Abstract
We have recently reported that, in the presence of light and a copper catalyst, nitrogen nucleophiles such as carbazoles and primary amides undergo C–N coupling with alkyl halides under mild conditions. In the present study, we establish that photoinduced, copper-catalyzed alkylation can also be applied to C–C bond formation, specifically, that the cyanation of unactivated secondary alkyl chlorides can be achieved at room temperature to afford nitriles, an important class of target molecules. Thus, in the presence of an inexpensive copper catalyst (CuI; no ligand co-additive) and a readily available light source (UVC compact fluorescent light bulb), a wide array of alkyl halides undergo cyanation in good yield. Our initial mechanistic studies are consistent with the hypothesis that an excited state of [Cu(CN)2]− may play a role, via single electron transfer, in this process. This investigation provides a rare example of a transition metal-catalyzed cyanation of an alkyl halide, as well as the first illustrations of photoinduced, copper-catalyzed alkylation with either a carbon nucleophile or a secondary alkyl chloride.
INTRODUCTION
The development of metal-catalyzed cross-coupling reactions has had a profound impact on the synthesis of organic molecules.1 The use of catalysts based on earth-abundant metals is attractive, and remarkable progress has been described in achieving a wide variety of bond constructions with the aid of copper catalysts.2 Elevated temperatures and added ligands are typically required.
We have recently established that, with the aid of light, an array of copper-catalyzed cross-couplings can be achieved under unusually mild conditions (−40 to 30 °C) without the need for an added ligand. In the case of couplings with aryl electrophiles, we have demonstrated the viability of nitrogen, sulfur, and oxygen nucleophiles as reaction partners,3,4 and, in the case of alkyl electrophiles, we have described the use of nitrogen nucleophiles.5,6 On the basis of one of our working mechanistic hypotheses for these processes (see below), the photophysical properties of a copper–nucleophile complex may play a key role in determining the feasibility of such transformations. Consequently, it was not clear at the outset whether the expansion of this mode of reactivity to the alkylation of carbon-based nucleophiles would be possible.
Organic compounds that bear a cyano group serve as important intermediates and endpoints in organic chemistry.7,8 Nucleophilic substitution of an alkyl electrophile with cyanide anion through an SN2 pathway is an attractive approach to the synthesis of nitriles, although undesired side reactions such as elimination can intervene, especially in the case of less reactive electrophiles. For unactivated secondary alkyl chlorides, we are not aware of cyanations that proceed efficiently at a temperature below 75 °C.9
Although a few studies have described the use of transition metals to catalyze the cyanation of alkyl halides, to the best of our knowledge these methods have been limited to benzylic chlorides.10 We therefore decided to investigate the possibility that a copper catalyst in combination with light could expand the scope of such metal-catalyzed cyanations to include challenging substrates such as unactivated secondary alkyl chlorides. In this report, we establish that copper and light do indeed enable cyanations of these electrophiles at room temperature (eq 1), thus expanding the scope of photoinduced, copper- catalyzed bond formation to include the alkylation of carbon-centered nucleophiles as well as the use of secondary chlorides as electrophiles.
 |
RESULTS AND DISCUSSION
Upon investigating a variety of reaction parameters, we have determined that the cyanation of an unactivated secondary alkyl chloride proceeds in good yield at room temperature with [N(n-Bu)4][CN] (TBACN) as the cyanide source, CuI as the catalyst, and irradiation with 15-watt UVC compact fluorescent light bulbs11 (Table 1, entry 1).12 This photoinduced, copper-catalyzed cyanation can even be achieved at 0 °C (entry 2; 60 h), whereas the thermal (no CuI and no light) cyanation of this substrate with TBACN in CH3CN requires heating to 92 °C (82% yield after 24 h). Under our standard conditions but in the absence of CuI and/or light, essentially no carbon–carbon bond formation is observed (entries 3–5). Use of a photoreactor establishes that irradiation at 254 nm is more effective than at 300 or 350 nm (entries 6–8). Substituting CuI with a variety of other Cu(I) or Cu(II) complexes, as well as with Cu nanopowder, leads to a significantly lower yield (entries 9–14), as does replacement of TBACN with other cyanide sources (entries 15–17). The amount of CuI can be reduced to 2.5% (but not to 1.0%; entries 18–20), and the reaction time can be shortened to 12 h (entry 21), without substantially diminishing product formation. Use of a smaller excess of TBACN results in a small loss in yield (entry 22). Although the cyanation method is somewhat sensitive to air (entry 23), it is not sensitive to moisture (entry 24).
Table 1.
Photoinduced, copper-catalyzed cyanation of an unactivated secondary alkyl chloride: Effect of reaction parametersa

