Abstract
The objective of this study was to improve the solubility and bioavailability of curcumin by a new curcumin dripping pills (Cur-DPs) formulation using melt mixing methods. The optimal formulation consisted of Polyethoxylated 40 hydrogenated castor oil (Cremophor RH40), Poloxamer 188, and Polyethylene glycol 4000 (PEG 4000). Differential scanning calorimetry (DSC), powder X-ray diffraction (PXRD), and Fourier-transform infrared spectroscopy (FT-IR) were used to verify the forming of Cur-DPs. All the physical characterization information proved the formation of Cur-DPs, and the results demonstrated the superiority of the dripping pills in dissolution rates. The pharmacokinetic study of Cur-DPs was performed in rats compared to the pure curcumin suspension. The oral bioavailability of poorly water-soluble curcumin was successfully improved by CUR-DPs. And the stability of prepared Cur-DP was also in a good state in 3 months. These results identified the Cur-DPs was an effective new approach for pharmaceutical application.
KEY WORDS: curcumin, dripping pills, oral bioavailability, physicochemical properties, stability
INTRODUCTION
Curcumin (1, 7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione, Fig. 1), a hydrophobic polyphenol derived from the rhizome of turmeric (Curcuma longa) (1), is widely used as a natural yellow pigment in the food industries with its coloring, flavoring, and preservative utility (2). In recent years, curcumin has attracted widespread attention because of its good biological activities and pharmacological actions, such as anti-inflammatory, anti-angiogenic, anti-tumor, anti-oxidation, especially for its anti-carcinogenic potential (3,4). What’s more, curcumin shows no significant toxicity in human at several grams orally daily for months (5).
Fig. 1.
The chemical structure of curcumin
However, according to the Biopharmaceutics Classification System (BCS), curcumin belongs to a class II drug that is poorly water-soluble but highly permeable (6,7). It was reported that the solubility of curcumin in aqueous solution (pH 5.0) was only 11 ng/ml (8) corresponding to the low oral bioavailability (only 1% in rats) (9). So it is very difficult for curcumin to be applied in clinical treatment, and the promising therapeutic potential of curcumin could often be limited by its poor solubility and low bioavailability. Therefore, it is urgent to develop a suitable formulation to increase its solubility and systemic bioavailability. To our knowledge, a number of methods have been developed to improve the oral absorption of curcumin such as microemulsions (10), liposomes (11), nanoparticles (12), and so on.
Among all the solubilization methods, dripping pills technology is a promising method to enhance the dissolution rates and improve the oral bioavailability of a drug. The process of dripping pill formation is similar to that of preparing a solid dispersion by melting. Dripping pills are prepared by blending herbal extracts and the matrix under thermal conditions and dripping the mixture into a cooling liquid in which the droplets are insoluble. The process of pill formation prepared solid dispersion in a pill shape, the drug can existed in the state of separate molecules, amorphous particles, and then solubility can be improved to the great extent (13). Dripping pills have already been introduced to Chinese medicine to enhance their pharmaceutical effects by increasing the dissolution and subsequent bioavailability for a long time. For example, Compound Danshen dripping pills (14,15) and Qishenyiqi dripping pills (16) are available on the market. Herbal extracts and matrix were melted together to make a solution of dripping pills, the herbal extracts are distributed in molecular form or turn into very fine, tiny crystals within the matrix, which are easily absorbed with a higher bioavailability as well as rapid biological activity. What’s more, the detail characteristics of dripping pills are as follows: (1) the device used for producing dripping pills are simple and easy to operate; (2) the dripping pills are all with accurate dose, stable quality, and high bioavailability; (3) the dripping pills prepared by solid dispersion technique can be absorbed quickly.
The objective of our study was to design, prepare, and characterize a curcumin dripping pills (Cur-DPs) using Cremophor RH40, Poloxamer 188, and Polyethylene glycol 4000 as carriers. The physicochemical properties of dripping pills were characterized by differential scanning calorimetry (DSC), powder X-ray diffraction (PXRD), and Fourier-transform infrared spectroscopy (FT-IR), aiming to confirm the existing state of the drug in dripping pills. Finally, the dissolution behavior and pharmacokinetic study in rats of curcumin dripping pills were compared with pure drug to prove the superiority of curcumin dripping pills. After the experiment, the stability of prepared Cur-DPs was also determined.
METHODS AND MATERIALS
Materials and Instrumentation
Curcumin (Analytical reagent, >98%) was obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China); Cremophor RH40, Poloxamer 188, and Polyethylene glycol 4000 were purchased from BASF Co., Ltd. (Germany) and Tianjin Guangfu Chemical Research Institute (Tianjin, China). All other chemicals were of analytical grade, and used without any further purification. Distilled water was used throughout the study. The ZRS-8G dissolution tester was obtained from Tianjin Haiyida Technology Co., Ltd. (Tianjin, China).
