Abstract
Regulatory Tcells (Tregs) limit contact between Dendritic cells (DCs) and conventional T cells (Tcons), decreasing the formation of aggregates as well as down-modulating the expression of co-stimulatory molecules by DCs, thus decreasing DC immunogenicity and abrogating T-cell activation. Despite the importance of this Treg-cell function, the capacity of Tregs from term and preterm neonates to suppress DCs, and the suppressive mechanisms they use, are still undefined. We found that, relative to adult Tregs, activated Tregs from human neonates expressed lower FOXP3 and CTLA-4, but contained higher levels of cAMP. We developed an in vitro model in which Treg function was measured at a physiological ratio of 1 Treg for 10 Tcon and 1 monocyte-derived DC, as Treg target. Term and preterm Tregs failed to suppress the formation of DC-Tcon aggregates, in contrast to naïve and memory Tregs from adults. However, neonatal Tregs diminished DC and Tcon activation as well as actin polymerization at the immunological synapses. In addition, CTLA-4 and cAMP were the main suppressive molecules used by neonatal Treg. Altogether, both preterm and term neonatal Tregs appear less functional than adult Tregs, and this defect is consistent with the general impairment of CD4 cell function in newborns.
Introduction
From early development, the fetal immune system learns to tolerate self-antigens as well as maternal antigens that are transferred across the placenta. Regulatory T cells (Tregs) are one of the critical mediators involved in this process [1, 2]. Tregs mediate their suppressive action by acting directly on antigen-presenting cells, such as dendritic cells (DC). Treg preferentially localize to DC aggregates to prevent T-cell activation both in vivo and in vitro [3, 4]. The formation of Treg-DC conjugates also suggests that DCs are the primary targets of Treg suppression [5-7]. Cytotoxic T-lymphocyte antigen-4 (CTLA-4), cyclic adenosine monophosphate (cAMP) and membrane-bound transforming growth factor-β (TGF-β) are the major contact-dependent mediators for suppression of conventional T-cell (Tcon) activation and DC maturation [8-10]. CTLA-4 and cAMP down-modulate the expression of the co-stimulatory molecules CD80 and CD86 by DC, thus decreasing DC immunogenicity and abrogating their activation of T cells [3, 10-12]. Likewise, TGF-β decreases the differentiation of DCs and their capacity to secrete the Th1-polarizing cytokine IL-12 [13].
Although Tregs from full term and preterm neonates control activation of Tcon [14-19], the capacity of neonate Treg to suppress DCs, and the suppressive mechanisms they use, are still undefined. Furthermore, the effect of prematurity on this aspect of Treg function has not been determined. We are particularly interested in late preterm neonates, a group that has been poorly studied. These neonates (32-36 weeks of gestation) have higher morbidities particularly those involving inflammation as a major component such as respiratory distress. They also have higher rates of hospitalization than term babies [20]. In addition, late prematurity is associated with significant increases in persistent asthma [21, 22]. Therefore, we asked whether Tregs from term and late preterm neonates could suppress DC function.
Results
Demographic and clinical characteristics of the study subjects
Seventeen full term neonates (“term”) (average 39w of gestational age, GA) and 15 late preterm (“preterm”) neonates (35w of GA) were studied (Table 1); in addition, 15 healthy adults (>18 years) were included. As expected, term neonates had a larger mean birth weight (3,519g) than preterm neonates (2,714g; p=0.0001). Birth weight positively correlated with GA in all neonates (p=0.0001, r=0.7). There were no significant differences among the groups regarding gender, race or method of delivery.
Table 1.
| Term (n=17) | Preterm (n=15) | |
|---|---|---|
| Gestational age (Weeks ± SEM) | 39±0.8 | 35±1* |
| Birth Weight (grams ± SEM) | 3,519±483 | 2,714±478* |
| Sex (% Male) | 66 | 47 |
| Race (% White) | 69 | 60 |
| Delivery mode (% Vaginal) | 88 | 73 |
| Use of antenatal steroids (%) | 0 | 0 |
p=0.0001
Treg-cell frequency is decreased and expression of CD45RA is higher in neonates
The frequency of peripheral Treg (CD4+CD25+CD127Low/−) was significantly lower in term (median: 2.1%) and preterm neonates (2.8%) compared to adults (4.5%) (p<0.05; Supporting Information Fig. 1A-B). Proportion of FOXP3+ cells within the sorted populations was similar in adult and neonates (Supporting Information Fig. 1C). Likewise, Treg-cell frequencies were lower in term neonates compared to preterm neonates (unpaired t test; p=0.04). In all neonates combined, Treg percentages were inversely correlated with GA (p=0.04, r=−0.4). As expected, the frequency of naive Treg was increased in term and preterm Treg compared to adult Treg, but was similar in both neonate groups (Fig. 1A).
Figure 1. Activated Tregs from preterm and term neonates express lower FOXP3 and CTLA-4 than adult Tregs, but contain higher levels of cAMP.
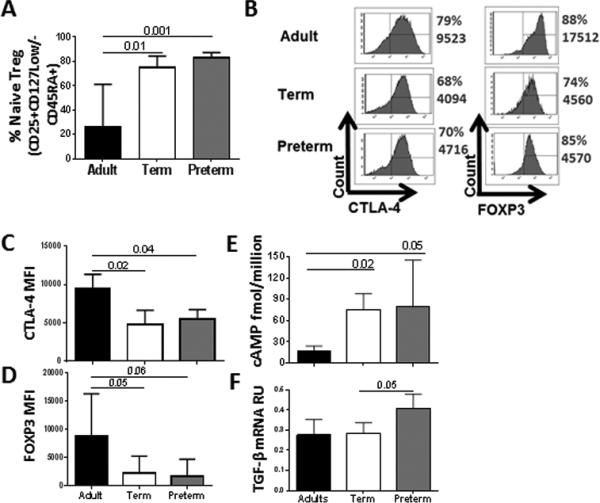
Percentage of resting Tregs expressing CD45RA+ within the Treg-cell population (CD4+CD25+CD127Low/) was determined by flow cytometry. (A) Percentage of naïve Treg cells in peripheral blood from adults (n=7) and cord blood from preterm (n=7) and term (n=11) neonates.
(B-F) Tregs from preterm (n=7) and term (n=6) neonates were isolated by cells sorting and activated with CD3/CD28 beads. Adults (n=6) were included as control group. (B) Intracellular CTLA-4 and FOXP3 expression in Treg was determined by flow cytometry. Histogram figures from one representative experiment of four independent experiments from each group of donor show the percentage and MFI of CTLA-4 and FOXP3 in Treg post-activation. (C) CTLA-4 and (B) FOXP3 expression was quantified by flow cytometry in four independent experiments. (E) cAMP concentration was determined by ELISA. Data from one experiment performed are shown. (F) TGF-β mRNA level was determined by qPCR. Values were normalized to ubiquitin-conjugating enzyme. Data from one experiment performed are shown. (A-F) Significant differences between groups were calculated using Kruskal-Wallis test. Bars graphs show the median and interquartile range.
