Abstract
Purpose
Isolated local retroperitoneal recurrence (RPR) after radical nephrectomy (RN) for renal cell carcinoma (RCC) poses a therapeutic challenge. We investigated the outcomes of patients with localized RPR treated with surgical resection.
Methods
This was a retrospective single-institutional study of 102 patients with RPR treated with surgery from 1990-2014. Demographics, clinical and pathological features, location of RPR, perioperative complications were reported using descriptive statistics. Recurrence free survival (RFS) and cancer-specific survival (CSS) were studied using univariate and multivariate analyses.
Results
Median age at RPR diagnosis was 55 years (IQR 49-64). Sixty-two (60.8%) patients were pT3-4 and 20 (19.6%) were pN1. No patients had distant metastatic disease at time of RPR surgery. Median time from nephrectomy to RPR diagnosis was 19 months (IQR 5-38.8). The median size of resected RPR was 4.5cm (IQR 2.7-7). Median follow up after RPR surgery was 32 months (IQR 16-57). Metastatic progression was observed in 60 (58.8%) patients after RPR surgery. Neoadjuvant and salvage systemic therapy were administered in 46 (45.1%) and 48 (47.1%) patients, respectively. On multivariate analysis, pathological nodal stage at original nephrectomy and maximum diameter of RPR were identified as independent risk factors for cancer specific death.
Conclusion
Clinico-pathological factors at the time of nephrectomy as well as RPR surgery are important prognosticators. Aggressive surgical resection offers potential cure in a substantial number of patients with RPR with acceptable complications, and still plays a dominant role in the management of isolated locally recurrent RCC.
Keywords: retroperitoneal recurrence, renal cell carcinoma, local recurrence, radical nephrectomy, renal fossa, lymph node, adrenal
INTRODUCTION
Renal cell carcinoma (RCC) is an increasingly common malignancy. Even with curative RN, 20-40% of patients develop metastatic disease.1-5 Of these, untreated patients have a poor 5-year survival rate of <20% with a median survival of 6-12 months.1 Localized retroperitoneal recurrence (RPR) for RCC is a rare event that occurs in 1-3% of patients after RN.6 Treatment of RPR represents a significant surgical and therapeutic challenge, as patients are at high risk for overt metastatic disease and overall prognosis could be poor. 2
The data on natural history, patient outcomes and prognostic factors associated with RPR are limited and to date there is no standard management strategy. Earlier series have reported small subsets of patients with relatively long term survival, however such surgery has been associated with significant morbidity and mortality.7-10 In the era of targeted therapy for locally advanced and metastatic RCC, treatment paradigms using combinations of medical and surgical therapies in patients diagnosed with localized recurrence after nephrectomy are paramount in maximizing oncological outcome.9
Our study objective was to assess the surgical and oncological outcomes of patients undergoing surgical resection of RPR and to identify prognostic factors for survival after surgical resection.
PATIENTS AND METHODS
The University of Texas MD Anderson Cancer Center institutional review board approved the current study. From 1990 to 2014, we identified 102 patients who underwent prior radical nephrectomy for RCC and had subsequent isolated RPR that was managed by surgical resection. We defined RPR as pathologically proven RCC in the soft tissue/renal fossa including the psoas muscle, ipsilateral adrenal gland or ipsilateral retroperitoneal lymph nodes. Patients with non-RCC pathology or detectable distant metastatic disease at the time of RPR surgery were excluded. Patients treated by partial nephrectomy or ablative therapies were also excluded.
We assessed patient demographics, Charlson Comorbidity Index11, tumor pathology, time to local and/or distant progression, location of RPR, perioperative complications (using Clavien-Dindo system12), and outcomes. Recurrence after RPR surgery was defined as any radiological evidence of local and/or distant metastatic disease. The use of systemic therapy before or after RPR surgery was also recorded. We defined neoadjuvant systemic therapy as therapy given between time of radical nephrectomy and RPR surgery, and salvage systemic therapy as therapy given after recurrence following RPR surgery. Adjuvant therapy was not used in this study. RCC staging was assigned using the AJCC 2010 classification.13
Initial diagnosis of RPR was based on computerized tomography (CT) or magnetic resonance imaging (MRI) performed in the context of regular follow-up or due to local and/or systemic symptoms. Restaging at the time of suspected progression included comprehensive physical and laboratory evaluation, CT chest, CT or MRI abdomen and pelvis and nuclear bone imaging. MRI brain was done as clinical indicated. Follow-up consisted of history, physical examination, serum chemistry and liver function tests. Radiological evaluation with CT of chest, CT or MRI abdomen and pelvis were performed in all patients every 3-6 months for the first 2 years after RPR surgery and every 6-12 months thereafter.
At RPR surgery, retroperitoneal lymph node dissection (RPLND) was performed either in isolation or with adrenalectomy and/or soft tissue resection depending on the recurrence pattern in the retroperitoneum, and at the surgeon's discretion. RPLND involved removal of at least the para-aortic nodal tissue from the crus of the diaphragm to the bifurcation of the aorta for left-sided tumors, and para-caval and interaortocaval lymph nodes from the diaphragmatic crus to the bifurcation of the great vessels for right-sided tumors, and removal of any other suspicious lymph nodes.