| ||
|---|---|---|
|
| ||
| entry | change from the "standard conditions" | ield (%)b |
| 1 | none | 88 |
| 2 | 0 °C (60 h) | 85c |
| 3 | no CuI | <1 |
| 4 | no h□ | <1 |
| 5 | no CuI and no h□ | <1 |
| 6 | h□ (photoreactor at 254 nm) | 86 |
| 7 | h□ (photoreactor at 300 nm) | 15 |
| 8 | h□ (photoreactor at 350 nm) | 12 |
| 9 | CuBr, instead of CuI | 42 |
| 10 | CuCl, instead of CuI | 48 |
| 11 | Cu2O, instead of CuI | 32 |
| 12 | CuCl2, instead of CuI | 44 |
| 13 | Cu(OTf)2, instead of CuI | 42 |
| 14 | Cu nanopowder (60-80 nm), instead of CuI | 23 |
| 15 | NaCN, instead of TBACN | 25 |
| 16 | KCN, instead of TBACN | 27 |
| 17 | K4[Fe(CN)6], instead of TBACN | <1 |
| 18 | 5.0% CuI | 83 |
| 19 | 2.5% CuI | 78 |
| 20 | 1.0% CuI | 47 |
| 21 | 12 h | 82 |
| 22 | 1.2, instead of 1.6, equiv TBACN | 75 |
| 23 | under an atmosphere of air, instead of nitrogen | 32 |
| 24 | 0.1 equiv H2O added | 87 |
All data are the average of two or more experiments.
The yield was determined through GC analysis with the aid of a calibrated internal standard.
Yield of purified product: 85% (average of two runs).
Under our standard conditions, photoinduced, copper-catalyzed cyanation of an array of unactivated secondary alkyl chlorides proceeds in generally good yield at room temperature (Table 2).13 Both acyclic (entries 1–6) and cyclic (entries 7–9) electrophiles are suitable coupling partners. This method is remarkably effective in the case of very hindered electrophiles, including a secondary alkyl chloride that bears a t-butyl substituent (entry 3; substantially more sterically demanding than neopentyl chloride) and one in which both substituents are α-branched (entry 6); an attempted cyanation of the latter substrate under thermal conditions (1.6 equiv TBACN, DMF, 80 °C, 24 h) was unsuccessful (<5% yield at 90% conversion). Functional groups such as an olefin, a furan, a carbamate, and a dialkyl ether are compatible with the coupling conditions (entries 4–6, 8, and 9). Furthermore, when additives (1.0 equiv) that contain a terminal alkyne (1-octyne), a cis disubstituted olefin (4-decene), or an ester (ethyl heptanoate) are introduced to the reaction mixture, cross-coupling occurs smoothly; on the other hand, the presence of certain nitrogen heterocycles impedes carbon–carbon bond formation. On a gram-scale (1.3 g of product), the cross-coupling illustrated in entry 1 of Table 2 proceeds in 94% yield.
Table 2.
Photoinduced, copper-catalyzed cyanation of unactivated secondary alkyl chlorides: Scope