Preparation
The solubility of curcumin in Cremophor RH40 was above 90 mg/mL (17), and Poloxamer 188 and Polyethylene glycol 4000 were hydrosoluble and common excipients in the preparation of dripping pills. Above all, these three excipients were with low melting points and made it convenient and energy-saving for the whole procedure of preparing dripping pills. So Cremophor RH40, Poloxamer 188, and Polyethylene glycol 4000 were considered as carriers in the formulation. Curcumin dripping pills were prepared using melt mixing methods. Briefly, the mixture of Cremophor RH40, Poloxamer 188, and Polyethylene glycol 4000 (the carriers, 3:3:4, g/g) were heated in a water bath at 65°C to achieve complete melting initially. Curcumin (the ratios of curcumin to carriers were 1:3, 1:5 and 1:7, g/g) was then added to the melt and stirred for 0.5 h to obtain a clear homogeneous solution. The resulting homogeneous liquid was dripped to the cold liquid paraffin to form the Cur-DPs, and then the Cur-DPs were collected. In order to promote the dissolution of dripping pills, 8% (g/g) of sodium carboxymethyl starch was added to the formulation of dripping pills.
The physical mixture (PM) was obtained by mixing the same amount of curcumin and carriers in a mortar for 10 min. The samples were stored at 25°C before use.
Chromatographic Conditions
A Shimadzu HPLC system was used to determine the concentration of curcumin. The HPLC system consisted of two LC-20A pumps and a SPD-20A UV/VIS detector (Shimadzu, Kyoto, Japan) with a C18 analytical column (5 μm, 250 × 4.6 mm, Agela Technologies Inc, USA) at room temperature. The mobile phase was a mixture of acetonitrile:H2O (containing 5.0% glacial acetic acid) (60:40, v/v). The detected wavelength of curcumin was 428 nm. And the mobile phase was at a flow rate of 1.0 mL/min. The injected volume was 20 μL.
Dissolution Experiments
The dissolution Apparatus II (stirring paddle method) was used in the dissolution study with a ZRS-8G dissolution tester at a rotation speed of 100 rpm in 900 mL 0.2% (w/v) sodium dodecyl sulfate aqueous solution medium (SDS), adjusted pH to 2.0 (with glacial acetic acid) and 6.8 (with phosphate-buffered saline) respectively. The temperature was 37 ± 0.5°C. Samples of curcumin, physical mixture, Cur-DPs formulations corresponding to 9 mg of curcumin were placed in each vessel. At specific time intervals (that was 0, 5, 10, 15, 20, 30, 45, 60, and 120 min), 5 mL of aqueous solution was withdrawn from the release medium, filtered through a 0.45 μm syringe filter, the primary filtrate was rejected, then the subsequent filtrate was analyzed for curcumin content with the Shimadzu HPLC system. And the equal volume of fresh medium was added into dissolution vessel.
Physical Properties of Cur-DPs
Hardness
Ten dripping pills were used for the determination of hardness. First, the diameter of each dripping pill was measured and recorded. Then, all dripping pills were put on a flat surface and a glass slide was put above the dripping pills. Weights with appropriate weight were put on the glass slide. After 10 min, the diameter of each dripping pill was measured again.
Friability
Take a sample of Cur-DPs corresponding to 6.5 g. The dripping pills should be carefully dedusted prior to testing. Then, dripping pills were accurately weighed, and placed in the drum of friability tester. The drum was rotated 100 times, and the dripping pills were removed. Any loose dust from the dripping pills were removed as before. Dripping pills were accurately weighed again and the weight loss was calculated.
Weight Variation
Twenty Cur-DPs were weighed precisely and the average weight was calculated. Then, 20 intact dripping pills were weighed individually. The weight of each dripping pill and the average weight were compared.
Physical Characterization
Differential Scanning Calorimetry
The thermal behavior of curcumin, PM, and Cur-DPs were conducted on a Shimadzu DSC-60 thermal analyzer (Shimadzu, Japan). Samples about 6 mg were packed in an indium-sealed pan which was used to calibrate for the temperature scale and energy. Samples were heated over a temperature gradient (from 40 to 200°C at a heating rate of 10°C/min) under the nitrogen purge gas flow rate of 25 mL/min.
