Abstract
Background
Adults with alcohol use disorders (AUDs) show different behavioral and neurological functioning during emotional processing tasks from healthy controls. Adults with a family history (FHP) of AUD also show different activation in limbic brain areas, such as the amygdala. However, it is unclear if this pattern exists during adolescence before any episodes of heavy alcohol use.
Objectives
We hypothesized that the amygdalar response to subliminally-presented fearful faces would be reduced in FHP adolescents compared to peers who were family history negative (FHN) for AUD.
Method
An adapted Masked Faces paradigm was used to examine blood oxygen level-dependent response to subliminal fearful vs. neutral faces in 14 FHP (6 females, 8 males) and 15 FHN (6 females, 9 males) youth, ages 11–15 years. Both FHP and FHN youth had no history of heavy alcohol consumption.
Results
A significant difference was seen between groups in the left superior parietal lobule FHN youth showed deactivation to fearful and neutral masked faces compared to baseline, whereas FHP youth showed deactivation only to fearful masked faces. No significant differences in amygdalar activation were seen between groups.
Conclusion
The left superior parietal lobule is part of the fronto-parietal network, which has been implicated in attentional control. Lack of reduced neural activity to neutral faces among FHP youth may represent differences in suppressing attention networks to less salient emotional stimuli, or perhaps, a higher threshold of saliency for emotional stimuli among at-risk youth.
Keywords: Adolescence, amygdala, at-risk youth, brain, fear, fMRI, parietal, substance use disorders
Introduction
Adults with alcohol use disorders (AUDs) have shown differences in decoding emotional faces (1,2), and altered activation in affective limbic brain areas during emotional processing tasks (3,4), compared with their peers. Abstinent men with AUDs show less activation in limbic areas, including the amygdala, when viewing positive or negative emotional faces compared to controls (3) and less activation of the anterior cingulate cortex (ACC) while evaluating facial expression intensity (4). One possibility for these findings is that heavy alcohol use may have neurotoxic effects on limbic neurocircuitry, leading to differences in emotional processing between individuals with and without AUDs. Alternatively, it may be that altered processing of emotionally salient information leads individuals to seek external sources of coping, such as alcohol. For example, early abstinent adults with AUDs report worse emotional regulation, including less emotional awareness and more impulsivity, compared with adult social drinkers (5). However, it is unknown if these patterns exist prior to alcohol abuse, and thereby may represent a behavioral and neurobiological risk for heavy alcohol use.
To disentangle the effects of alcohol-induced neurotoxicity on emotional processing from pre-morbid vulnerability, it is important to study individuals prior to heavy alcohol use. Youth with a family history of alcoholism (FHP) are at heightened risk for developing AUDs (6,7) compared to youth without such family history (FHN). Similar to adults with AUDs, FHP young adults show less amygdalar activation in response to emotional faces compared to controls (8). Furthermore, during emotion recognition, older FHP adolescents and young adults with personal alcohol and drug use histories have decreased activation in the right middle temporal gyrus (9). Differential limbic response to emotionally salient stimuli may contribute to alterations in socio-emotional processing, that could be associated with depressive and anxiety symptoms, negative emotionality, as well as interpersonal difficulties in FHP individuals, features that have been reported in offspring of parents with AUDs (10,11). It is possible that socio-emotional problems and difficulties with affect regulation may lead to alcohol use as a coping strategy, and thus increase risk for alcohol abuse. However, given that previous studies included older participants who had experience with alcohol/drugs, it is unclear whether these findings were the result of family history risk or substance-related neurotoxicity.
There is growing evidence of differences in brain functioning during tasks of executive functioning and decision-making among FHP adolescents prior to onset of heavy alcohol use (12–15), but emotional processing in this age range has not been examined as extensively. Only one study has investigated the neural substrates of emotional processing in these adolescents using a verbal task and showed that resilient and vulnerable FHP youth (who differed on age of alcohol use onset, ever being drunk, and self-reported drinking problems) showed different activation in frontal and limbic regions compared to controls (16). Again, vulnerable FHP youth were not free of heavy alcohol use, confounding potential interpretations.
The neural correlates of emotional processing are complex, yet the amygdala has been most commonly implicated in conscious and unconscious processing of emotional faces (17–20), especially negatively valenced expressions, such as fear (21–24). Unconscious, or subliminal, processing of emotional stimuli may represent automaticity of limbic neural functions (i.e. bottom-up processing in the amygdala) that interact with regulatory functions (i.e. top-down processing via the reciprocal connections between the amygdala and prefrontal areas (25)). Amygdalar activation for subliminal emotional stimuli has been thought of as a bottom-up process for vigilance of threat (24). Adolescence is a time of neural development of limbic and regulatory functions (26,27). Similar to adults, amygdalar response to subliminal (or masked) emotional faces has been found in adolescents (18,28); however, differences have been noted, such as greater amygdalar response to facial expressions compared to adults (29,30), and a left, rather than right, lateralizing amygdalar pathway in response to masked faces (18). It is not known, however, if differences in these systems exist between FHP and FHN youth.
