Abstract
Introduction
This review assesses the impact of prevention interventions for people living with HIV on HIV-related mortality, morbidity, retention in care, quality of life, and prevention of ongoing HIV transmission in resource-limited settings (RLSs).
Methods
We conducted a systematic review of studies reporting the results of prevention interventions for people living with HIV in RLS published between January 2000 and August 2014. Standardized methods of searching and data abstraction were used.
Results
Ninety-two studies met the eligibility criteria: 24 articles related to adherence counseling and support, 13 on risk reduction education and condom provision, 19 on partner HIV testing and counseling, 14 on provision of family planning services, and 22 on assessment and treatment of other sexually transmitted infections. Findings indicate good evidence that adherence counseling and sexually transmitted infection treatment can have a high impact on morbidity, whereas risk reduction education, partner HIV testing and counseling, and family planning counseling can prevent transmission of HIV. More limited evidence was found to support the impact of these interventions on retention in care and quality of life. Most studies did not report cost information, making it difficult to draw conclusions about the cost-effectiveness of these interventions.
Conclusions
This evidence suggests that these prevention interventions, if brought to sufficient scale and coverage, can help support and optimize the impact of core treatment and prevention interventions in RLS. Further operational research with more rigorous study designs, and ideally with biomarkers and costing information, is needed to determine the best model for providing these interventions in RLS.
Keywords: HIV/AIDS, resource-limited settings, HIV prevention, people living with HIV, positive health, dignity, prevention
INTRODUCTION
Globally, an estimated 35 million people were living with HIV (PLHIV) at the end of 2013, of which 32.6 million live in low- and middle-income countries.1 Expansion of antiretroviral treatment (ART) services for PLHIV has led to a 22% decrease in HIV-related mortality since 2009. Although the number of people newly infected with HIV continues to decline, an estimated 2.1 million individuals acquired HIV in 2013.1 Assisting PLHIV to maintain good health and avoid transmission is essential to a sustainable effective HIV response.
Prevention interventions integrated into the routine HIV care offered in facility and community settings can both protect the health of PLHIV and promote shared responsibility for prevention of HIV transmission.2 These interventions take a holistic approach to meeting the needs of PLHIV by offering a comprehensive range of behavioral and biomedical interventions aimed at reducing the morbidity and mortality experienced by HIV-positive individuals and reducing the risk of transmission to HIV-negative partner(s) and infants. They also provide the support that PLHIV need to protect their physical and mental health while equipping them with the knowledge and skills necessary to protect the health of their partner(s) and families. As such, these interventions promote the link between respecting the human rights of PLHIV to access comprehensive health services and engaging PLHIV as equal partners in efforts to curb the HIV epidemic.2 Finally, these prevention interventions for PLHIV—also known as Prevention with Positives; Positive Prevention; or Positive Health, Dignity, and Prevention—provide a supportive foundation for optimizing high-impact prevention interventions including ART, prevention of mother-to-child transmission (PMTCT), male circumcision, and HIV testing targeted to individuals at highest risk of infection.
The US Government’s President’s Emergency Plan for AIDS Relief (PEPFAR) defines the minimum package of prevention interventions for PLHIV as including the following 5 components: (1) adherence counseling and support, (2) risk reduction education and condom provision, (3) HIV serostatus disclosure counseling and partner HIV testing and counseling (HTC), (4) family planning (FP) counseling and services, and (5) assessment and treatment of other sexually transmitted infections (STIs).3 This package of services is consistent with guidelines issued by the World Health Organization.4 Systematic integration of all 5 prevention interventions for PLHIV into existing HIV care and treatment programs in facility and community settings can improve PLHIV’s access to the full range of services, ensure that these services are consistently delivered and documented, and that services delivered meet the basic standards of quality necessary to achieve the desired outcomes.
Although evidence exists on the effectiveness of prevention interventions for PLHIV in US domestic settings,5,6 the data from resource-limited settings (RLSs) are lacking.7 Many donor agencies are experiencing reduced funding levels, requiring that resources be strategically prioritized to invest in high-impact evidence-based activities. The purpose of this systematic review is to summarize the evidence for the 5 prevention interventions for PLHIV in RLSs to provide guidance to national program managers and planners on how best to use their limited HIV prevention, care, and treatment resources to maximally impact the HIV epidemic. This article is part of a series of 12 systematic reviews published in this supplement that address the likely impact of HIV care and support interventions on the following clinical outcomes: (1) mortality, (2) morbidity, (3) retention in HIV care, (4) quality of life, and (5) prevention of ongoing HIV transmission. This article also describes data on the cost-effectiveness of the 5 prevention interventions, where available.
METHODS
Relevant literature was identified through database searches of PubMed, MEDLINE, Embase, Cumulative Index to Nursing and Allied Health Literature, Sociological Abstracts, and African Index Medicus using a standard set of search terms (see the Introduction Paper for general search terms and Table 1 for intervention definitions and search terms). Search results were imported into EndNote X4, a bibliographic citation management software, and duplicate citations deleted. Article titles were then reviewed and clearly irrelevant citations removed. Any article that appeared to contain relevant information during these electronic searches was retrieved and the bibliography was searched for additional references. In addition, the bibliographies of relevant systematic reviews, meta-analyses, or current guidelines were also examined to identify potentially relevant articles. Once a pool of potentially eligible studies was created, the full-text article was retrieved. Final inclusion/exclusion of studies was based on a thorough reading of the full-text article by at least 2 study authors.
TABLE 1.
Intervention Definitions and Key Word Search Terms for Prevention Interventions for PLHIV
| Intervention | Definition | Key Word Search Terms |
|---|---|---|
| Prevention interventions for PLHIV | A package of behavioral and biomedical HIV prevention interventions offered to PLHIV to prevent HIV-related morbidity and mortality, reduce onward transmission of HIV, and retain PLHIV in HIV care and treatment services | Positive prevention + HIV Prevention with Positives + HIV Positive health, dignity, and prevention |
| Adherence counseling and support | Interventions that support PLHIV to maintain optimal levels of adherence to prophylactic medications (eg, cotrimoxazole, isoniazid) and/or therapeutic regimens (eg, antiretrovirals) | HIV positive + adherence counseling PLHIV + adherence counseling |
| HIV serostatus disclosure counseling and partner HTC | Offering counseling to PLHIV on how they can safely disclose their HIV status to their sex partner(s) and how they can get their partner (s) tested for HIV. Partner testing can be integrated into other clinical services accessed by PLHIV (eg, antenatal, HIV care and treatment, TB) or offered through home- or community-based approaches | HIV positive + disclosure counseling PLHIV + disclosure counseling HIV + index partner testing HIV + couples testing and counseling |
| FP counseling and services | Assessing the reproductive intentions and fertility desires of PLHIV and then providing, as indicated, either FP counseling and contraceptives (either directly or through referral) OR safer pregnancy counseling and referral to PMTCT services | HIV positive + safe pregnancy counseling HIV positive + FP PLHIV + safe pregnancy counseling PLHIV + FP |
| Risk reduction education and condom provision | Ongoing counseling offered to PLHIV to support them in reducing their HIV risk behavior. Counseling typically includes messages on correct and consistent condom use at every sexual encounter, reducing number of sex partner(s), and alcohol reduction/elimination. In addition, PLHIV should be provided with an adequate supply of condoms (male or female) at every encounter with a provider or counselor in either a facility or community setting | PLHIV + safer sex HIV positive + safer sex Risk reduction counseling + PLHIV Risk reduction counseling + HIV positive HIV positive + condom distribution PLHIV + condom distribution Alcohol counseling + PLHIV Alcohol counseling + HIV positive |
| STI assessment and treatment | Assessing PLHIV for the signs and symptoms of other STIs [eg, vaginal/penile discharge, genital ulcers, and (for women) abdominal pain] and providing syndromic management according to national treatment algorithms for all patients who report symptoms and/or where symptoms are detected during a genital examination | PLHIV + STI assessment HIV positive + STI assessment PLHIV + syndromic management HIV positive + syndromic management PLHIV + STI treatment HIV positive + STI treatment |
To be eligible for inclusion, articles had to (1) report on 1 of the 5 prevention interventions, (2) specifically target PLHIV or HIV-serodiscordant couples, (3) be conducted in a RLS (based on categories of low-income and lower-middle income economies as defined by the World Bank), (4) include a quantitative comparison of individuals or groups who received the intervention vs. those who did not, or a comparison of individuals or groups before and after receiving the intervention, (5) present data on at least 1 of the 5 outcomes of interest (mortality, morbidity, retention in care, quality of life, and prevention of HIV transmission), and (6) be published between January 1, 2000 and August 30, 2014. Articles were also included if they provided information on costing or cost-effectiveness of 1 of the 5 prevention interventions in RLSs. Only articles published in English in a peer-reviewed journal were included in the review. Articles published in other languages and/or conference abstracts were not included.
