Abstract
Treatment of pain with morphine and its congeners in sickle cell anemia is suboptimal, warranting the need for analgesics devoid of side effects, addiction and tolerance liability. Small-molecule nociceptin opioid receptor ligands show analgesic efficacy in acute and chronic pain models. We show that AT-200, a high affinity nociceptin opioid receptor agonist with low efficacy at the mu opioid receptor, ameliorated chronic and hypoxia/reoxygenation-induced mechanical, thermal and deep tissue/musculoskeletal hyperalgesia in HbSS-BERK sickle mice. The antinociceptive effect of AT-200 was antagonized by SB-612111, a nociceptin opioid receptor antagonist, but not naloxone, a non-selective mu opioid receptor antagonist. Daily 7-day treatment with AT-200 did not develop tolerance and showed a sustained anti-nociceptive effect, which improved over time and led to reduced plasma serum amyloid protein, neuropeptides, inflammatory cytokines and mast cell activation in the periphery. These data suggest that AT-200 ameliorates pain in sickle mice via the nociceptin opioid receptor by reducing inflammation and mast cell activation without causing tolerance. Thus, nociceptin opioid receptor agonists are promising drugs for treating pain in sickle cell anemia.
Introduction
Sickle cell anemia (SCA) is associated with unpredictable recurrent acute vaso-occlusive (VOC) pain episodes (“crises”) and chronic pain, which can begin early and continue to increase, leading to poor quality of life and shortened survival.1,2 Opioid treatment is widely used to treat pain in SCA, but remains a suboptimal approach due to side-effects including somatic and psychiatric co-morbidities, dependence liability, and opioid hyperalgesia.3–5 Relatively higher doses of morphine are required to treat sickle pain as compared to other conditions, which may increase the risk of side-effects. Morphine is also known to increase inflammation via the activation of toll-like receptor 4 (TLR4).6 We found that TLR4 expression is increased in mast cells and morphine stimulated the release of substance P (SP) from mast cells and promoted SP- and capsaicin-induced neurogenic inflammation in sickle mice.7 Therefore, while used for treating pain in SCA, morphine may exacerbate existing mast cell activation, neurogenic inflammation and pain.
Morphine and its congeners exert their anti-nociceptive effect via the mu opioid receptor (MOP/R). In addition to MOP/R, the opioid receptor family includes the nociceptin opioid receptor (NOP/R) which is involved in nociceptive signaling via its endogenous peptide ligand nociceptin/orphanin FQ (N/OFQ).8 NOP/Rs are found in the dorsal root ganglion (DRG), spinal cord and supraspinal regions of the brain involved in nociception.9–11 Agonists and antagonists of NOP/R have been demonstrated to influence pain at the peripheral, spinal and supraspinal level in rodent models of neuropathic and inflammatory pain.12–14
The anti-nociceptive role of spinal NOP/Rs is suggested to result from an inhibitory action on SP-mediated nociception via second-order neurons.15 SP levels are increased in the serum of patients with SCA and in sickle mice compared to healthy subjects and control mice, respectively.16,17 The endogenous NOP/R peptide agonist N/OFQ has been shown to inhibit the release of SP and calcitonin gene-related peptide (CGRP) from sensory nerve endings.18 Chemically-induced release of neuropeptides from mast cells and capsaicin-sensitive afferent nerve terminals were also attenuated by N/OFQ.19 Mast cells are tissue-resident inflammatory cells, which can release pro-inflammatory cytokines, neuropeptides and proteases such as tryptase, upon degranulation/activation.20,21 Tryptase released from mast cells activates protease activated receptor-2 (PAR-2) on peripheral nerve terminals stimulating the release of neuropeptides and pain.22,23 Neuropeptide SP, in turn, activates mast cells, leading to a vicious cycle of mast cell and neural activation, resulting in chronic pain. We found that sickle mice show mast cell activation and mast cell-dependent neurogenic inflammation and pain.7 Since NOP/R agonists appear to block neuropeptide release from peripheral nerve endings and mast cells, we examined the possibility of using small-molecule NOP/R agonist AT-200 to treat pain in SCA.
We used transgenic homozygous HbSS-BERK mice, expressing sickle hemoglobin, which show characteristic features of experimental pain observed in human SCA.16,24,25 We demonstrate the anti-nociceptive activity of a small-molecule NOP/R agonist AT-200 (formerly SR14150),26 and the mechanism of its action in sickle mice.