Since FHP individuals have altered emotional brain processing and are at heightened risk for developing AUDs, our goal was to investigate FHP youth prior to heavy alcohol use to disentangle pre-morbid vulnerability and alcohol’s effects on emotional brain functioning. A better understanding of differential emotion processing among at-risk youth will help identify affective behavioral and neurobiological risk factors for the development of AUDs. This is especially important as neural systems involved in emotional processing are developing during adolescence, suggesting heightened plasticity of these systems and possible response to intervention during this time of life. Based on findings of different amygdalar activation in response to negatively valenced emotional facial expressions among at-risk (8) and abstinent adults with AUDs (3), we hypothesized reduced activation in the amygdala among FHP adolescents prior to onset of AUD compared to FHN peers in response to subliminal fearful faces.
Methods
Participants
Fourteen FHP (6 females, 8 males) and 15 FHN (6 females, 9 males) youth, ages 11–15 years, were recruited through community advertisements and mailings. Following written consent and assent, separate structured telephone interviews were conducted with both the youth and one of their parents. Interviews consisted of the Diagnostic Interview Schedule for Children Predictive Scales (DISC-PS-4.32 b) (31,32), the Family History Assessment Module (FHAM) (33), the Brief Lifetime version of the Customary Drinking and Drug Use Record (34), and the Structured Clinical Interview (SCI) (35). For the current study, we excluded adolescents with left-handedness, as assessed by the Edinburgh Handedness Inventory (36), current (past year) diagnosis of a DSM-IV psychiatric disorder, absence of family history information, heavy alcohol and/or substance use (>10 lifetime alcoholic drinks or >2 drinks/occasion1, >5 uses of marijuana, any other drug use, or>4 cigarettes per day), neurological illness, significant head trauma (loss of consciousness >2 minutes), serious medical problems, learning disability, prenatal exposure to drugs or alcohol, reported history of psychotic disorders in biological parents (i.e. schizophrenia or bipolar I), irremovable metal, and pregnancy. All procedures were in accordance with the ethical standards of the Oregon Health & Science University (OHSU) Institutional Review Board. Youth were compensated monetarily for completing the study procedures. The participating parents were also compensated monetarily for completing the phone screen.
Family history of alcohol and substance use disorders
Although it is difficult to disentangle genetic and environmental effects, categorizing individuals based on first, or first and second degree relatives with an AUD, has been shown to be a valid predictor of alcohol use vulnerability and future dependence (37). Thus, the FHAM was administered during the structured telephone interview with both the youth and one of their biological parents to assess the presence of AUDs in first and second degree relatives. Based on the information provided on the FHAM, FHP youth were defined as those with a history of AUDs for at least one biological parent or two or more second degree relatives on either the maternal or paternal side of the family; youth with no alcohol or substance use disorders among relatives were considered FHN (37).
Demographic characteristics
To ensure comparable participant characteristics between groups, demographic information was assessed. General intelligence was evaluated using the Wechsler Abbreviated Scale of Intelligence (38). Grade point average (GPA) was collected from all youth as a measure of academic performance. Pubertal maturation at the time of the magnetic resonance imaging (MRI) scan was assessed using Petersen’s Pubertal Development Scale (39), which has a range of 1–4, with lower scores reflecting less pubertal maturation. Finally, the Hollingshead Index of Social Position (40), which uses parents’ education and employment level, was used to calculate youths’ socioeconomic status.