A systematic process was then used to assess the quality of published studies and the likely impact of each intervention on the 5 clinical outcomes. Each article was read in its entirety and data were abstracted using a systematic coding form. Information abstracted from each article included study characteristics (eg, design, time period conducted, setting, and number of participants) and key findings. For each article, authors rated the internal validity of the study (eg, amount of bias, confounding, attrition, and other factors that could affect the interpretation of the study findings) and the external validity or generalizability of the study findings. For each article, the overall quality of evidence was rated as strong, medium, or weak. Once all articles were reviewed, the overall quality of evidence and expected impact of the intervention by outcome was determined based on the magnitude of effect demonstrated in individual studies, the quality of the body of evidence (all included studies), and consistency across all studies reviewed. The overall quality of evidence was then rated as good, fair, or poor and the expected impact of the intervention on each of the 5 clinical outcomes was rated as high, moderate, low, or uncertain. See the introductory article in this supplement for more details on how the various ratings were defined and determined.8
RESULTS
Of the 4132 articles identified through the search, many were eliminated based on a title and abstract review (Fig. 1). Three hundred eighty-seven abstracts were selected for review, of which 158 full-text articles were further evaluated. Of those, 92 were deemed to meet the eligibility criteria including 24 articles related to adherence counseling and support, 13 on risk reduction education and condom provision, 19 on disclosure and partner testing, 14 on provision of FP services, and 22 on STI assessment and treatment. The outcomes addressed within each intervention are summarized in Figure 1. These articles were used to generate the observations and conclusions described below and in Tables S1–S3 (see Supplemental Digital Content, http://links.lww.com/QAI/A646).
FIGURE 1.
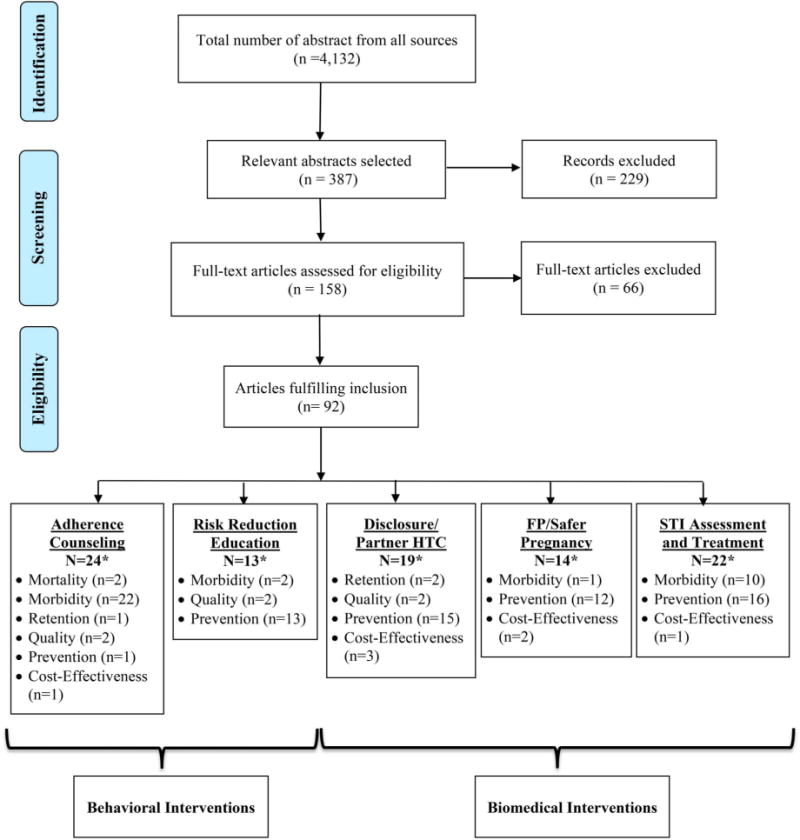
Disposition of citations during the search and screening process in the systematic review of prevention interventions for people living with HIV in RLSs. *Some articles reported multiple outcomes.
Adherence Counseling and Support
Twenty-four studies reporting on adherence counseling and support interventions were identified (see Table S2, Supplemental Digital Content, http://links.lww.com/QAI/A646).9–31,93 Two of the studies reported mortality as an outcome,9,10 22 reported morbidity,9–30 1 on retention in care,19 2 on quality of life,15,20 and 1 on prevention of HIV transmission,31 with some studies reporting on multiple outcomes. One article included information on the costs associated with providing adherence counseling and support.93 Most of the intervention studies were conducted in sub-Saharan Africa (19), followed by Asia (3) and South America (2).
Only 2 studies, with conflicting results, reported the effect of adherence counseling and support on mortality.9,10 In a randomized control trial from South Africa, survival was significantly better with directly observed therapy compared with self-administered therapy.9 However, another randomized control trial from Nigeria found no significant difference in mortality after 48 weeks of follow-up comparing partnerassisted therapy and standard of care.10 Thus, the overall body evidence for the effect of adherence counseling and support interventions on mortality is poor and the expected impact remains uncertain (see Table S3, Supplemental Digital Content, http://links.lww.com/QAI/A646).
In contrast, this review found good evidence that adherence counseling and support interventions can have a high impact on improving HIV-related morbidity measured through both adherence (self-report or through pill counts)10,11,13,16,20,21,23,24 and virologic suppression.10,12,13,15,17 Effective interventions include provider-delivered adherence counseling,13,15,16,24 food supplementation,11 treatment supporters,10 adherence support from community health workers,12,20,21 and mobile phone text messages.17,23 The evidence for directly observed therapy was mixed with some studies finding a positive association with adherence and clinical outcomes22,26 and other studies finding no association.9,14
Only 1 study, with null findings reported the effect of adherence support on retention in care.19 In a trial from Tanzania, Mugusi et al found no difference in the number of patients who stopped attending the clinic for more than 3 months comparing participants who received regular adherence counseling, those who received regular counseling plus a calendar, and those who received regular counseling and a treatment assistant.19 Overall, the body of evidence on the effect of adherence counseling on retention in care is poor and the overall impact remains uncertain.
Two studies, both with weak designs, found that adherence counseling and support was associated with improvements in the quality of life experienced by PLHIV including the amount of social support they received and a reduction in their depressive symptoms.15,20 These studies provide a fair amount of evidence that adherence counseling can have a moderate impact on the quality of life among HIV-infected adults, although additional research is needed.
Finally, only 1 small study examined the effect of adherence counseling on prevention of HIV transmission.31 This study, conducted among 60 women living with HIV (WLHIV), found that motivational interviewing was associated with higher rates of self-reported adherence and condom use.31 However, given the paucity of data, the overall body of evidence on the effect of adherence counseling on prevention of HIV transmission is poor and the expected impact remains uncertain. Additional research will be needed to determine if adherence counseling can lead to reductions in HIV risk behavior.
The cost-effectiveness of providing adherence counseling and support was reported in 1 cohort study from South Africa (see Table S3, Supplemental Digital Content, http://links.lww.com/QAI/A646). This study, conducted among 6833 HIV-infected adults, found that increasing patients’ ART adherence was associated with reduced hospitalization costs and lower mean monthly direct health care costs.93 Additional information is needed to assess the cost-effectiveness of the specific interventions for providing adherence counseling and support described above.
Risk Reduction Education and Condom Provision
Thirteen studies were identified that reported the effectiveness of risk reduction education interventions (see Table S1, Supplemental Digital Content, http://links.lww.com/QAI/A646).32–44 Two of the studies reported morbidity as an outcome,32,33 2 reported quality of life,34,35 and 13 reported prevention of HIV transmission.32–44 Most of the studies were conducted in sub-Saharan Africa (11), followed by South America (1) and Asia (1).
Only 2 studies, reporting conflicting results, reported the impact of risk reduction education on morbidity. A small randomized control trial conducted among 120 HIV-positive pregnant women attending primary care clinics in South Africa found that a 4-session risk reduction and coping intervention was associated with a significant reduction in STI incidence.33 However, a much larger randomized control trial in South Africa conducted among 1480 pregnant women found no intervention effects on STI incidence.32 Given these mixed findings, the evidence for the ability of risk reduction interventions to reduce STI incidence and other biologic outcomes is poor and the impact remains uncertain.
