Abstract
Multiple reports indicate that epidermal growth factor receptor (EGFR) mutations are associated with lepidic-pattern lung adenocarcinoma, and that KRAS mutations are associated with invasive mucinous adenocarcinoma. We sought to investigate the association between EGFR and KRAS mutations and specific morphologic characteristics, such as predominant histologic subtype and mucinous features. Clinical data for 864 patients with resected lung adenocarcinoma that underwent molecular testing for EGFR and KRAS mutations were collected. Histologic subtyping was performed according to the IASLC/ATS/ERS lung adenocarcinoma classification, with attention given to signet-ring cell feature and extracellular mucin. EGFR mutations were detected using a polymerase chain reaction–based sizing assay, KRAS mutations were detected using Sanger sequencing, and ALK expression was detected using immunohistochemistry. Invasive mucinous adenocarcinoma was associated with KRAS mutation (P<0.001). Among invasive mucinous adenocarcinomas with KRAS mutation, a pure mucinous pattern was more common than a mixed mucinous/nonmucinous pattern (P=0.002). Invasive mucinous adenocarcinoma was associated with KRAS transition mutations (G→A) but not transversion mutations (G→T or G→C) compared to non-mucinous tumors (P=0.009). The lepidic-predominant group was associated with EGFR mutation compared to nonlepidic-predominant tumors (P=0.011). Extracellular mucin was associated with KRAS mutation (P<0.001), whereas signet-ring cell feature was not associated with EGFR or KRAS mutation (P=0.517). ALK expression was associated with signet-ring cell feature (P=0.001) but not with extracellular mucin (P=0.089). Our study shows that histologic patterns of mucin in lung adenocarcinoma - including invasive mucinous adenocarcinoma and extracellular mucin - are associated with KRAS mutation.
Keywords: lung, adenocarcinoma, subtype, mutation, mucin
INTRODUCTIONS
Activating mutations in the tyrosine kinase domain of epidermal growth factor receptor (EGFR) can predict sensitivity to EGFR tyrosine kinase inhibitors (TKIs) in patients with non-small cell lung cancer (NSCLC).1–3 Such mutations are most frequently observed in adenocarcinomas, in women, in never smokers, and in Asian patients.1–5 Previous reports indicate that EGFR mutation is associated with lung adenocarcinoma with lepidic-pattern, formerly known as bronchioloalveolar carcinoma (BAC) pattern.5–8 This has led to the hypothesis that tumors with lepidic (formerly BAC) pattern may be associated with EGFR mutation, and that lepidic pattern may predict responses to TKIs.9–11 KRAS is one of the downstream molecules in the EGFR signaling pathway,12, 13 but EGFR and KRAS mutations are mutually exclusive.4–6 In contrast to EGFR mutations, KRAS mutations predict primary resistance to TKI treatment in patients with NSCLC,14, 15 and they are associated with a history of cigarette smoking and with poor prognosis.5, 16–18 Interestingly, KRAS transversion mutations (G→T or G→C) are more common in ever smokers (because of the bulky carcinogens in cigarette smoke), whereas KRAS transition mutations (G→A) are more common in never smokers.19, 20 KRAS mutation has been reported to be associated with invasive mucinous adenocarcinoma, formerly known as mucinous BAC.21–25 EGFR mutations are detected in 20% to 50% and KRAS mutations are detected in 10% to 40% of adenocarcinomas.4, 5, 16, 18, 26 Therefore, approximately half of adenocarcinomas potentially have either EGFR or KRAS mutations, the presence of which influences clinical decisions regarding TKI treatment. It has recently been reported that anaplastic lymphoma kinase (ALK) rearrangement is associated with mucinous features, such as signet-ring cell feature and extracellular mucin, in lung adenocarcinoma.27–29 However, the relationship between KRAS mutations and these mucinous features are not well-documented.
A new lung adenocarcinoma classification that is based on predominant histologic patterns was proposed by the International Association for the Study of Lung Cancer (IASLC), American Thoracic Society (ATS), and European Respiratory Society (ERS) in 2011. This classification clearly emphasizes the prognostic significance of histologic subtypes,30 which have been validated in independent cohorts.31–34 However, the associations between the new classification of histologic subtypes and molecular features have not been thoroughly investigated.
We sought to investigate the associations between histologic subtypes (according to the new classification) and EGFR/KRAS mutations and to determine whether mucinous features, such as signet-ring cell feature and extracellular mucin, are associated with EGFR and KRAS mutations in patients with lung adenocarcinoma.
MATERIALS AND METHODS
Patients
Institutional review board approval was obtained for this retrospective study. Clinical data on 864 patients with lung adenocarcinoma who underwent surgical resection and molecular testing for EGFR and KRAS mutations at Memorial Sloan-Kettering Cancer Center between 2002 and 2009 were collected through the prospectively maintained Thoracic Service database. Patients were staged according to the seventh edition of the American Joint Committee on Cancer TNM Staging Manual.35
Histologic Evaluation
All available hematoxylin and eosin (H&E) - stained slides from study patients were reviewed jointly by two pathologists (K.K. and W.D.T.) using an Olympus BX51 microscope (Olympus, Tokyo, Japan) with a standard 22-mm diameter eyepiece. Histologic subtyping was performed according to the IASLC/ATS/ERS lung adenocarcinoma classification.30 Each tumor was reviewed by means of comprehensive histologic subtyping, with the percentage of each histologic component recorded in 5% increments.31 Tumors were classified as adenocarcinoma in situ (AIS), minimally invasive adenocarcinoma (MIA), and invasive adenocarcinoma, which was further divided into lepidic-predominant, papillary-predominant, acinar-predominant, micropapillary-predominant, and solid-predominant, as well as the variant forms invasive mucinous and colloid-predominant.
AIS and MIA were divided into nonmucinous, mucinous, and mixed mucinous/nonmucinous. Invasive mucinous adenocarcinoma was defined as a tumor with goblet or columnar cells, with abundant intracellular mucin (Fig. 1A) and with lepidic, acinar, papillary, or micropapillary pattern. Invasive mucinous adenocarcinoma was divided into two groups: pure mucinous (>90% invasive mucinous pattern) and mixed mucinous/nonmucinous (≥10% of each component).30 Colloid-predominant adenocarcinoma was defined as a tumor with mucin pools within airspaces, with destruction of alveolar walls (Fig. 1B).30
FIGURE 1.