Powder X-Ray Diffraction
X-ray powder diffraction pattern of pure curcumin, PM, and Cur-DPs were performed at room temperature with a Y-2000 Automated X-ray diffractometer system (Drigc, China). Monochromatic Cu Kα-radiation (λ = 1.5406 Å) was obtained with a nickel-filtration, and a system of diverging, receiving, and scattering slides were 1° and 0.2 mm. The patterns were recorded on a quartz plate at a tube voltage of 30 kV and a current of 20 mA. The data were collected in step scan mode over a 2θ range of 5–45° using a step size of 0.06° at a scan speed of 1 s/step. The peak intensities and 2θ values of Cur-DPs patterns were compared to those of the curcumin and PM in order to evaluate the forming of Cur-DPs in the samples.
Fourier-Transform Infrared Spectroscopy
FT-IR spectra for samples were obtained using a FT-IR spectrometer-8400S (Shimadzu, Japan). The FT-IR spectra were obtained in the region of 400–4000 cm−1 at a resolution of 4.0 cm−1 and 20 co-added scans. The samples were prepared in KBr disks by means of mixing samples and KBr thoroughly in a ratio of 1:100.
Pharmacokinetic Study
All the following animal experiments met the requirements of the National Act of the People’s Republic of China for the use of experimental animals. One week was used for rats to adapt to the experiment. Ten healthy male Sprague-Dawley rats (250 ± 15 g) were used for the study. They were randomized to be separated into two groups with free access to food and water. All the rats were fasted for 12 h prior to drug administration and supplied water only. For the control group, pure curcumin was suspended in aqueous sodium carboxymethyl cellulose solution (1%, w/v) and given orally (1000 mg/kg). For the experiment group, Cur-DPs formulation was administered orally (400 mg/kg). Blood samples were taken from the rats’ eye socket by collecting blood at pre-determined interval (0, 15, 30, 60, 120, 180, 240, 300, and 360 min) after administration. The blood samples were collected in tubes containing heparin to prevent blood clotting. Then, the blood samples were quickly centrifuged at 10,000 rpm to prepare plasma samples. The plasma samples were kept frozen at −20°C until analyzed.
Extraction method: The plasma was thawed before analyzing. A 0.6-mL volume of ethanol was added to 0.2 mL plasma and vortexed for 5 min. Then, the mixture was centrifuged at 10000 rpm for 10 min. The concentration of curcumin in the supernatant was determined by HPLC method reported above.
Stability Studies
After all the above experiments, the Cur-DPs were stored, in a stability tester, from light at 25°C and with 75% of relative humidity. The stability studies of Cur-DPs were determined after 3 months.
RESULTS AND DISCUSSION
Preparation of Dripping Pills
The forming of dripping pills could be affected by numerous factors: the temperature of melting liquid, the condensate temperature, the dripping distance, the dripping speed. The roundness (the ratio of the longest diameter and the shortest diameter) was set as an index to judge the dripping pill.
The Temperature of Melting Liquid
The influences of melting liquid temperature on forming the dripping pills were shown in Table I. Under the experimental conditions, the higher the temperature was, the closer the roundness was to 1. When the temperature was too low, the melting liquid may adhere to the dripping pill device, causing a waste of drug. Also, considering the stability of the formulation, 70°C was chosen as the best temperature.
Table I.
The Influences of Melting Liquid Temperature on Forming the Dripping Pills
| Melting liquid temperature (°C) | The roundness (r) | RSD (%) |
|---|---|---|
| 60°C | 1.09 | 5.8 |
| 70°C | 1.06 | 2.5 |
| 80°C | 1.07 | 3.8 |
The Condensate Temperature
The liquid paraffin was lyophilized before the experiment. And during the whole procedure, the liquid paraffin was maintained at about 5–10°C. A too-high temperature may cause the trailing phenomenon (the shape of the prepared dripping pill was not completely spherical but was like a water droplet with a pointed projection in the surface) to form a spindle ball of dripping pills.
The Dripping Distance
On one hand, when dripping distance was too short, the dripping droplets were easy to float on the surface of the condensate due to the surface tension, sinking slowly to form a spindle ball, which affects the roundness of the dripping pills; on the other hand, when the dripping distance was too long, part of the droplets in the system may be separated into small droplets because of gravity, thus affecting the weight variation of dripping pills. So 7 cm of the dripping distance was chosen in the study.
The Dripping Speed
The influences of dripping speed on forming the dripping pills were shown in Table II. As it is shown, when the dripping speed was too fast, the drug adhered in the cooling column to a certain extent, thus affecting the roundness of the dropping pill and weight variation; when the dripping speed was too slow, the dripping pills may join together; 20 ± 3 drips/min was chosen as the best dripping speed.
Table II.