Recurrence free survival (RFS) was defined as time from RPR surgery to a diagnosis of local or distant recurrence or last follow-up. Patients who were alive with NED at their last follow-up were censored on that date. Cancer-specific survival (CSS) was defined as time from RPR surgery to death from RCC or last follow-up. The two patients who died postoperatively were counted as cancer-specific deaths. Patients who were alive at their last follow-up were censored on that date. The Kaplan-Meier method14 was used to estimate RFS and CSS, and survival differences were assessed with log-rank statistic. Univariate and multivariate survival analysis were performed using Cox proportional hazard regression model. Statistical significance in this study was considered at p ≤ 0.05. All analyses were performed with SPSS®,version 22.
RESULTS
Analysis at time of radical nephrectomy
A total of 102 patients were identified as having a RPR of RCC after RN, and were surgically treated between 1990 and 2014. Eight-six (84.3%) patients underwent radical nephrectomy at outside institutions and were subsequently referred to our institution for RPR surgery. Median time from nephrectomy to RPR diagnosis was 19 months (IQR 5-38.8). At nephrectomy, 62 (60.8%) patients were pT3-4 and 20 (19.6%) patients were pN1. Table 1 shows other patient demographics and pathological features after RN.
Table 1.
Clinical and pathological characteristics in 102 patients with isolated ipsilateral RPR at time of original radical nephrectomy
| N (%) or Median (IQR) | |
|---|---|
| All patients | 102 (100) |
| Age, years | 55 (49-64) |
| Gender | |
| Female | 73 (71.6) |
| Male | 29 (28.4) |
| Race | |
| White | 83 (81.4) |
| Non-White | 19 (18.6) |
| Laterality of prior nephrectomy | |
| Right | 71 (68.9) |
| Left | 31 (30.1) |
| Prior nephrectomy type | |
| Open | 81 (79.4) |
| Laparoscopic | 20 (19.6) |
| Robotic | 1 (1) |
| Prior nephrectomy done at outside institution | |
| Yes | 86 (84.3) |
| No | 16 (15.7) |
| Original tumor diameter at prior nephrectomy, cm | 8 (5.3-10.3) |
| Pathological T-stage at time of prior nephrectomy | |
| T1 | 20 (19.6) |
| T2 | 20 (19.6) |
| T3a | 45 (44.1) |
| T3b | 13 (12.7) |
| T3c | 1 (1) |
| T4 | 3 (3) |
| Pathological N-stage at time of prior nephrectomy | |
| Nx/N0 | 82 (80.4) |
| N1 | 20 (19.6) |
| Histology at time of prior nephrectomy | |
| Clear Cell | 66 (64.7) |
| Non-Clear Cell | 36 (35.3) |
| Fuhrman grade at time of prior nephrectomy | |
| 1-2 | 28 (27.4) |
| 3-4 | 74 (72.6) |
| Sarcomatoid de-differentiation at time of prior nephrectomy | |
| Yes | 9 (8.7) |
| No | 93 (91.3) |
| Necrosis | |
| Yes | 20 (20.6) |
| No | 81 (79.4) |
| Adrenalectomy at time of prior nephrectomy | |
| Yes | 38 (37.3) |
| No | 64 (62.7) |
| RPLND at time of prior nephrectomy | |
| Yes | 27 (26.4) |
| No | 75 (73.6) |
| Positive surgical margin at time of prior nephrectomy | |
| Yes | 14 (13.7) |
| No | 88 (86.3) |
Analysis at time of retroperitoneal recurrence surgery
Table 2 shows patient demographics and pathological features after RPR surgery. Of the 102 RPR, 49 were in soft tissue/renal fossa, 41 were in ipsilateral lymph nodes, and 12 were in the ipsilateral adrenal gland. All patients had complete extirpation of the RPR with grossly negative margins. Median size of resected RPR was 4.5cm(IQR 2.7-7). In the RPR specimens, surgical margins were microscopically positive in 12(11.8%) patients and predominantly occurred in soft tissue recurrence within the renal fossa 8/12(66.6%). Of the 20 patients that had pN1 at radical nephrectomy, 14 recurred within the retroperitoneal lymph nodes, 4 recurred in soft tissue and 2 recurred in the ipsilateral adrenal gland. Median follow up after RPR surgery was 32 months(IQR 16-57). Table 3 displays intraoperative details and postoperative outcomes, including complications. Two patients died of multi-organ failure on postoperative days 43 and 45, respectively, and were counted as Grade 5 complications.
Table 2.
Clinical and Pathological characteristics in 102 patients at time of RPR surgery
| N (%) or Median (IQR) | |
|---|---|
| All patients | 102 (100) |
| Follow up after RPR surgery, months | 32 (16-57) |
| ECOG PS | |
| 0 | 71 (69.6) |
| 1 | 23 (22.5) |
| 2 | 7 (6.9) |
| Charlson Comorbidity Index | |
| 1-3 | 59 (57.8) |
| 4-6 | 25 (24.5) |
| >6 | 18 (17.7) |
| Symptoms at presentation | |
| Yes | 42 (41.2) |
| No | 60 (58.8) |
| Type of RPR | |
| Soft Tissue | 49 (48) |
| Lymph nodes | 41 (40.2) |
| Adrenal | 12 (11.8) |
| Size of RPR, cm | 4.5 (2.7-7) |
| Number of lymph nodes resected | 18 (4-24) |
| Positive margin of RPR tumor | |
| Yes | 12 (11.8) |
| No | 90 (88.2) |
| Serum hemoglobin | |
| Normal | 44 (43.1) |
| Abnormal (male <14; female <12) | 58 (56.9) |
| Serum platelets | |
| Normal | 96 (94.1) |
| Abnormal (>440 K/uL) | 6 (5.9) |
| Serum creatinine | |
| Normal | 54 (53.1) |
| Abnormal (>1.2mg/dL) | 48 (46.9) |
| Alkaline phosphatase | |
| Normal | 89 (87.3) |
| Abnormal (>126 IU/L) | 13 (12.7) |
| Serum lactate dehydrogenase | |
| Normal | 88 (86.3) |
| Abnormal (>650 IU/L) | 15 (14.7) |
| Corrected calcium | |
| Normal | 93 (91.5) |
| Abnormal (>10.2mg/dL) | 9 (8.5) |
| Systemic therapy | |
| None | 31 (30) |
| Before RPR surgery (neoadjuvant) | 46 (45.1) |
| Following recurrence after RPR surgery (salvage) | 48 (47.1) |
| Progression to metastasis (M1) after RPR surgery | |
| Yes | 60 (58.8) |
| No | 42 (41.2) |
Table 3.