| ||
|---|---|---|
|
| ||
| entry | electrophile | yield (%)a |
| 1 |

|
93 |
| 2 |

|
84 |
| 3 |

|
84 |
| 4 |
|
84 |
| 5 |

|
74 |
| 6 |

|
93 |
| 7 |

|
79 |
| 8 |

|
68 |
| 9 |

|
86 |
Yield of purified product (average of two experiments).
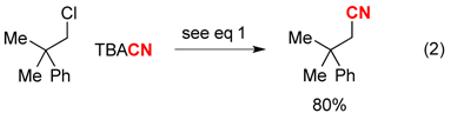
This new pathway for carbon–carbon bond formation is not limited to the cyanation of unactivated secondary alkyl chlorides. For example, under our standard conditions, neophyl chloride, a poor substrate for SN2 reactions, can be converted to the target nitrile in 80% yield (eq 2); no product formation (<1%) was observed in the absence of light or of copper, or under thermal conditions (DMF, 80 °C, 24 h). We have also determined that an unactivated tertiary alkyl chloride can serve as a useful coupling partner, thereby generating an all-carbon quaternary center (eq 3);14 an effort to accomplish this transformation under thermal conditions failed to furnish the desired product (<1% yield; DMF, 80 °C, 24 h).15 In a competition experiment, we have established that a tertiary alkyl chloride undergoes cyanation more rapidly than a secondary alkyl chloride, likely due to the greater stability of the more highly substituted radical (eq 4; see the mechanistic discussion below).
 |
 |
 |
The conditions that we have developed for the photoinduced, copper-catalyzed cyanation of alkyl chlorides can be applied without modification to unactivated secondary alkyl bromides (Table 3);16 for these electrophiles, under our standard conditions but in the absence either of copper or of light, a small amount of cyanation occurs (the catalyzed process is at least five times faster). A nitrile, an ester, and a carbamate are compatible with the coupling method. In a competition study, we have determined that an alkyl bromide reacts much more rapidly than does an alkyl chloride (eq 5).
Table 3.
Photoinduced, copper-catalyzed cyanation of unactivated secondary alkyl bromides: Scope

| ||
|---|---|---|
|
| ||
| entry | electrophile | yield (%)a |
| 1 |

|
84 |
| 2 |

|
82 |
| 3 |
|
82 |
| 4 |

|
92 |
| 5 |

|
84 |
Yield of purified product (average of two experiments).
 |
Mechanistic observations
Figure 1 provides an outline of one of the possible pathways for photoinduced, copper-catalyzed cyanations of unactivated secondary alkyl chlorides.3,5,17 In this scenario, a Cu(I)-cyanide adduct (A) undergoes photoexcitation to afford an excited-state complex (B) that engages in an electron-transfer reaction with the alkyl halide to furnish a Cu(II)-cyanide adduct (C) and an alkyl radical, which next combine to provide the nitrile and a Cu(I)-halide complex (D).17 Reaction of Cu(I)-halide complex D with TBACN then regenerates Cu(I)-cyanide adduct A.
Figure 1.

Outline of one of the possible pathways for photoinduced, copper-catalyzed cyanations of unactivated secondary alkyl chlorides (for the sake of simplicity, the copper complexes are illustrated as neutral species).
During our reaction-development efforts, we determined that, while CuCl displays activity as a catalyst for the photoinduced cyanation of a secondary alkyl chloride, carbon–carbon bond formation is more efficient in the presence of CuI (Table 1, entries 1 and 10). We have confirmed that similar behavior is observed when cyclohexyl chloride is employed as the electrophile (Table 4, entries 1 and 2); the addition of TBAI to CuCl furnishes results that are comparable to CuI alone (entries 2 and 3).18 Because copper(I) halides undergo ligand substitution in the presence of TBACN (see below), we hypothesized that the enhanced effectiveness of CuI relative to CuCl might arise from the transient formation of an alkyl iodide through the reaction of iodide anion with the alkyl chloride,19 which could provide a pathway for carbon–carbon bond formation that parallels direct reaction of the alkyl chloride.
Table 4.
Cyanation of Cyclohexyl Halides