Activated Treg from neonates express lower FOXP3 and CTLA-4 but contain higher levels of cAMP
Resting Tregs are poorly suppressive, however, they gain potent suppressive properties after activation in vitro [14, 18]. In particular, resting Tregs exhibit lower expression of suppressive molecules than Tregs activated in vitro [23, 24]. Likewise, peripheral blood Treg express low levels of inhibitory molecules, such as CTLA-4 or GITR are poorly suppressive compared to tissue Treg [24, 25]. We therefore pre-activated Tregs before performing functional assays. We first evaluated the purity of Treg (CD25+CD127−) after sorting by intracellular staining of FOXP3. Purity of the sorted populations was ≥ 90% (Supporting Information Fig. 1C). Then, we compared the phenotype of activated Tregs in the different groups of subjects. The percentages of Treg expressing CTLA-4 (76%) and FOXP3 (78%) were similar in all groups (Fig 1B). While the mean fluorescence intensity (MFI) for FOXP3 and CTLA-4 in gated Treg was low for both term and preterm Tregs compared to adult Tregs (Fig 1C-1D), the CTLA-4 and FOXP3 expression per cell were similar in term and preterm neonatal Tregs. As expected, CTLA-4 MFI positively correlated with FOXP3 MFI (p=0.01, r=0.5). The percentages of Treg expressing CD25 and Ki67 were not different in neonates and adults (data not shown). As shown in Fig. 1E, intracellular cAMP concentration was higher in term and preterm Tregs than in adult Tregs, but similar in Tregs from both neonate groups. TGF-β mRNA levels were not different among neonatal groups and adults; however, preterm Tregs expressed slightly higher levels of TGF-β mRNA than term Tregs (Fig. 1F).
Neonate Treg fail to suppress the formation of DC-Tcon aggregates
Murine Tregs inhibit stable contacts between DC and CD4+ T-cells, or compete with Tcon to form aggregates with DC [3]. We thus compared the capacity of Treg from adults and neonates to disrupt DC-Tcon aggregates. We used in all assays the same adult allogeneic DC as targets, which were cultured with Tcon autologous to Treg. Importantly, the ratio of DC:Tcon:Treg (1:10:1) mimics physiological conditions [26]. Tcons were labeled with CellTrace™ Violet and HLA-DR expression was used to identify DC. DC:Tcon (HLA-DR+ CellTrace™ Violet+) aggregates were identified using imaging flow cytometry (Fig. 2A). Initially, we characterized the formation of DC-Tcon aggregates in absence of Tregs and, as shown in Fig. 2B, the percentage of DC-Tcon aggregates was similar in the 3 groups of subjects (1.7-2.2% of the total events, p=0.4). In adult and term neonate samples, 60 to 66% of these DC-Tcon aggregates contained 1 Tcon per aggregate while the percentage of aggregates containing 2 and > 3 Tcons was ~27% and ~7% respectively (data not shown). In preterm neonates, the percentage of DC-Tcon aggregates containing 1 Tcon was significantly higher than in adults (81% vs. 63%, p=0.008), while the fraction of aggregates with 2 (17% vs. 30%, p=0.009) and ≥ 3 (2% vs. 7%, p=0.007) Tcons was diminished compared to adults.
Figure 2. Treg from term and preterm neonates fail to suppress formation of DC-Tcon aggregates.
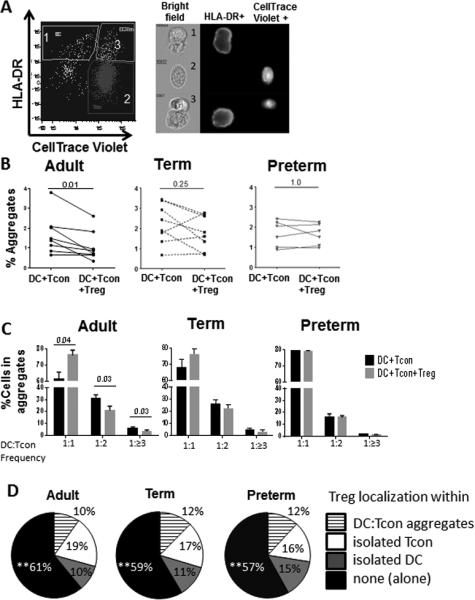
(A-D) Activated Tregs from peripheral blood from adults (n=8) and cord blood from preterm (n=6) and term (n=8) neonates were added or not to DC:Tcon cultures at a 1:10:1 (DC:Tcon:Treg) ratio and cultured for 24h. A) Gating strategy: Using imaging flow cytometry, 3 populations can be visualized (1) isolated DCs (HLA-DR+ cells), (2) isolated Tcons (CellTrace™-Violet+ cells) and (3) DC:Tcon aggregates (CellTraceTM-Violet+ HLA−DR+ population). B) Percentage of DC:Tcon aggregates in the total population was quantified by flow cytometry as shown in A in presence or absence of Tregs from adults, term and preterm babies. Each dot shows represent a single donor. C) The percentage of Tcons interacting with each DC in absence and presence of adult and neonate Tregs was quantified using the Spot count feature. Bars graphs show the median and interquartile range of DC:Tcon frequency from adult, term and preterm neonates cocultures. D) Pie charts show the percentage of Tregs interacting with DC:Tcon aggregates, isolated Tcons, or isolated DCs, or found alone. (B-D) Results are representative of five independent experiments performed. Treg suppression was calculated using Wilcoxon tests.
Adult Tregs significantly decreased the percentage of these DC-Tcon aggregates (see Supporting Information Fig. 2A for the gating strategy and Fig 2B left panel for data summary), in contrast to Tregs from term and preterm neonates (Fig 2B, middle and right panel). To evaluate whether the differences between adult and neonate Tregs were caused by the different percentage of naïve or memory Treg in these subsets, we purified adult naïve (CD45RA+) and memory (CD45RA−) Tregs and determined their functionality. Purity of naïve and memory Treg after sorting was ≥ 90% (Supporting Information Fig. 2B). As shown in Supporting Information Figure 3A and Figure 2B, both subsets of adult Tregs were able to decrease the formation of DC-Tcon aggregates, in contrast to neonatal Tregs.