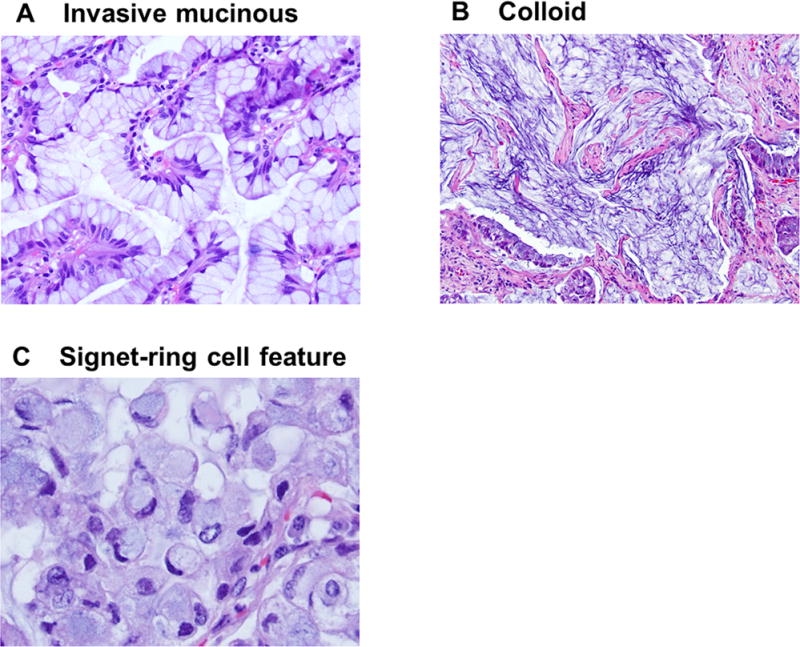
Mucinous subtypes and features. (A) Invasive mucinous adenocarcinoma. Goblet or columnar tumor cells with abundant intracellular mucin. (B) Colloid-predominant adenocarcinoma. A tumor has a mucin pool within tumoral stroma, with destruction of the alveolar wall. (C) Signet-ring cell feature. Tumor cells have abundant intracellular mucin and a crescentic nucleus displaced toward one end of the cells.
On the basis of the presence of mucinous pattern (according to the IASLC/ATS/ERS lung adenocarcinoma classification), the histologic subtypes were grouped into two categories: nonmucinous subtype (composed of nonmucinous AIS, nonmucinous MIA, and nonmucinous invasive adenocarcinoma) and mucinous subtype (composed of mucinous AIS, mucinous MIA, invasive mucinous adenocarcinoma, and colloid-predominant adenocarcinoma).
The percentage of cribriform pattern,36 which our group has recently published as a distinct histologic pattern in acinar predominant subtype with poor prognosis in stage I lung adenocarcinoma,37 was also recorded in 5% increments, and designations of cribriform-predominant subtype were made using criteria similar to the IASLC/ATS/ERS classification.
We also evaluated two additional mucinous features: signet-ring cell feature and extracellular mucin. Both of these mucinous features were reported to be positive for histochemical mucin stain.29 Signet-ring cell feature is characterized by abundant intracellular mucin and a crescentic nucleus displaced toward one end of the cell (Fig. 1C), and represent a cytologic change that can occur in multiple histologic subtypes of invasive adenocarcinoma (acinar, papillary, micropapillary, and solid predominant), the percentage of signet-ring cell feature was recorded regardless of histologic subtype of each tumor, and this feature was recorded as being present when any percentage of signet-ring cell features was found. Extracellular mucin is characterized by abundant mucin pools in tumoral alveolar or intraglandular spaces, which can also occur in multiple histologic subtypes including lepidic (Fig. 2A), acinar (Fig. 2B), papillary (Fig. 2C), and micropapillary pattern (Fig. 2D). Since extracellular mucin can occur in most tumors with mucinous subtypes (invasive mucinous and colloid-predominant), to delineate extracellular mucin as an additional category that is independent from mucinous subtypes, extracellular mucin was recorded to be present only in tumors with nonmucinous subtypes. Extracellular mucin was considered to be positive when observed in ≥10% of the tumor.
FIGURE 2.
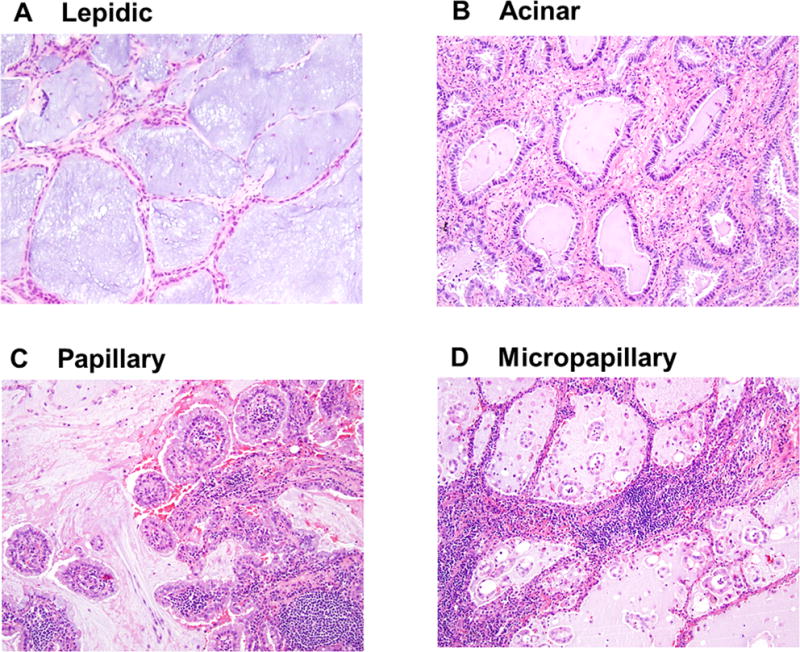
Extracellular mucin in each histologic pattern. Extracellular mucin in (A) lepidic, (B) acinar, (C) papillary, and (D) micropapillary pattern.
Mitotic counts were investigated using high-power fields (HPFs) at ×400 magnification (0.237 mm2 field of view). Mitoses were counted at 50 HPFs in areas with the highest mitotic activity and were assessed as the average number of mitotic figures per 10 HPFs.38–40 In addition, we investigated the following histologic factors: visceral pleural invasion (classified as absent [PL0] or present [PL1, PL2, and PL3]),35 lymphatic invasion, vascular invasion, and tumor necrosis.
Analysis of Mutations
EGFR exon 19 deletion and exon 21 L858R mutation were detected using a polymerase chain reaction - based assay, as previously described.41 KRAS exon 2 mutation was detected by Sanger sequencing.14
Tissue Microarray
Formalin-fixed, paraffin-embedded tumor specimens were used for tissue microarray construction. In brief, six representative tumor areas were marked on H&E-stained slides, and cylindrical 0.6-mm tissue cores were arrayed from the corresponding paraffin blocks into a recipient block using an automated tissue arrayer (ATA-27; Beecher Instruments, Sun Prairie, WI), resulting in 15 tissue microarray blocks. From each tissue microarray, 4-μm-thick paraffin sections were prepared for immunohistochemical analysis. In total, 677 cases with adequate cores were available for immunohistochemical analysis. On average, 5.6 tumor cores per patient were available for analysis.
Immunohistochemical Analysis and Scoring of ALK
Since it was not possible to study all patients in this cohort for ALK rearrangements by fluorescence in situ hybridization (FISH), we used immunohistochemistry as a surrogate as it has been shown in multiple studies to correlate highly with FISH results.42–44 In brief, 4-μm sections from the tissue microarray blocks were deparaffinized in xylene and dehydrated in graded alcohols. The standard avidin-biotin complex peroxidase technique was used for immunohistochemical staining of anti-ALK antibody (clone 5A4; Adcam; diluted at 1:30). Sections were stained using a Ventana Discovery XT automated immunohistochemical stainer (Ventana, Tucson, AZ), in accordance with the manufacturer’s guidelines. Diaminobenzidine was used as the chromogen, and hematoxylin was used as the nuclear counterstain. Positive control tissues were stained in parallel with the study cases.