| |||
|---|---|---|---|
|
| |||
| entry | X | Y | yield (%)a |
| 1 | CI | CuCI | 34 |
| 2 | CI | Cul | 82 |
| 3 | CI | CuCI + TBAI | 78 |
| 4 | I | none | 26 |
| 5 | I | none (no light) | <1 |
| 6 | CI | TBAI | 5 |
| 7 | CI | [Cu(CN)2]TBA | 26 |
| 8 | CI | [Cu(CN)2]TBA+TBAI | 76 |
The yield was determined through GC analysis with the aid of a calibrated internal standard (average of two runs).
The in situ generation of an alkyl iodide could enhance the efficiency of the pathway outlined in Figure 1 by, for example, facilitating electron transfer from excited-state complex B to the alkyl halide. At the same time, we also considered the possibility that the transient formation of an alkyl iodide might open the door to a copper-free cyanation pathway (e.g., eq 6), since homolysis of an alkyl–I bond can occur upon irradiation at 254 nm.20,21,22,23
 |
Indeed, we have determined that, upon irradiation, cyclohexyl iodide reacts with TBACN to generate the corresponding nitrile in modest yield (26%; Table 4, entry 4); a control reaction in the absence of light demonstrates that this cyanation does not arise from a simple SN2 displacement (Table 4, entry 5).24 However, we have also established that, under our standard conditions for photoinduced cyanation, replacement of CuI with TBAI does not lead to a substantial quantity of the nitrile (Table 4, entry 6 vs. entry 2),25 thereby confirming the critical role of copper. Thus, in situ formation of an alkyl iodide, followed by copper-free, photoinduced formation of the nitrile (eq 6), is likely at most a minor contributor to the enhanced catalytic activity of CuI relative to CuCl (Table 4, entries 1 and 2).
We decided to explore the possible intermediacy of an organic radical in photoinduced, copper-catalyzed cyanations (e.g., Figure 1). In order to avoid complications that might arise from the formation and direct photochemistry of an alkyl iodide, we chose to employ CuCl as a catalyst in our investigation, while recognizing that it would afford a modest yield. In particular, we have examined the cyanation of the unactivated secondary alkyl chloride illustrated in eq 7;26 the radical derived from this electrophile has been reported to furnish a 2:1 endo:exo mixture of [3.3.0] bicyclic compounds upon cyclization.27 When we subject this electrophile to photoinduced, copper-catalyzed cyanation, we also obtain a 2:1 endo:exo mixture of products, consistent with a common radical intermediate.
 |
Although our working mechanistic hypothesis (Figure 1) does not invoke a radical-chain pathway, a variety of photoinitiated nucleophilic substitution processes have been described that are believed to proceed through a chain mechanism.21 To gain insight into whether a chain process might be operative under our cyanation conditions, we determined the quantum yield for the reaction of cyclohexyl chloride with TBACN.28 The observed value of 0.030 (.002) does not suggest a chain pathway.
In order to obtain information about what copper complexes might be present under our standard coupling conditions, we have analyzed by electrospray ionization mass spectrometry (ESI–MS) the cyanation of cyclohexyl chloride. After 30 min of irradiation, anionic species were detected with m/z = 115 and 117, which can be assigned to 63CuI(CN)2− and 65CuI(CN)2−, respectively. When a mixture of CuI (or CuCl) and TBACN (1:10) was independently analyzed by ESI–MS, the same two species were observed.
The absorption spectrum of [Cu(CN)2]TBA29 reveals stronger absorbance at 254 nm (ε254 = 1.5 × 103 M−1 cm−1) than at 300 and 350 nm (Figure 2). In view of our observation that irradiation at 254 nm leads to more effective coupling than does irradiation at 300 or 350 nm (Table 1, entries 6–8), this spectrum is consistent with the hypothesis that excitation of [Cu(CN)2]− may play a key role in the photoinduced, copper-catalyzed process, for example by serving as a reductant (Figure 1).30 Under a variety of conditions, a common photoredox catalyst, Ir(ppy)3,3e,31 does not facilitate cyanation (eq 8), also consistent with the hypothesis that the role of copper likely extends beyond electron transfer (Figure 1).32
Figure 2.