In addition to decreasing the number of aggregates, adult Tregs significantly decreased the number of Tcon per aggregate, as the percentage of aggregates containing 2, 3 or more Tcons per DC diminished, while the percentage of aggregates containing only 1 Tcon per DC increased (Fig 2C, left panel). Again, Tregs from term and preterm neonates did not modify the number of Tcon per DC in the aggregates that formed (Fig 2C, middle and right panel). Using a fluorescent label (Calcein AM FITC) to track Tregs localization, we analyzed whether Tregs preferentially localized within the DC:Tcon aggregates or formed aggregates with DC alone. The majority of Treg were found alone (close to 60%), and the rest of the Treg were found either in DC:Tcon aggregates, or formed aggregates with DCs or Tcons (Fig. 2D). Importantly, similar repartition was found for adult, term and preterm Tregs.
Neonatal Treg suppress Tcon activation and DC maturation within the aggregates
Tregs suppress DCs function by down-regulating the expression of CD83, CD80, CD86 and CD40 on DC [2, 27]. Likewise, Tregs diminish Tcon proliferation and their expression of molecules associated with proliferation/ activation such as CD134 [27-29]. Although neonate Tregs did not impede the formation of DC-Tcon aggregates, we next investigated whether they might decrease DC and T cell activation in the aggregates. In pilot experiments, we found that Tregs consistently down-regulated DC expression of CD83 following LPS stimulation, whereas the effect of Treg on CD86 and CD40 expression was more variable (data not shown). We therefore used CD83 expression as our main read-out of DC activation.
In absence of Tregs, Tcons from adult, term and preterm neonates expressed similar levels of CD134 when cultured with DCs. As expected, almost all Tcons (adult, term and preterm neonates) present in the aggregates expressed CD134 (~85-90%) while Tcons that were not in contact with DCs did not (~3-6%), and this was true for adult and neonatal Tcons (% CD134 T-cells in aggregates vs. isolated; p= 0.01 for each group). Likewise, in absence of Tregs, CD83 expression by DCs was modestly, but significantly increased in aggregates with Tcons from adult, term and preterm samples (75-82%), compared to isolated DCs (55-62%) (%CD83 DC in aggregates vs. isolated; p= 0.03 for each group).
Next, we analyzed whether Treg suppressed CD134 and CD83 expression in DC:Tcon aggregates. Similar to adult Tregs, addition of Tregs from term and preterm neonates significantly decreased the percentage of CD134+ Tcons as well as the frequency of CD83+ DCs (see Supporting Information Fig. 4A and 4B for gating strategy and representative examples, and Fig. 3A-B for data summary). These results were similar when CD134 MFI and CD83 MFI were analyzed (data not shown). The amounts by which term and preterm Treg reduced CD134 and CD83 expression were similar. Tregs did not suppress expression of CD134 and CD83 in isolated Tcons and DCs respectively (data not shown). We also analyzed whether purified naïve and memory Tregs from adults were comparable to neonate Treg in terms of suppression of Tcon activation and DC maturation within the aggregates. As shown in Figure 3A and 3B and Supporting Information Figure 3B and 3C, similar suppression was seen in all Treg groups.
Figure 3. Tregs from term and preterm neonates suppress Tcon activation, DC maturation and actin polymerization in DC:Tcon aggregates.
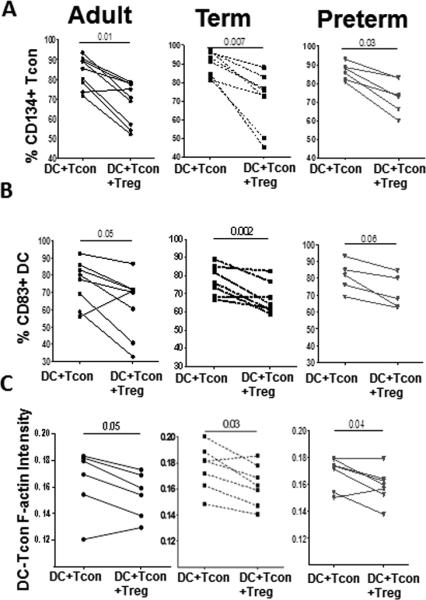
Activated Tregs from peripheral blood from adults (n=8) and cord blood from preterm (n=6) and term (n=8) neonates were added or not to DC:Tcon cultures at a 1:10:1 (DC:Tcon:Treg) ratio and cultured for 24h. (A) Percentages of CD134+ Tcon, (B) CD83+ DC in aggregates and C) actin polymerization at the IS in presence or absence of Treg were determined by imaging flow cytometry. (A-C) Results are representative of five independent experiments performed. Each dot shows represent a single donor. Treg suppression was analyzed using Wilcoxon tests.
Treg from term and preterm neonates decrease actin polymerization in Tcon:DC aggregates
We also investigated whether neonate Treg could inhibit the formation of an immunological synapse (IS) between Tcon and DC. We quantified polymerized actin at the IS after normalization on the total actin level of each aggregate, as depicted in Supporting Information Fig. 4C. Interestingly, Tregs from most of the adult, term and preterm neonates decreased actin polymerization (Fig 3C and Supporting Information Fig. 4D). In addition, naïve and memory adult Tregs reduced actin polymerization as much as term Tregs did ((Fig. 3C and Supporting Information Fig. 3D).
Treg from neonates use different mechanisms to suppress Tcon activation and DC maturation
Treg control DC interactions with Tcon or CD8+ T-cells through CTLA-4 or cAMP mechanisms [30, 31]. We therefore tested the involvement of these molecules, as well as that of TGF-β, in Treg suppressive activity by blocking individually or simultaneously these three effector molecules. To determine the role of cAMP in Treg suppression, we decreased their intracellular levels of cAMP using an adenylyl cyclase inhibitor (ddADA) before co-culturing them with DC and Tcon. Treatment of Tregs with ddADA significantly decreased Treg cAMP content to levels comparable to those in Tcon (Supporting Information Fig. 4A). Anti-CTLA-4 and anti-TGF-β blocking antibodies acted on Treg, as they did not alter Tcon and DC activation in absence of Tregs (Supporting Information Fig. 4B). Simultaneous blockade of CTLA-4, TGF-β and cAMP resulted in a substantial decrease of suppression by adult Tregs, inhibiting almost completely Treg suppression of the formation of DC-Tcon aggregates (Supporting Information Fig. 5A). These blockades also rescinded the effect Treg exerted on the composition of the aggregates (Supporting Information Fig. 5B). Individual blockades were not sufficient to significantly decrease adult Tregs suppression in these assays (data not shown).