Imaging procedures
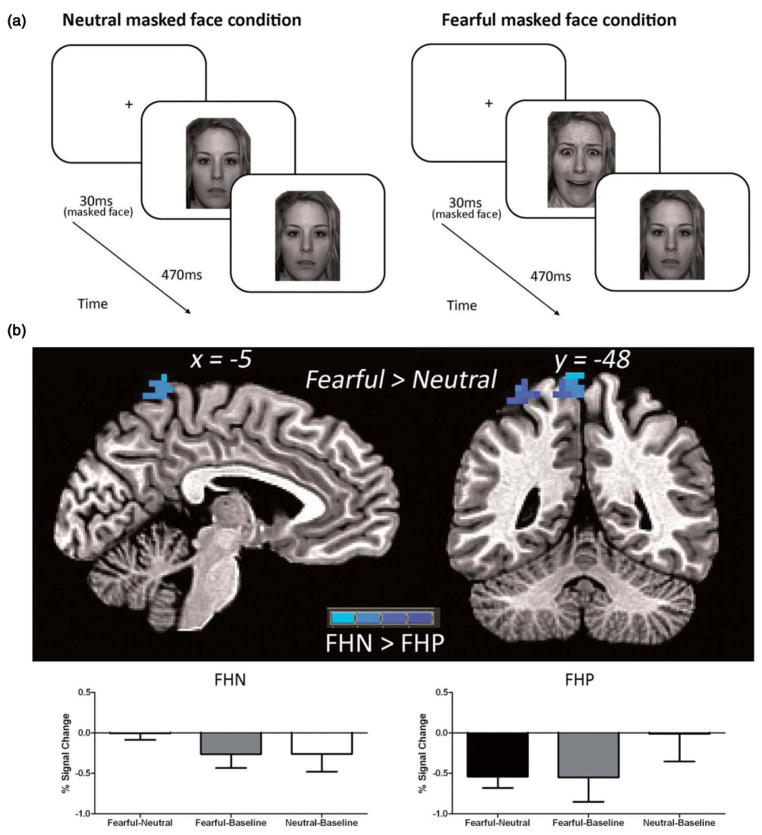
Image acquisition was conducted on a 3.0 Tesla Siemens Magnetom Tim Trio system at OHSU’s Advanced Imaging Research Center. Whole-brain, high-resolution T1-weighted MPRAGE images were acquired in the sagittal plane (13). A T2*-weighted echo planar blood oxygen level-dependent (BOLD) sequence was used to collect whole-brain functional images in the axial plane oblique to the anterior-commissure/ posterior-commissure (TR =2000 ms, TE =30 ms, field of view =240 mm, flip angle =90°, 33 slices no gap, resolution =3.75 × 3.75 × 3.8 mm, time of acquisition ~5:20) during an adapted Masked Faces paradigm (24). This task was utilized to investigate brain activity to subliminal fearful stimuli and consisted of two runs during which two 30 second blocks of negatively valenced subliminal fearful facial expressions and two 30 second blocks of neutral subliminal facial stimuli were presented. Masked neutral or fearful facial expressions appeared for 30 ms, while the facial masks appeared for 470 ms following the masked faces (see Figure 2a). A total of 60 masked faces were presented in each 30 second block. The two runs were counterbalanced for neutral and fearful masked emotional faces, such that one run started with neutral and the other with fearful blocks. Counterbalancing was done within each group, with approximately half the group receiving neutral blocks first. Fifteen seconds per run of unmodeled fixation between task blocks were included in the estimated baseline model (see below). Subjects were debriefed with a questionnaire following the functional magnetic resonance imaging (fMRI) task and asked whether or not they saw any of the subliminal masked faces; however, subjective ratings of emotional valence for facial stimuli were not collected. Data on this questionnaire was missing for one FHP participant. While both FHP and FHN youth indicated, at times, that they were able to visually perceive fearful subliminal masked faces during the task, there were no group differences (U(26) =79.0, Z =−1.28, p =0.41). Responses indicating visual perception of fearful subliminal masked faces most likely reflect false positive errors completing the questionnaire rather than true detection of the masked fearful face stimuli.
Figure 2.
(a) Participants were shown varying stimuli that consisted of either masked neutral or fearful facial expressions that appeared for 30 ms, while facial masks appeared for 470 ms following the masked faces. Masks were always a neutral facial expression. (b) There was a significant difference between the FHP and FHN groups in the left superior parietal lobule for the Fearful-Neutral contrast. Simple effects analysis showed that FHN adolescents showed deactivation in this region for both fearful and neutral blocks compared to baseline, whereas FHP youth only showed deactivation in the left superior parietal lobe for fearful blocks.
Image processing
Using Analysis of Functional NeuroImages (AFNI) (41), data processing included slice timing correction, motion correction, masking, co-registration of functional to anatomical images, spatial smoothing using a Gaussian filter (full-width half maximum =6 mm kernel), and signal normalization. TRs that showed >2.5 mm or 2.5° in any of six displacement or rotational parameters were censored from the subsequent analyses, resulting in one FHP participant requiring 10 time-points to be censored. Analysis of the root mean square of motion indicated no differences in movement between groups for the remaining TRs (t(27) =1.25, p =0.22). A general linear model was used to correlate time series data with a vector representing the task design. This was done to create regressors of interest for subliminal facial stimuli in the negatively valenced and neutral blocks, while accounting for the delay of the hemodynamic response and covarying for motion and linear trends (42). The fit coefficients derived from fitting the time series data to the model represented the BOLD response, which was contrasted between the fearful facial stimuli and baseline, neutral facial stimuli and baseline, and fearful-neutral facial stimuli. Functional data sets were resampled into 3 mm3 voxels and transformed into standard Talairach coordinates (43).