Absorption spectrum of [Cu(CN)2]TBA in CH3CN at r.t.
 |
Finally, we have examined the chemical competence of [Cu(CN)2]TBA under our coupling conditions by irradiating it in the presence of cyclohexyl chloride. Cyanation proceeds in 14% yield in the absence of TBAI, whereas the addition of TBAI leads to a substantial improvement in coupling efficiency (98% yield) (eq 9); no product is formed in the absence of light. These data for stoichiometric reactions of [Cu(CN)2]TBA are consistent with our observation that the presence of iodide anion is beneficial, although not absolutely required, for copper-catalyzed cyanations of alkyl chlorides (Table 4, entries 1 and 2). Similarly, photoinduced cyanations in which [Cu(CN)2]TBA is employed as a catalyst are less effective under iodide-free conditions than when iodide is added (Table 4, entries 7 and 8; cf. entries 1 and 3).
 |
CONCLUSION
We have substantially expanded the scope of photoinduced, copper-catalyzed cross-couplings of alkyl electrophiles: with respect to the nucleophile, we have shown for the first time that a carbon nucleophile can be employed; with respect to the electrophile, we have provided the first examples of the use of secondary alkyl chlorides as electrophiles. More specifically, we have established that, with a combination of light and a copper catalyst, the cyanation of a variety of unactivated secondary alkyl chlorides, including very sterically demanding substrates, proceeds in good yield under unusually mild conditions (room temperature) to generate nitriles, a useful class of organic molecules. An inexpensive pre-catalyst (CuI) and light source are used, no added ligand is necessary, the method is versatile (e.g., secondary alkyl bromides, a very hindered primary alkyl chloride, and an unactivated tertiary alkyl chloride serve as suitable electrophiles under the same conditions), and the cyanation can even be achieved at 0 °C. Initial mechanistic observations are consistent with possible roles for [Cu(CN)2]−*, alkyl iodides, and alkyl radicals as intermediates. Further efforts are underway to exploit the unusual reactivity provided by copper and light to accomplish new families of bond constructions for organic synthesis.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Junwon Choi, Nathaniel T. Kadunce, Dr. Wesley Sattler, Dr. David VanderVelde, Dr. Scott C. Virgil, Paul Walton, and Dr. Daniel T. Ziegler for experimental assistance and for helpful discussions, and we thank the Gordon and Betty Moore Foundation, the National Science Foundation (graduate research fellowship to T.S.R.), and the NIH (NIGMS: R01 GM109194) for funding.
Footnotes
Supporting Information
Experimental procedures and compound characterization data. The Supporting Information is available free of charge on the ACS Publications website at http://pubs.acs.org.
Notes
The authors declare no competing financial interests.
REFERENCES
- (1).(a) Colacot T, editor. New Trends in Cross-Coupling. RSC; Cambridge, UK: 2015. For recent monographs with leading references, see: [Google Scholar]; (b) de Meijere A, Oestreich M, editors. Metal-Catalyzed Cross-Coupling Reactions and More. Wiley–VCH; Weinheim, Germany: 2014. [Google Scholar]
- (2).Evano G, Blanchard N, editors. Copper-Mediated Cross-Coupling Reactions. John Wiley & Sons; Hoboken, NJ: 2013. For a recent monograph, see: [Google Scholar]
- (3).(a) Creutz SE, Lotito KJ, Fu GC, Peters JC. Science. 2012;338:647–651. doi: 10.1126/science.1226458. C–N. [DOI] [PubMed] [Google Scholar]; (b) Uyeda C, Tan Y, Fu GC, Peters JC. J. Am. Chem. Soc. 2013;135:9548–9552. doi: 10.1021/ja404050f. C–S. [DOI] [PubMed] [Google Scholar]; (c) Ziegler DT, Choi J, Muñoz-Molina JM, Bissember AC, Peters JC, Fu GC. J. Am. Chem. Soc. 2013;135:13107–13112. doi: 10.1021/ja4060806. C–N. [DOI] [PubMed] [Google Scholar]; (d) Tan Y, Muñoz-Molina JM, Fu GC, Peters JC. Chem. Sci. 2014;5:2831–2835. C–O. [Google Scholar]; (e) Yoo W-J, Tsukamoto T, Kobayashi S. Org. Lett. 2015;17:3640–3642. doi: 10.1021/acs.orglett.5b01645. For a related study, see: [DOI] [PubMed] [Google Scholar]