The Influences of Dripping Speed on Forming the Dripping Pills
| The dripping speed (drips/min) | The roundness (r) | RSD (%) |
|---|---|---|
| 30 ± 3 | 1.15 | 6.4 |
| 20 ± 3 | 1.06 | 4.0 |
| 10 ± 3 | 1.12 | 7.0 |
In summary, the best condition for preparing dripping pills were as follows: melting liquid temperature, 70°C; the condensate temperature, 10°C; dripping distance, 7 cm; dripping speed, 20 ± 3 drips/min.
Dissolution Experiments
The dissolution profiles of Cur-DPs with different ratios of drug to carriers were shown in Fig. 2. It is clear that when the ratio of curcumin and carriers was 1:3, the dissolution rate of curcumin was not reach 70%, greatly below the dissolution rate of other ratios. What’s more, when the ratio turns to be 1:5 or 1:7, the dissolution behaviors were in similar tendency. So the ratio of 1:5 was chosen as the optimal ratio. Therefore, the dripping pill possessed an average weight of 27 mg, and the content of curcumin was maintained at 4.5 mg per pill (Fig. 3).
Fig. 2.
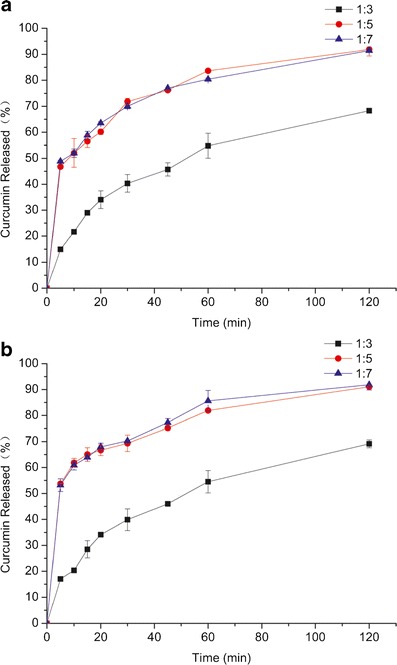
The dissolution profiles of Cur-DPs with different ratios in different medium (a pH 2.0, b pH 6.8)
Fig. 3.

The appearance of Cur-DPs
Dissolution profiles of curcumin from all curcumin formulations were shown in Fig. 4. The dissolution profiles of curcumin in medium with pH 2.0 were similar to the medium with pH 6.8. Only about 10% of curcumin was dissolved in the pure curcumin. The dissolution rate of physical mixture was slightly higher than that of pure curcumin. Compared to physical mixture, Cur-DPs showed a significantly higher dissolution rate. The dissolution rate of Cur-DPs without Cremophor RH40 or Poloxamer 188 was still much slower than Cur-DPs, which were to say, the dissolution of Cur-DPs was the highest of all formulations. The dissolution may reach about 80% at 60 min. Meanwhile, the great majority of curcumin from pure curcumin and physical mixture was floating in the dissolution medium, showed delayed dissolution behaviors. In Cur-DPs, curcumin was uniformly dispersed in the carriers, and the wettability and solubility of curcumin were improved by combination Poloxamer 188 with Cremophor RH40. What’s more, the poorly soluble particles of curcumin were surrounded tightly by the water-soluble carriers. The carriers were dissolved quickly in dissolution medium, then curcumin will form a homogeneous suspension of micro-particles when agitated slightly and come in contact with the dissolution medium closely, as s result, the dissolution process was increased.
Fig. 4.
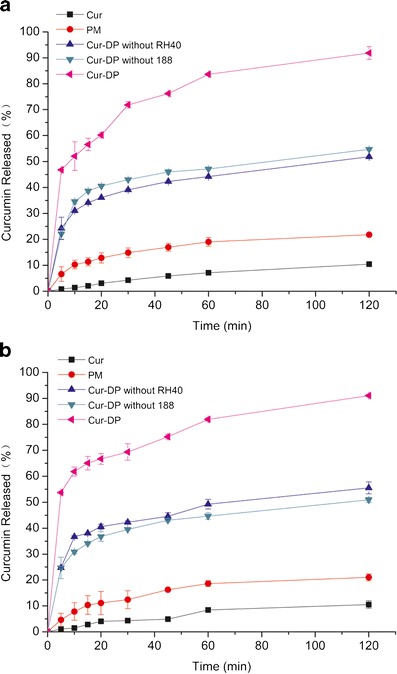
The dissolution profile of curcumin in different medium (a pH 2.0, b pH 6.8) from curcumin, physical mixture (PM), Cur-DPs without RH40, Cur-DPs without 188, and Cur-DPs
In summary, the dissolution rate of the drug was significantly increased by the formulation of Cur-DPs. All of the mentioned characteristics promoted the dissolution of curcumin.