ALK expression was recorded as the intensity of tumor cells with cytoplasmic-positive immunostaining in each tumor core. The intensity of staining was classified as no staining, weakly positive (faint cytoplasmic staining), moderately positive (moderate granular cytoplasmic staining), and strongly positive (strong granular cytoplasmic staining).42–44 ALK expression was divided into two groups: negative (no staining – weakly positive) and positive (moderately – strongly positive).43, 44
Statistical Analysis
Associations between clinicopathologic variables and histologic findings were analyzed using Fisher’s exact test (for categorical variables) and the Wilcoxon test (for continuous variables). Two-sided P < 0.05 was considered to indicate statistical significance. All analyses were performed using SAS statistical software (version 9.2; SAS Institute, Cary, NC).
RESULTS
Patient Demographic Characteristics
The clinical demographic characteristics for all 864 patients are outlined in Table 1. The median age was 69 years (range, 23 to 96 years); 63% of patients were women. Most patients were white (91%), followed by Asian (5%) and African-American (4%). Most patients were former smokers (67%); 14% were current smokers. Most patients had pathologic stage I disease (77%), followed by stage II (13%) and stage III (11%).
TABLE 1.
Patient demographics
| Variable | n (%) |
|---|---|
| Age, median (range) | 69 (23–96) |
| Sex | |
| Female | 541 (63) |
| Male | 323 (37) |
| Race | |
| Asian | 41 (5) |
| African-American | 33 (4) |
| White | 790 (91) |
| Smoking | |
| Never | 160 (19) |
| Ever | 704 (81) |
| Pathologic TNM stage | |
| I | 663 (77) |
| II | 109 (13) |
| III | 92 (11) |
Histologic Subtypes and Mucinous Features
In total, 42 tumors (5%) had mucinous subtypes: 1 mucinous MIA (0.1%), 36 invasive mucinous adenocarcinomas (4%), and 5 colloid-predominant tumors (0.6%). Of the invasive mucinous adenocarcinomas, 20 were pure mucinous, and 16 were mixed mucinous/nonmucinous. No mucinous or mixed mucinous/nonmucinous AIS were identified. There were 822 tumors with nonmucinous subtypes (95%): 2 nonmucinous AIS (0.2%), 31 nonmucinous MIA (4%), 97 lepidic-predominant (11%), 300 acinar-predominant (35%), 151 papillary-predominant (17%), 78 micropapillary-predominant (9%), and 163 solid-predominant tumors (19%) (Table 2). Among acinar-predominant tumors, 34 showed cribriform-predominant pattern.
TABLE 2.
Associations between EGFR/KRAS mutations and histologic subtypes
| Histologic subtype | Total | Mutation, n (%)
|
||
|---|---|---|---|---|
| Wild-type | EGFR | KRAS | ||
| Mucinous subtypes | 42 | 18 (43) | 0 (0) | 24 (57) |
| Mucinous AIS | 0 | 0 (0) | 0 (0) | 0 (0) |
| Mucinous MIA | 1 | 0 (0) | 0 (0) | 1 (100) |
| Invasive mucinous | 36 | 14 (39) | 0 (0) | 22 (61) |
| Colloid | 5 | 4 (80) | 0 (0) | 1 (20) |
| Non-mucinous subtypes | 822 | 491 (60) | 127 (15) | 204 (25) |
| Nonmucinous AIS | 2 | 1 (50) | 0 (0) | 1 (50) |
| Nonmucinous MIA | 31 | 22 (71) | 4 (13) | 5 (16) |
| Lepidic | 97 | 49 (50) | 27 (28) | 21 (22) |
| Acinar | 300 | 180 (60) | 54 (18) | 66 (22) |
| Papillary | 151 | 76 (50) | 29 (19) | 46 (30) |
| Micropapillary | 78 | 49 (63) | 7 (9) | 22 (28) |
| Solid | 163 | 114 (70) | 6 (4) | 43 (26) |
| Total | 864 | 509 (59) | 127 (15) | 228 (26) |
EGFR, epidermal growth factor receptor; AIS, adenocarcinoma in situ;
MIA, minimally invasive adenocarcinoma
Signet-ring cell features were identified in 69 tumors (8%): 2 lepidic-predominant, 31 acinar-predominant, 9 papillary-predominant, 4 micropapillary-predominant, 16 solid-predominant, 5 invasive mucinous, and 2 colloid-predominant (Fig. 3A). Extracellular mucin was identified in 116 tumors (13%): 2 nonmucinous MIA, 11 lepidic-predominant, 56 acinar-predominant (including 6 cribriform predomiant), 26 papillary-predominant, 6 micropapillary-predominant and 15 solid-predominant tumors (Fig. 3B).
FIGURE 3.
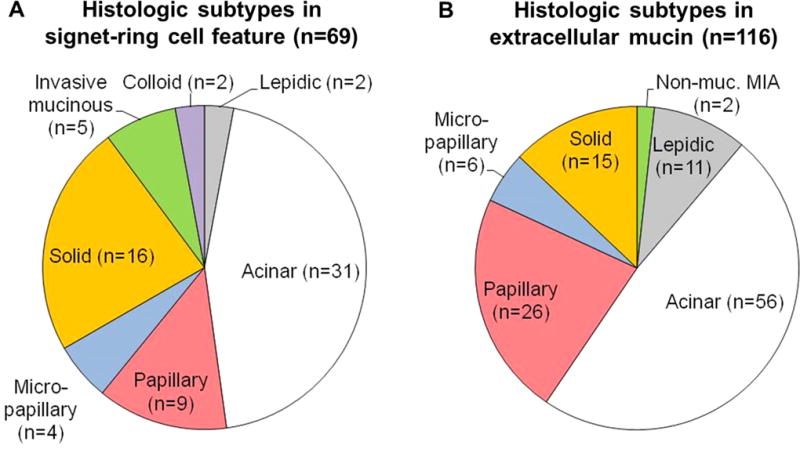
Mucinous features and their predominant subtypes. (A) Signet-ring cell features were identified in 69 tumors (5%): 2 lepidic-predominant, 31 acinar-predominant, 9 papillary-predominant, 4 micropapillary-predominant, 16 solid-predominant, 5 invasive mucinous, and 2 colloid-predominant. (B) Extracellular mucin was identified in 116 tumors (13%): 2 nonmucinous MIA, 11 lepidic-predominant, 56 acinar-predominant, 26 papillary-predominant, 6 micropapillary-predominant, and 15 solid-predominant.
Associations between Clinicopathologic Characteristics and Mucinous Patterns
Invasive mucinous adenocarcinoma was associated with non-Asian race (P=0.045) - in particular, with African-American race (P=0.044). In addition, invasive mucinous adenocarcinoma was associated with a lower rate of nodal metastases (P=0.031), less lymphatic invasion (P<0.001), less vascular invasion (P=0.011), absence of necrosis (P=0.030), and lower mitotic count (P<0.001). Signet-ring cell features were associated with higher stage (P=0.037) and presence of lymphatic invasion (P=0.032). Extracellular mucin was associated with history of smoking (P=0.014), higher rate of nodal metastases (P=0.012), and higher stage (P=0.019) and had a tendency to be associated with non-Asian race (P=0.055).