Statistical analyses
Analyses were performed in PASW Statistics 18 (PASW, Chicago, IL, USA) and AFNI. Data normality was assessed using Shapiro-Wilk. Nonparametric statistics were used when normality was violated. Demographic data were analyzed using independent samples t-tests, Mann-Whitney U-test, and χ2. To examine amygdala BOLD response to faces, one-sample t-tests were performed for whole-brain BOLD response for each face stimuli vs. baseline for both groups. To examine BOLD response to subliminal fearful vs. neutral faces, one-sample t-tests were performed for whole-brain BOLD response for negatively valenced stimuli vs. neutral stimuli for each group. To best represent task-related activity, individual group maps were thresholded at p<0.05 and then combined to form a map of task-related brain activity for the entire sample. Significant activation differences between FHP and FHN youth during brain response to fearful vs. neutral subliminal faces was then assessed in this voxel thresholded task-related activity map using independent samples t-test. To control for Type I error, a family-wise approach was used by applying Monte Carlo simulation using both a voxel and cluster threshold (44), based on the mask of task-related activity. Only clusters with a voxel threshold of p<0.05 and 83 contiguous (α<0.05) 3 mm3 voxels were considered significant.
Results
Participant demographics for FHP and FHN can be found in Table 1. Groups were predominately Caucasian (χ2 =0.45, p =0.50), and matched on age (t(27) =−0.10, p =0.92), IQ (t(26) =1.10, p =0.28), GPA (t(27) =1.08, p =0.29), pubertal status (U =98.5, Z =−0.29, p =0.78), and socioeconomic status (SES) (t(27) =−1.60, p =0.12).
Table 1.
Participant demographics for FHP and FHN youth.
| Demographic characteristics | FHN (n =15) | FHP (n =14) | χ2 | t/U | p |
|---|---|---|---|---|---|
| Female (n) | 6 | 6 | 0.02 | 0.88 | |
| Caucasian (%) | 93.30 | 85.70 | 0.45 | 0.50 | |
| Age | 13.67 (1.60) | 13.73 (1.49) | −0.10 | 0.92 | |
| Pubertal statusa | 2.62 (0.84) | 2.70 (0.78) | 98.5 | 0.78 | |
| IQb | 119.86 (10.42)c | 115.93 (8.37) | 1.10 | 0.28 | |
| GPAd | 3.56 (0.40) | 3.40 (0.40) | 1.08 | 0.29 | |
| SESe | 22.33 (10.73) | 29.21 (12.36) | −1.60 | 0.12 | |
| Alcohol use | n =0 | n =2f | |||
| Cigarette use | n =0 | n =3g | |||
| Marijuana use | n =0 | n =1h |
Mean (SD), unless otherwise indicated.
Mean score of Pubertal Development Scale, range of 1–4, with 1 indicating pre-puberty and 4 indicating post-puberty.
Wechsler Abbreviated Scale of Intelligence (WASI), standard score.
n =14 because WASI score was not obtained for one participant.
Grade Point Average.
Hollingshead Index of Social Position, range of 11–77, with higher scores indicating lower SES.
One youth reported consuming three drinks once in his lifetime; one other youth reported drinking one drink on two occasions in her lifetime.
Two youth had smoked cigarettes twice in their lifetime, and one youth had smoked once in his lifetime.
One youth had smoked marijuana once in his lifetime.
Imaging
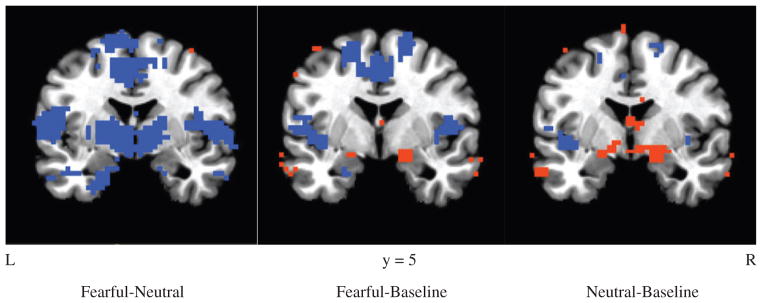
For both face stimuli conditions (fearful-baseline and neutral-baseline contrasts), amygdalar activation was seen in both FHN and FHP groups (Figure 1). For subliminal fearful vs. neutral faces (e.g. fearful-neutral contrast) similar patterns of brain activation were seen for both FHN and FHP subjects, including deactivation of limbic regions such as the insula, ACC, and orbital frontal cortex (OFC). In addition, a significant difference was also seen between the groups for the fearful-neutral contrast in the left superior parietal lobule [107 voxels; coordinates: x =−5, y =−47, z =72] (Figure 2 and Table 2). FHN youth showed deactivation in this region during both fearful and neutral blocks. FHP youth only showed deactivation in the left superior parietal lobe during masked fearful faces, but did not show deactivation during the neutral blocks. A region of interest (ROI) analysis indicated no significant group differences in amygdalar activation in either right or left amygdala for fearful-baseline, neutral-baseline, or fearful-neutral contrasts (p/α <0.05, ≥10 voxels). Within each group, amygdalar brain activity in the fearful-neutral contrast, was also absent at a voxel threshold of p<0.05, although there was some sub-threshold deactivation present in FHN youth at p =0.10.
Figure 1.