Two studies, a randomized control trial and an observational study, reported the effect of a group-based intervention on the quality of life experienced by PLHIV. Both studies found improved coping and social support after a risk reduction intervention.34,35 These studies provide fair evidence that risk reduction interventions may moderately improve the quality of life for PLHIV, although additional research is needed.
Although the evidence is mixed, 13 studies including several well-conducted randomized control trials and observational studies indicate that risk reduction interventions are associated with lower rates of self-reported sexual risk behaviors.32–44 This is particularly true when the education is provided during multiple high-intensity sessions.35–41,43 Only 1 randomized controlled trial focused on alcohol reduction counseling. This study, conducted among PLHIV reporting hazardous or binge drinking in Kenya, found that cognitive-behavioral therapy was associated with fewer drinking days and fewer mean drinks per day.42 The collective findings from the 13 studies provide good evidence that risk reduction interventions can have a high impact in reducing self-reported sexual risk behavior and preventing onward transmission of HIV.
Disclosure Counseling and Partner HTC
Nineteen studies were identified that reported the effectiveness of interventions to increase partner HTC (see Table S2, Supplemental Digital Content, http://links.lww.com/QAI/A646).45–59,94–97 Two of the studies reported retention in care as an outcome,45,46 2 reported quality of life,47,48 and 15 reported prevention of HIV transmission.45,46,48–59,94 In addition, 3 studies included information on the costs associated with providing partner HTC.95–97 All 16 intervention studies were conducted in sub-Saharan Africa.
Within antenatal programs, 2 studies provide fair evidence that couples and index partner testing can lead to improved retention of the pregnant mother in PMTCT programs.45,46 Women who received couples HTC were more likely to use nevirapine45 and to deliver in a hospital and return for the 18-month postnatal follow-up visit46 than women who received individual HTC. However, because only a few studies included retention as an outcome, the expected impact of couples HTC on improving retention in care remains uncertain.
Two studies found no significant association between partner testing and improved quality of life among HIV-infected pregnant women attending antenatal clinics.47,48 In a randomized control trial from Tanzania, there was no significant difference in rates of depression comparing a nurse-facilitated psychosocial support group to standard of care.47 A study from Zambia found no significant difference in the reported number of adverse events between women who tested as a couple compared with those who tested individually.48 Although this study provides some reassurance that couples HTC is not associated with an increased risk of experiencing an adverse event, additional research is needed. Overall, the body of evidence for the effect of couples and partner HTC on quality of life is poor and the expected impact remains uncertain.
Fifteen studies included prevention of onward HIV transmission as an outcome.45,46,48–59,94 In 6 of these studies, couples and partners HTC was associated with better uptake of interventions for PMTCT,45,50,56 reduced rates of vertical transmission,49 and reductions in self-reported sexual risk behavior46,58 among pregnant women attending antenatal services. Six other studies reported innovative strategies to increase uptake of partner HTC outside of antenatal settings.51,53–55,57,94 A study in Uganda found that counselor-facilitated disclosure was associated with increased disclosure rates among HIV-infected adults in a serodiscordant relationship.54 In addition, home-based index partner testing was associated with increased uptake of couples and partner HIV testing in Uganda55,57 and Kenya.94 Partner notification and contact tracing was also associated with increased rates of partner HIV testing among individuals newly diagnosed with HIV in Malawi51 and Cameroon.53 In the study from Cameroon, an average of 3.2 index cases were interviewed for every 1 case of HIV identified, suggesting that partner notification strategies may be a highly effective strategy for identifying new cases of HIV.53 The remaining 3 studies found null effects for the association between couples and partner HTC and prevention of HIV transmission.48,52,59 Altogether, these studies provide good evidence that partner notification, contact tracing, and various forms of indexpatient HTC can have a high impact on preventing both vertical and horizontal transmission of HIV.
Three studies reported cost-effectiveness information. In 1 analysis from a prospective cohort study, the total program cost for couples HTC was more expensive (US $44,013) than individual HTC (US $42,528), but couples HTC averted a greater number of infant infections (91 vs. 88).95 A study from Malawi compared the cost-effectiveness of 3 partner notification strategies: provider notification (provider attempts to notify indexes’ locatable partners), contract notification (index given 1 week to notify partners then provider attempts notification), and passive referral (index is encouraged to notify partners, standard of care). The costs per new case identified were $36 for provider notification, $18 for contract notification, and $8 for passive referral and the costs per partner tested were $19 (provider), $9 (contract), and $4 (passive).96 Similarly, findings from another costing study indicate that contact tracing could be a cheaper approach to detecting an undiagnosed HIV infection than both client-initiated and provider-initiated testing in rural settings with low-to-moderate HIV prevalence.97
FP Counseling and Services
Fourteen studies reported the provision of FP education and services (see Table S2, Supplemental Digital Content, http://links.lww.com/QAI/A646).60–71,98,99 One study reported morbidity,61 whereas 12 studies reported prevention of HIV transmission.60–71 In addition, 2 studies reported the costs associated with providing FP services.98,99 All 12 intervention studies were conducted in sub-Saharan Africa. FP interventions included a facilitated referral model,61 integration of FP services into HIV clinical care,62,64–68 an education video to promote long-acting methods of contraception,63,70,71 and group education sessions.69
Only 1 study reported the morbidity associated with hormonal contraceptive use among WLHIV.60 In this study, clinical disease progression was more common among hormonal contraceptive users compared with intrauterine device (IUD) users.60 Given the paucity of data, the overall body of evidence for the effect of contraceptive use on morbidity is poor and the expected impact remains uncertain (see Table S3, Supplemental Digital Content, http://links.lww.com/QAI/A646).
Prevention of unintended pregnancy through voluntary contraceptive use is a key component of efforts to prevent mother-to-child HIV transmission. Twelve studies reported the effects of FP counseling on contraceptive uptake and prevention of unintended pregnancy.60–71 Most of these studies reported significant increases in contraceptive uptake as a result of the intervention,61,62,65–68,70,71 although 2 studies reported null effects.64,69 Of the 4 studies that included pregnancy incidence as an outcome, 2 found a significant reduction in pregnancy incidence,68,71 and 2 found no change in pregnancy incidence as a result of the intervention.62,65 Taken together, these studies provide good evidence that FP interventions can have a high impact on improving contraceptive uptake but the impact on pregnancy incidence remains uncertain.
Cost analysis indicates that preventing unintended births among HIV-infected women with FP services would cost US $63 per birth averted globally.98 In a randomized control trial from Kenya, integration of FP services into HIV clinical care was associated with an average marginal cost of US $841 per site, US $48 per female patient, and US $1368 for each pregnancy averted.99
STI Assessment and Treatment
Twenty-two studies reported the assessment and treatment of other STIs among individuals living with HIV (see Table S2, Supplemental Digital Content, http://links.lww.com/QAI/A646).72–92,100 Ten studies reported morbidity,72–81 whereas 16 reported prevention of HIV transmission.73,76–78,81–92 In addition, 1 study reported the costs associated with STI treatment.100 Fourteen of the intervention studies were conducted in sub-Saharan Africa, 3 in South America, and 4 at multiple sites.
A systematic review of 37 studies highlights the degree to which PLHIV are coinfected with other STIs.101 In this review, the overall mean point prevalence for confirmed STI was 16.3% (SD = 16.4), and the median STI prevalence in PLHIV was 12.4%. The most common STIs were trichomoniasis (median: 18.8%), syphilis (9.5%), gonorrhea (9.5%), and chlamydia (5%). STI prevalence was greatest at the time of HIV diagnosis but once enrolled in care, there was no difference in STI prevalence between PLHIV receiving and not receiving ART.