Associations between EGFR/KRAS Mutations and Histologic Subtypes or Mucinous Features
In total, 127 tumors (15%) had EGFR mutations, and 228 (26%) had KRAS mutations. The associations between mucinous and nonmucinous subtypes and EGFR and KRAS mutations are summarized in Table 2. No mucinous subtype tumors had EGFR mutations. Mucinous subtype was significantly associated with KRAS mutation, compared with EGFR mutation (P<0.001) and EGFR/KRAS wild-type (P<0.001). One mucinous MIA had KRAS mutation. Of the 36 invasive mucinous adenocarcinomas, 22 (61%) had KRAS mutations, and none had EGFR mutations. Invasive mucinous adenocarcinomas were significantly associated with KRAS mutation, compared with EGFR mutation (P<0.001) and EGFR/KRAS wild-type (P<0.001). Among invasive mucinous adenocarcinomas, pure mucinous tumors were significantly more likely to have KRAS mutations, compared with mixed mucinous/nonmucinous tumors (85% vs. 31%; P=0.002) (Fig. 4). Of the 5 colloid-predominant tumors, 1 had KRAS mutation, and none had EGFR mutation.
FIGURE 4.
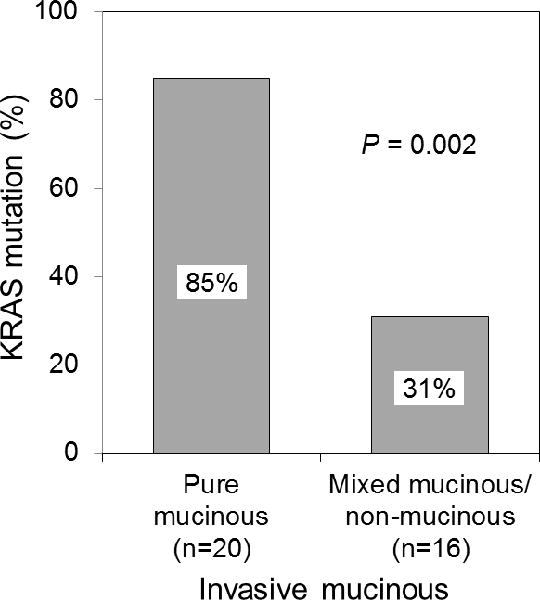
Associations between KRAS mutation and invasive mucinous adenocarcinoma (pure mucinous vs. mixed mucinous/nonmucinous). Among invasive mucinous adenocarcinomas (n=36), pure mucinous tumors were significantly more likely to have KRAS mutations, compared with mixed mucinous/nonmucinous tumors (85% vs. 31%).
Of the 2 tumors with nonmucinous AIS, 1 had KRAS mutation. Of the 31 tumors with nonmucinous MIA, 4 (13%) had EGFR mutations, and 5 (16%) had KRAS mutations. Of the 97 lepidic-predominant invasive adenocarcinomas, 27 (28%) had EGFR mutations, and 21 (22%) had KRAS mutations. Nonmucinous AIS/MIA was not associated with EGFR and KRAS mutations (P=0.49). Because of the similarities in their morphologic appearances, nonmucinous AIS, nonmucinous MIA, and lepidic-predominant invasive adenocarcinoma were combined into the lepidic-predominant group for the following analysis. The associations between EGFR/KRAS mutations and predominant subtypes/mucinous features among nonmucinous subtype tumors are summarized in Table 3. Tumors in the lepidic-predominant group (n=130) were more likely to have EGFR mutations, compared with tumors with the other predominant subtypes (P=0.011 [EGFR mutation vs. EGFR/KRAS wild-type] and P=0.011 [EGFR mutation vs. KRAS mutation]). Papillary-predominant tumors were less likely to have EGFR/KRAS wild-type (P=0.032). Solid-predominant tumors were more likely to have KRAS mutations (P<0.001) or EGFR/KRAS wild-type (P<0.001) compared to EGFR mutation. However, acinar-predominant, micropapillary-predominant, and cribriform-predominant tumors were not associated with EGFR and KRAS mutations (P=0.18, P=0.23 and P=0.11, respectively).
TABLE 3.
Associations between EGFR/KRAS mutations and histologic subtypes/mucinous features
| Variable | Mutation, n (%)
|
P-value (overall) |
P-value (pairwise comparison)
|
||||
|---|---|---|---|---|---|---|---|
| Wild-type | EGFR | KRAS | EGFR vs. wild-type | KRAS vs. wild-type | EGFR vs. KRAS | ||
| Histologic subtype | |||||||
| Lepidic predominant group | 72 (55) | 31 (24) | 27 (21) | 0.019 | 0.011 | 0.72 | 0.011 |
| Nonlepidic | 419 (60) | 96 (14) | 177 (26) | ||||
| Acinar | 180 (60) | 54 (18) | 66 (22) | 0.18 | |||
| Nonacinar | 311 (60) | 73 (14) | 138 (26) | ||||
| Papillary | 76 (50) | 29 (19) | 46 (30) | 0.032 | 0.063 | 0.029 | 1.00 |
| Nonpapillary | 415 (62) | 98 (15) | 158 (24) | ||||
| Micropapillary | 49 (63) | 7 (9) | 22 (28) | 0.23 | |||
| Nonmicropapillary | 442 (59) | 120 (16) | 182 (24) | ||||
| Solid | 114 (70) | 6 (4) | 43 (26) | <0.001 | <0.001 | 0.62 | <0.001 |
| Nonsolid | 377 (57) | 121 (18) | 161 (24) | ||||
| Cribriform | 26 (77) | 4 (12) | 4 (12) | 0.11 | |||
| Noncribriform | 465 (59) | 123 (16) | 200 (25) | ||||
| Mucinous feature | |||||||
| Signet-ring cell feature | 35 (56) | 8 (13) | 19 (31) | 0.52 | |||
| Non–signet-ring cell feature | 456 (60) | 119 (16) | 185 (24) | ||||
| Extracellular mucin | 58 (50) | 7 (6) | 51 (44) | <0.001 | 0.050 | <0.001 | <0.001 |
| Nonextracellular mucin | 433 (61) | 120 (17) | 153 (22) | ||||
Lepidic-predominant group includes nonmucinous adenocarcinoma in situ, nonmucinous minimally invasive adenocarcinoma, and lepidic-predominant invasive adenocarcinoma.
Significant P-values are shown in bold.
EGFR, epidermal growth factor receptor
Of the 116 tumors with extracellular mucin, 7 (6%) had EGFR mutations, and 51 (44%) had KRAS mutations (Table 3). Tumors with extracellular mucin were more likely to have KRAS mutations, compared with tumors without extracellular mucin (P<0.001 [KRAS mutation vs. EGFR/KRAS wild-type] and P<0.001 [KRAS mutation vs. EGFR mutation]). Of note, KRAS mutations were most frequent in tumors with ≥50% extracellular mucin, followed by tumors with 10% to 49% extracellular mucin and those with <10% extracellular mucin (62% vs. 40% vs. 22%; P<0.001) (Fig. 5). However, the presence of signet-ring cell features was not associated with EGFR or KRAS mutations (P=0.52).
FIGURE 5.