We next studied the mechanisms used by neonate Treg to suppress actin polymerization, and the activation and maturation of Tcon and DC in the remaining aggregates. The combined blockade of CTLA-4, TGF-β and cAMP significantly inhibited Treg suppression for all read-outs (Fig. 4 panels A-C). This simultaneous blockade almost completely inhibited suppression of CD134 expression (~85-90%) by both groups of neonate Tregs (Fig. 4A). In contrast, Treg suppression of actin polymerization and CD83 expression were significantly but only partially inhibited by simultaneous blockade (~50-80%) (Fig. 4B and 4C), suggesting that other mechanisms of suppression are also used by neonate Treg to control DC activation. Term and preterm Tregs suppressive activity was similarly inhibited by this combined blockade.
Figure 4. Tregs mainly use cAMP and CTLA-4 to suppress interactions of DC:Tcon and activation of Tcons and DCs.
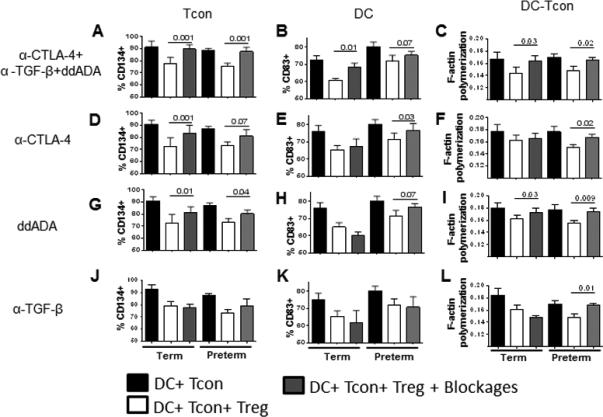
Activated Tregs from from preterm (n=5) and term (n=7) neonates were added or not to DC:Tcon cultures at a 1:10:1 (DC:Tcon:Treg) ratio and cultured for 24h. Cultures were incubated in presence or absence of blockades (anti-CTLA-4, anti-TGF-β and ddADA). Percentage of suppression of CD134+ Tcon (left panels), CD83+ DC (middle panels) and actin polymerization (right panels) was determined by imaging flow cytometry and calculated as percentage related to untreated Tregs. (A-C) shows the combined blockades. (D-L) show individual blockades. (A-L) Results are representative of five independent experiments performed. Bars graphs show the median and interquartile range from term and preterm cocultures. Treg suppression was analyzed using Wilcoxon tests.
We next evaluated the contribution of each mechanism, in both groups of neonates. CTLA-4 and cAMP were critical for preterm Treg to suppress CD83. In contrast, term Tregs appeared to need both molecules to suppress CD83, as individual blockades had only a minor effect (Fig. 4E, H, K). For actin polymerization, the pattern was similar and individual blockade of CTLA-4, cAMP and TGF-β significantly affected preterm Treg function, while they did not affect term Treg function (Fig. 4E, H, K). Both term and preterm Treg needed CTLA-4 and cAMP to suppress Tcon CD134 expression (Fig. 4D, G, J). Therefore, Tregs use different mechanisms to suppress each target. These mechanisms could work independently or synergistically and may vary between term and preterm neonates.
Discussion
Fetal Treg actively promote self-tolerance, as well as tolerance to non-inherited antigens on chimeric maternal cells that reside in fetal tissues [1]. Term neonates have a high frequency of CD45RA+ Tregs due to the absence of external Ag exposure [17, 32]. After birth, Tregs gradually switch from a naïve to a memory phenotype within the first 16-18 months of life [32, 33]. Due to the higher expression of homing molecules, memory Tregs are more potent than naïve Tregs in vivo; however, in vitro, naïve Tregs appear as potent to suppress Tcon as memory Treg [24, 34, 35]. In agreement with these studies, we and other authors have reported that Treg from term neonates suppress Tcon proliferation in vitro at levels similar to those of adult, which are a mix of naïve/memory cells [14-16]. However, we recently reported that Treg from preterm newborns were less suppressive than those from term neonates, although the proportion of naïve Treg was similar in both groups [16].
Importantly, although DC are likely the most important target for Treg in vivo [5, 6], the capacity and the mechanisms of human term and preterm Treg to suppress DC has not been studied, and this was the aim of the present study. Mice Treg suppress DC in vitro by inhibiting the initial formation of DC-Tcon aggregates, due to Treg being more mobile and out-competing Tcon [3]. In addition, Treg diminish the formation of stable contacts between antigen-activated T-cells and DC in vivo [5-7]. In agreement with these models, we found that adult Tregs decreased the number of DC-Tcon aggregates and modified their composition, thereby decreasing the number of Tcon in contact with DC. Larger numbers of clusters were previously reported by Onishi et al, [3]. This discrepancy could be explained by several technical differences, such as differing times of analysis and DC-Tcon-Treg ratios. Indeed, they used a (1:1:1) DC-Tcon-Treg ratio, whereas we used a more physiological ratio of 1:10:1. Additionally, these authors used confocal microscopy, which does not allow precise quantification. Of note, we previously quantified the percentage of DC-Tcon clusters using flow cytometry and imaging flow cytometry side by side, and found similar results for either whatever the technique [27].
In contrast to adult Tregs, preterm and term Tregs did not affect the number or composition of DC-Tcon aggregates. Although we did not measure Treg expression of LFA-1 and Neuropilin-1, molecules associated with Treg-DC interaction [3, 7], our data do not show a difference in localization for neonate Treg compared to adult Treg. Furthermore, neonate T-cells to express similar levels of LFA-1 as adult T-cells [36-38]. Importantly, naïve adult Treg also suppress DC-Tcon aggregates, suggesting that the defect in neonate Treg is not solely due to their mainly naïve phenotype. The reason for this intrinsic difference is currently unknown, but could be related with the origin of Treg. Early in life, the naïve T cell compartment contains a high fraction of recent thymic emigrants (RTE), compared to naïve T-cells from adults [39]. Interestingly, studies in different murine models have shown that both CD8 and Treg RTE are less functional than their counterparts that have been in the periphery for a while, suggesting that complete T cell functionality requires postthymic maturation [40, 41]. Furthermore, in adults, the naïve T cell compartment may also include antigen-experienced cells that have reverted to a naive phenotype [42].
Resting Treg from term neonates express lower levels of both FOXP3 and CTLA-4 than in adult Treg [24]. CTLA-4 is likely important for adult Treg to suppress the formation of DC:Tcon aggregates, although there is redundancy with cAMP and TGF-β, as we showed that the combined blockade of all 3 mechanisms was necessary to abrogate adult Treg suppression (Supporting Information Fig. 5). Since FOXP3 modulates a multitude of genes, it is possible that the low expression of FOXP3 in neonate Tregs is involved in this defect. The molecular mechanisms underlying these alterations have not been elucidated. However, one mechanism could be the reduced expression of NFAT1 by cord blood T-cells, because NFAT1 is a critical transcription factor necessary for up-regulation of multiple molecules including CTLA-4 and FOXP3 [43, 44]. Future studies will be needed to clarify these differences between adult and neonate Tregs.