Ten studies reported the effect of STI assessment and treatment on HIV-related morbidity.72–81 These studies include several well-conducted randomized control trials and observational studies that provide good evidence that STI treatment can reduce the morbidity experienced by PLHIV. A single 2 g dose of metronidazole was found to effectively treat Trichomoniasis vaginalis in both HIV-positive and negative women.72 However, persistent infection was associated with being on a nevirapine-based ART regimen and having a concurrent bacterial vaginosis infection.72 Similarly, penicillin therapy appeared to be effective at treating incident cases of syphilis in HIV-serodiscordant couples, irrespective of HIV status.74 Although treatment of HSV-2 with nucleoside analogs such as acyclovir and valacyclovir has been shown to reduce the occurrence of genital ulcers among PLHIV,73 it is unclear if these medications impact ulcer healing.77,78 Acyclovir treatment, however, may reduce the risk of HIV disease progression (defined as CD4 <200 cells/mm3 or WHO clinical stage 4) by as much as 16%.75,79,80
Sixteen studies reported the effect of STI assessment and treatment on the prevention of HIV transmission,73,76–78,81–92 including 15 studies on how coinfection with another STI can impact the infectiousness of HIV76–78,81–92 and 1 study directly assessing the impact of HSV-2 treatment on HIV incidence.73 Two of these studies indicate that genital HSV-2 is associated with increased cervicovaginal, seminal, and plasma HIV-1 RNA viral load concentrations.76,81 Treatment of HSV-2 with valacyclovir and acyclovir seems to reduce the frequency of lesional HIV-1 RNA shedding77,78,84 and plasma HIV-1 viral load concentrations.73,83,85,89,90 These drugs were also associated with reductions in seminal78,91 and rectal92 HIV-1 RNA plasma detection in men but not the frequency of cervical–vaginal shedding in women.78,85 A large multisite intervention trial conducted among HIV-serodiscordant couples failed to find an association between acyclovir treatment and a reduction in the risk of HIV transmission.73 However, the study did find a reduction in plasma HIV-1 RNA levels and a 73% reduction in the occurrence of genital ulcers as a result of HSV-2 treatment.73 Similarly, treatment of T. vaginalis was not associated with reductions in genital tract viral load.82,87 Taken together, these findings provide good evidence that STI assessment and treatment is likely to have a low impact on preventing HIV incidence at either the individual or population level.
One study from South Africa presented cost information on HSV-2–suppressive therapy.100 The costs of this therapy were estimated to be about US $737 per life year gained (95% confidence interval: $373 to $2489) if ART eligibility criteria were set at CD4 count <350 cells per microliter. These costs compare favorably with the estimated cost-effectiveness of ART in South Africa (~US $1200 per life year gained), suggesting that HSV-2–suppressive therapy may be a cost-effective intervention for delaying HIV disease progression among PLHIV not yet eligible for ART.
DISCUSSION
This systematic review summarizes the evidence for 5 interventions that comprise the minimum package of prevention interventions for PLHIV as defined by PEPFAR.3 These interventions include (1) adherence counseling and support, (2) partner HTC, (3) FP counseling and services, (4) risk reduction education, including an alcohol screening, and condom provision, and (5) assessment and treatment of other STIs. Findings from this review provide good evidence that adherence counseling and support and STI assessment and treatment can have a high impact on the morbidity experienced by PLHIV enrolled in HIV care. In addition, this review found good evidence that risk reduction education, partner HTC, and FP counseling can prevent onward transmission of HIV. More limited evidence was found to support the efficacy of these interventions at impacting mortality, retention in care, and quality of life. Most studies did not report cost information, making it difficult to draw conclusions about the cost-effectiveness of these interventions.
These findings are consistent with a randomized control trial conducted in Tanzania, Kenya, and Namibia, which found that integrating this package of prevention services into the routine care offered to PLHIV was associated with lower rates of unprotected vaginal sex and higher rates of contraceptive use (P. Bachanas, personal communication, February 19, 2015). Further research is needed, however, to determine the synergistic effects of providing all 5 of the prevention interventions together as a package instead of 5 separate interventions.
Programmatic Considerations
Results of this review indicate that prevention interventions for PLHIV can be effectively delivered by health care providers in health facilities5,29 and by lay or peer counselors in clinic and community settings.37,43 Many implementation challenges remain, however, to ensure that all PLHIV have access to effective prevention services. First, coordination and collaboration across multiple disciplines including care and treatment, prevention, and HTC will be required to integrate HIV prevention into the routine care offered to PLHIV in both clinic and community settings. Collaboration with other key stakeholders including health care workers with “real-world” implementation experience, technical advisors from international agencies, and PLHIV will also be necessary to ensure the continuity and sustainability of prevention programs for PLHIV. National HIV prevention, care, and treatment guidelines and policies may need to be revised to include prevention activities for PLHIV. In addition, national plans for implementation of prevention interventions for PLHIV may need to be developed. These plans should include clear and measurable objectives for integration, articulate plans for training facility and community-based service providers, describe methods for documenting and reporting provision of prevention interventions for PLHIV, and include strategies for ensuring that prevention interventions are implemented with high quality and fidelity.
At the facility and community level, programs will need an adequate number of trained staff to deliver prevention interventions for PLHIV. In addition, task-shifting some responsibilities to lay counselors, expert patients, and PLHIV volunteers is a potentially cost-effective and supportive model for delivering prevention counseling and partner and couples testing to HIV-positive patients. With appropriate supportive supervision from health care providers, lay counselors and patient volunteers can provide more in-depth counseling on prevention services and help clients set and meet prevention goals. Programs will also need to ensure a regular supply of necessary commodities including condoms, STI drugs, contraceptives, and HIV rapid test kits to ensure that providers are able to deliver prevention interventions for PLHIV. Finally, it is important that providers are able to document and monitor the prevention services they deliver to patients. Revising existing registers and patient records may be necessary to allow documentation of these services. Programs should also be encouraged to use their routine program data to continually improve the quality of services they offer to their HIV-infected clients.
Research Gaps
Prevention interventions were most effective when targeted to patients known to be at risk for nonadherence and/or continued high-risk behavior and when a focus on developing practical management skills was included as part of the intervention.8,102 There is now a need to identify how to tailor these prevention interventions to settings and populations in which HIV transmission is occurring. This includes patients who experience difficulty in reducing their HIV risk behaviors, young people (aged 15–24) who account for nearly one-third of individuals estimated to be newly infected with HIV globally,1 and key populations including men who have sex with men, sex workers, transgender people, and people who inject drugs.
Many of the FP interventions were associated with significant increases in uptake of highly effective contraceptive methods. These uptake rates, however, did not always translate into reduced incidence of unintended pregnancy primarily because of high levels of discontinuation, method switching, and user failure. Further operational research is needed to determine strategies for helping women maintain their contraceptive method of choice or to safely switch methods without compromising the efficacy of the current method to prevent unintended pregnancy.
In addition, concerns have been raised about hormonal contraceptive use among women living with and at risk for HIV.103 Several systematic reviews indicate that hormonal contraceptive use is safe in WLHIV and is not associated with HIV disease progression,104,105 female-to-male HIV transmission,106 or reduced ART efficacy.107 However, the data on hormonal contraceptive use and the risk of HIV acquisition among HIV-negative women in serodiscordant relationships are more difficult to interpret. Some studies indicate that progestin-only injectables are associated with an increased HIV-acquisition risk, whereas other studies report nonsignificant findings.108 A large clinical trial to provide further evidence on this issue is being planned. In the meantime, women at high risk for HIV acquisition, including women in serodiscordant relationships, should be counseled about the importance of using condoms in addition to progestin-only injectables to protect against unintended pregnancy and the risk of acquiring and transmitting HIV and other STIs.109
Although several studies found that treatment of HSV-2 seems to reduce the frequency of lesional HIV-1 RNA shedding77,78,84 and plasma HIV-1 viral load,73,83,85,89,90 a large multisite intervention trial failed to find an association between daily acyclovir treatment and reduced risk of HIV transmission.73 These findings highlight the unknown benefits of suppressing HIV in the genital tract, because the exact mechanism by which HIV transmission occurs, including the concentration of HIV required for transmission, remains unknown.78 Further research in this area is needed to determine what role, if any, that STI treatment may play in preventing HIV transmission.
Limitations of the Current Review
The ability to draw conclusions regarding the effectiveness of prevention interventions for PLHIV is complicated by the fact that these interventions varied widely in terms of target populations (eg, men, women, couples), intensities, modalities (provider-delivered, counselor-delivered, delivered to individuals, delivered to groups, etc.), and indicators (eg, condom use at last sex vs. condom use during the past 3 months, self-reported adherence using a visual analog scale vs. pill counts, etc.). The lack of standard indicators limits the ability to compare findings across studies and to draw conclusions regarding the strength of the evidence base.
Furthermore, weak study designs and an overreliance on self-reported data limit the generalizability of the findings. Further operational research, with more rigorous study designs and ideally with biomarkers, is needed to determine the best model for providing HIV prevention services to PLHIV in both facility and community settings. These studies should answer questions related to “how” services should be delivered, “who” should deliver these services, and “where” to reach PLHIV with these services including those traditionally underserved in facility-based settings (eg, young men, sex workers, men who have sex with men, etc.). In addition, delivery of prevention interventions for PLHIV in communities has not been rigorously evaluated and community-based programs have not been brought to scale.