Contrasts are provided for each condition (fearful – neutral, fearful – baseline, neutral – baseline, p<0.05) across both FHP and FHN groups. Amygdalar activation was seen in both FHN and FHP groups for the fearful-baseline and neutral-baseline contrasts. Red color reflect regions in which activation was greater for the first term in the subtraction, while blue reflects areas of activation that were greater for the second term.
Table 2.
Results of fMRI for FHN>FHP youth during fearful>neutral contrast.
| Cluster | Talairach* | Voxels | Region | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| 1 | −5 | −47 | 72 | 106 | Left superior parietal lobule |
Peak coordinates.
Discussion
The current study shows deactivation of limbic regions, including the insula, ACC, and OFC to the fearful compared to neutral condition in both FHP and FHN youth. Differences in activation between FHP and FHN youth were found in the left superior parietal lobule. Specifically, FHN youth showed deactivation to both fearful and neutral masked faces compared to baseline, whereas FHP youth showed deactivation only to fearful masked faces but not to neutral masked faces. Consistent with previous research, youth showed activation in the amygdala for both neutral and fearful faces compared to baseline (29,30,45); however, contrary to our hypothesis, there were no differences in amygdalar activation between groups.
The left superior parietal lobule is part of a larger brain network, known as the fronto-parietal network, implicated in attentional control (46–48). Further, recent evidence suggests the fronto-parietal network plays a role in modulation of emotional faces through direct and indirect connections with subcortical pathways, such as the amygdala (48–50); albeit, these researchers found activation, rather than deactivation, in the fronto-parietal network among adult samples. The role of the superior parietal lobule in the fronto-parietal network in modulation of emotional faces has not been studied among adolescents, but compared to FHN peers, FHP youth show stronger association for pathways between the right superior parietal lobule and left middle frontal gyrus on a spatial working memory task (51). Additionally, on a visual working memory task, FHP youth show less fronto-parietal connectivity between the posterior parietal cortex and the dorsolateral prefrontal cortex (52). These latter findings suggest broader differences in this network in FHP compared to FHN youth.
Adolescence is a time when cognitive control and emotional regulatory systems are undergoing development and emotional stimuli have heightened saliency (29). Although not highly valenced, even neutral facial expressions are thought to be emotionally salient, particularly during adolescence (45). A lag in the development of neural systems underlying cognitive control relative to the development of limbic systems underlying emotional processes during adolescence has been thought to explain emotional sensitivity and risk-taking behavior (29). Thus, deactivation in attention to emotional stimuli, via the fronto-parietal network, might be adaptive during this time of heightened emotional saliency and developing cognitive control. Additionally, it may be that even emotional stimuli with low saliency, such as neutral faces, can elicit a deactivation response in attention networks for normal developing adolescents. This idea is supported in the current study, as FHN youth showed expected deactivation of attention circuitry via the fronto-parietal network to both highly valenced fearful faces and the less emotionally salient neutral faces. However, FHP youth displayed fronto-parietal deactivation to only highly emotional fearful faces and not to neutral faces.
There are several possible explanations for the differences noted in the fronto-parietal network between FHP and FHN youth when presented with subtle emotional stimuli. Lack of reduced neural activity for neutral faces among FHP youth may represent a higher threshold of saliency for emotional stimuli among these adolescents, and thus different neural processing for ambiguous stimuli. Alternatively, FHP youth may be deficient at suppressing attention networks to less salient emotional stimuli. Inefficiency in suppressing attention to subtle and ambiguous affective stimuli may represent risk for problems with emotional regulation (5) and socio-emotional difficulties (53) often seen among abstinent adults with AUDs. On the other hand, lack of reduced activation in the fronto-parietal network to less salient emotional stimuli may represent more efficient processing among FHP youth compared to FHN peers, such that FHP adolescents are able to process subtle emotional stimuli without suppressing this network. This could represent a neurobiological marker of resilience in at-risk youth, as other neural phenotypes reflecting resilience from heavy alcohol use have been reported (54,55).
The lack of greater amygdalar activation to fearful-neutral blocks was surprising given research supporting amygdalar activity in response to negatively valenced masked faces during similar tasks for adults (17,19–24) and adolescents (18,28). Diminished amygdalar response has been found with increasing demands on attention (56); however, it is unlikely the task in the current study taxed attention enough to explain lack of amygdalar response. Pine et al. (57) also did not find amygdala activation among adolescents using a masked faces task, which they primarily attributed to signal dropout, although significant amygdalar activity seen during the fearful-baseline and neutral-baseline contrasts in the current study rules out this possibility. Rather, given previous research suggesting that neutral faces are processed differently during adolescence than adulthood (45), it is possible that valence and/or arousal to masked fearful and neutral faces was not distinct enough to elicit differences in amygdalar activity between the two different task blocks.
Overall, the current study, although preliminary due to small sample size, offers support that FHP youth show altered activation in the superior parietal lobule, part of the fronto-parietal network, in response to a masked faces task compared to FHN peers. Future research should further investigate the functional connectivity between subcortical areas involved in emotional processing and fronto-parietal brain regions involved with attention in adolescents, as well as the role of atypical functioning in these networks to risk for developing AUDs.