Surgical parameters and perioperative complications in 102 patients who underwent RPR surgery.
| N (%) or Median (IQR) | |
|---|---|
| All patients | 102 (100) |
| Surgical Approach for RPR surgery | |
| Open | 99 (97.1) |
| Laparoscopic | 3 (2.9) |
| Patients with complications post RPR surgery | |
| Yes | 46 (45.1) |
| No | 56 (54.9) |
| Complications - highest Clavien grade | |
| 1 | 16 (15.7) |
| 2 | 15 (14.7) |
| 3 | 12 (11.7) |
| 4 | 1 (1) |
| 5 | 2 (2) |
| Estimated blood loss, cc | 700 (450-1350) |
| Operating time, hours | 3.5 (2.4-4.8) |
| Length of Stay, days | 7 (5-10) |
Outcomes and predictors of RFS and CSS
Metastatic progression was observed in 60(58.8%) of patients after RPR surgery. After resection, 42(41.2%) patients remained NED to the time of last follow up(median 32 months, IQR 16-57). Two of the patients died of myocardial infarction unrelated to surgery or metastatic RCC. Of the 60 patients that recurred after RPR surgery, 10 had local recurrence only, 43 had distant recurrences only(20 in multiple sites), and 7 had both local and distant recurrences. Of the 10 patients that recurred locally, only 3 had microscopic positive margins at RPR resection. Sixteen patients underwent further metastasectomy for RCC progression. Of these, 4 patients remained NED, 8 died of metastatic disease and 4 remained alive with metastatic disease until date of last follow-up.
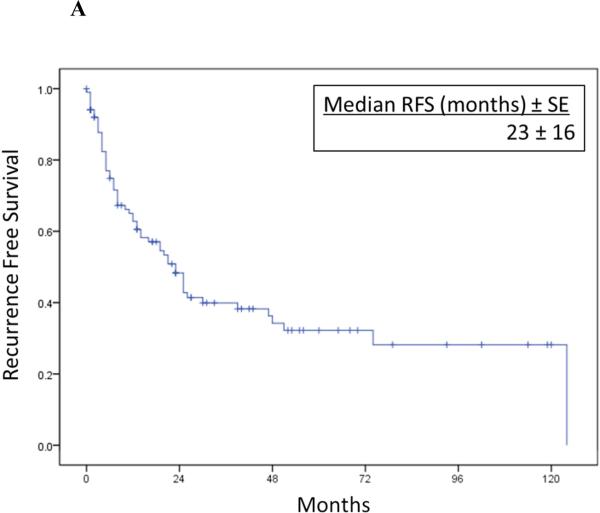
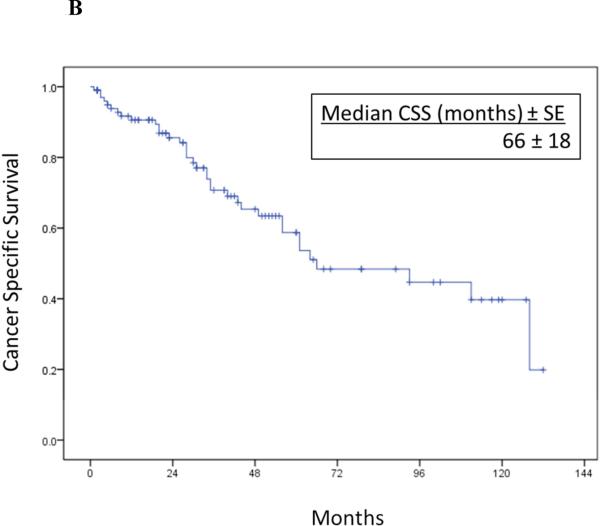
Overall RFS and CSS after RPR surgery are illustrated in Figure 1. Median RFS was 23 months(95%CI 16.4-29.6) and median CSS was 66 months(95%CI 29.9-102.1). One, 3 and 5-year cancer specific survival rates were 92%, 71% and 52%, respectively.
Figure 1A-B.
Overall recurrence-free survival (1A) and cancer-specific survival (1B) after resection of isolated RPR in 102 patients.