Although neonate Tregs failed to suppress the formation of DC:Tcon aggregates, they decreased DC and Tcon activation and actin polymerization at the immunological synapse. Furthermore, our data demonstrate that cord blood Tregs function is not significantly modified by prematurity. However, regarding the mechanism of Treg suppression, a more redundant profile was exhibited by preterm Tregs (using CTLA-4 and cAMP, and in some case TGF-β) than the more synergistic profile exhibited by term Tregs. Evaluating the mechanisms used by neonate Treg in our DC-Tcon model, CTLA-4 interaction with its ligands also decreased DC maturation, which is in agreement with previous studies of adult Treg [3, 27]. Although the level of CTLA-4 per cell was low in activated term and preterm Treg compared to those in adult Treg, blockade of CTLA-4 in neonate Treg abrogated their suppressive activity.
cAMP suppresses the cytoskeleton and formation of the immunological synapse through direct phosphorylation of some molecules such as monomeric actin [45]. Treg can influx cAMP in target cells through gap junctions [46, 47], causing a significant decrease in actin polymerization as well as diminished levels of genes involved in cell cycle and cytokine secretion [45, 46]. A new finding of our study is that term and late preterm Tregs have higher concentrations of cAMP than adult Tregs. This high cAMP content probably helps to compensate the low CTLA-4 and FOXP3 levels. High cAMP was reported in resting PBMC from term neonates compared to adults, but these previous studies did not specifically analyze T-cells or Treg [48, 49]. In murine Tregs, FOXP3 keep the adenylate cyclase 9/cAMP pathway active [50]. However, since neonatal Tregs have lower expression of FOXP3, this suggests that cAMP levels are also regulated by FOXP3-independent pathways. In contrast to CTLA-4 and cAMP, our experiments did not suggest that TGF-β suppressed DC function. TGF-β expressed on the surface of Treg might inhibit Tcon through a direct Treg- Tcon contact within the aggregate [51].
In conclusion, our data demonstrate that neonatal Tregs have reduced capacity to suppress DC function, but they are still able to decrease Tcon activation, DC maturation and actin polymerization in the aggregates. This defect is consistent with lower CD4 function in newborns [52, 53]. Importantly, we also found functional differences between late preterm and term neonates, although the percentage of naïve Treg were similar in these two groups. These data imply a developmentally regulated maturation of Treg function, and suggest that the differences that we found between adult and neonate are not simply due to the increased proportion of naïve Tregs in neonates. Decreased capacity to control DC activation may contribute to the exacerbated inflammatory diseases seen in neonates, particularly those associated with severe chorioamionitis [54]. Future experiments will be important to clearly identify the in vivo functionality of neonatal Tregs.
Methods
The authors acknowledge the recently published Minimum Information about T cell Assays (MIATA) Guidelines [55] and have provided detailed information in accordance with MIATA.
Patient Recruitment
Mothers who delivered late-preterm infants (320-366 weeks gestation) or term infants (390-406 weeks gestation) were consented under a protocol approved by the IRB of Good Samaritan Hospital and Cincinnati Children's Hospital Medical Center, Cincinnati, OH. The samples are from a subset of a larger study, which enrolled 580 infants. Infants exposed to histologic chorioamnionitis and antenatal steroids were excluded. Peripheral blood from healthy adults was collected at the Hoxworth Blood Center (Cincinnati, OH).
Collection of umbilical cord blood and adult peripheral blood
Following delivery of the infant and before delivery of the placenta, the umbilical cord vein was cannulated. Adult peripheral blood was likewise collected into citrate phosphate dextrose pouch. Cord blood mononuclear cells (CBMCs) and adult peripheral blood mononuclear cells (PBMCs) were isolated with Ficoll-Hypaque gradients (GE Healthcare, Fairfield, CT). Aliquots of PBMC and CBMC were frozen in fetal calf serum (FCS) with 10% dimethyl sulfide and stored in liquid nitrogen.
Regulatory and conventional T-cells purification and activation
Resting CD4+ T-cells were purified from mononuclear cells by negative selection using magnetic beads (Miltenyi-Biotec, Auburn, CA). Purified CD4+ T-cells were stained with anti-CD8-FITC (RPT-T8), anti-CD25-allophycocyanin (M-A251) (BD-Biosciences, San Jose, CA), anti-CD45RA-PB (MEM-56) (Life technologies, NY) and anti–CD127-PE (eBioRDR5; Beckman-Coulter, Fullerton, CA). Treg (CD8−CD25HighCD127Neg) and Tcon (CD8−CD25−CD127High) were isolated by cell sorting using a FACSAria cell sorter (BD-Biosciences). Naïve and memory Tregs from adult were sorted as CD45RA+ and CD45RA− respectively. Purified Treg and Tcon were activated for 4 days with anti–CD3/CD28 beads (Invitrogen, Grand Island, NY) at a 1:1 bead-cell ratio, in RPMI medium supplemented with 100 U/mL penicillin, 10 μg/mL streptomycin, 2mM glutamine, 10mM HEPES, 10% FCS (Life-Technologies, Grand Island, NY), and 100 U/mL IL-2 (National Institutes of Health AIDS Research and Reference Reagent Program, Bethesda, MD).
CTLA-4 and FOXP3 expression in activated Tregs
Activated Tregs were stained with LIVE/DEAD® Fixable Dead Cell Stain kit (Invitrogen, Grand Island, NY), anti-CD4-AF700 (RPA-T4) and anti-CD25-allophycocyanin (BD-Biosciences) for surface marker. Then, cells were stained for intracellular markers using anti-CD152-PE (CTLA-4; 14D3), anti-Ki67-PerCP-Cy5.5 (B56) (BD-Biosciences) and anti-FOXP3-Pacific Blue (PCH101) (eBioscience). Flow cytometry analysis was performed using FACSDiva software version 6.1.2 (BD-Biosciences).
TGF-β expression in activated Tregs
mRNA isolation and cDNA synthesis from activated Tregs were performed using μMACS™ and multiMACS™ Kits (Miltenyi-Biotec) respectively. The cDNA was used in quantitative RT-PCR reactions using SYBR Green and TGF-β-1 primers (QuantiTect Primer Assays, Qiagen, Valencia, CA). Expression levels were normalized by Δct using ubiquitin-conjugating enzyme as the housekeeping gene.
Intracellular cAMP quantification
Intracellular cAMP levels were quantified in cell lysates of activated T-cells using a commercially available assay (cAMP Direct Biotrak EIA; GE Healthcare Biosciences) with a sensitivity limit of 12.5 fmol.