Acknowledgments
Grant support for the authors of this study was provided by R01 AA017664 (Nagel), and a pilot grant to Nagel from the Portland Alcohol Research Center (P60 AA010760 [Crabbe]). A special thanks to Emily Maxwell, Troy Lubianski, and Christy Irvine for their assistance in data collection and data entry.
Footnotes
One youth with variable reporting later reported having had 3 drinks on an occasion.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
References
- 1.Maurage P, Campanella S, Philippot P, Martin S, de Timary P. Face processing in chronic alcoholism: a specific deficit for emotional features. Alcoholism: Clin Experim Res. 2008;32:600–606. doi: 10.1111/j.1530-0277.2007.00611.x. [DOI] [PubMed] [Google Scholar]
- 2.Philippot P, Kornreich C, Blairy S, Baert I, Den Dulk A, Le Bon O, Streel E, et al. Alcoholics’ deficits in the decoding of emotional facial expression. Alcoholism: Clin Experim Res. 1999;23:1031–1038. [PubMed] [Google Scholar]
- 3.Marinkovic K, Oscar-Berman M, Urban T, O’Reilly CE, Howard JA, Sawyer K, Harris GJ. Alcoholism and dampened temporal limbic activation to emotional faces. Alcoholism: Clin Experim Res. 2009;33:1880–1892. doi: 10.1111/j.1530-0277.2009.01026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salloum JB, Ramchandani VA, Bodurka J, Rawlings R, Momenan R, George D, Hommer DW. Blunted rostral anterior cingulate response during a simplified decoding task of negative emotional facial expressions in alcoholic patients. Alcoholism: Clin Experim Res. 2007;31:1490–1504. doi: 10.1111/j.1530-0277.2007.00447.x. [DOI] [PubMed] [Google Scholar]
- 5.Fox HC, Hong KA, Sinha R. Difficulties in emotion regulation and impulse control in recently abstinent alcoholics compared with social drinkers. Addictive Behav. 2008;33:388–394. doi: 10.1016/j.addbeh.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Dawson DA, Harford TC, Grant BF. Family history as a predictor of alcohol dependence. Alcoholism: Clin Experim Res. 1992;16:572–575. doi: 10.1111/j.1530-0277.1992.tb01419.x. [DOI] [PubMed] [Google Scholar]
- 7.Schuckit MA. Genetics of the risk for alcoholism. Am J Addictions/ Am Acad Psychiatrists Alcoholism Addictions. 2000;9:103–112. doi: 10.1080/10550490050173172. [DOI] [PubMed] [Google Scholar]
- 8.Glahn DC, Lovallo WR, Fox PT. Reduced amygdala activation in young adults at high risk of alcoholism: studies from the Oklahoma family health patterns project. Biolog Psychiatry. 2007;61:1306–1309. doi: 10.1016/j.biopsych.2006.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hill SY, Kostelnik B, Holmes B, Goradia D, McDermott M, Diwadkar V, Keshavan M. fMRI BOLD response to the eyes task in offspring from multiplex alcohol dependence families. Alcoholism: Clin Experim Res. 2007;31:2028–2035. doi: 10.1111/j.1530-0277.2007.00535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sher KJ, Walitzer KS, Wood PK, Brent EE. Characteristics of children of alcoholics: putative risk factors, substance use and abuse, and psychopathology. J Abnormal Psychol. 1991;100:427–448. doi: 10.1037//0021-843x.100.4.427. [DOI] [PubMed] [Google Scholar]
- 11.Elkins IJ, McGue M, Malone S, Iacono WG. The effect of parental alcohol and drug disorders on adolescent personality. Am J Psychiatry. 2004;161:670–676. doi: 10.1176/appi.ajp.161.4.670. [DOI] [PubMed] [Google Scholar]
- 12.Cservenka A, Herting MM, Nagel BJ. Atypical frontal lobe activity during verbal working memory in youth with a family history of alcoholism. Drug Alcohol Depend. 2012;123:98–104. doi: 10.1016/j.drugalcdep.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cservenka A, Nagel BJ. Risky decision-making: an fMRI study of youth at high risk for alcoholism. Alcoholism: Clin Experim Res. 2012;36:604–615. doi: 10.1111/j.1530-0277.2011.01650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silveri MM, Rogowska J, McCaffrey A, Yurgelun-Todd DA. Adolescents at risk for alcohol abuse demonstrate altered frontal lobe activation during stroop performance. Alcoholism: Clin Experim Res. 2011;35:218–228. doi: 10.1111/j.1530-0277.2010.01337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spadoni AD, Norman AL, Schweinsburg AD, Tapert SF. Effects of family history of alcohol use disorders on spatial working memory BOLD response in adolescents. Alcoholism: Clin Experim Res. 2008;32:1135–1145. doi: 10.1111/j.1530-0277.2008.00694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heitzeg MM, Nigg JT, Yau WY, Zubieta JK, Zucker RA. Affective circuitry and risk for alcoholism in late adolescence: differences in frontostriatal responses between vulnerable and resilient children of alcoholic parents. Alcoholism: Clin Experim Res. 2008;32:414–426. doi: 10.1111/j.1530-0277.2007.00605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Breiter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL, Buckner RL, Strauss MM, et al. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17:875–887. doi: 10.1016/s0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- 18.Killgore WD, Yurgelun-Todd DA. Cerebral correlates of amygdala responses during non-conscious perception of facial affect in adolescent and pre-adolescent children. Cognit Neurosci. 2010;1:33–43. doi: 10.1080/17588920903243957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wright CI, Martis B, Shin LM, Fischer H, Rauch SL. Enhanced amygdala responses to emotional versus neutral schematic facial expressions. Neuroreport. 2002;13:785–790. doi: 10.1097/00001756-200205070-00010. [DOI] [PubMed] [Google Scholar]
- 20.Yang TT, Menon V, Eliez S, Blasey C, White CD, Reid AJ, Gotlib IH, Reiss AL. Amygdalar activation associated with positive and negative facial expressions. Neuroreport. 2002;13:1737–1741. doi: 10.1097/00001756-200210070-00009. [DOI] [PubMed] [Google Scholar]
- 21.Kim MJ, Loucks RA, Neta M, Davis FC, Oler JA, Mazzulla EC, Whalen PJ. Behind the mask: the influence of mask-type on amygdala response to fearful faces. Soc Cognit Affective Neurosci. 2010;5:363–368. doi: 10.1093/scan/nsq014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morris JS, Frith CD, Perrett DI, Rowland D, Young AW, Calder AJ, Dolan RJ. A differential neural response in the human amygdala to fearful and happy facial expressions. Nature. 1996;383:812–815. doi: 10.1038/383812a0. [DOI] [PubMed] [Google Scholar]
- 23.Suslow T, Ohrmann P, Bauer J, Rauch AV, Schwindt W, Arolt V, Heindel W, Kugel H. Amygdala activation during masked presentation of emotional faces predicts conscious detection of threat-related faces. Brain Cognition. 2006;61:243–248. doi: 10.1016/j.bandc.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 24.Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J Neurosci. 1998;18:411–418. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim MJ, Loucks RA, Palmer AL, Brown AC, Solomon KM, Marchante AN, Whalen PJ. The structural and functional connectivity of the amygdala: from normal emotion to pathological anxiety. Behav Brain Res. 2011;223:403–410. doi: 10.1016/j.bbr.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ernst M, Pine DS, Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psycholog Med. 2006;36:299–312. doi: 10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dahl RE. Affect regulation, brain development, and behavioral/ emotional health in adolescence. CNS Spectrums. 2001;6:60–72. doi: 10.1017/s1092852900022884. [DOI] [PubMed] [Google Scholar]
- 28.Killgore WD, Yurgelun-Todd DA. Unconscious processing of facial affect in children and adolescents. Soc Neurosci. 2007;2:28–47. doi: 10.1080/17470910701214186. [DOI] [PubMed] [Google Scholar]
- 29.Somerville LH, Jones RM, Casey BJ. A time of change: behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain Cognit. 2010;72:124–133. doi: 10.1016/j.bandc.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guyer AE, Monk CS, McClure-Tone EB, Nelson EE, Roberson-Nay R, Adler AD, Fromm SJ, et al. A developmental examination of amygdala response to facial expressions. J Cognit Neurosci. 2008;20:1565–1582. doi: 10.1162/jocn.2008.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoven CW, Duarte CS, Lucas CP, Wu P, Mandell DJ, Goodwin RD, Cohen M, et al. Psychopathology among New York city public school children 6 months after September 11. Arch Gen Psychiatry. 2005;62:545–552. doi: 10.1001/archpsyc.62.5.545. [DOI] [PubMed] [Google Scholar]
- 32.Lucas CP, Zhang H, Fisher PW, Shaffer D, Regier DA, Narrow WE, Bourdon K, et al. The DISC Predictive Scales (DPS): efficiently screening for diagnoses. J Am Acad Child Adolesc Psychiatry. 2001;40:443–449. doi: 10.1097/00004583-200104000-00013. [DOI] [PubMed] [Google Scholar]
- 33.Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, Hesselbrock VM, et al. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcoholism: Clin Exp Res. 1995;19:1018–1023. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- 34.Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): a measure of adolescent alcohol and drug involvement. J Stud Alcohol. 1998;59:427–438. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- 35.Brown SA, Myers MG, Mott MA, Vik PW. Correlates of success following treatment for adolescent substance abuse. Appl Prevent Psychol. 1994;3:61–73. [Google Scholar]