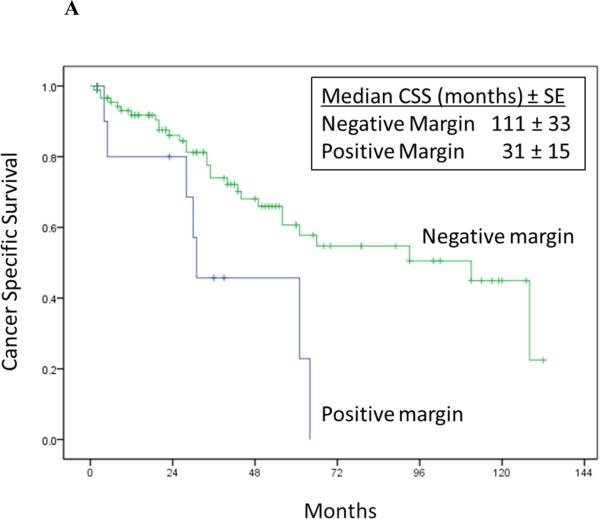
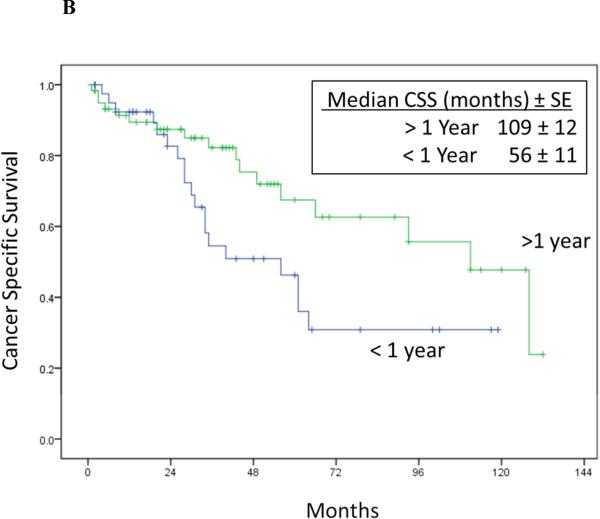
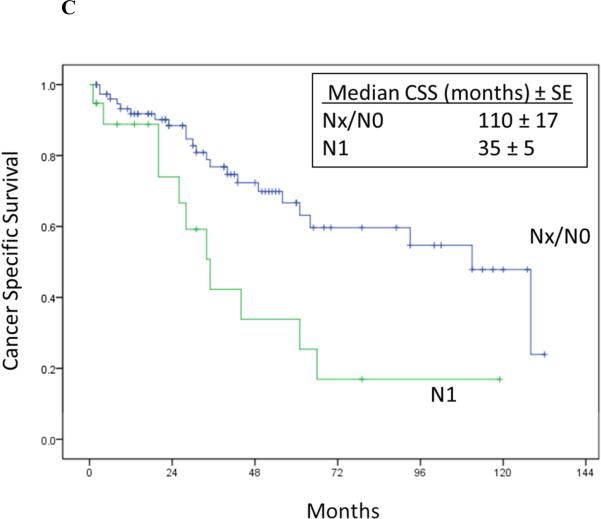
Univariate Cox proportional hazards regression analysis showed pN1 stage at prior nephrectomy, time to recurrence < 1 year after RPR surgery, maximum diameter of RPR mass, positive margin at RPR surgery, and abnormal hemoglobin were associated with increased risk of cancer specific death after RPR surgery (Table 4 and Figure 2).
Table 4.
Univariate Cox regression analysis of cancer specific mortality after RPR surgery
| Univariate | ||
|---|---|---|
| HR (95% CI) | P Value | |
| Variables at time of RN | ||
| pT Stage (pT3-4 versus pT1-2) | 1.48 (0.70-3.12) | 0.299 |
| pN Stage (pN1 versus pNO/Nx) | 2.72 (1.31-5.62) | 0.007 |
| Size of Tumor (per cm) | 1.02 (0.95-1.10) | 0.580 |
| Histology (Clear Cell versus Non-Clear Cell) | 1.04 (0.95-1.10) | 0.580 |
| Sarcomatoid (Yes versus No) | 1.73 (0.66-4.51) | 0.915 |
| Necrosis (Yes versus No) | 1.12 (0.45-2.73) | 0.580 |
| Positive margin at nephrectomy (Yes versus No) | 0.96 (0.45-2.02) | 0.917 |
| Fuhrman grade (Grade 3-4 versus 1-2) | 1.74 (0.71-4.24) | 0.223 |
| Variables at time of RPR surgery | ||
| Age (per year) | 0.99 (0.96-1.03) | 0.960 |
| Gender (Male versus Female) | 1.32 (0.62-2.83) | 0.462 |
| Race (White versus Non-White) | 1.47 (0.56-3.82) | 0.425 |
| ECOG PS | ||
| 0 | Reference | |
| 1 | 1.07 (0.47-2.42) | 0.860 |
| >1 | 2.29 (0.77-6.80) | 0.134 |
| Time to recurrence after RN < 1 year (Yes versus No) | 1.98 (1.01-3.86) | 0.045 |
| Location of relapse | ||
| Soft Tissue | Reference | |
| Lymph nodes | 1.04 (0.52-2.09) | 0.904 |
| Adrenal gland | 0.45 (0.13-1.53) | 0.201 |
| Maximum diameter of RPR tumor (per cm) | 1.17 (1.09-1.26) | <0.001 |
| Positive margin of RPR tumor (Yes versus No) | 2.79 (1.20-6.49) | <0.017 |
| Abnormal Hemoglobin (Yes versus No) | 2.13 (1.07-4.24) | 0.031 |
| Abnormal Platelets (Yes versus No) | 1.20 (0.28-5.03) | 0.801 |
| Abnormal Alkaline Phosphatase (Yes versus No) | 0.75 (0.26-2.14) | 0.593 |
| Abnormal LDH (Yes versus No) | 0.72 (0.25-2.06) | 0.544 |
| Abnormal Corrected Calcium (Yes versus No) | 2.25 (0.48-10.48) | 0.301 |
Figure 2A-D.