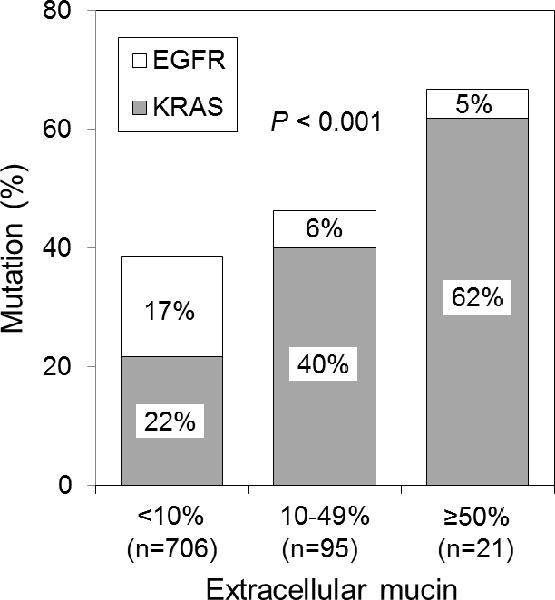
Associations between KRAS mutation and percentage of extracellular mucin. KRAS mutations were most frequent in tumors with ≥50% extracellular mucin (n=21), followed by tumors with 10% to 49% extracellular mucin and those with <10% extracellular mucin (62% vs. 40% vs. 22%).
Associations between EGFR and KRAS Mutation Types and Histologic Subtypes and Mucinous Features
Of the 127 tumors with EGFR mutations, 64 (50%) had exon 19 deletions, and 63 (50%) had exon 21 L858R mutations. However, no associations were found between any of the nonmucinous subtypes and EGFR mutation types.
Of the 228 tumors with KRAS mutations, 170 (75%) had transversion mutations (G→T or G→C), and 58 (25%) had transition mutations (G→A). KRAS transversion mutations were more common in ever smokers (76%; 166/218), whereas KRAS transition mutations were more common in never smokers (60%; 6/10, P=0.019). KRAS transition mutations were more frequently observed in invasive mucinous adenocarcinoma (50%; 11/22) compared to non-mucinous subtype tumors (23%; 46/204, P=0.009). Extracellular mucin was not associated with KRAS mutation types (P=0.36).
Associations between ALK Expression and Histologic Subtypes, Mucinous Features, or Clinical Characteristics
Positive ALK expression was identified in 29 of the 677 tumors (4%) that underwent ALK immunohistochemical analysis. Among ALK positive tumors, diffuse positivity (>50% of tumor area) was identified in half of the tumor cores in tissue microarrays. Of the EGFR/KRAS wild-type tumors in this cohort (n=400), positive ALK expression was identified in 27 (7%). In contrast, of the tumors with EGFR mutations (n=97), positive ALK expression was identified in only 1 (1%). In addition, of the tumors with KRAS mutations (n=180), positive ALK expression was identified in only 1 (0.6%).
The associations between histologic subtypes and mucinous features and ALK expression are summarized in Table 4. Of the tumors with signet-ring cell features (n=54), 8 (15%) had positive ALK expression. Signet-ring cell features were significantly associated with positive ALK expression (P=0.001). In addition, positive ALK expression was identified in 12% (3/26) of cribriform-predominant tumors (P=0.095) and in 7% (9/126) of tumors with extracellular mucin (P=0.089). Mucinous subtype was not associated with ALK expression, compared with nonmucinous subtype (P=0.18).
TABLE 4.
Associations between ALK expression and histologic subtypes/mucinous features
| Variable | Total, no. | ALK expression, no. (%)
|
P-value | |
|---|---|---|---|---|
| Positive | Negative | |||
| Histologic subtype | ||||
| Mucinous subtype | 35 | 3 (9) | 32 (91) | 0.18 |
| Nonmucinous subtype | 642 | 26 (4) | 616 (96) | |
| Cribriform pattern | ||||
| Cribriform-predominant | 26 | 3 (12) | 23 (88) | 0.095 |
| Noncribriform-predominant | 651 | 26 (4) | 625 (96) | |
| Signet-ring cell features | ||||
| Positive | 54 | 8 (15) | 46 (85) | 0.001 |
| Negative | 623 | 21 (3) | 602 (97) | |
| Extracellular mucin | ||||
| Positive | 126 | 9 (7) | 117 (93) | 0.089 |
| Negative | 551 | 20 (4) | 531 (96) | |
Significant P-values are shown in bold.
ALK, anaplastic lymphoma kinase
ALK expression was not associated with patient sex (P=0.85) or age (P=0.85). In this cohort, ALK expression was more frequently observed in never smokers (7%; 8/120) than in ever smokers (4%; 21/557) but the difference was not statistically significant (P=0.21).
DISCUSSION
In this study, we have demonstrated that specific histologic subtypes and mucinous features are associated with EGFR and KRAS mutations. (1) KRAS mutations are associated with invasive mucinous adenocarcinoma and extracellular mucin. (2) Among invasive mucinous adenocarcinomas, pure mucinous tumors are more likely to have KRAS mutations, compared with mixed mucinous/nonmucinous tumors. (3) Among tumors with KRAS mutations, invasive mucinous adenocarcinoma is associated with transition mutations, rather than transversion mutations. (4) EGFR mutations are associated with the lepidic-predominant group (nonmucinous AIS, nonmucinous MIA, and lepidic-predominant invasive adenocarcinoma).
KRAS mutation is associated with the subset of mucinous adenocarcinomas formerly classified as mucinous BAC21–25 (now called invasive mucinous adenocarcinoma, or mucinous AIS or mucinous MIA).30 This is supported by a recent study that demonstrated invasive mucinous adenocarcinoma classified according to the IASLC/ATS/ERS classification was associated with KRAS mutation.45 We hypothesized that other mucinous features might also be associated with KRAS mutation, and identified that invasive mucinous adenocarcinoma was significantly associated with KRAS mutation and a complete absence of EGFR mutation. More interestingly, KRAS mutations were significantly more frequently detected in pure invasive mucinous adenocarcinomas (85%) than in mixed mucinous/nonmucinous tumors (31%). This finding supports the practical value of subclassifying invasive mucinous adenocarcinoma as either pure mucinous or mixed mucinous/nonmucinous. KRAS transition mutations have been reported to occur frequently in never smokers with lung adenocarcinoma.19, 20 This association was confirmed in our study, and KRAS transition mutations were more common in invasive mucinous adenocarcinomas than in nonmucinous subtypes. With regard to the other mucinous subtypes, 1 mucinous MIA tumor had KRAS mutation. Of the 5 colloid-predominant adenocarcinomas, 1 had KRAS mutation, and none had EGFR mutation. However, this finding was based on a small number of cases; thus, further investigation, with more cases, is warranted.
The clinical and pathologic significance of extracellular mucin in lung adenocarcinoma has not yet been investigated. In our study, extracellular mucin was identified in 13% of tumors. KRAS mutations were detected in 44% of tumors with extracellular mucin. Of note, of the tumors with ≥50% extracellular mucin, KRAS mutations were detected in 62%, indicating a significant association between extracellular mucin and KRAS mutation. Whether extracellular mucin in lung adenocarcinomas is associated with resistance to TKI treatment may require additional investigation.