Physical Properties of Cur-DPs
The prepared Cur-DPs exhibited good roundness and hardness, the weight loss was less than 1% in the experiment of friability, none were cracked, cleaved, or broken. And each dripping pill weight was within the limits of weight variation, nothing was beyond the limit.
Characterization of Cur-DPs
Differential Scanning Calorimetry
The DSC thermograms of pure curcumin, carriers, physical mixture, and Cur-DPs were shown in Fig. 5. The obvious endothermic peak of curcumin emerged around 183°C is also the melting point of curcumin. This was corresponding to the result of the previous study (18). The melting peak of carriers was in the region of 50–60°C. For the physical mixture curve, the drug endothermic peak could also be tracked around 180°C with less intensity, and the carriers’ peaks were also presented. All the peaks in the physical mixture corresponded to their intrinsic melting points. The physical mixture of curcumin and carriers revealed some similarities with the crystals of the free curcumin and carriers indicating that the physical mixture system was simply mixed and both of the components exist in the form of crystalline. Therefore, the existence of the curcumin melting peak in the physical mixture could also suggest that no amorphous state was formed in this system. In the thermograms of Cur-DPs, the complete disappearance of curcumin was detected which suggested that the drug existed in the form of amorphous and dispersed in the carriers uniformly. The DSC results suggested that curcumin was successfully dispersed into the carriers and existed in the amorphous state.
Fig. 5.
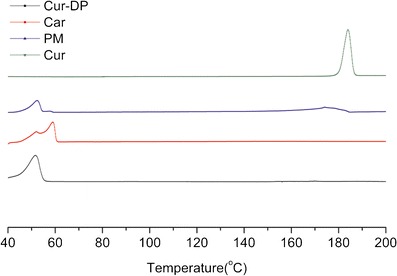
DSC thermograms of curcumin (Cur), carriers (Car), curcumin/carriers physical mixtures (PM) and Cur-DPs
Powder X-Ray Diffraction
The PXRD patterns of pure curcumin, carriers, physical mixture, and Cur-DPs were shown in Fig. 6. The PXRD spectrogram of curcumin exhibited several intensely sharp peaks, which could be attributed to the high crystallinity of curcumin. The characteristic peaks of curcumin were also observed in the diffraction pattern of physical mixture with lower intensity. Due to the high concentration of carriers compared to curcumin, the characteristic peaks of curcumin were partially, but not all present in the physical mixture. These results indicated that the curcumin was still partly in the crystalline form in the physical mixture. Compared to the curves of pure curcumin and the physical mixture, there was an important difference in Cur-DPs, those peaks of curcumin completely disappeared, which suggested an amorphous state of curcumin existed in the Cur-DPs. The result of the PXRD was inconsistent with that of DSC.
Fig. 6.
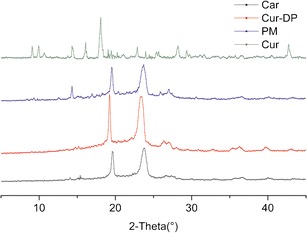
PXRD thermograms of curcumin (Cur), carriers (Car), curcumin/carriers physical mixtures (PM) and Cur-DPs
Fourier-Transform Infrared Spectroscopy
The FT-IR spectra of curcumin, carriers, physical mixture, and Cur-DPs were shown in Fig. 7. In FT-IR technology, the atom forming chemical bonds or functional groups were in the state of vibration, the infrared spectrum of different chemical bonds or functional groups were located in a different wavenumber. So the presence of a peak at a specific wavenumber can directly reflect the presence of a specific chemical bond (7). In contrast, if specific interactions took place between curcumin and carriers, new peaks or a shift of existing peaks would appear. The sharp absorption band at 3510 cm−1 in the crystalline curcumin spectrum could be attributed to phenolic OH stretching. The other wavenumber of obvious peaks in the curve of curcumin were 1507 cm−1 and 1278 cm−1. What’s more, there is a prominent stretching band at 1627 cm−1 owing to the carbonyl in curcumin consistent with the formation of a keto-enol tautomer stabilized by a strong intramolecular hydrogen bond. The spectra of physical mixture were the combination of curcumin and the carriers. From this result, it was shown that the curcumin and the carriers were mixed physically, and no interaction occurred. However, at the spectrum of Cur-DPs, the peak at wavenumber 1627 cm−1 disappeared; it might be anticipated that the carbonyl of curcumin formed a strong hydrogen bond with hydroxyl in the carriers. Curcumin was dispersed into the carriers and formed Cur-DPs.
Fig. 7.