Allogeneic DC Suppression assays
To diminish inter-individual variability, the DC used in for all experiments were from a single adult healthy donor. CD14+ monocytes were isolated by positive selection (CD14 microbeads, Miltenyi-Biotec) and cryopreserved in aliquots until differentiation for use as target cells in suppression assays. Monocyte-derived DC were generated by culturing isolated monocytes for 4 days in complete RPMI medium with 500 U/ml rhIL-4 and 1000 U/ml rhGM-CSF (Peprotech-Inc, Rocky Hill, NJ).
DC-Tcon Suppression assays
Allogeneic DC were co-cultured for 24h with activated autologous Tcon labeled with 0.5 uM CellTrace™-Violet (Molecular-Probes) plus anti-CD3 (10ug/ml, UCHT1) in the presence or absence of autologous Treg labeled with 0.3 uM Calcein-AM-FITC (Molecular Probes). A ratio of 1 DC, 10 Tcon and 1 Treg was used.
Co-cultures were stained with anti-HLA-DR-BV570 (L243, Biolegend, San Diego, CA), anti-CD83-PE-Cy7 (HB15e, eBiosciences) and anti-CD134-PerCP/Cy5.5 (OX40, Ber-ACT35, Biolegend) for 30 minutes at 4°C. Cells were then fixed with 2% methanol-free formaldehyde for 30 minutes, washed and stained with Phaloidine AF-647 (Invitrogen) in 0.3% saponin for 30 min at 4°C. The samples were acquired on the ImageStream and analyzed using the IDEAS Software version 5.0 (Seattle, WA). HLA-DR fluorescence signal from Treg and Tcon after activation was removed with the software analysis. To count the number of Tcon interacting with each DC in each aggregate, the number of CellTrace™ Violet+ cells per HLA-DR+ cell were quantified using the Spot count feature. To quantify the actin polymerization at the immunological synapse (IS), we used a mask to define the specific area of interface in an image and calculated the intensity of actin polymerization. Actin intensity at the synapse was normalized based on the total actin intensity in the overall aggregate using the following formula-actin intensity at the IS= actin at the interface/total actin.
Blockade of Treg activity
In some experiments, co-cultures were performed in the presence of anti-CTLA-4 (10μg/ml, BNI3, Beckman-Coulter) and anti-TGF-β (10μg/ml, 9016.2, Sigma-Aldrich, St. Louis, MO). In other experiments, Tregs were pre-treated with 2',5'-dideoxyadenosine (ddADA, 200uM, Sigma), an inhibitor of adenylate cyclase, for 24h. In addition, to evaluate if CTLA-4, TGF-β and cAMP blockade synergized, co-cultures were performed in some experiments in presence of all three inhibitors. Combined blockade was compared to individual blockades.
Data analysis
Comparisons among 3 or more groups were analyzed by Kruskal-Wallis and Dunn's test for multiple comparisons. Comparison of 2 groups was analyzed by Mann Whitney test or Wilcoxon tests using GraphPad Prism, version 5.0 for Windows (La Jolla, CA).
Acknowledgements
We would like to thank Manuel Alvarez Jr. and Casey B. Wells for help with cord blood collection and processing, and the Hatton Research Center at Good Samaritan Hospital, particularly Rita Doerger RN, Peggy Walsh RN, Donna Lambers MD, Laurie Bambrick RN, Karen Henderson and Thomas Panke MD for help with recruitment and placenta processing. Likewise, we like to thank the Center for Excellence in Molecular Hematology Grant 1P30DK090971-01 and the Digestive Health Center Grant AR47363. This work was supported by NIH grant U01 HL101800 (to CAC and AHJ) and HL97064 (to AHJ and SGK).
Footnotes
Conflict of interest
The authors declare no financial or commercial conflict of interest.
References
- 1.Mold JE, Michaelsson J, Burt TD, Muench MO, Beckerman KP, Busch MP, Lee TH, et al. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science. 2008;322:1562–1565. doi: 10.1126/science.1164511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Misra N, Bayry J, Lacroix-Desmazes S, Kazatchkine MD, Kaveri SV. Cutting edge: human CD4+CD25+ T cells restrain the maturation and antigen-presenting function of dendritic cells. J Immunol. 2004;172:4676–4680. doi: 10.4049/jimmunol.172.8.4676. [DOI] [PubMed] [Google Scholar]
- 3.Onishi Y, Fehervari Z, Yamaguchi T, Sakaguchi S. Foxp3+ natural regulatory T cells preferentially form aggregates on dendritic cells in vitro and actively inhibit their maturation. Proc Natl Acad Sci U S A. 2008;105:10113–10118. doi: 10.1073/pnas.0711106105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang Q, Krummel MF. Imaging the function of regulatory T cells in vivo. Curr Opin Immunol. 2006;18:496–502. doi: 10.1016/j.coi.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 5.Tang Q, Adams JY, Tooley AJ, Bi M, Fife BT, Serra P, Santamaria P, et al. Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice. Nat Immunol. 2006;7:83–92. doi: 10.1038/ni1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tadokoro CE, Shakhar G, Shen S, Ding Y, Lino AC, Maraver A, Lafaille JJ, et al. Regulatory T cells inhibit stable contacts between CD4+ T cells and dendritic cells in vivo. J Exp Med. 2006;203:505–511. doi: 10.1084/jem.20050783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sarris M, Andersen KG, Randow F, Mayr L, Betz AG. Neuropilin-1 expression on regulatory T cells enhances their interactions with dendritic cells during antigen recognition. Immunity. 2008;28:402–413. doi: 10.1016/j.immuni.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sojka DK, Huang YH, Fowell DJ. Mechanisms of regulatory T-cell suppression - a diverse arsenal for a moving target. Immunology. 2008;124:13–22. doi: 10.1111/j.1365-2567.2008.02813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakamura K, Kitani A, Strober W. Cell contact-dependent immunosuppression by CD4(+)CD25(+) regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J Exp Med. 2001;194:629–644. doi: 10.1084/jem.194.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fassbender M, Gerlitzki B, Ullrich N, Lupp C, Klein M, Radsak MP, Schmitt E, et al. Cyclic adenosine monophosphate and IL-10 coordinately contribute to nTreg cell-mediated suppression of dendritic cell activation. Cell Immunol. 2010;265:91–96. doi: 10.1016/j.cellimm.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 11.Borsellino G, Kleinewietfeld M, Di Mitri D, Sternjak A, Diamantini A, Giometto R, Hopner S, et al. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood. 2007;110:1225–1232. doi: 10.1182/blood-2006-12-064527. [DOI] [PubMed] [Google Scholar]