Recent studies have reported that ALK rearrangement is associated with mucinous features, such as signet-ring cell feature and extracellular mucin, and cribriform pattern in lung adenocarcinoma27–29; however, the associations between EGFR and KRAS mutations and these mucinous features have not been thoroughly investigated. In our study, signet-ring cell features were not associated with EGFR or KRAS mutations. Although our study did not specifically assess for ALK rearrangement, ALK expression was used as a surrogate method. Based on ALK status by immunohistochemistry (using monoclonal antibody clone 5A4), signet-ring cell features were associated with positive ALK expression. Tumors with extracellular mucin more frequently had positive ALK expression, although the association was not statistically significant. In studies of Japanese cohorts, cribriform pattern was associated with ALK rearrangement in lung adenocarcinoma.29, 46 In contrast, a study from the United States did not identify an association between cribriform pattern and ALK rearrangement.47 Therefore, it is not clear whether cribriform pattern is universally associated with ALK rearrangement. In the current study, cribriform-predominant tumors had more frequent ALK expression although the association was not statistically significant. One limitation of these findings is that, in our study, ALK rearrangement was not confirmed by FISH. However, a strong association between ALK immunohistochemical staining and ALK FISH- which has led to proposals to use immunohistochemical analysis as a screening method for ALK rearrangement - has been demonstrated in recent studies. Of note, anti-ALK monoclonal antibody 5A4, which was also used in our study, has demonstrated 95% to 100% sensitivity and specificity for the identification of tumors with ALK rearrangement confirmed by FISH in NSCLC.42–44 EGFR and KRAS mutations and ALK rearrangement have been reported to be mutually exclusive.43, 48 However, several studies have demonstrated that a very small number of tumors (<1%) concomitantly harbor EGFR or KRAS mutations and ALK rearrangement in NSCLC.42, 49, 50 In our study, positive ALK expression was identified in 1% of tumors with EGFR mutations and in 0.6% of tumors with KRAS mutations.
EGFR and KRAS mutations are detected even in preinvasive lesions, such as atypical adenomatous hyperplasia and AIS.51–53 In our study, EGFR mutations were identified in nonmucinous MIA, and KRAS mutations were identified in nonmucinous AIS and mucinous and nonmucinous MIA. EGFR mutations were associated with nonmucinous lepidic-predominant growth. This finding is compatible with previous studies that reported an association between EGFR mutations and presence of lepidic (formerly BAC) features.5–8 In the current study, furthermore, solid-predominant tumors were associated with KRAS mutations compared EGFR mutations, however; acinar-, papillary- and micropapillary-predominant tumors were not associated with EGFR and KRAS mutations. In the previous study from our group using a smaller cohort (n=100) of lung adenocarcinoma, papillary and micropapillary-predominant tumors were associated with EGFR mutation.54 In the recent study from our group using lung adenocarcinoma samples (n=180), in which mutation analyses were performed by Sequenom Mass ARRAY system (Sequenom), KRAS mutation was associated with solid-predominant tumors, and EGFR mutation was associated with lepidic, papillary and acinar-predominant tumors.55 There would be 2 possible explanations of the discrepancy regarding the association between predominant histologic subtype and mutation status: 1) inter-observer variability and 2) differences in mutation detection method. The reproducibility of histologic subtyping for lung adenocarcinoma was assessed by experienced pulmonary pathologists from multiple countries, with respect to predominant pattern, which reported good reproducibility (kappa score 0.77±0.07) in identifying predominant subtypes with typical cases.56 However, the limitations on this study were that the cases were reviewed using a micro-photographic image-based method evaluating selected images of tumors but actual tumor slides were not reviewed. Therefore, to confirm reproducibility of the new classification, further investigation is needed using actual tumor slides. Differences in mutation detection methods (direct sequencing vs. Sequenom anlaysis) could also have some impact on the study comparing morphologic finding to mutation status. Sequenom Mass ARRAY system (Sequenom) is suitable for more sensitive and broader mutation screening than direct sequencing.57
In summary, our study has demonstrated that mucinous histologic patterns - particularly invasive mucinous adenocarcinoma and extracellular mucin - are associated with KRAS mutations and specific clinicopathologic features. From a clinical standpoint, our findings suggest that there are some tendencies for associations between molecular characteristics and lung adenocarcinoma subtypes. However, the histologic-molecular associations are not 100% specific, so it is difficult to reliably predict the molecular status of EGFR and KRAS mutations and ALK rearrangement on the basis of histologic features alone. Therefore, molecular testing is still necessary, even in tumors for which a strong association between histologic type and EGFR or KRAS mutation or ALK rearrangement status has been demonstrated.
Acknowledgments
We thank Drs. Marc Ladanyi and Natasha Rekhtman for their comments on this manuscript; Joe Dycoco for his help with the lung adenocarcinoma database in the Division of Thoracic Service, Department of Surgery; and David Sewell for his editorial assistance.
Source of Funding: This work was supported, in part, by William H. Goodwin and Alice Goodwin, the Commonwealth Foundation for Cancer Research and the Experimental Therapeutics Center; the National Cancer Institute (grants R21CA164568 and R21CA164585); and the U.S. Department of Defense (grant LC110202).
Footnotes
Conflicts of Interest: All authors affirm no actual or potential conflicts of interest, including any financial, personal, or other relationships with other people or organizations.
References
- 1.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350(21):2129–39. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 2.Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: Correlation with clinical response to gefitinib therapy. Science. 2004;304(5676):1497–500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 3.Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A. 2004;101(36):13306–11. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shigematsu H, Lin L, Takahashi T, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst. 2005;97(5):339–46. doi: 10.1093/jnci/dji055. [DOI] [PubMed] [Google Scholar]
- 5.Tam IY, Chung LP, Suen WS, et al. Distinct epidermal growth factor receptor and KRAS mutation patterns in non-small cell lung cancer patients with different tobacco exposure and clinicopathologic features. Clin Cancer Res. 2006;12(5):1647–53. doi: 10.1158/1078-0432.CCR-05-1981. [DOI] [PubMed] [Google Scholar]