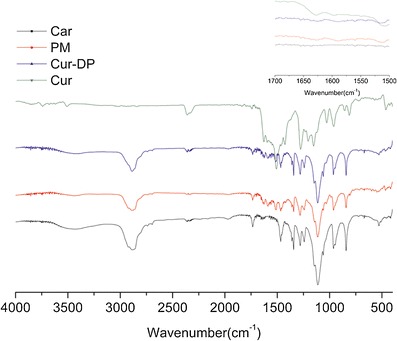
FT-IR thermograms of curcumin (Cur), carriers (Car), curcumin/carriers physical mixtures (PM) and Cur-DPs
Pharmacokinetic Study
The calibration curve of curcumin in rat plasma was linear in the range 0.10–4.00 μg/ml plasma. The calibration line of curcumin was y = 45, 627x + 111.04 with r2 = 0.9994. The mean plasma concentration-time curves of curcumin in rats after oral administration of curcumin suspension and the Cur-DPs were shown in Fig. 8. The pharmacokinetic parameters were shown in Table III. The plasma concentration of the Cur-DPs at each time point was compared with that of curcumin. These results showed that the peak plasma concentration (Cmax) values of curcumin for Cur-DPs was 3.62 ± 0.74 μg/mL; about 4.0 times higher compared to 0.95 ± 0.12 μg/mL for curcumin suspension. The area under the plasma concentration-time curve of Cur-DPs was 9.01 ± 1.52 μg h/mL also significantly greater than 3.48 ± 0.59 μg h/mL of curcumin suspension (p < 0.05). The result indicated that the bioavailability of curcumin in Cur-DPs was improved significantly after its oral administration in rats.
Fig. 8.
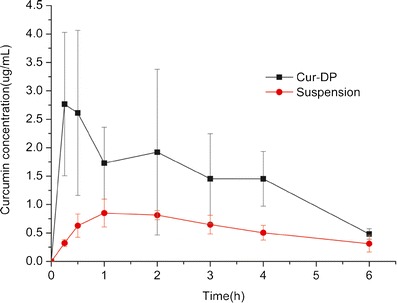
Plasma concentration-time profile of curcumin in rats after oral administration of curcumin suspension and Cur-DPs (mean ± SD, n = 5)
Table III.
Comparison of Pharmacokinetic Parameters Between Cur-DPs (400 mg/kg of Body Weight) and Curcumin Suspension (1000 mg/kg of Body Weight) (Mean ± SD; n = 5)
| Group | Cur-DPs | Suspension |
|---|---|---|
| T 1/2 (h) | 3.36 ± 2.14 | 3.08 ± 1.26 |
| C max (μg/mL) | 2.62 ± 0.74 | 0.95 ± 0.12 |
| AUC (μg h/mL) | 9.01 ± 1.52 | 3.48 ± 0.59 |
| T max (h) | 0.75 ± 0.71 | 1.40 ± 0.55 |
| Mean retention time (MRT) (h) | 4.44 ± 1.67 | 5.00 ± 1.92 |
The reasons for the improvement of the bioavailability of curcumin and the multiple peaks may be as follows: (1) Due to the lower capacity for the hydrogen-bonding and dipole interactions, curcumin has low solubility. Cremophor RH40 is a non-ionic solubilizing enhancer; with the addition of Cremophor RH40, the solute-solvent interactions can be qualitatively as well as quantitatively changed to improve the curcumin solubility (19). As a permeation enhancer, Cremophor RH40 can also improve the absorption of drugs by increasing its paracellular and transcellular transports via the modification of the cell membrane. This surfactant can be used to increase bioavailability of drugs by solubilizing of poorly soluble compounds and by increasing of cell membrane fluidity (20). What’s more, Cremophor RH40 has been practically used for the enhancement of hydrophobic drugs solubility and their micelle formation in the industrial field (20); (2) Since Poloxamer 188 was an absorption enhancer, the gastrointestinal tract peristalsis was made slow by it, then the retention time of curcumin in gastrointestinal tract was prolonged, the absorption and bioavailability of curcumin was increased; hence, the carriers used in our Cur-DPs formulation enhance the solubility and permeability for curcumin to reach the systemic circulation. (3) Due to rapid cooling procedure of dripping pills, the viscosity of the solution was increased and solidified. The drug molecules which dispersed in the solution cannot conglomerate into large crystals. As a result, drug micro-particles were formed. These micro-particles have a rapidly dissolvable property within body fluids and a better bioavailability. (4) The multiple peaks of absorption may be caused by enterohepatic circulation of curcumin, that is, under the role of intestinal bacterial enzyme, the metabolites were hydrolyzed into prototype drugs, and then were again absorbed into the liver (10).
