(83)90230-9. [DOI] [PubMed] [Google Scholar]
- 7.Wikipedia on hemoglobin (http://en.wikipedia.org/wiki/Hemoglobin).
- 8.Iwata S, et al. Complete structure of the 11-subunit bovine mitochondrial cytochrome bc1 complex. Science. 1998;281:64–71. doi: 10.1126/science.281.5373.64. [DOI] [PubMed] [Google Scholar]
- 9.Zhang J, et al. Structure of electron transfer flavoprotein-ubiquinone oxidoreductase and electron transfer to the mitochondrial ubiquinone pool. Proc Natl Acad Sci. 2006;103(44):16212–7. doi: 10.1073/pnas.0604567103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang W, et al. A manganese(IV)/iron(III) cofactorin Chlamydia trachomatis ribonucleotide Reductase. Science. 2007;316:1188. doi: 10.1126/science.1141179. [DOI] [PubMed] [Google Scholar]
- 11.Campbell NRC, Hasinoff BB. Iron supplements: a common cause of drug interactions. Br J Clin Pharmacol. 1991;31:251–5. doi: 10.1111/j.1365-2125.1991.tb05525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.http://medimoon.com on “Which salt is better ferrous gluconate or ferrous sulfate?”
- 13.Chinese Pharmacopeias, Monograph on aspirin.
- 14.Spectrophotometric analysis of aspirin, visible spectroscopy, Purdue University Instrument Van Project. 1993.
- 15.Fujita Y, Mori I, Fujita K, Kitano S, Tanaka T. A color reaction of 1,2-Diphenols based on colored complex formation with phenylfluoron and iron(III) and its application the assay of catecholamines in pharmaceutical preparation. Chem Pharm Bull. 1985;33(12):5385–8392. doi: 10.1248/cpb.33.5385. [DOI] [PubMed] [Google Scholar]
- 16.Zhang XL, Su H, Yao ZX, Lan YL. Monitoring aspirin and salicylic acid in synthesis systems of aspirin by ultraviolet spectroscopy. Chin J Spectrosc Lab. 2011;28(4):1911–5. [Google Scholar]
- 17.Oláh J, Mulholland AJ, Harvey JN. Understanding the determinants of selectivity in drug metabolism through modeling of dextromethorphan oxidation by cytochrome P450. PNAS. 2011;108(15):6050–5. doi: 10.1073/pnas.1010194108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang J. Water based biological and photochemical batteries, US Patent 8,871,370. 2014.