- 36.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 37.Stoltenberg SF, Mudd SA, Blow FC, Hill EM. Evaluating measures of family history of alcoholism: density versus dichotomy. Addiction (UK) 1998;93:1511–1520. doi: 10.1046/j.1360-0443.1998.931015117.x. [DOI] [PubMed] [Google Scholar]
- 38.Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- 39.Petersen AC, Crockett L, Tobin-Richard M, Boxer A. Measuring pubertal status: reliability and validity of a self-report measure. Pennsylvania State University; University Park: 1985. [Google Scholar]
- 40.Hollingshead AB. Two factor index of social position. New Haven, CT: 1957. [Google Scholar]
- 41.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 42.Cohen MS. Parametric analysis of fMRI data using linear systems methods. NeuroImage. 1997;6:93–103. doi: 10.1006/nimg.1997.0278. [DOI] [PubMed] [Google Scholar]
- 43.Talairach J, Tournoux P. Three-dimensional Coplanar stereotaxic atlas of the human brain proportional system: an approach to cerebral imaging. New York: Thieme; 1988. [Google Scholar]
- 44.Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- 45.Thomas KM, Drevets WC, Whalen PJ, Eccard CH, Dahl RE, Ryan ND, Casey BJ. Amygdala response to facial expressions in children and adults. Biolog Psychiatry. 2001;49:309–316. doi: 10.1016/s0006-3223(00)01066-0. [DOI] [PubMed] [Google Scholar]
- 46.Culham JC, Brandt SA, Cavanagh P, Kanwisher NG, Dale AM, Tootell RB. Cortical fMRI activation produced by attentive tracking of moving targets. J Neurophysiol. 1998;80:2657–2670. doi: 10.1152/jn.1998.80.5.2657. [DOI] [PubMed] [Google Scholar]
- 47.Gitelman DR, Nobre AC, Parrish TB, LaBar KS, Kim YH, Meyer JR, Mesulam M. A large-scale distributed network for covert spatial attention: further anatomical delineation based on stringent behavioural and cognitive controls. Brain. 1999;122:1093–1106. doi: 10.1093/brain/122.6.1093. [DOI] [PubMed] [Google Scholar]
- 48.Pessoa L, Kastner S, Ungerleider LG. Attentional control of the processing of neural and emotional stimuli. Brain Res Cognit Brain Res. 2002;15:31–45. doi: 10.1016/s0926-6410(02)00214-8. [DOI] [PubMed] [Google Scholar]
- 49.Amting JM, Greening SG, Mitchell DG. Multiple mechanisms of consciousness: the neural correlates of emotional awareness. J Neurosci. 2010;30:10039–10047. doi: 10.1523/JNEUROSCI.6434-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vuilleumier P, Pourtois G. Distributed and interactive brain mechanisms during emotion face perception: evidence from functional neuroimaging. Neuropsychologia. 2007;45:174–194. doi: 10.1016/j.neuropsychologia.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 51.Spadoni AD, Simmons AN, Yang TT, Tapert SF. Family history of alcohol use disorders and neuromaturation: a functional connectivity study with adolescents. Am J Drug Alcohol Abuse. 2013;39:356–364. doi: 10.3109/00952990.2013.818680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wetherill RR, Bava S, Thompson WK, Boucquey V, Pulido C, Yang TT, Tapert SF. Frontoparietal connectivity in substance-naive youth with and without a family history of alcoholism. Brain Res. 2012;1432:66–73. doi: 10.1016/j.brainres.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thoma P, Friedmann C, Suchan B. Empathy and social problem solving in alcohol dependence, mood disorders and selected personality disorders. Neurosci Biobehav Rev. 2013;37:448–470. doi: 10.1016/j.neubiorev.2013.01.024. [DOI] [PubMed] [Google Scholar]
- 54.Weiland BJ, Nigg JT, Welsh RC, Yau WY, Zubieta JK, Zucker RA, Heitzeg MM. Resiliency in adolescents at high risk for substance abuse: flexible adaptation via subthalamic nucleus and linkage to drinking and drug use in early adulthood. Alcoholism: Clin Experim Res. 2012;36:1355–1364. doi: 10.1111/j.1530-0277.2012.01741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yau WY, Zubieta JK, Weiland BJ, Samudra PG, Zucker RA, Heitzeg MM. Nucleus accumbens response to incentive stimuli anticipation in children of alcoholics: relationships with precursive behavioral risk and lifetime alcohol use. J Neurosci. 2012;32:2544–2551. doi: 10.1523/JNEUROSCI.1390-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pessoa L, McKenna M, Gutierrez E, Ungerleider LG. Neural processing of emotional faces requires attention. Proc Natl Acad Sci USA. 2002;99:11458–11463. doi: 10.1073/pnas.172403899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pine DS, Grun J, Zarahn E, Fyer A, Koda V, Li W, Szeszko PR, et al. Cortical brain regions engaged by masked emotional faces in adolescents and adults: an fMRI study. Emotion. 2001;1:137–147. doi: 10.1037/1528-3542.1.2.137. [DOI] [PubMed] [Google Scholar]