Stability Studies
The stability studies of Cur-DPs were determined after 3 months. Stability studies on the physical characteristics of the Cur-DPs were performed through DSC, PXRD, and FT-IR spectroscopy. Dissolution experiments were also carried out. All the profiles were shown in Fig. 9. As we see from the data, the curcumin from the Cur-DPs formulations still remained in amorphous state. It is also clear that the dissolution properties are still much alike with those of newly prepared dripping pills. These profiles suggested that the formulations remained stable under these conditions.
Fig. 9.
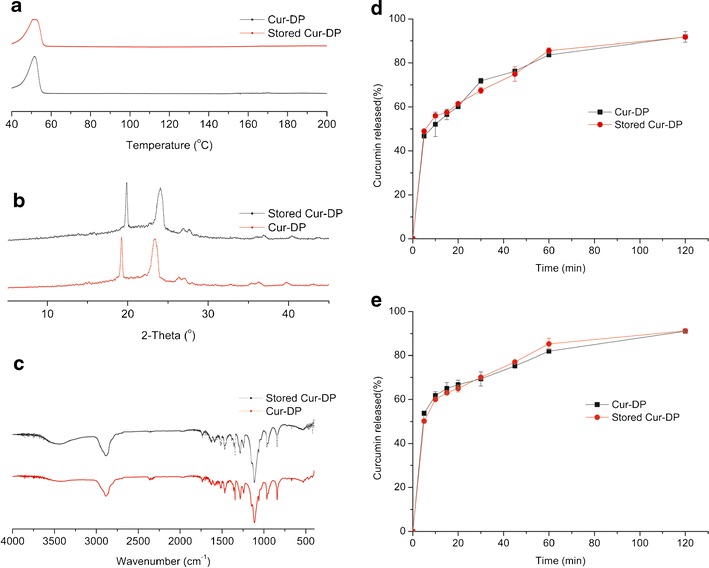
Stability studies a DSC thermograms of Cur-DPs and the stored Cur-DPs; b PXRD thermograms of Cur-DPs and the stored Cur-DPs; c FT-IR thermograms of Cur-DPs and the stored Cur-DPs; d The dissolution profiles of Cur-DPs and the stored Cur-DPs (pH 2.0); e The dissolution profiles of Cur-DPs and the stored Cur-DPs (pH 6.8)
CONCLUSIONS
The present study confirmed that the blends of Cremophor RH40, Poloxamer 188, and Polyethylene glycol 4000 are appropriate carriers for the preparation of curcumin dripping pills by melt mixing. DSC, PXRD, and FT-IR were used to confirm the transformation of curcumin from the crystalline to the amorphous state. The proposed Cur-DPs formulation successfully improved the absorption of curcumin compared with that of the pure curcumin. Our studies proved that the developed Cur-DPs is a promising and convenient method for improving oral bioavailability of drugs with poor aqueous solubility and the technique of preparing dripping pills is very suitable for industrial production.
ACKNOWLEDGMENTS
This work was supported by the Medical and Engineering Science Research Center of Hebei University (No. BM201109), Hebei Provincial Natural Science Foundation of China—Shijiazhuang Pharmaceutical Group (CSPC) Foundation (No. H2013201274) and the Top Young Talents Program of Hebei Province.
REFERENCES
- 1.López-Lázaro M. Anticancer and carcinogenic properties of curcumin: considerations for its clinical development as a cancer chemopreventive and chemotherapeutic agent. Mol Nutr Food Res. 2008;52(suppl 1):S103–27. doi: 10.1002/mnfr.200700238. [DOI] [PubMed] [Google Scholar]
- 2.Hu L, Jia Y, Niu F, Jia Z, Yang X, Jiao K. Preparation and enhancement of oral bioavailability of curcumin using microemulsions vehicle. J Agric Food Chem. 2012;60(29):7137–41. doi: 10.1021/jf204078t. [DOI] [PubMed] [Google Scholar]
- 3.John MK, Xie H, Bell EC, Liang D. Development and pharmacokinetic evaluation of a curcumin co-solvent formulation. Anticancer Res. 2013;33(10):4285–91. [PubMed] [Google Scholar]
- 4.Yoysungnoen P, Wirachwong P, Changtam C, Suksamrarn A, Patumraj S. Anti-cancer and anti-angiogenic effects of curcumin and tetrahydrocurcumin on implanted hepatocellular carcinoma in nude mice. World J Gastroenterol. 2008;14(13):2003–9. doi: 10.3748/wjg.14.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu H, Huang Q. Improving the oral bioavailability of curcumin using novel organogel-based nanoemulsions. J Agric Food Chem. 2012;60(21):5373–9. doi: 10.1021/jf300609p. [DOI] [PubMed] [Google Scholar]
- 6.Visser MR, Baert L, Klooster G, Schueller L, Geldof M, Vanwelkenhuysen I, et al. Inulin solid dispersion technology to improve the absorption of the BCS Class IV drug TMC240. Eur J Pharm Biopharm. 2010;74(2):233–8. doi: 10.1016/j.ejpb.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Wan S, Sun Y, Qi X, Tan F. Improved bioavailability of poorly water-soluble drug curcumin in cellulose acetate solid dispersion. AAPS PharmSciTech. 2012;13(1):159–66. doi: 10.1208/s12249-011-9732-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tønnesen HH, Másson M, Loftsson T. Studies of curcumin and curcuminoids. XXVII. Cyclodextrin complexation: solubility, chemical and photochemical stability. Int J Pharm. 2002;244(1–2):127–35. doi: 10.1016/S0378-5173(02)00323-X. [DOI] [PubMed] [Google Scholar]