Finally, most studies included in this review did not report cost information. As a result, it is difficult to determine the cost-effectiveness of these prevention interventions and services. Adherence counseling and support and STI assessment and treatment were associated with improved clinical outcomes including achievement of viral suppression and prevention of treatment failure. Interventions that reduce treatment failure are likely to be cost saving since they reduce the need for more expensive second-line antiretroviral medications and prevent the costs associated with treating opportunistic infections. To test this premise, future studies should include a costing component to determine whether these interventions are cost-effective.
CONCLUSIONS
In summary, there is good but not perfect evidence supporting each of the 5 interventions included in the minimum package of prevention interventions for PLHIV. Adherence counseling and support and STI assessment and treatment were associated with positive impacts on the morbidity experienced by PLHIV. In addition, risk reduction education, partner HTC, and FP counseling had positive impacts on preventing both horizontal and vertical transmission of HIV. This evidence suggests that these interventions, if brought to sufficient scale and coverage, can help support and optimize other high-impact interventions (eg, HTC, treatment as prevention, and PMTCT). Further operational research with more rigorous study designs, and ideally with biomarkers and costing information, is needed to determine the best model for providing these prevention services to PLHIV in RLSs.
Supplementary Material
Acknowledgments
The authors thank Gail Bang and Emily Weyant for their assistance with the literature search for this review, Margo Sloan and Kelly Woodland for their help in reading and reviewing articles for possible inclusion, and Naomi Bock and Janet Moore for their thoughtful suggestions on how to improve the manuscript.
Supported by the US President’s Emergency Plan for AIDS Relief (PEPFAR) through the US Department of State Office of the US Global AIDS Coordinator and Health Diplomacy, the US Centers for Disease Control and Prevention, the US Department of Defense, and the US Agency for International Development.
Footnotes
The authors have no conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.jaids.com).
The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the official positions of the US Department of State Office of the US Global AIDS Coordinator and Health Diplomacy, the Centers for Disease Control and Prevention, the US Agency for International Development, or the Naval Health Research Center.
References
- 1.World Health Organization. Global Update on the Health Sector Response to HIV, 2014. Geneva, Switzerland: 2014. Available at: http://apps.who.int/iris/bitstream/10665/128494/1/9789241507585_eng.pdf?ua=1. [Google Scholar]
- 2.Positive Health, Dignity and Prevention: Operational Guidelines. Geneva, Switzerland: 2013. Joint Programme on HIV/AIDS (UNAIDS) and the Global Network of People Living with HIV (GNP+) Available at: http://www.unaids.org/en/media/unaids/contentassets/documents/unaidspublication/2013/20130802_Positive_Health_Dignity_Prevention_Operational_Guidelines.pdf. [Google Scholar]
- 3.Guidance for the Prevention of Sexually Transmitted HIV Infections. Washington, DC: 2011. President’s Emergency Plan for AIDS Relief (PEPFAR) Available at: http://www.pepfar.gov/documents/organization/171303.pdf. [Google Scholar]
- 4.World Health Organization (WHO) Essential Prevention and Care Interventions for Adults and Adolescents Living with HIV in Resource-Limited Settings. Geneva, Switzerland: 2008. Available at: http://www.who.int/hiv/pub/guidelines/EP/en/index.html. [Google Scholar]
- 5.Crepaz N, Lyles CM, Wolitski RJ, et al. Do prevention interventions reduce HIV risk behaviours among people living with HIV? A metaanalytic review of controlled trials. AIDS. 2006;20:143–157. doi: 10.1097/01.aids.0000196166.48518.a0. [DOI] [PubMed] [Google Scholar]
- 6.Myers J, Shade S, Rose C, et al. Interventions delivered in clinical settings are effective in reducing risk of HIV transmission among people living with HIV: results from the Health Resources and Services Administration (HRSA)’s Special Projects of National Significance initiative. AIDS Behav. 2010;14:483–492. doi: 10.1007/s10461-010-9679-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kennedy C, Medley A, Sweat M, et al. Behavioural interventions for HIV-positive prevention in developing countries: a systematic review and meta-analysis. Bull World Health Organ. 2010;88:615–623. doi: 10.2471/BLT.09.068213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaplan JE, Hamm TE, Forhan S, et al. The impact of HIV care and support interventions on key outcomes in low and middle-income countries: a literature review–introduction. J Acquir Immune Defic Syndr. 2015 doi: 10.1097/QAI.0000000000000495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nachega JB, Chaisson RE, Goliath R, et al. Randomized controlled trial of trained patient-nominated treatment supporters providing partial directly observed antiretroviral therapy. AIDS. 2010;24:1273–1280. doi: 10.1097/QAD.0b013e328339e20e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taiwo BO, Idoko JA, Welty LJ, et al. Assessing the viorologic and adherence benefits of patient-selected HIV treatment partners in a resource-limited setting. J Acquir Immune Defic Syndr. 2010;54:85–92. doi: 10.1097/01.qai.0000371678.25873.1c. [DOI] [PubMed] [Google Scholar]
- 11.Cantrell RA, Sinkala M, Megazinni K, et al. A pilot study of food supplementation to improve adherence to antiretroviral therapy among food-insecure adults in Lusaka, Zambia. JAIDS. 2008;49:190–195. doi: 10.1097/QAI.0b013e31818455d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang LW, Kagaayi J, Nakigozi G, et al. Effect of peer health workers on AIDS care in Rakai, Uganda: a cluster-randomized trial. PLoS One. 2010;5:e10923. doi: 10.1371/journal.pone.0010923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chung MH, Richardson BA, Tapia K, et al. A randomized controlled trial comparing the effects of counselling and alarm device on HAART adherence and virologic outcomes. PLoS Med. 2011;8:e1000422. doi: 10.1371/journal.pmed.1000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Idoko JA, Agbaji O, Agaba P, et al. Direct observation therapy-highly active antiretroviral therapy in a resource-limited setting: the use of community treatment support can be effective. Int J STD AIDS. 2007;18:760–763. doi: 10.1258/095646207782212252. [DOI] [PubMed] [Google Scholar]
- 15.Khachani I, Harmouche H, Ammouri W, et al. Impact of a psychoeducative intervention on adherence to HAART among low-literacy patients in a resource-limited setting: the case of an Arab country— Morocco. J Int Assoc Physicians AIDS Care (Chic) 2012;11:47–56. doi: 10.1177/1545109710397891. [DOI] [PubMed] [Google Scholar]
- 16.Kunutsor S, Walley J, Muchuro S, et al. Improving adherence to antiretroviral therapy in sub-Saharan African HIV-positive populations: an enhanced adherence package. AIDS Care. 2012;24:1308–1315. doi: 10.1080/09540121.2012.661833. [DOI] [PubMed] [Google Scholar]
- 17.Lester RT, Ritvo R, Mills E, et al. Effects of a mobile phone short message service on antiretroviral treatment adherence in Kenya (WelTel Kenya1): a randomised trial. Lancet. 2010;376:1838–1845. doi: 10.1016/S0140-6736(10)61997-6. [DOI] [PubMed] [Google Scholar]
- 18.Mbuagbaw L, Thabane L, Ongolo-Zogo P, et al. The Cameroon Mobile Phone SMS (CAMPS) trial: a randomized trial of text messaging versus usual care for adherence to antiretroviral therapy. PLoS One. 2012;7:e46909. doi: 10.1371/journal.pone.0046909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mugusi F, Mugusi S, Bakari M, et al. Enhancing adherence to antiretroviral therapy at the HIV clinic in resource constrained countries; the Tanzanian experience. Trop Med Int Health. 2009;14:1226–1232. doi: 10.1111/j.1365-3156.2009.02359.x. [DOI] [PubMed] [Google Scholar]