Methods
Mice
Experiments were performed following approval from the Institutional Animal Care and Use Committee. We used transgenic HbSS-BERK mice (designated ‘sickle’) that are homozygous for knockout of both α and b murine globins, but carry the transgenes for human α and βS (hemoglobin S). Control HbAA-BERK mice (designated ‘control’) are also knockout for both α and β murine globins and express normal human hemoglobin A. Sickle mice recapitulate the genetic, hematologic and pathological features of the human SCA.27 Sickle mice are challenging to breed. Pups can die in utero or postnatally, resulting in few surviving adults and fewer male mice. Breeder mice and pups are fed “Sickle Cell Mouse Diet” (TestDiet, St Louis, MO, USA) up to 10–12 weeks and the regular Rodent Diet (Harlan Laboratories, Hayward, CA, USA) thereafter. We and others found that sickle mice start developing hyperalgesia after three months of age, which continues to increase up to ten months and also show central sensitization.16,25,28,29 Features of experimental pain in sickle mice are similar to those observed clinically, including, mechanical, thermal and deep tissue hyperalgesia.16,24,25,28 In addition to hyperalgesia, sickle mice also show inflammation, endothelial dysfunction, mast cell activation and increased cutaneous SP and CGRP.7,16,30 Mice were genotyped for the presence of human α and β/βS hemoglobins and absence of mouse α and b hemoglobins (Transnetyx), and phenotyped for human HbA (control mice) or single band of HbS (homozygous sickle).7 Control and sickle mice show hematocrit values of 46.64±2.8 and 32.5±6.3 (P=0.0005), white blood cell counts of 5.3 K/uL±1.7 and 17.2 K/uL±7.9 (P=0.003), and reticulocyte percentage levels of 5.7±1.9 and 65.6±6 (P=0.0000), respectively.
Drugs
AT-200 [1-(1-cyclooctylpiperidin-4-yl)-indolin-2-one], formerly called SR14150, was synthesized as a hydrochloride salt using previously reported procedures by Zaveri et al.26 AT-200 is a high affinity NOP/R ligand, with more than 20-fold binding selectivity over MOP/R, and partial agonist activity at NOP/R, as determined by a functional assay measuring the stimulation of [35 S]GTPγS.26 AT-200 also has low efficacy partial agonist activity at MOP/R.26 AT-200 was dissolved in 1–2% dimethylsulfoxide and 0.5% aqueous hydroxypropylcellulose, and administered subcutaneously (s.c.) at doses of 5, 10 and 20 mg/kg. Morphine sulfate (Eli Lilly & Co., Indianapolis, IN, USA) was administered s.c. at a dose of 20 mg/kg, based on our previous study showing that 10 mg/kg dose was not effective; in fact, a 20 mg/kg dose was effective in this model.16
Treatment of mice with AT-200
Chronic treatment with AT-200: mice were injected with 10 mg/kg s.c. AT-200 or vehicle daily for seven days and tested for pain behaviors 30 min after drug administration on days 1, 3, 5 and 7 and on day 8 (24 h after the last injection).
Co-administration of Naloxone or SB-612111: mice were injected with 1 mg/kg s.c. Naloxone or 10 mg/kg s.c. SB-612111 (synthesized in the Zaveri laboratory31) ten minutes prior to injecting with 10 mg/kg s.c. AT-200. Hyperalgesia was measured at 30, 60, 90, 180, and 240 min, and 24 h after the administration of AT-200.
Hypoxia/reoxygenation
Mice were exposed to hypoxia with 8% O2 and 92% N2 for 3 hours followed by re-oxygenation (H/R) at room air for 1 h, as previously described by us.25
Detailed descriptions of the following methods are provided in the Online Supplementary Appendix.
Somatosensory testing for pain behaviors
The following behavioral measures were performed in a quiet, temperature-controlled room:16,25
–mechanical paw withdrawal threshold and frequency;
–grip force to determine deep tissue/musculoskeletal pain;
–withdrawal responses to heat stimuli;
–withdrawal responses to cold stimuli;
–balance and motor co-ordination
–cutaneous blood flow;
–cutaneous mast cell staining;
–laser scanning confocal microscopy (LSCM) of skin
Statistical analysis
The inferential test in Figure 1 is a repeated measures two-way ANOVA to determine the effect of time and/or dose of AT-200. For Figures 2–4, two type of inferential tests were used: 1) for comparisons of base-line levels with other time points, one-way ANOVA followed by paired ‘t’ tests were used; 2) comparisons of vehicle and AT-200 at individual time points were based on ANOVA followed by an unpaired t-test for individual comparisons along with additional comparisons as indicated between vehicle and other (morphine or NOP/OR antagonists) treatment and comparisons of pain measures at hypoxia/re-oxygenation and later time points. A two-way ANOVA is used in Figure 5 to determine the effect of genotype and/or treatment followed by an unpaired t-test within treatment category or within genotype. Bonferroni correction was used to adjust for multiple pairwise comparison in all the analyses. P<0.05 was considered significant. Data were analyzed using Prism 5 software (GraphPad, San Diego, CA, USA).