- 12.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 13.Lyakh LA, Sanford M, Chekol S, Young HA, Roberts AB. TGF-beta and vitamin D3 utilize distinct pathways to suppress IL-12 production and modulate rapid differentiation of human monocytes into CD83+ dendritic cells. J Immunol. 2005;174:2061–2070. doi: 10.4049/jimmunol.174.4.2061. [DOI] [PubMed] [Google Scholar]
- 14.Wing K, Lindgren S, Kollberg G, Lundgren A, Harris RA, Rudin A, Lundin S, et al. CD4 T cell activation by myelin oligodendrocyte glycoprotein is suppressed by adult but not cord blood CD25+ T cells. Eur J Immunol. 2003;33:579–587. doi: 10.1002/eji.200323701. [DOI] [PubMed] [Google Scholar]
- 15.Seddiki N, Santner-Nanan B, Tangye SG, Alexander SI, Solomon M, Lee S, Nanan R, et al. Persistence of naive CD45RA+ regulatory T cells in adult life. Blood. 2006;107:2830–2838. doi: 10.1182/blood-2005-06-2403. [DOI] [PubMed] [Google Scholar]
- 16.Rueda CM, Wells CB, Gisslen T, Jobe AH, Kallapur SG, Chougnet CA. Effect of chorioamnionitis on regulatory T cells in moderate/late preterm neonates. Hum Immunol. 2015;76:65–73. doi: 10.1016/j.humimm.2014.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ng WF, Duggan PJ, Ponchel F, Matarese G, Lombardi G, Edwards AD, Isaacs JD, et al. Human CD4(+)CD25(+) cells: a naturally occurring population of regulatory T cells. Blood. 2001;98:2736–2744. doi: 10.1182/blood.v98.9.2736. [DOI] [PubMed] [Google Scholar]
- 18.Mayer E, Bannert C, Gruber S, Klunker S, Spittler A, Akdis CA, Szepfalusi Z, et al. Cord blood derived CD4+ CD25(high) T cells become functional regulatory T cells upon antigen encounter. PLoS One. 2012;7:e29355. doi: 10.1371/journal.pone.0029355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steinborn A, Engst M, Haensch GM, Mahnke K, Schmitt E, Meuer S, Sohn C. Small for gestational age (SGA) neonates show reduced suppressive activity of their regulatory T cells. Clin Immunol. 2010;134:188–197. doi: 10.1016/j.clim.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 20.Raju TN, Higgins RD, Stark AR, Leveno KJ. Optimizing care and outcome for late-preterm (near-term) infants: a summary of the workshop sponsored by the National Institute of Child Health and Human Development. Pediatrics. 2006;118:1207–1214. doi: 10.1542/peds.2006-0018. [DOI] [PubMed] [Google Scholar]
- 21.Goyal NK, Fiks AG, Lorch SA. Association of late-preterm birth with asthma in young children: practice-based study. Pediatrics. 2011;128:e830–838. doi: 10.1542/peds.2011-0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harju M, Keski-Nisula L, Georgiadis L, Raisanen S, Gissler M, Heinonen S. The burden of childhood asthma and late preterm and early term births. J Pediatr. 2014;164:295–299. doi: 10.1016/j.jpeds.2013.09.057. [DOI] [PubMed] [Google Scholar]
- 23.Kolar P, Knieke K, Hegel JK, Quandt D, Burmester GR, Hoff H, Brunner-Weinzierl MC. CTLA-4 (CD152) controls homeostasis and suppressive capacity of regulatory T cells in mice. Arthritis Rheum. 2009;60:123–132. doi: 10.1002/art.24181. [DOI] [PubMed] [Google Scholar]
- 24.Fujimaki W, Takahashi N, Ohnuma K, Nagatsu M, Kurosawa H, Yoshida S, Dang NH, et al. Comparative study of regulatory T cell function of human CD25CD4 T cells from thymocytes, cord blood, and adult peripheral blood. Clin Dev Immunol. 2008;2008:305859. doi: 10.1155/2008/305859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simark-Mattsson C, Dahlgren U, Roos K. CD4+CD25+ T lymphocytes in human tonsils suppress the proliferation of CD4+CD25- tonsil cells. Scand J Immunol. 2002;55:606–611. doi: 10.1046/j.1365-3083.2002.01095.x. [DOI] [PubMed] [Google Scholar]
- 26.Presicce P, Orsborn K, King E, Pratt J, Fichtenbaum CJ, Chougnet CA. Frequency of circulating regulatory T cells increases during chronic HIV infection and is largely controlled by highly active antiretroviral therapy. PLoS One. 2011;6:e28118. doi: 10.1371/journal.pone.0028118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moreno-Fernandez ME, Joedicke JJ, Chougnet CA. Regulatory T Cells Diminish HIV Infection in Dendritic Cells - Conventional CD4(+) T Cell Clusters. Front Immunol. 2014;5:199. doi: 10.3389/fimmu.2014.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shaw JM, Hunt PW, Critchfield JW, McConnell DH, Garcia JC, Pollard RB, Somsouk M, et al. Increased frequency of regulatory T cells accompanies increased immune activation in rectal mucosae of HIV-positive noncontrollers. J Virol. 2011;85:11422–11434. doi: 10.1128/JVI.05608-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Godfrey WR, Ge YG, Spoden DJ, Levine BL, June CH, Blazar BR, Porter SB. In vitro-expanded human CD4(+)CD25(+) T-regulatory cells can markedly inhibit allogeneic dendritic cell-stimulated MLR cultures. Blood. 2004;104:453–461. doi: 10.1182/blood-2004-01-0151. [DOI] [PubMed] [Google Scholar]
- 30.Oderup C, Cederbom L, Makowska A, Cilio CM, Ivars F. Cytotoxic T lymphocyte antigen-4-dependent down-modulation of costimulatory molecules on dendritic cells in CD4+ CD25+ regulatory T-cell-mediated suppression. Immunology. 2006;118:240–249. doi: 10.1111/j.1365-2567.2006.02362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ring S, Karakhanova S, Johnson T, Enk AH, Mahnke K. Gap junctions between regulatory T cells and dendritic cells prevent sensitization of CD8(+) T cells. J Allergy Clin Immunol. 2010;125:237–246. e231–237. doi: 10.1016/j.jaci.2009.10.025. [DOI] [PubMed] [Google Scholar]