- 6.Marchetti A, Martella C, Felicioni L, et al. EGFR mutations in non-small-cell lung cancer: analysis of a large series of cases and development of a rapid and sensitive method for diagnostic screening with potential implications on pharmacologic treatment. J Clin Oncol. 2005;23(4):857–65. doi: 10.1200/JCO.2005.08.043. [DOI] [PubMed] [Google Scholar]
- 7.Hsieh RK, Lim KH, Kuo HT, Tzen CY, Huang MJ. Female sex and bronchioloalveolar pathologic subtype predict EGFR mutations in non-small cell lung cancer. Chest. 2005;128(1):317–21. doi: 10.1378/chest.128.1.317. [DOI] [PubMed] [Google Scholar]
- 8.Blons H, Cote JF, Le Corre D, et al. Epidermal growth factor receptor mutation in lung cancer are linked to bronchioloalveolar differentiation. Am J Surg Pathol. 2006;30(10):1309–15. doi: 10.1097/01.pas.0000213285.65907.31. [DOI] [PubMed] [Google Scholar]
- 9.Miller VA, Kris MG, Shah N, et al. Bronchioloalveolar pathologic subtype and smoking history predict sensitivity to gefitinib in advanced non-small-cell lung cancer. J Clin Oncol. 2004;22(6):1103–9. doi: 10.1200/JCO.2004.08.158. [DOI] [PubMed] [Google Scholar]
- 10.Kim YH, Ishii G, Goto K, et al. Dominant papillary subtype is a significant predictor of the response to gefitinib in adenocarcinoma of the lung. Clin Cancer Res. 2004;10(21):7311–7. doi: 10.1158/1078-0432.CCR-04-0811. [DOI] [PubMed] [Google Scholar]
- 11.Zakowski MF, Hussain S, Pao W, et al. Morphologic features of adenocarcinoma of the lung predictive of response to the epidermal growth factor receptor kinase inhibitors erlotinib and gefitinib. Arch Pathol Lab Med. 2009;133(3):470–7. doi: 10.1043/1543-2165-133.3.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitsudomi T, Yatabe Y. Mutations of the epidermal growth factor receptor gene and related genes as determinants of epidermal growth factor receptor tyrosine kinase inhibitors sensitivity in lung cancer. Cancer Sci. 2007;98(12):1817–24. doi: 10.1111/j.1349-7006.2007.00607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riely GJ, Marks J, Pao W. KRAS mutations in non-small cell lung cancer. Proc Am Thorac Soc. 2009;6(2):201–5. doi: 10.1513/pats.200809-107LC. [DOI] [PubMed] [Google Scholar]
- 14.Pao W, Wang TY, Riely GJ, et al. KRAS mutations and primary resistance of lung adenocarcinomas to gefitinib or erlotinib. PLoS Med. 2005;2(1):e17. doi: 10.1371/journal.pmed.0020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eberhard DA, Johnson BE, Amler LC, et al. Mutations in the epidermal growth factor receptor and in KRAS are predictive and prognostic indicators in patients with non-small-cell lung cancer treated with chemotherapy alone and in combination with erlotinib. J Clin Oncol. 2005;23(25):5900–9. doi: 10.1200/JCO.2005.02.857. [DOI] [PubMed] [Google Scholar]
- 16.Ahrendt SA, Decker PA, Alawi EA, et al. Cigarette smoking is strongly associated with mutation of the K-ras gene in patients with primary adenocarcinoma of the lung. Cancer. 2001;92(6):1525–30. doi: 10.1002/1097-0142(20010915)92:6<1525::aid-cncr1478>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 17.Mascaux C, Iannino N, Martin B, et al. The role of RAS oncogene in survival of patients with lung cancer: a systematic review of the literature with meta-analysis. Br J Cancer. 2005;92(1):131–9. doi: 10.1038/sj.bjc.6602258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marks JL, Broderick S, Zhou Q, et al. Prognostic and therapeutic implications of EGFR and KRAS mutations in resected lung adenocarcinoma. J Thorac Oncol. 2008;3(2):111–6. doi: 10.1097/JTO.0b013e318160c607. [DOI] [PubMed] [Google Scholar]
- 19.Riely GJ, Kris MG, Rosenbaum D, et al. Frequency and distinctive spectrum of KRAS mutations in never smokers with lung adenocarcinoma. Clin Cancer Res. 2008;14(18):5731–4. doi: 10.1158/1078-0432.CCR-08-0646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dogan S, Shen R, Ang DC, et al. Molecular epidemiology of EGFR and KRAS mutations in 3,026 lung adenocarcinomas: higher susceptibility of women to smoking-related KRAS-mutant cancers. Clin Cancer Res. 2012;18(22):6169–77. doi: 10.1158/1078-0432.CCR-11-3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marchetti A, Buttitta F, Pellegrini S, et al. Bronchioloalveolar lung carcinomas: K-ras mutations are constant events in the mucinous subtype. J Pathol. 1996;179(3):254–9. doi: 10.1002/(SICI)1096-9896(199607)179:3<254::AID-PATH589>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 22.Finberg KE, Sequist LV, Joshi VA, et al. Mucinous differentiation correlates with absence of EGFR mutation and presence of KRAS mutation in lung adenocarcinomas with bronchioloalveolar features. J Mol Diagn. 2007;9(3):320–6. doi: 10.2353/jmoldx.2007.060182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Casali C, Rossi G, Marchioni A, et al. A single institution-based retrospective study of surgically treated bronchioloalveolar adenocarcinoma of the lung: clinicopathologic analysis, molecular features, and possible pitfalls in routine practice. J Thorac Oncol. 2010;5(6):830–6. doi: 10.1097/jto.0b013e3181d60ff5. [DOI] [PubMed] [Google Scholar]
- 24.Hata A, Katakami N, Fujita S, et al. Frequency of EGFR and KRAS mutations in Japanese patients with lung adenocarcinoma with features of the mucinous subtype of bronchioloalveolar carcinoma. J Thorac Oncol. 2010;5(8):1197–200. doi: 10.1097/JTO.0b013e3181e2a2bc. [DOI] [PubMed] [Google Scholar]
- 25.Kakegawa S, Shimizu K, Sugano M, et al. Clinicopathological features of lung adenocarcinoma with KRAS mutations. Cancer. 2011;117(18):4257–66. doi: 10.1002/cncr.26010. [DOI] [PubMed] [Google Scholar]
- 26.Paik PK, Arcila ME, Fara M, et al. Clinical characteristics of patients with lung adenocarcinomas harboring BRAF mutations. J Clin Oncol. 2011;29(15):2046–51. doi: 10.1200/JCO.2010.33.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inamura K, Takeuchi K, Togashi Y, et al. EML4-ALK lung cancers are characterized by rare other mutations, a TTF-1 cell lineage, an acinar histology, and young onset. Mod Pathol. 2009;22(4):508–15. doi: 10.1038/modpathol.2009.2. [DOI] [PubMed] [Google Scholar]
- 28.Rodig SJ, Mino-Kenudson M, Dacic S, et al. Unique clinicopathologic features characterize ALK-rearranged lung adenocarcinoma in the western population. Clin Cancer Res. 2009;15(16):5216–23. doi: 10.1158/1078-0432.CCR-09-0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jokoji R, Yamasaki T, Minami S, et al. Combination of morphological feature analysis and immunohistochemistry is useful for screening of EML4-ALK-positive lung adenocarcinoma. J Clin Pathol. 2010;63(12):1066–70. doi: 10.1136/jcp.2010.081166. [DOI] [PubMed] [Google Scholar]
- 30.Travis WD, Brambilla E, Noguchi M, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6(2):244–85. doi: 10.1097/JTO.0b013e318206a221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoshizawa A, Motoi N, Riely GJ, et al. Impact of proposed IASLC/ATS/ERS classification of lung adenocarcinoma: prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases. Mod Pathol. 2011;24(5):653–64. doi: 10.1038/modpathol.2010.232. [DOI] [PubMed] [Google Scholar]
- 32.Russell PA, Wainer Z, Wright GM, et al. Does lung adenocarcinoma subtype predict patient survival?: A clinicopathologic study based on the new International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary lung adenocarcinoma classification. J Thorac Oncol. 2011;6(9):1496–504. doi: 10.1097/JTO.0b013e318221f701. [DOI] [PubMed] [Google Scholar]