- 9.Yang KY, Lin LC, Tseng TY, Wang SC, Tsai TH. Oral bioavailability of curcumin in rat and the herbal analysis from Curcumalonga by LC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;853(1–2):183–9. doi: 10.1016/j.jchromb.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 10.Xiao Y, Chen X, Yang L, Zhu X, Zou L, Meng F, et al. Preparation and oral bioavailability study of curcuminoid-loaded microemulsion. J Agric Food Chem. 2013;61(15):3654–60. doi: 10.1021/jf400002x. [DOI] [PubMed] [Google Scholar]
- 11.Saengkrit N, Saesoo S, Srinuanchai W, Phunpee S, Ruktanonchai UR. Influence of curcumin-loaded cationic liposome on anticancer activity for cervical cancer therapy. Colloids Surf B: Biointerfaces. 2014;114:349–356. doi: 10.1016/j.colsurfb.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Francis AP, Murthy PB, Devas T. Bis-demethoxy curcumin analog nanoparticles: synthesis, characterization, and anticancer activity in vitro. J Nanosci Nanotechnol. 2014;14(7):4865–73. doi: 10.1166/jnn.2014.9219. [DOI] [PubMed] [Google Scholar]
- 13.Vo CL, Park C, Lee BJ. Current trends and future perspectives of solid dispersions containing poorly water-soluble drugs. Eur J Pharm Biopharm. 2013;85(3 Pt B):799–813. doi: 10.1016/j.ejpb.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 14.Chu Y, Zhang L, Wang XY, Guo JH, Guo ZX, Ma XH. The effect of compound danshen dripping pills, a Chinese herb medicine, on the pharmacokinetics and pharmacodynamics of warfarin in rats. J Ethnopharmacol. 2011;137(3):1457–61. doi: 10.1016/j.jep.2011.08.035. [DOI] [PubMed] [Google Scholar]
- 15.Hatfield MJ, Tsurkan LG, Hyatt JL, Edwards CC, Lemoff A, Jeffries C, et al. Modulation of esterified drug metabolism by tanshinones from Salvia miltiorrhiza (“Danshen”) J Nat Prod. 2013;76(1):36–44. doi: 10.1021/np300628a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cao H, Zhai J, Mu W, Lei X, Cao H, Liu C, et al. Use of comparative effectiveness research for similar Chinese patent medicine for angina pectoris of coronary heart disease: a new approach based on patient-important outcomes. Trials. 2014;15:84. doi: 10.1186/1745-6215-15-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jang DJ, Kim ST, Lee K, Oh E. Improved bioavailability and antiasthmatic efficacy of poorly soluble curcumin-solid dispersion granules obtained using fluid bed granulation. Biomed Mater Eng. 2014;24(1):413–29. doi: 10.3233/BME-130826. [DOI] [PubMed] [Google Scholar]
- 18.Arya P, Pathak K. Assessing the viability of microsponges as gastroretentive drug delivery system of curcumin: optimization and pharmacokinetics. Int J Pharm. 2014;460(1–2):1–12. doi: 10.1016/j.ijpharm.2013.10.045. [DOI] [PubMed] [Google Scholar]
- 19.Narang AS, Delmarre D, Gao D. Stabledrugencapsulation in micelles and microemulsions. Int J Pharm. 2007;345(1–2):9–25. doi: 10.1016/j.ijpharm.2007.08.057. [DOI] [PubMed] [Google Scholar]
- 20.Horvát S, Fehér A, Wolburg H, Sipos P, Veszelka S, Tóth A, et al. Sodium hyaluronate as a mucoadhesive component in nasal formulation enhances delivery of molecules to brain tissue. Eur J Pharm Biopharm. 2009;72(1):252–9. doi: 10.1016/j.ejpb.2008.10.009. [DOI] [PubMed] [Google Scholar]