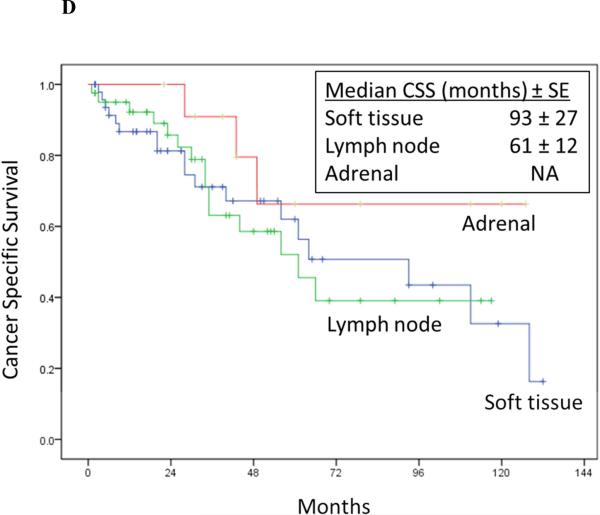
Cancer-specific survival stratified by RPR surgical margin status (Log rank p = 0.012) (2A), time to RPR (Log rank p = 0.04) (2B), nodal status at RN (Log rank p = 0.005) (2C), and location of RPR recurrence (Log rank p = 0.370) (2D).
On multivariate Cox proportional hazards regression analysis, only pN1 stage at prior nephrectomy (HR:4.08; 95%CI, 1.89-8.83; p<0.001) and maximum diameter of RPR mass (HR:1.21; 95%CI, 1.12-2.32; p < 0.001) remained as independent risk factors for cancer specific death after RPR surgery (Table 5).
Table 5.
Multivariate Cox regression analysis of cancer specific mortality after RPR surgery
| Multivariate | ||
|---|---|---|
| HR (95% CI) | P Value | |
| pN Stage at Nephrectomy (pN1 versus pNO/Nx) | 4.08 (1.89-8.83) | <0.001 |
| Maximum diameter of RPR tumor (per cm) | 1.21 (1.12-1.32) | <0.001 |
| Time to recurrence after RN < 1 year (Yes versus No) | 1.77 (0.85-3.69) | 0.124 |
| Positive pathological margin of RPR tumor (Yes versus No) | 2.09 (0.78-5.65) | 0.143 |
| Abnormal Hemoglobin (Yes versus No) | 1.45 (0.69-3.09) | 0.324 |
Use of systemic therapy
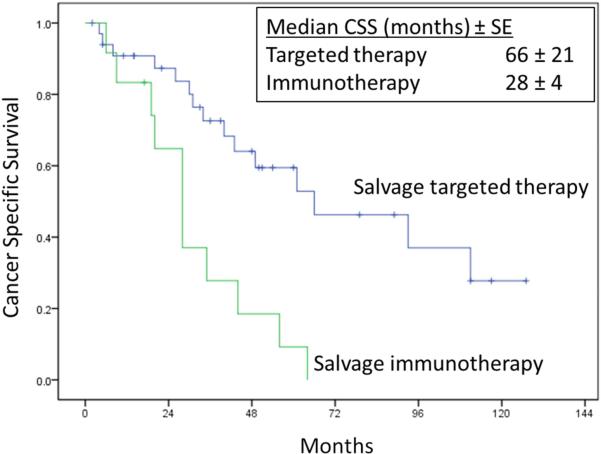
Neoadjuvant and salvage systemic therapy of any type (immunotherapy or targeted therapy) were administered in 46(45.1%) and 48(47.1%) patients, respectively. Of these patients, 21/46(45.7%) and 36/48(75%) patients received targeted neoadjuvant and salvage therapy, respectively. In general, treatment regimens included immunotherapy prior to 2004 and targeted therapy after 2004. Four(3.8%) patients had concurrent distant metastasis at the time of initial RPR diagnosis and were all treated with neoadjuvant therapy prior to RPR surgery. Two out of four patients had pathological confirmed contralateral adrenal metastases and underwent simultaneous resection of the contralateral adrenal gland at RPR surgery. The two other patients had a metastasis to a supraclavicular node and to the mediastinum, respectively, and both patients had complete radiological resolution of their distant metastasis with systemic therapy prior to undergoing resection of persistent residual RPR. Hence, all 102 patients had no detectable evidence of distant metastatic disease at time of RPR surgery and were clinically NED after RPR surgery. When comparing CSS in patients that received immunotherapy versus targeted therapy in the salvage setting, there was a significant survival benefit in those that received targeted therapy (Figure 3).
Figure 3.
Cancer specific survival in 48 patients post-RPR surgery receiving salvage systemic targeted therapy (n=35) versus salvage systemic immunotherapy (n=13) (Log rank p = 0.001)
DISCUSSION
Isolated RPR after RN is a rare event, and if left untreated, has an unfavorable outcome. Historical data have previously shown that when recurrence of RCC occurs within the retroperitoneum, up to 86% patients die within 1 year.15 Despite increased survival and improved response rates with targeted therapy for metastatic RCC, median overall survival continues to be less than 2 years.16 Local recurrence after RN occurs in up to 3% cases after RN and presents a management challenge. In the current study, we report the largest single institutional experience of aggressive surgical resection for localized RPR after RN.