- 33.Yoshizawa A, Sumiyoshi S, Sonobe M, et al. Validation of the IASLC/ATS/ERS lung adenocarcinoma classification for prognosis and association with EGFR and KRAS gene mutations: analysis of 440 Japanese patients. J Thorac Oncol. 2013;8(1):52–61. doi: 10.1097/JTO.0b013e3182769aa8. [DOI] [PubMed] [Google Scholar]
- 34.Russell PA, Barnett SA, Walkiewicz M, et al. Correlation of mutation status and survival with predominant histologic subtype according to the new IASLC/ATS/ERS lung adenocarcinoma classification in stage III (N2) patients. J Thorac Oncol. 2013;8(4):461–8. doi: 10.1097/JTO.0b013e3182828fb8. [DOI] [PubMed] [Google Scholar]
- 35.Edge SB, Byrd DR, Compton CC, et al. American Joint Committee on Cancer Cancer Staging Manual. 7th. New York, NY: Springer; 2009. pp. 253–270. [Google Scholar]
- 36.Mackinnon AC, Jr, Luevano A, de Araujo LC, et al. Cribriform adenocarcinoma of the lung: clinicopathologic, immunohistochemical, and molecular analysis of 15 cases of a distinctive morphologic subtype of lung adenocarcinoma. Mod Pathol. 2014 Jan 3; doi: 10.1038/modpathol.2013.227. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 37.Kadota K, Yeh YC, Sima CS, et al. The cribriform pattern identifies a subset of acinar predominant tumors with poor prognosis in patients with stage I lung adenocarcinoma: a conceptual proposal to classify cribriform predominant tumors as a distinct histologic subtype. Mod Pathol. 2013 Nov 1; doi: 10.1038/modpathol.2013.188. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kadota K, Suzuki K, Colovos C, et al. A nuclear grading system is a strong predictor of survival in epitheloid diffuse malignant pleural mesothelioma. Mod Pathol. 2012;25(2):260–71. doi: 10.1038/modpathol.2011.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kadota K, Suzuki K, Kachala SS, et al. A grading system combining architectural features and mitotic count predicts recurrence in stage I lung adenocarcinoma. Mod Pathol. 2012;25(8):1117–27. doi: 10.1038/modpathol.2012.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Travis WD, Rush W, Flieder DB, et al. Survival analysis of 200 pulmonary neuroendocrine tumors with clarification of criteria for atypical carcinoid and its separation from typical carcinoid. Am J Surg Pathol. 1998;22(8):934–44. doi: 10.1097/00000478-199808000-00003. [DOI] [PubMed] [Google Scholar]
- 41.Pan Q, Pao W, Ladanyi M. Rapid polymerase chain reaction-based detection of epidermal growth factor receptor gene mutations in lung adenocarcinomas. J Mol Diagn. 2005;7(3):396–403. doi: 10.1016/S1525-1578(10)60569-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McLeer-Florin A, Moro-Sibilot D, Melis A, et al. Dual IHC and FISH Testing for ALK Gene Rearrangement in Lung Adenocarcinomas in a Routine Practice: A French Study. J Thorac Oncol. 2012;7(2):348–54. doi: 10.1097/JTO.0b013e3182381535. [DOI] [PubMed] [Google Scholar]
- 43.Paik JH, Choi C-M, Kim H, et al. Clinicopathologic implication of ALK rearrangement in surgically resected lung cancer: A proposal of diagnostic algorithm for ALK-rearranged adenocarcinoma. Lung Cancer. 2012;76(3):403–9. doi: 10.1016/j.lungcan.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 44.Paik JH, Choe G, Kim H, et al. Screening of anaplastic lymphoma kinase rearrangement by immunohistochemistry in non-small cell lung cancer: correlation with fluorescence In situ hybridization. J Thorac Oncol. 2011;6(3):466–72. doi: 10.1097/JTO.0b013e31820b82e8. [DOI] [PubMed] [Google Scholar]
- 45.Tsuta K, Kawago M, Inoue E, et al. The utility of the proposed IASLC/ATS/ERS lung adenocarcinoma subtypes for disease prognosis and correlation of driver gene alterations. Lung Cancer. 2013;81(3):371–6. doi: 10.1016/j.lungcan.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 46.Yoshida A, Tsuta K, Nakamura H, et al. Comprehensive histologic analysis of ALK-rearranged lung carcinomas. Am J Surg Pathol. 2011;35(8):1226–34. doi: 10.1097/PAS.0b013e3182233e06. [DOI] [PubMed] [Google Scholar]
- 47.Nishino M, Klepeis VE, Yeap BY, et al. Histologic and cytomorphologic features of ALK-rearranged lung adenocarcinomas. Mod Pathol. 2012;25(11):1462–72. doi: 10.1038/modpathol.2012.109. [DOI] [PubMed] [Google Scholar]
- 48.Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical Features and Outcome of Patients With Non–Small-Cell Lung Cancer Who Harbor EML4-ALK. J Clin Oncol. 2009;27(26):4247–53. doi: 10.1200/JCO.2009.22.6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koivunen JP, Mermel C, Zejnullahu K, et al. EML4-ALK fusion gene and efficacy of an ALK kinase inhibitor in lung cancer. Clin Cancer Res. 2008;14(13):4275–83. doi: 10.1158/1078-0432.CCR-08-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martelli MP, Sozzi G, Hernandez L, et al. EML4-ALK rearrangement in non-small cell lung cancer and non-tumor lung tissues. Am J Pathol. 2009;174(2):661–70. doi: 10.2353/ajpath.2009.080755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sakamoto H, Shimizu J, Horio Y, et al. Disproportionate representation of KRAS gene mutation in atypical adenomatous hyperplasia, but even distribution of EGFR gene mutation from preinvasive to invasive adenocarcinomas. J Pathol. 2007;212(3):287–94. doi: 10.1002/path.2165. [DOI] [PubMed] [Google Scholar]
- 52.Ikeda K, Nomori H, Ohba Y, et al. Epidermal growth factor receptor mutations in multicentric lung adenocarcinomas and atypical adenomatous hyperplasias. J Thorac Oncol. 2008;3(5):467–71. doi: 10.1097/JTO.0b013e31816b4b14. [DOI] [PubMed] [Google Scholar]
- 53.Yoshida Y, Shibata T, Kokubu A, et al. Mutations of the epidermal growth factor receptor gene in atypical adenomatous hyperplasia and bronchioloalveolar carcinoma of the lung. Lung Cancer. 2005;50(1):1–8. doi: 10.1016/j.lungcan.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 54.Motoi N, Szoke J, Riely GJ, et al. Lung adenocarcinoma: modification of the 2004 WHO mixed subtype to include the major histologic subtype suggests correlations between papillary and micropapillary adenocarcinoma subtypes, EGFR mutations and gene expression analysis. Am J Surg Pathol. 2008;32(6):810–27. doi: 10.1097/PAS.0b013e31815cb162. [DOI] [PubMed] [Google Scholar]
- 55.Rekhtman N, Ang DC, Riely GJ, Ladanyi M, Moreira AL. KRAS mutations are associated with solid growth pattern and tumor-infiltrating leukocytes in lung adenocarcinoma. Mod Pathol. 2013;26(10):1307–19. doi: 10.1038/modpathol.2013.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thunnissen E, Beasley MB, Borczuk AC, et al. Reproducibility of histopathological subtypes and invasion in pulmonary adenocarcinoma. An international interobserver study. Mod Pathol. 2012;25(12):1574–83. doi: 10.1038/modpathol.2012.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arcila M, Lau C, Nafa K, Ladanyi M. Detection of KRAS and BRAF mutations in colorectal carcinoma roles for high-sensitivity locked nucleic acid-PCR sequencing and broad-spectrum mass spectrometry genotyping. J Mol Diagn. 2011;13(1):64–73. doi: 10.1016/j.jmoldx.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]