Figure 1.
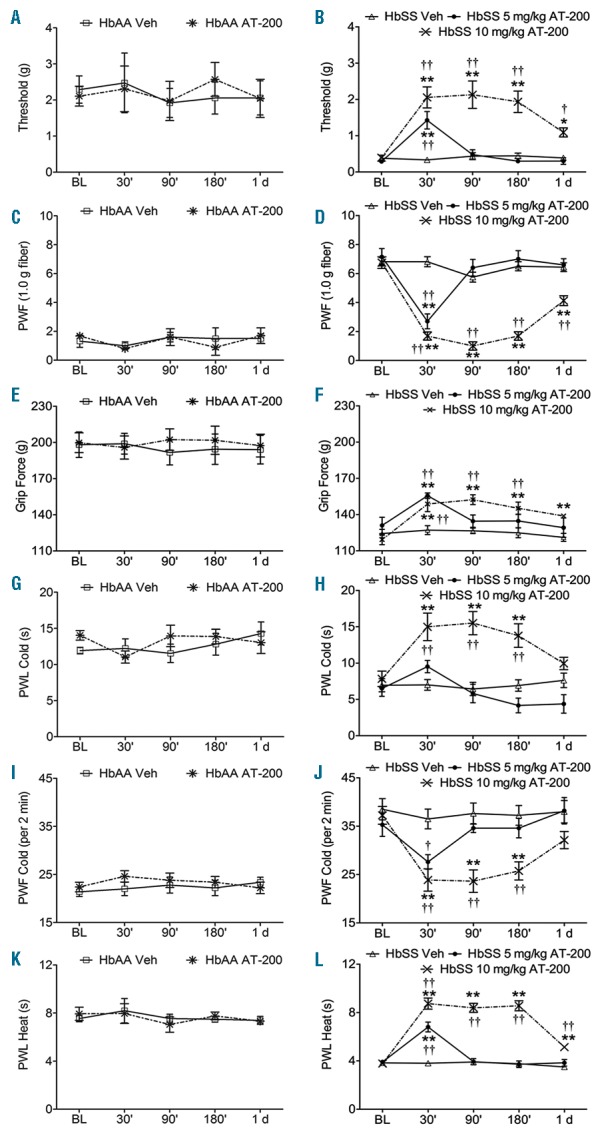
AT-200 ameliorates chronic mechanical, deep tissue and thermal hyperalgesia. Sickle mice were injected subcutaneously with 10 mg/kg AT-200, 5 mg/kg AT-200 or vehicle, while control mice were injected subcutaneously with 10 mg/kg AT-200 or vehicle. The sensitivity to mechanical stimuli (A–D), deep tissue hyperalgesia (E and F), and thermal hyperalgesia (G–L) were measured over the indicated time period. (A and B) Mechanical withdrawal threshold using von Frey monofilaments, a measure of cutaneous nociception is shown. Lower thresholds are indicative of increased sensitivity to cutaneous nociception. (C and D) PWF in response to 10 applications of a 9.8 mN (1.0 g) von Frey monofilament suggestive of mechanical hyperalgesia. A higher PWF indicates increased nociception. (E and F) Grip force measurements indicating deep tissue/musculoskeletal pain are shown. Lower grip force suggests increased deep tissue hyperalgesia. (G and H) PWL and (I and J) PWF on a cold plate maintained at 4 ± 1°C. A lower PWL and higher PWF in a 2-min period on a cold plate are indicative of increased sensitivity to cold-induced nociception. (K and L) PWL to a heat stimulus. Shorter PWL (in seconds) in response to heat stimulus is indicative of increased heat sensitivity. Sickle mice treated with 10 mg/kg of AT-200 showed significant analgesic response at all the time points tested compared to control mice. However, sickle mice treated with 5 mg/kg of AT-200 did not show sustained anti-nociceptive effect. Statistical significance was calculated by comparing each value to BL (*) and between vehicle and AT-200 (†). *P<0.05 and **P<0.005 compared to BL; and †P<0.05 and ††P<0.005 compared to vehicle for that time point. Mean age of mice ± SEM in months were, HbAA-BERK vehicle (n=12) and HbAA-BERK AT-200 (n=12), 17.2 ± 1.6; HbSS-BERK vehicle (n=16), 21.2 ± 1.2, and HbSS-BERK AT-200, 20.7 ± 1.4 (10 mg/kg; n=16); 10.8 ± 1.9 (5 mg/kg; n=10). BL: baseline; PWF: paw withdrawal frequency; PWL: paw withdrawal frequency; Veh: vehicle.