Despite its retrospective nature, our study highlights several important principles in managing this controversial and challenging cohort of patients. Our study reinforces the role of aggressive surgical resection of local RCC recurrence as it can achieve long-term cure in a substantial proportion of patients and reinforces previous literature promoting surgical management when feasible (Table 6). In our series, 42(41.2%) remained NED after RPR surgery without any further therapies and an additional 21(20.6%) patients were alive with disease until the time of last follow-up.
Table 6.
Oncological outcomes and risk factors for cancer specific death after resection of RPR after radical nephrectomy in the literature.
| References | No. Patients | Time of Study | RPR Location | Follow-up (months) | No. Patients who had RPR surgery (%) | Recurrence/Progression (%) | Death from Cancer (%) | NED (%) | 1, 3, 5 Year CSS (%) | Risk Factors for cancer-specific death |
|---|---|---|---|---|---|---|---|---|---|---|
| Current study | 102 | 1990-2014 | RPR | 32 | 102 (100) | 60 (58.8) | 33 (32.4) | 40 (39.2) | 92, 71, 52 | †N-stage, recurrent tumor size |
| *Russell et al.27 | 22 | 1993-2012 | LN | 22.3 | 22 (100) | 10 (45%) | 2 (9) | 12 (54.5) | NA | NA |
| *Paparel et al.26 | 72 | Not specified | RPR | 26.4 | 46 (66) | NA | 28 (38) | 12 (17) | 74, 55, 46 | Time to recurrence, surgical resection of RPR |
| Margulis et al.9 | 54 | 1990-2007 | RPR | 41 | 54 (100) | 35 (65) | 23 (43) | 11 (20) | NA | †Positive surgical margin at RPR resection, recurrent tumor size, LDH |
| Master et al.17 | 14 | 1990-2003 | Soft tissue | 34 | 14 (100) | 9 (64) | 9 (64) | NA | 86, 40, 30 | NA |
| Schrodter et al.10 | 13 | 1991-2000 | Soft tissue | 36.9 | 13 (100) | 8 (61) | 7 (54) | 5 (38) | NA ,56, NA | Time to recurrence, recurrent tumor size |
| Itano et al.7 | 30 | 1970-1998 | Soft tissue | 39 | 10 (33) | 8 (80) | 25 (83) | 2 (20) | 66, 40, 28 | Surgical resection of RPR |
| Sandhu et al.6 | 16 | 1994-2004 | RPR | 12 | 14 (87) | 11 (68) | 5 (36) | 5 (31) | NA | NA |
| Bruno et al.28 | 34 | 1989-2004 | RPR | 16.9 | 16 (47) | NA | 26 (76) | 3 (9) | 63, 31,18 | Surgical resection of RPR |
| Boorjian et al.29 | 15 | 1970-2004 | Soft tissue, LN | 18 | 15 (100) | 10 (67) | 6 (40) | 5 (33) | **65, 35 | NA |
| Tanguay et al.30 | 16 | 1983-1994 | Soft tissue | 23.5 | 16 (100) | 10 (62) | 4 (25) | 6 (38) | NA | NA |
| Esrig et al.8 | 11 | 1973-1990 | Soft tissue | NA | 10 (91) | 7 (63) | 4 (36) | 4 (36) | 55, 36, NA | NA |
Multi-institutional study
CSS (%) at 2 and 4 years respectively
Multivariate Cox regression analysis (vs Univariate Cox regression analysis)
Retroperitoneal recurrence (RPR) defined as soft tissue, ipsilateral adrenal gland, ipsilateral lymph nodes (LN)
NA, not applicable
NED, no evidence of disease
In addition, we identified several clinicopathological prognostic factors associated with RCC recurrence and cancer-specific survival after RPR surgery. On multivariate analysis, pathological nodal stage at time of radical nephrectomy and size of RPR were identified as adverse prognostic indicators in our cohort. Harboring either risk factor significantly impacted CSS and may help identify patients that most benefit from aggressive surgery.
It is noteworthy that 39% of the cohort was originally diagnosed with pT1-2 renal tumors at nephrectomy, but still experienced a RPR, emphasizing the importance of follow-up even in potentially ‘low-risk’ patients after RN. Furthermore, 59% of patients in our series had no symptoms (local or systemic), and the diagnosis of recurrence was based solely on abdominal imaging. We found that RPR size was an independent prognostic factor for survival on multivariate analysis, with larger recurrent tumors associated with more deaths. All these factors put together again reinforce the need for careful surveillance with appropriate imaging after RN, with the goal of identifying recurrences when they are still small in size and asymptomatic.