- 20.Muñoz M, Finnegan K, Zeladita J, et al. Community-based DOTHAART accompaniment in an urban resource-poor setting. AIDS Behav. 2010;14:721–730. doi: 10.1007/s10461-009-9559-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nyamathi A, Hanson AY, Salem BE, et al. Impact of a rural village women (Asha) intervention on adherence to antiretroviral therapy in southern India. Nurs Res. 2012;61:353–362. doi: 10.1097/NNR.0b013e31825fe3ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pearson CR, Micek MA, Simoni JM, et al. Randomized control trial of peer-delivered, modified directly observed therapy for HAART in Mozambique. JAIDS. 2007;46:238–244. doi: 10.1097/QAI.0b013e318153f7ba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pop-Eleches C, Thirumurthy H, Habyarimana JP, et al. Mobile phone technologies improve adherence to antiretroviral treatment in a resource-limited setting: a randomized controlled trial of text message reminders. AIDS. 2011;25:825–834. doi: 10.1097/QAD.0b013e32834380c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sabin LL, DeSilva MB, Hamer DH, et al. Using electronic drug monitor feedback to improve adherence to antiretroviral therapy among HIV-positive patients in China. AIDS Behav. 2010;14:580–589. doi: 10.1007/s10461-009-9615-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sampaio-Sa M, Page-Shafer K, Bangsberg DR, et al. 100% adherence study: educational workshops vs. video sessions to improve adherence among ART-naive patients in Salvador, Brazil. AIDS Behav. 2008;12(suppl 4):S54–S62. doi: 10.1007/s10461-008-9414-0. [DOI] [PubMed] [Google Scholar]
- 26.Sarna A, Luchters S, Geibel S, et al. Short- and long-term efficacy of modified directly observed antiretroviral treatment in Mombasa, Kenya: a randomized trial. J Acquir Immune Defic Syndr. 2008;48:611–619. doi: 10.1097/QAI.0b013e3181806bf1. [DOI] [PubMed] [Google Scholar]
- 27.Siedner MJ, Lankowski A, Haberer JE, et al. Rethinking the “Pre” in pre-therapy counseling: no benefit of additional visits prior to therapy on adherence or viremia in Ugandans initiating ARVs. PLoS One. 2012;7:e39894. doi: 10.1371/journal.pone.0039894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simoni JM, Chen WT, Huh D, et al. A preliminary randomized controlled trial of a nurse-delivered medication adherence intervention among HIV-positive outpatients initiating antiretroviral therapy in Beijing, China. AIDS Behav. 2011;15:919–929. doi: 10.1007/s10461-010-9828-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Torpey K, Kabaso M, Mutale L, et al. Adherence support workers: a way to address human resource constraints in antiretroviral treatment programs in the public health settings in Zambia. PLoS One. 2008;3:e2204. doi: 10.1371/journal.pone.0002204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Loggerenberg F, Grant AD, Naidoo K, et al. Individualised motivational counselling to enhance adherence to antiretroviral therapy is not superior to didactic counselling in South African patients: findings of the CAPRISA 058 randomised controlled trial. AIDS Behav. 2015;19(1):145–56. doi: 10.1007/s10461-014-0763-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holstad MM, Essien JE, Ekong E, et al. Motivational groups support adherence to antiretroviral therapy and use of risk reduction behaviors in HIV positive Nigerian women: a pilot study. Afr J Reprod Health. 2012;16:14–27. [PMC free article] [PubMed] [Google Scholar]
- 32.Maman S, Moodley D, McNaughton-Reyes HL, et al. Efficacy of enhanced HIV counseling for risk reduction during pregnancy and in the postpartum period: a randomized control trial. PLoS One. 2014;9:e97092. doi: 10.1371/journal.pone.0097092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saleh-Onoya D, Reddy P, Ruiter R, et al. Condom use promotion among isiXhosa speaking women living with HIV in the Western Cape Province, South Africa: a pilot study. AIDS Care. 2009;21:817–825. doi: 10.1080/09540120802537823. [DOI] [PubMed] [Google Scholar]
- 34.Futterman D, Shea J, Besser M, et al. Mamekhaya: a pilot study combining a cognitive-behavioral intervention and mentor mothers with PMTCT services in South Africa. AIDS Care. 2010;22:1093–1100. doi: 10.1080/09540121003600352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olley BO. Improving well-being through psycho-education among voluntary counseling and testing seekers in Nigeria: a controlled outcome study. AIDS Care. 2006;18:1025–1031. doi: 10.1080/09540120600568756. [DOI] [PubMed] [Google Scholar]
- 36.Apondi R, Bunnell R, Ekwaru JP, et al. Sexual behavior and HIV transmission risk of Ugandan adults taking antiretroviral therapy: 3 year follow-up. AIDS. 2011;25:1317–1327. doi: 10.1097/QAD.0b013e328347f775. [DOI] [PubMed] [Google Scholar]
- 37.Cornman DH, Kiene SM, Christie S, et al. Clinic-based intervention reduces unprotected sexual behavior among HIV-infected patients in KwaZulu-Natal, South Africa: results of a pilot study. JAIDS. 2008;48:553–560. doi: 10.1097/QAI.0b013e31817bebd7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.da Silveria MF, dos Santos IS. Impact of an educational intervention to promote condom use among the male partners of HIV-positive women. J Eval Clin Pract. 2006;12:102–111. doi: 10.1111/j.1365-2753.2005.00626.x. [DOI] [PubMed] [Google Scholar]
- 39.Jones DL, Weiss SM, Bhat GJ, et al. Influencing sexual practices among HIV-positive Zambian women. AIDS Care. 2006;18:629–634. doi: 10.1080/09540120500415371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jones DL, Ross D, Weiss SM, et al. Influence of partner participation on sexual risk behavior reduction among HIV-positive Zambian women. J Urban Health. 2005;82:92–99. doi: 10.1093/jurban/jti111. [DOI] [PubMed] [Google Scholar]
- 41.Lightfoor MA, Kasirye R, Comulada WS, et al. Efficacy of a culturally adapted intervention for youth living with HIV in Uganda. Prev Sci. 2007;8:271–273. doi: 10.1007/s11121-007-0074-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Papas RK, Sidle JE, Gakinya BN, et al. Treatment outcomes of a stage 1 cognitive-behavioral trial to reduce alcohol use among human immunodeficiency virus-infected out-patients in western Kenya. Addiction. 2011;106:2156–2166. doi: 10.1111/j.1360-0443.2011.03518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peltzer K, Tabane C, Matseke G, et al. Lay counsellor-based risk reduction intervention with HIV-positive diagnosed patients at public HIV counseling and testing sites in Mpumalanga, South Africa. Eval Program Plann. 2010;33:379–385. doi: 10.1016/j.evalprogplan.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 44.Rongkavilit C, Naar-King S, Wang B, et al. Motivational interviewing targeting risk behaviors for youth living with HIV in Thailand. AIDS Behav. 2013;17:2063–2074. doi: 10.1007/s10461-013-0407-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Farquhar C, Kiarie JN, Richardson BA, et al. Antenatal couple counseling increases uptake of interventions to prevent HIV-1 transmission. J Acquir Immune Defic Syndr. 2004;37:1620–1626. doi: 10.1097/00126334-200412150-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kalembo FW, Zgambo M, Mulaga AN, et al. Association between male partner involvement and the uptake of prevention of mother-to-child transmission of HIV (PMTCT) interventions in Mwanza district, Malawi: a retrospective cohort study. PLoS One. 2013;8:e66517. doi: 10.1371/journal.pone.0066517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaaya SF, Blander J, Antelman G, et al. Randomized controlled trial evaluating the effect of an interactive group counseling intervention for HIV-positive women on prenatal depression and disclosure of HIV status. AIDS Care. 2013;25:854–862. doi: 10.1080/09540121.2013.763891. [DOI] [PubMed] [Google Scholar]
- 48.Semrau K, Kuhn L, Vwalika C, et al. Women in couples antenatal HIV counseling and testing are not more likely to report adverse social events. AIDS. 2005;19:603–609. doi: 10.1097/01.aids.0000163937.07026.a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aluisio A, Richardson B, Bosire R, et al. Male antenatal attendance and HIV testing are associated with decreased infant HIV infection and increased HIV-free survival. JAIDS. 2010;56:76–82. doi: 10.1097/QAI.0b013e3181fdb4c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Becker S, Mlay R, Schwandt H, et al. Comparing couples’ and individual voluntary counseling and testing for HIV at antenatal clinics in Tanzania: a randomized trial. AIDS Behav. 2010;14:558–566. doi: 10.1007/s10461-009-9607-1. [DOI] [PubMed] [Google Scholar]