- (4).Sagadevan A, Hwang KC. Adv. Synth. Catal. 2012;354:3421–3427. For independent work by Hwang (C–C), see: [Google Scholar]
- (5).(a) Bissember AC, Lundgren RJ, Creutz SE, Peters JC, Fu GC. Angew. Chem., Int. Ed. 2013;52:5129–5133. doi: 10.1002/anie.201301202. Carbazoles as nucleophiles: [DOI] [PubMed] [Google Scholar]; (b) Do H-Q, Bachman S, Bissember AC, Peters JC, Fu GC. J. Am. Chem. Soc. 2014;136:2162–2167. doi: 10.1021/ja4126609. Amides as nucleophiles. [DOI] [PubMed] [Google Scholar]
- (6).(a) Majek M, von Wangelin A. J. Angew. Chem., Int. Ed. 2013;52:5919–5921. doi: 10.1002/anie.201301843. [DOI] [PubMed] [Google Scholar]; (b) Paria S, Reiser O. ChemCatChem. 2014;6:2477–2483. [Google Scholar]
- (7).(a) Murahashi S-I, editor. Science of Synthesis. Vol. 19. Georg Thieme Verlag; Stuttgart, Germany: 2004. For leading references, see: [Google Scholar]; (b) Fleming FF, Yao L, Ravikumar PC, Funk L, Shook BC. J. Med. Chem. 2010;53:7902–7917. doi: 10.1021/jm100762r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8). Vildagliptin and verapamil are two examples of alkyl nitriles that serve as pharmaceutical drugs.
- (9).(a) Smiley RA, Arnold C. J. Org. Chem. 1960;25:257–258. For example, see: (189 °C; DMSO). 2-Chlorobutane and 2-chlorooctane were cyanated in 69% and 70% yield, respectively. [Google Scholar]; (b) Cook FL, Bowers CW, Liotta CL. J. Org. Chem. 1974;39:3416–3418. (83 °C; CH3CN/18-crown-6). 2-Chlorooctane was cyanated in 78% yield (based on recovered starting material; 10 days), whereas cyclohexyl chloride did not furnish any of the desired product. [Google Scholar]; (c) Shaw JE, Hsia DY, Parries GS, Sawyer TK. J. Org. Chem. 1978;43:1017–1018. (80 °C; HMPA). 2-Chlorooctane was cyanated in 87% yield. [Google Scholar]; (d) Reddy MS, Rajan ST, Eswaraiah S, Satyanarayana R. Improved Process for Manufacture of Pregabalin. Patent WO 2009/001372 A2, Dec 31, 2008. A secondary alkyl chloride was cyanated in ~70% yield. [Google Scholar]
- (10).(a) Satoh Y, Obora Y. RSC Adv. 2014;4:15736–15739. For example, see: Nickel catalyst/60 °C. [Google Scholar]; (b) Ren Y, Dong C, Zhao S, Sun Y, Wang J, Ma J, Hou C. Copper catalyst/180 °C. Tetrahedron Lett. 2012;53:2825–2827. [Google Scholar]; (c) Ren Y, Yan M, Zhao S, Sun Y, Wang J, Yin W, Liu Z. Tetrahedron Lett. 2011;52:5107–5109. Palladium catalyst/140 °C. [Google Scholar]; (d) Zieger HE, Wo S. J. Org. Chem. 1994;59:3838–3840. Titanium catalyst/0 °C (benzhydryl chloride): [Google Scholar]
- (11). The UVC bulbs can be purchased from a retailer such as Amazon.com ($15).
- (12).(a) Notes: The temperature of the reaction mixture during the course of the cross-coupling has been determined to be ≤25 °C.; (b) A very small amount (~2%) of reduction of the alkyl chloride (Cl→H) is observed.
- (13). An aryl halide (iodide) can also undergo cyanation, albeit in modest yield.
- (14).(a) Notes: In the absence of light and/or CuI, no cyanation was observed.; (b) This method is not yet general: under the current conditions, more hindered tertiary alkyl chlorides react more slowly. We have not yet pursued optimization of this process.
- (15).Reetz MT, Chatziiosifidis I. Angew. Chem., Int. Ed. 1981;20:1017–1018. The cyanation of a tertiary alkyl chloride under SN1 conditions has been described. For example, see: [Google Scholar]
- (16). In preliminary studies under our standard conditions, an unactivated secondary alkyl fluoride and an unactivated secondary alkyl tosylate did not serve as suitable coupling partners.