Reoperation after ipsilateral nephrectomy has previously been associated with significant morbidity in patients with RPR.17 In our series, even though 46(45.1%) patients experienced postoperative complications, the majority experienced either no or minor complications(Clavien grade 1-2). Still, 15(14.7%) experienced grade 3 complications or higher(including 2 grade 5 complications), indicating the importance of performing this type of surgery in a specialized referral center. Our median length of stay after RPR surgery was 7 days. In our series, 97% of cases were all performed through an open approach. Previous studies have reported the feasibility of minimally invasive approaches with laparoscopic resection of RPR, however, the numbers in these series were extremely small.18, 19
In our current series of 102 patients, 45.1% received neoadjuvant and 47.1% salvage systemic therapy after RPR surgery. Of these, 21/102(20.5%) and 35/102(34.3%) patients received targeted neoadjuvant and salvage therapy after RPR surgery, respectively. Median recurrence free survival in our cohort was 23 months, and a median cancer-specific survival of 66 months. In our cohort, 60/102(59%) patients progressed to metastatic disease after RPR surgery of which 48(80%) received salvage systemic therapy. When comparing CSS in patients that received immunotherapy versus targeted therapy in the salvage setting, there was a significant survival benefit in those that received targeted therapy (Figure 3). However, true analysis of this effect is not possible due to the retrospective nature of our study, and the heterogeneity of our patient population, where multiple agents and non-standardized treatments were used. We report 1, 3 and 5-year CSS of 92%, 71% and 52% which appear to be more favorable to previous reports in the literature (Table 6). This improvement in survival compared to other series may reflect the availability, tolerability and greater use of targeted therapy compared with immunotherapy reflected in multiple studies, and could be partially related to selection bias and small patient numbers.20-25
Paparel et al reported on a multi-institutional study (involving over 12 centers with 72 patients) examining the role of surgery in local RCC recurrence after radical nephrectomy. The authors report 1, 3 and 5-year cancer specific survival of 74%, 55% and 46%, respectively. They noted, on univariate analysis only, that time to recurrence and surgical intervention remained independent predictive factors for cancer specific mortality.26 However, this study included a significant number of patients (30%) with concomitant distant metastases at time of RPR. In contrast, all cases in our study were performed at a single institution, and no patients had detectable distant metastases at the time of RPR surgery. In addition, in our study, all patients underwent surgical resection of their local recurrence, in comparison to only 66% in Paparel's cohort. Finally, our mean follow-up was significantly longer at 43 months (versus 26.4) with a median of 32 months (IQR 16-57).
Another recent multi-institutional study by Russell et al examined 22 patients with isolated ipsilateral nodal recurrence for RCC after radical nephrectomy. All patients underwent complete surgical excision of localized nodal recurrence. Of these, 46% progressed to metastatic disease with a median progression free survival of 12.7 months.27 In our series, we report a larger subgroup of patients(41/102 patients) with localized lymph node recurrence undergoing complete surgical excision. The overall median recurrence free survival was 23 months and we noted no significant differences in CSS where we stratified by location of recurrence (renal fossa/soft tissue, lymph node or adrenal) within the retroperitoneum (Figure 2D).
There are some limitations to our study worth mentioning. This is a retrospective analysis of a highly selected patient population, treated in a single tertiary referral center. Our cohort was heterogeneous, and included patients with soft tissue, lymph node and adrenal recurrence. It is likely that these sites of recurrence have a different etiology and biologic behavior, which we cannot account for in our study, although survival was not significantly different between these groups. In addition, all patients in our study underwent surgical resection and a non-surgical group was not available for comparison. However, one can argue that patients with RPR who did not have surgery are more likely to have poor performance status, unresectable disease, or distant metastatic disease, precluding the performance of a curative RPR surgery. Furthermore, true effects of targeted neoadjuvant or salvage therapy on survival cannot be ascertained due to variations in treatment regimens, and lack of uniformity in the indication as to which patients received neoadjuvant therapy. On the other hand, our series is the largest single institutional report of surgical treatment of RPR to date, reflecting contemporary outcomes of a high-volume tertiary referral center in the targeted therapy era.
CONCLUSION
In the absence of distant metastatic disease, aggressive surgical resection of RPR after radical nephrectomy is feasible in selected patients, with acceptable complications, and is potentially curative in more than 40% of patients. Pathological nodal stage at original nephrectomy and size of resected RPR are independent risk factors for CSS. Further studies are needed to examine the potential utility and impact of targeted neoadjuvant therapy in this patient group.
Key of Abbreviations
- RCC
Renal cell carcinoma
- CT
computerized tomography
- MRI
magnetic resonance imaging
- RPLND
retroperitoneal lymph node dissection
- RFS
recurrence free survival
- CSS
cancer-specific survival
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: Jose A. Karam acted as a one-time consultant for Pfizer in 2013, which is unrelated to the current submitted manuscript.