- 51.Brown LB, Miller W, Kamanga G, et al. HIV partner notification is effective and feasible in sub-Saharan Africa: opportunities for HIV treatment and prevention. JAIDS. 2011;56:437–442. doi: 10.1097/qai.0b013e318202bf7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Conkling M, Shutes EL, Karita E, et al. Couples’ voluntary counseling and testing and nevirapine use in antenatal clinics in two African capitals: a prospective cohort study. J Int AIDS Soc. 2010;13:10. doi: 10.1186/1758-2652-13-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Henley C, Forgwei G, Welty T, et al. Scale-up and case-finding effectiveness of an HIV partner services program in Cameroon: an innovative HIV prevention intervention for developing countries. Sex Transm Dis. 2013;40:909–914. doi: 10.1097/OLQ.0000000000000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kairania R, Gray RH, Kiwanuka N, et al. Disclosure of HIV results among discordant couples in Rakai, Uganda: a facilitated couple counselling approach. AIDS Care. 2010;22:1041–1051. doi: 10.1080/09540121003602226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lugada E, Levin J, Abang B, et al. Comparison of home and clinic-based HIV testing among household members of persons taking antiretroviral therapy in Uganda: results from a randomized trial. JAIDS. 2010;55:245–252. doi: 10.1097/QAI.0b013e3181e9e069. [DOI] [PubMed] [Google Scholar]
- 56.Msuya SE, Mbizvo EM, Hussain A, et al. Low male partner participation in antenatal HIV counselling and testing in northern Tanzania: implications for preventive programs. AIDS Care. 2008;20:700–709. doi: 10.1080/09540120701687059. [DOI] [PubMed] [Google Scholar]
- 57.Were WA, Mermin JH, Wamai N, et al. Undiagnosed HIV infection and couple HIV discordance among household members of HIV-infected people receiving antiretroviral therapy in Uganda. J Acquir Immune Defic Syndr. 2006;43:91–95. doi: 10.1097/01.qai.0000225021.81384.28. [DOI] [PubMed] [Google Scholar]
- 58.Rosenberg NE, Pettifor AE, De Bruyn G, et al. HIV testing and counseling leads to immediate consistent condom use among South African stable HIV-discordant couples. JAIDS. 2012;62:226–233. doi: 10.1097/QAI.0b013e31827971ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Skogmar S, Shakely D, Lans M, et al. Effect of antiretroviral treatment and counselling on disclosure of HIV-serostatus in Johannesburg, South Africa. AIDS Care. 2006;18:725–730. doi: 10.1080/09540120500307248. [DOI] [PubMed] [Google Scholar]
- 60.Stringer EM, Kaseba C, Levy J, et al. A randomized trial of the intrauterine contraceptive device vs hormonal contraception in women who are infected with the human immunodeficiency virus. Am J Obstet Gynecol. 2007;197:144.e1–144.e8. doi: 10.1016/j.ajog.2007.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baumgartner JN, Green M, Weaver MA, et al. Integrating family planning services into HIV care and treatment clinics in Tanzania: evaluation of a facilitated referral model. Health Policy Plan. 2014;29(5):570–9. doi: 10.1093/heapol/czt043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grossman D, Onono M, Newmann SJ, et al. Integration of family planning services into HIV care and treatment in Kenya: a cluster-randomized trial. AIDS. 2013;27(suppl 1):S77–S85. doi: 10.1097/QAD.0000000000000035. [DOI] [PubMed] [Google Scholar]
- 63.Haddad L, Wall KM, Vwalika B, et al. Contraceptive discontinuation and switching among couples receiving integrated HIV and family planning services in Lusaka, Zambia. AIDS. 2013;27(suppl 1):S93–S103. doi: 10.1097/QAD.0000000000000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hoke T, Harries J, Crede S, et al. Expanding contraceptive options for PMTCT clients: a mixed methods implementation study in Cape Town, South Africa. Reprod Health. 2014;11:3. doi: 10.1186/1742-4755-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kosgei RJ, Lubano KM, Shen C, et al. Impact of integrated family planning and HIV care services on contraceptive use and pregnancy outcomes: a retrospective cohort study. J Acquir Immune Defic Syndr. 2011;58:e121–e126. doi: 10.1097/QAI.0b013e318237ca80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mark K, Menizen-Derr J, Stephenson R, et al. Contraception among HIV concordant and discordant couples in Zambia: a randomized controlled trial. J Women’s Health (Larchmt) 2007;16:1200–1210. doi: 10.1089/jwh.2006.0238. [DOI] [PubMed] [Google Scholar]
- 67.McCarraher DR, Vance G, Gwarzo U, et al. Changes in contraceptive use following integration of family planning into ART Services in Cross River State, Nigeria. Stud Fam Plann. 2011;42:283–290. doi: 10.1111/j.1728-4465.2011.00291.x. [DOI] [PubMed] [Google Scholar]
- 68.Ngure K, Heffron R, Mugo N, et al. Successful increase in contraceptive uptake among Kenyan HIV-1-serodiscordant couples enrolled in an HIV-1 prevention trial. AIDS. 2009;23(suppl 1):S89–S95. doi: 10.1097/01.aids.0000363781.50580.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sarnquist CC, Moyo P, Stranix-Chibanda L, et al. Integrating family planning and prevention of mother to child HIV transmission in Zimbabwe. Contraception. 2014;89:209–214. doi: 10.1016/j.contraception.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stephenson R, Vwalika B, Greenberg L, et al. A randomized controlled trial to promote long-term contraceptive use among HIV-serodiscordant and concordant positive couples in Zambia. J Women’s Health (Larchmt) 2011;20:567–574. doi: 10.1089/jwh.2010.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wall KM, Vwalika B, Haddad L, et al. Impact of long-term contraceptive promotion on incident pregnancy: a randomized controlled trial among HIV-positive couples in Lusaka, Zambia. J Acquir Immune Defic Syndr. 2013;63:86–95. doi: 10.1097/QAI.0b013e31827ee19c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Balkus JE, Richardson BA, Mochache V, et al. A prospective cohort study comparing the effect of single-dose 2 g metronidazole on Trichomonas vaginalis infection in HIV-seropositive versus HIV-seronegative women. Sex Transm Dis. 2013;40:499–505. doi: 10.1097/OLQ.0b013e31828fce34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Celum C, Wald A, Lingappa J, et al. Acyclovir and transmission of HIV-1 from persons infected with HIV-1 and HSV-2. N Engl J Med. 2010;362:427–439. doi: 10.1056/NEJMoa0904849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dionne-Odom J, Karita E, Kilembe W, et al. Syphilis treatment response among HIV-discordant couples in Zambia and Rwanda. Clin Infect Dis. 2013;56:1829–1837. doi: 10.1093/cid/cit146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lingappa JR, Baeten JM, Wald A, et al. Daily aciclovir for HIV-1 disease progression in people dually infected with HIV-1 and herpes simplex virus type 2: a randomised placebo-controlled trial. Lancet. 2010;375:824–833. doi: 10.1016/S0140-6736(09)62038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mayaud P, Nagot N, Konaté I, et al. ANRS 1285 Study Group Effect of HIV-1 and antiretroviral therapy on herpes simplex virus type 2: a prospective study in African women. Sex Transm Infect. 2008;84:332–337. doi: 10.1136/sti.2008.030692. [DOI] [PubMed] [Google Scholar]
- 77.Paz-Bailey G, Sternberg M, Puren AJ, et al. Improvement in healing and reduction in HIV shedding with episodic acyclovir therapy as part of syndromic management among men: a randomized, controlled trial. J Infect Dis. 2009;200:1039–1049. doi: 10.1086/605647. [DOI] [PubMed] [Google Scholar]
- 78.Phiri S, Hoffman IF, Weiss HA, et al. Impact of aciclovir on ulcer healing, lesional, genital and plasma HIV-1 RNA among patients with genital ulcer disease in Malawi. Sex Transm Infect. 2010;86:345–352. doi: 10.1136/sti.2009.041814. [DOI] [PubMed] [Google Scholar]