Figure 2.
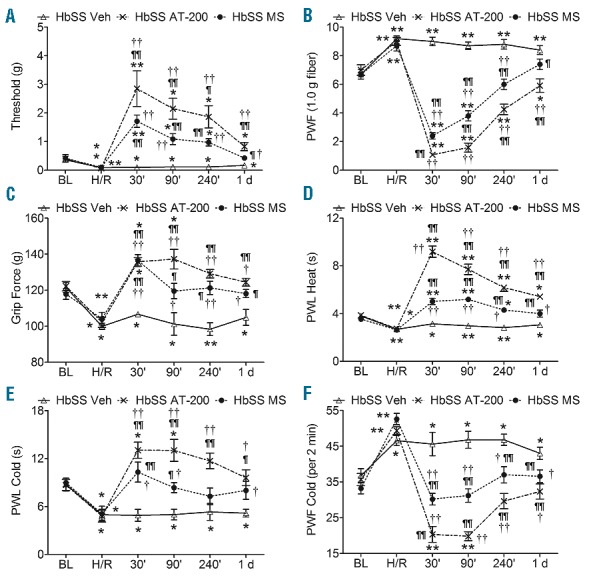
AT-200 attenuates hypoxia/reoxygenation evoked pain. Sickle mice were exposed to 3 h of hypoxia and 1 h of reoxygenation at room air and treated with vehicle, AT-200, or morphine. Sensory testing was performed at baseline, immediately following H/R and after drug treatments at indicated times. (A and B) mechanical threshold, and supra-threshold; (C) deep tissue hyperalgesia, and (D–F) thermal sensitivity to heat and cold. Open triangles with solid lines, crosses with dashed lines, and solid circles with dashed lines represent vehicle, AT-200, and morphine treatment, respectively. Statistical significance was calculated by comparing each value with BL (*) or with H/R (¶); and between vehicle and AT-200 (†). *P<0.05 and **P<0.005 compared to BL; ¶P< 0.05 and ¶¶P<0.005 compared to H/R; and †P<0.05 and ††P<0.005 compared to vehicle for that timepoint. Mean age of mice ± SEM in months were, HbSS-BERK Vehicle (n=10), 20.9±1.7, and HbSS-BERK AT-200 (n=12), 21.2±1.7, and HbSS-BERK MS (n=10), 21.4±1.6. BL, baseline; H/R: hypoxia/reoxygenation; Veh: vehicle; MS: morphine sulfate; PWF: paw withdrawal frequency; PWL: paw withdrawal latency.
Figure 4.
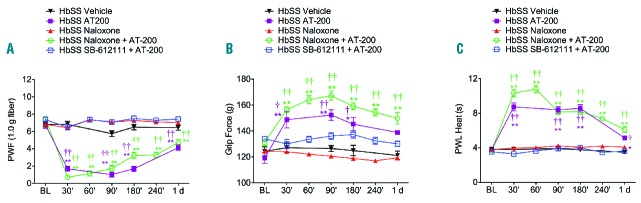
Nociceptin receptor mediates the analgesic effect of AT-200. HbSS-BERK mice were injected with naloxone (1 mg/kg), a non-selective opioid receptor antagonist or SB-612111 (10 mg/kg), a selective NOP receptor antagonist, 10 min before injecting AT-200 (10 mg/kg) or were administered vehicle, AT-200 or naloxone alone. Measures of (A) PWF, (B) Grip Force, and (C) PWL to heat are shown. SB-612111 antagonized AT-200–induced anti-nociceptive effect, but naloxone did not. Data are shown as mean ± SEM from 5–7 HbSS-BERK mice per treatment. Statistical significance was calculated by comparing each value to BL values (*), between vehicle and treatment groups (†).*P<0.05 and **P<0.005 compared to BL; †P<0.05 and ††P<0.005 compared to vehicle for that time point. Mean age of mice ± SEM in months were, HbSS-BERK Naloxone (n=6), 6.2 ± 0.08; HbSS-BERK SB-612111 + AT-200 (n=7), 5.42 ± 0.06; and HbSS-BERK Naloxone + AT-200 (n=7), 5.5 ± 0.09.