REFERENCES
- 1.Flanigan RC. Cytoreductive nephrectomy in metastatic renal cancer. Curr Urol Rep. 2003;4:36. doi: 10.1007/s11934-003-0055-6. [DOI] [PubMed] [Google Scholar]
- 2.Pantuck AJ, Zisman A, Belldegrun AS. The changing natural history of renal cell carcinoma. J Urol. 2001;166:1611. [PubMed] [Google Scholar]
- 3.Swanson DA. Surgery for metastases of renal cell carcinoma. Scand J Surg. 2004;93:150. doi: 10.1177/145749690409300211. [DOI] [PubMed] [Google Scholar]
- 4.Hollingsworth JM, Miller DC, Daignault S, et al. Five-year survival after surgical treatment for kidney cancer: a population-based competing risk analysis. Cancer. 2007;109:1763. doi: 10.1002/cncr.22600. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen CT, Lane BR, Kaouk JH, et al. Surgical salvage of renal cell carcinoma recurrence after thermal ablative therapy. J Urol. 2008;180:104. doi: 10.1016/j.juro.2008.03.046. [DOI] [PubMed] [Google Scholar]
- 6.Sandhu SS, Symes A, A'Hern R, et al. Surgical excision of isolated renal-bed recurrence after radical nephrectomy for renal cell carcinoma. BJU Int. 2005;95:522. doi: 10.1111/j.1464-410X.2005.05331.x. [DOI] [PubMed] [Google Scholar]
- 7.Itano NB, Blute ML, Spotts B, et al. Outcome of isolated renal cell carcinoma fossa recurrence after nephrectomy. J Urol. 2000;164:322. [PubMed] [Google Scholar]
- 8.Esrig D, Ahlering TE, Lieskovsky G, et al. Experience with fossa recurrence of renal cell carcinoma. J Urol. 1992;147:1491. doi: 10.1016/s0022-5347(17)37605-x. [DOI] [PubMed] [Google Scholar]
- 9.Margulis V, McDonald M, Tamboli P, et al. Predictors of oncological outcome after resection of locally recurrent renal cell carcinoma. J Urol. 2009;181:2044. doi: 10.1016/j.juro.2009.01.043. [DOI] [PubMed] [Google Scholar]
- 10.Schrodter S, Hakenberg OW, Manseck A, et al. Outcome of surgical treatment of isolated local recurrence after radical nephrectomy for renal cell carcinoma. J Urol. 2002;167:1630. [PubMed] [Google Scholar]
- 11.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 12.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of. 6336 patients and results of a survey. Ann Surg. 2004;240:205. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edge SB, American Joint Committee on Cancer . American Cancer Society.: AJCC cancer staging handbook : from the AJCC cancer staging manual. 7th ed. Springer; New York: 2010. p. xix.p. 718. [DOI] [PubMed] [Google Scholar]
- 14.Kaplan EL, Meier P. Nonparametric Estimation from Incomplete Observations. In: Kotz S, Johnson N, editors. Breakthroughs in Statistics. Springer; New York: 1992. pp. 319–337. [Google Scholar]
- 15.Dekernion JB, Ramming KP, Smith RB. The natural history of metastatic renal cell carcinoma: a computer analysis. J Urol. 1978;120:148. doi: 10.1016/s0022-5347(17)57082-2. [DOI] [PubMed] [Google Scholar]
- 16.Heng DY, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol. 2009;27:5794. doi: 10.1200/JCO.2008.21.4809. [DOI] [PubMed] [Google Scholar]
- 17.Master VA, Gottschalk AR, Kane C, et al. Management of isolated renal fossa recurrence following radical nephrectomy. J Urol. 2005;174:473. doi: 10.1097/01.ju.0000165574.62188.d0. [DOI] [PubMed] [Google Scholar]
- 18.Abel EJ, Karam JA, Carrasco A, et al. Laparoscopic adrenalectomy for metachronous metastases after ipsilateral nephrectomy for renal-cell carcinoma. J Endourol. 2011;25:1323. doi: 10.1089/end.2011.0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yohannan J, Feng T, Berkowitz J, et al. Laparoscopic resection of local recurrence after previous radical nephrectomy for clinically localized renal-cell carcinoma: perioperative outcomes and initial observations. J Endourol. 2010;24:1609. doi: 10.1089/end.2010.0051. [DOI] [PubMed] [Google Scholar]
- 20.Di Lorenzo G, Autorino R, Sternberg CN. Metastatic Renal Cell Carcinoma: Recent Advances in the Targeted Therapy Era. European Urology. 2009;56:959. doi: 10.1016/j.eururo.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Escudier B, Bellmunt J, Negrier S, et al. Phase III trial of bevacizumab plus interferon alfa-2a in patients with metastatic renal cell carcinoma (AVOREN): final analysis of overall survival. J Clin Oncol. 2010;28:2144. doi: 10.1200/JCO.2009.26.7849. [DOI] [PubMed] [Google Scholar]
- 22.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in Advanced Clear-Cell Renal-Cell Carcinoma. New England Journal of Medicine. 2007;356:125. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 23.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, Interferon Alfa, or Both for Advanced Renal-Cell Carcinoma. New England Journal of Medicine. 2007;356:2271. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 24.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus Interferon Alfa in Metastatic Renal-Cell Carcinoma. New England Journal of Medicine. 2007;356:115. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 25.Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28:1061. doi: 10.1200/JCO.2009.23.9764. [DOI] [PubMed] [Google Scholar]
- 26.Paparel P, Bigot P, Matillon X, et al. Local recurrence after radical nephrectomy for kidney cancer: management and prediction of outcomes. a multi-institutional study. J Surg Oncol. 2014;109:126. doi: 10.1002/jso.23473. [DOI] [PubMed] [Google Scholar]
- 27.Russell CM, Espiritu PN, Kassouf W, et al. Surgical outcomes in the management of isolated nodal recurrences: a multicenter, international retrospective cohort. J Urol. 2014;192:350. doi: 10.1016/j.juro.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 28.Bruno JJ, 2nd, Snyder ME, Motzer RJ, et al. Renal cell carcinoma local recurrences: impact of surgical treatment and concomitant metastasis on survival. BJU Int. 2006;97:933. doi: 10.1111/j.1464-410X.2006.06076.x. [DOI] [PubMed] [Google Scholar]
- 29.Boorjian SA, Crispen PL, Lohse CM, et al. Surgical resection of isolated retroperitoneal lymph node recurrence of renal cell carcinoma following nephrectomy. J Urol. 2008;180:99. doi: 10.1016/j.juro.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 30.Tanguay S, Pisters LL, Lawrence DD, et al. Therapy of locally recurrent renal cell carcinoma after nephrectomy. J Urol. 1996;155:26. [PubMed] [Google Scholar]