- 79.Reynolds SJ, Makumbi F, Newell K, et al. Effect of daily aciclovir on HIV disease progression in individuals in Rakai, Uganda, co-infected with HIV-1 and herpes simplex virus type 2: a randomised, double-blind placebo-controlled trial. Lancet Infect Dis. 2012;12:441–448. doi: 10.1016/S1473-3099(12)70037-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Roxby AC, Drake AL, Ongecha-Owuor F, et al. Effects of valacyclovir on markers of disease progression in postpartum women co-infected with HIV-1 and herpes simplex virus-2. PLoS One. 2012;7:e38622. doi: 10.1371/journal.pone.0038622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wolday D, Gebremariam Z, Mohammed Z, et al. The impact of syndromic treatment of sexually transmitted diseases on genital shedding of HIV-1. AIDS. 2004;18:781–785. doi: 10.1097/00002030-200403260-00009. [DOI] [PubMed] [Google Scholar]
- 82.Anderson BL, Firnhaber C, Liu T, et al. Effect of trichomoniasis therapy on genital HIV viral burden among African women. Sex Transm Dis. 2012;39:638–642. doi: 10.1097/OLQ.0b013e31825725ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Baeten JM, Strick LB, Lucchetti A, et al. Herpes simplex virus (HSV)-suppressive therapy decreases plasma and genital HIV-1 levels in HSV-2/HIV-1 coinfected women: a randomized, placebo-controlled, cross-over trial. J Infect Dis. 2008;198:1804–1808. doi: 10.1086/593214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Delany S, Mlaba N, Clayton T, et al. Impact of aciclovir on genital and plasma HIV-1 RNA in HSV-2/HIV-1 co-infected women: a randomized placebo-controlled trial in South Africa. AIDS. 2009;23:461–469. doi: 10.1097/QAD.0b013e32831db217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Drake AL, Roxby AC, Ongecha-Owuor F, et al. Valacyclovir suppressive therapy reduces plasma and breast milk HIV-1 RNA levels during pregnancy and postpartum: a randomized trial. J Infect Dis. 2012;205:366–375. doi: 10.1093/infdis/jir766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim HN, Wang J, Hughes J, et al. Effect of acyclovir on HIV-1 set point among herpes simplex virus type 2-seropositive persons during early HIV-1 infection. J Infect Dis. 2010;202:734–738. doi: 10.1086/655662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Masese LN, Graham SM, Gitau R, et al. A prospective study of vaginal trichomoniasis and HIV-1 shedding in women on antiretroviral therapy. BMC Infect Dis. 2011;11:307. doi: 10.1186/1471-2334-11-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mayaud P, Legoff J, Weiss HA, et al. Impact of acyclovir on genital and plasma HIV-1 RNA, genital herpes simplex virus type 2 DNA, and ulcer healing among HIV-1-infected African women with herpes ulcers: a randomized placebo-controlled trial. J Infect Dis. 2009;200:216–226. doi: 10.1086/599991. [DOI] [PubMed] [Google Scholar]
- 89.Mugwanya K, Baeten JM, Mugo NR, et al. High-dose valacyclovir HSV-2 suppression results in greater reduction in plasma HIV-1 levels compared with standard dose acyclovir among HIV-1/HSV-2 co-infected persons: a randomized, crossover trial. J Infect Dis. 2011;204:1912–1917. doi: 10.1093/infdis/jir649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nagot N, Ouédraogo A, Foulongne V, et al. Reduction of HIV-1 RNA levels with therapy to suppress herpes simplex virus. N Engl J Med. 2007;356:790–799. doi: 10.1056/NEJMoa062607. [DOI] [PubMed] [Google Scholar]
- 91.Zuckerman RA, Lucchetti A, Whittington WL, et al. HSV suppression reduces seminal HIV-1 levels in HIV-1/HSV-2 co-infected men who have sex with men. AIDS. 2009;23:479–483. doi: 10.1097/QAD.0b013e328326ca62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zuckerman RA, Lucchetti A, Whittington WL, et al. Herpes simplex virus (HSV) suppression with valacyclovir reduces rectal and blood plasma HIV-1 levels in HIV-1/HSV-2-seropositive men: a randomized, double-blind, placebo-controlled crossover trial. J Infect Dis. 2007;196:1500–1508. doi: 10.1086/522523. [DOI] [PubMed] [Google Scholar]
- 93.Nachega JB, Leisegang R, Bishai D, et al. Association of antiretroviral therapy adherence and health care costs. Ann Intern Med. 2010;152:18–25. doi: 10.7326/0003-4819-152-1-201001050-00006. [DOI] [PubMed] [Google Scholar]
- 94.Osoti AO, John-Stewart G, Kiarie J, et al. Home visits during pregnancy enhance male partner HIV counseling and testing in Kenya: a randomized clinical trial. AIDS. 2014;28:95–103. doi: 10.1097/QAD.0000000000000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.John FN, Farquhar C, Kiarie JN, et al. Cost effectiveness of couple counselling to enhance infant HIV-1 prevention. Int J STD AIDS. 2008;19:406–409. doi: 10.1258/ijsa.2008.007234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Armbruster B, Helleringer S, Kohler HP, et al. Exploring the relative costs of contact tracing in increasing HIV case-finding in sub-Saharan countries: the case of Likoma Island (Malawi) JAIDS. 2011;58:e29–e36. doi: 10.1097/QAI.0b013e31822a9fa8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rutstein SE, Brown LB, Biddle AK, et al. Cost-effectiveness of provider-based HIV partner notification in urban Malawi. Health Policy Plan. 2014;29:115–126. doi: 10.1093/heapol/czs140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Halperin DT, Stover J, Reynolds HW. Benefits and costs of expanding access to family planning programs to women living with HIV. AIDS. 2009;23(suppl 1):S123–S130. doi: 10.1097/01.aids.0000363785.73450.5a. [DOI] [PubMed] [Google Scholar]
- 99.Shade SB, Kevany S, Onono M, et al. Cost, cost-efficiency and cost-effectiveness of integrated family planning and HIV services. AIDS. 2013;27(suppl 1):S87–S92. doi: 10.1097/QAD.0000000000000038. [DOI] [PubMed] [Google Scholar]
- 100.Vickerman P, Devine A, Foss AM, et al. The cost-effectiveness of herpes simplex virus-2 suppressive therapy with daily aciclovir for delaying HIV disease progression among HIV-1-infected women in South Africa. Sex Transm Dis. 2011;38:401–409. doi: 10.1097/OLQ.0b013e31820b8bc8. [DOI] [PubMed] [Google Scholar]
- 101.Kalichman SC, Pellowski J, Turner C. Prevalence of sexually transmitted co-infections in people living with HIV/AIDS: systematic review with implications for using HIV treatments for prevention. Sex Transm Infect. 2011;87:183–190. doi: 10.1136/sti.2010.047514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Simoni J, Pearson C, Pantalone D, et al. Efficacy of interventions in improving highly active antiretroviral therapy adherence and HIV-1 RNA viral load: a meta-analytic review of randomized controlled trials. JAIDS. 2006;43(suppl 1):S23–S35. doi: 10.1097/01.qai.0000248342.05438.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Farley TM, Lusti-Narasimhan M. Hormonal contraception and risk of HIV acquisition: a difficult policy position in spite of incomplete evidence. Reprod Health Matters. 2012;20(39 suppl):41–47. doi: 10.1016/S0968-8080(12)39645-6. [DOI] [PubMed] [Google Scholar]
- 104.Curtis KM, Nanda K, Kapp N. Safety of hormonal and intrauterine methods of contraception for women with HIV/AIDS: a systematic review. AIDS. 2009;23(suppl 1):S55–S67. doi: 10.1097/01.aids.0000363778.58203.b6. [DOI] [PubMed] [Google Scholar]
- 105.Phillips SJ, Curtis KM, Polis CB. Effect of hormonal contraceptive methods on HIV disease progression: a systematic review. AIDS. 2013;27:787–794. doi: 10.1097/QAD.0b013e32835bb672. [DOI] [PubMed] [Google Scholar]
- 106.Polis CB, Phillips SJ, Curtis KM. Hormonal contraceptive use and female-to-male HIV transmission: a systematic review of the epidemiologic evidence. AIDS. 2013;27:493–505. doi: 10.1097/QAD.0b013e32835ad539. [DOI] [PubMed] [Google Scholar]
- 107.Drug Interactions Between Hormonal Contraceptive Methods and Antiretroviral Medications Used to Treat HIV. Washington, DC: 2014. President’s Emergency Plan for AIDS Relief (PEPFAR) Available at: http://www.usaid.gov/sites/default/files/documents/1864/HC_ART-Brief.pdf. [Google Scholar]
- 108.Polis CB, Phillips SJ, Curtis KM, et al. Hormonal contraceptive methods and risk of HIV acquisition in women: a systematic review of epidemiological evidence. Contraception. 2014;90:360–390. doi: 10.1016/j.contraception.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 109.World Health Organization. Hormonal Contraception and HIV: Technical Statement. Geneva, Switzerland: 2012. Available at: http://www.who.int/reproductivehealth/publications/family_planning/rhr_12_8/en/ [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