- 32.Grindebacke H, Stenstad H, Quiding-Jarbrink M, Waldenstrom J, Adlerberth I, Wold AE, Rudin A. Dynamic development of homing receptor expression and memory cell differentiation of infant CD4+CD25high regulatory T cells. J Immunol. 2009;183:4360–4370. doi: 10.4049/jimmunol.0901091. [DOI] [PubMed] [Google Scholar]
- 33.Dirix V, Vermeulen F, Mascart F. Maturation of CD4(+) Regulatory T Lymphocytes and of Cytokine Secretions in Infants Born Prematurely. J Clin Immunol. 2013;33:1126–1133. doi: 10.1007/s10875-013-9911-4. [DOI] [PubMed] [Google Scholar]
- 34.Thornton AM, Shevach EM. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J Immunol. 2000;164:183–190. doi: 10.4049/jimmunol.164.1.183. [DOI] [PubMed] [Google Scholar]
- 35.Menning A, Hopken UE, Siegmund K, Lipp M, Hamann A, Huehn J. Distinctive role of CCR7 in migration and functional activity of naive- and effector/memory-like Treg subsets. Eur J Immunol. 2007;37:1575–1583. doi: 10.1002/eji.200737201. [DOI] [PubMed] [Google Scholar]
- 36.Keever CA. Characterization of cord blood lymphocyte subpopulations. J Hematother. 1993;2:203–206. doi: 10.1089/scd.1.1993.2.203. [DOI] [PubMed] [Google Scholar]
- 37.Gerli R, Agea E, Muscat C, Tognellini R, Fiorucci G, Spinozzi F, Cernetti C, et al. Activation of cord T lymphocytes. III. Role of LFA-1/ICAM-1 and CD2/LFA-3 adhesion molecules in CD3-induced proliferative response. Cell Immunol. 1993;148:32–47. doi: 10.1006/cimm.1993.1089. [DOI] [PubMed] [Google Scholar]
- 38.Thornton CA, Capristo CC, Power LL, Holloway J, Popplewell EJ, Diaper ND, Warner JO. The effect of labor on neonatal T-cell phenotype and function. Pediatr Res. 2003;54:120–124. doi: 10.1203/01.PDR.0000069704.25043.BA. [DOI] [PubMed] [Google Scholar]
- 39.Lee MS, Hanspers K, Barker CS, Korn AP, McCune JM. Gene expression profiles during human CD4+ T cell differentiation. Int Immunol. 2004;16:1109–1124. doi: 10.1093/intimm/dxh112. [DOI] [PubMed] [Google Scholar]
- 40.Petzold C, Steinbronn N, Gereke M, Strasser RH, Sparwasser T, Bruder D, Geffers R, et al. Fluorochrome-based definition of naturally occurring Foxp3(+) regulatory T cells of intra- and extrathymic origin. Eur J Immunol. 2014;44:3632–3645. doi: 10.1002/eji.201444750. [DOI] [PubMed] [Google Scholar]
- 41.Makaroff LE, Hendricks DW, Niec RE, Fink PJ. Postthymic maturation influences the CD8 T cell response to antigen. Proc Natl Acad Sci U S A. 2009;106:4799–4804. doi: 10.1073/pnas.0812354106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hargreaves M, Bell EB. Identical expression of CD45R isoforms by CD45RC+ 'revertant' memory and CD45RC+ naive CD4 T cells. Immunology. 1997;91:323–330. doi: 10.1046/j.1365-2567.1997.00267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaminski BA, Kadereit S, Miller RE, Leahy P, Stein KR, Topa DA, Radivoyevitch T, et al. Reduced expression of NFAT-associated genes in UCB versus adult CD4+ T lymphocytes during primary stimulation. Blood. 2003;102:4608–4617. doi: 10.1182/blood-2003-05-1732. [DOI] [PubMed] [Google Scholar]
- 44.Vaeth M, Schliesser U, Muller G, Reissig S, Satoh K, Tuettenberg A, Jonuleit H, et al. Dependence on nuclear factor of activated T-cells (NFAT) levels discriminates conventional T cells from Foxp3+ regulatory T cells. Proc Natl Acad Sci U S A. 2012;109:16258–16263. doi: 10.1073/pnas.1203870109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Howe AK. Regulation of actin-based cell migration by cAMP/PKA. Biochim Biophys Acta. 2004;1692:159–174. doi: 10.1016/j.bbamcr.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 46.Moreno-Fernandez ME, Rueda CM, Velilla PA, Rugeles MT, Chougnet CA. cAMP during HIV infection: friend or foe? AIDS Res Hum Retroviruses. 2012;28:49–53. doi: 10.1089/aid.2011.0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moreno-Fernandez ME, Rueda CM, Rusie LK, Chougnet CA. Regulatory T cells control HIV replication in activated T cells through a cAMP-dependent mechanism. Blood. 2011;117:5372–5380. doi: 10.1182/blood-2010-12-323162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Levy O, Coughlin M, Cronstein BN, Roy RM, Desai A, Wessels MR. The adenosine system selectively inhibits TLR-mediated TNF-alpha production in the human newborn. J Immunol. 2006;177:1956–1966. doi: 10.4049/jimmunol.177.3.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol. 2007;7:379–390. doi: 10.1038/nri2075. [DOI] [PubMed] [Google Scholar]
- 50.Huang B, Zhao J, Lei Z, Shen S, Li D, Shen GX, Zhang GM, et al. miR-142-3p restricts cAMP production in CD4+CD25- T cells and CD4+CD25+ TREG cells by targeting AC9 mRNA. EMBO Rep. 2009;10:180–185. doi: 10.1038/embor.2008.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gorelik L, Flavell RA. Transforming growth factor-beta in T-cell biology. Nat Rev Immunol. 2002;2:46–53. doi: 10.1038/nri704. [DOI] [PubMed] [Google Scholar]
- 52.Han P, McDonald T, Hodge G. Potential immaturity of the T-cell and antigen-presenting cell interaction in cord blood with particular emphasis on the CD40-CD40 ligand costimulatory pathway. Immunology. 2004;113:26–34. doi: 10.1111/j.1365-2567.2004.01933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takahashi N, Imanishi K, Nishida H, Uchiyama T. Evidence for immunologic immaturity of cord blood T cells. Cord blood T cells are susceptible to tolerance induction to in vitro stimulation with a superantigen. J Immunol. 1995;155:5213–5219. [PubMed] [Google Scholar]
- 54.Kallapur SG, Presicce P, Rueda CM, Jobe AH, Chougnet CA. Fetal immune response to chorioamnionitis. Semin Reprod Med. 2014;32:56–67. doi: 10.1055/s-0033-1361823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Britten CM, Janetzki S, van der Burg SH, Huber C, Kalos M, Levitsky HI, Maecker HT, et al. Minimal information about T cell assays: the process of reaching the community of T cell immunologists in cancer and beyond. Cancer Immunol Immunother. 2011;60:15–22. doi: 10.1007/s00262-010-0940-z. [DOI] [PMC free article] [PubMed] [Google Scholar]