- (17). Note: We are also considering mechanisms that incorporate features such as out-of-cage chemistry of the alkyl radical, including carbon–carbon bond formation via reaction of the alkyl radical with a Cu(I)–CN complex.
- (18). Cyclohexyl chloride can be a challenging substrate for cyanation, including under “naked nucleophile” conditions (Reference 9b)
- (19). During the course of a catalyzed cyanation, we have observed the accumulation of only a trace (<1%) of the alkyl iodide.
- (20). Photolysis (254 nm) of cyclohexyl iodide in CH3CN leads to its gradual disappearance over 24 hours. In contrast, under the same conditions, cyclohexyl chloride can be recovered quantitatively after 24 hours (in the presence of TBAI (1.0 equiv), some cyclohexyl chloride is consumed (~25%)
- (21).Kropp PJ, Poindexter GS, Pienta NJ, Hamilton DC. J. Am. Chem. Soc. 1976;98:8135–8144. For an example and leading references, see: These authors report that, under certain conditions, a sequence of homolysis and then electron transfer can generate carbocationic intermediates. [Google Scholar]
- (22).Cho CH, Lee JY, Kim S. Synlett. 2009:81–84. The cyanation of alkyl iodides by diethylphosphoryl cyanide via a radical pathway has been described: [Google Scholar]
- (23).(a) Buden ME, Martin SE, Rossi RA. In: CRC Handbook of Organic Photochemistry and Photobiology. Griesbeck A, Oelgemoller M, Ghetti F, editors. CRC Press; Boca Raton, Florida: 2012. pp. 347–368. For reviews of photoinduced SRN1 reactions, see: [Google Scholar]; (b) Penenory AB, Argüello JE, Albini A, Fagnoni M. Handbook of Synthetic Photochemistry. Wiley–VCH; Weinheim, Germany: 2010. Chapter 10. [Google Scholar]; (c) Bowman WR. In: Photoinduced Electron Transfer. Fox MA, Chanon M, editors. Elsevier; Amsterdam: 1990. pp. 487–552. [Google Scholar]
- (24). In contrast, in the case of an acyclic secondary alkyl iodide, we have observed cyanation under the same conditions.
- (25). When entry 6 of Table 4 was conducted in the absence of light, essentially no cyanation (<1%) was observed.
- (26).(a) Notes: This alkyl chloride is stable to irradiation at 254 nm.; (b) If CuI is employed as the catalyst, at partial conversion we observe a trace of the two diastereomers of the [3.3.0] bicyclic primary alkyl iodide, which could be formed through Cl→I transhalogenation of the starting electrophile, homolysis of the C–I bond, radical cyclization, and then radical–radical recombination to generate a C–I bond.
- (27).Pandey G, Rao KSSP, Palit DK, Mittal JP. J. Org. Chem. 1998;63:3968–3978. [Google Scholar]
- (28).(a) Murov SL, Carmichael I, Hug GL. Handbook of Photochemistry. CRC Press; New York: 1993. pp. 298–313. The Hatchard–Parker method was employed. See: [Google Scholar]; (b) Bolton JR, Stefan MI, Shaw P-S, Lykke KR. J. Photochem. Photobiol. A: Chem. 2011;222:166–169. [Google Scholar]
- (29).Nilsson M. Acta Chem. Scand. B. 1982;36:125–126. [Google Scholar]
- (30).(a) Horväth A, Stevenson KL. Inorg. Chem. 1993;32:2225–2227. For examples of studies of the reactivity of [Cu(CN)2]−* in aqueous solution, see: Formation of an exciplex with halide ions: [Google Scholar]; (b) Kemecsi F, Wood CE, Horväth A, Stevenson KL. J. Photochem. Photobiol. A: Chem. 1995;89:121–125. Electron transfer. [Google Scholar]
- (31).rier CK, Rankic DA, MacMillan DWC. Chem. Rev. 2013;113:5322–5363. doi: 10.1021/cr300503r. For a recent review of photoredox catalysis, see: [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Bagal DB, Kachkovskyi G, Knorn M, Rawner T, Bhanage BM, Reiser O. Angew. Chem., Int. Ed. 2015;54:6999–7002. doi: 10.1002/anie.201501880. For a recent example, see: [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