Figure 5.
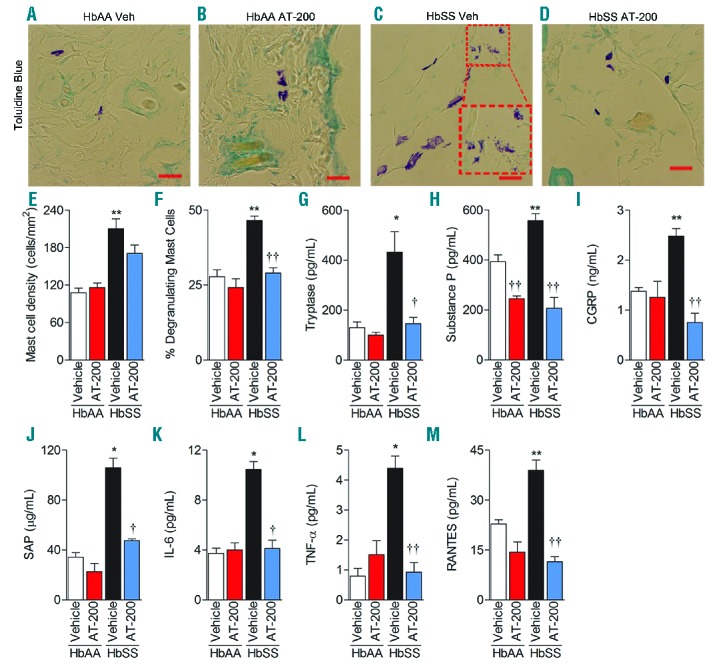
AT-200 treatment reduces mast cell degranulation and inflammation. Control and sickle mice were treated with vehicle or 10 mg/kg AT-200/day for seven days as described in Figure 3D–E, and their dorsal skin and serum/plasma were analyzed 24 h following the last injection on day 7 (A–D). Toluidine blue staining for mast cells in dorsal skin: Inset in (C) shows magnified degranulating mast cells. Original magnification X900; scale bar, 20 mm. Each image represents 12–16 reproducible images per mouse from 10–12 mice per treatment. Image acquisition information: Olympus IX70 microscope, 60x/0.45 objective lens, DP70 digital camera and DP70 Manager Software (Olympus) and Adobe Photoshop. (E) Mast cell density shown as means ± SEM per square millimeter of skin sections stained with Toluidine blue. (F) Percentage of degranulating mast cells shown as means ± SEM degranulating mast cells to the total number of mast cells per square millimeter. Plasma concentrations are shown in (G) Tryptase, (H) Substance P, (I) CGRP, (J) SAP, (K) IL-6, (L) TNF-α, and (M) RANTES.. Statistical significance was calculated by comparing each value to control HbAA-BERK mice (*) and between vehicle and AT-200 (†). *P<0.05 and **P< 0.005 compared to vehicle-treated HbAA-BERK; and †P<0.05 and ††P<0.005 compared to vehicle for that time point. Mean age of mice ± SEM in months were, HbAA-BERK Vehicle (n=4), 4.06 ± 0.29, HbAA-BERK AT-200 (n=4), 4.04 ± 0.29, HbSS-BERK Vehicle (n=4–7), 4.38 ± 0.32, and HbSS-BERK AT-200 (n=4–7), 4.41 ± 0.34. Veh: vehicle; CGRP: Calcitonin Gene-Related Protein; SAP: Serum Amyloid Protein; IL-6: Interleukin 6; TNF-α: Tumor Necrosis Factor alpha; RANTES: Regulated on Activation, Normal T-Cell Expressed and Secreted.
Results
AT-200 ameliorates hyperalgesia in sickle mice
Mechanical, deep tissue and thermal hyperalgesia increases with age in sickle mice16,25 similar to chronic pain in sickle patients.1,16,25,32 Therefore, older mice [approx. 18.5 months (mo)] showing increased sensitivity to different stimuli compared to young mice (approx. 4.4 mo) were used to examine the effectiveness of AT-200 (Online Supplementary Figure S1). The two-way repeated measures ANOVA shows no significance for comparison with control, whereas different doses of AT-200 in sickle mice show significant interaction over time for all behavioral measures (Table 1).
Table 1.
Statistical analysis of two-way repeated measures ANOVA for comparing behavioral response to AT-200 treatment under normoxia.
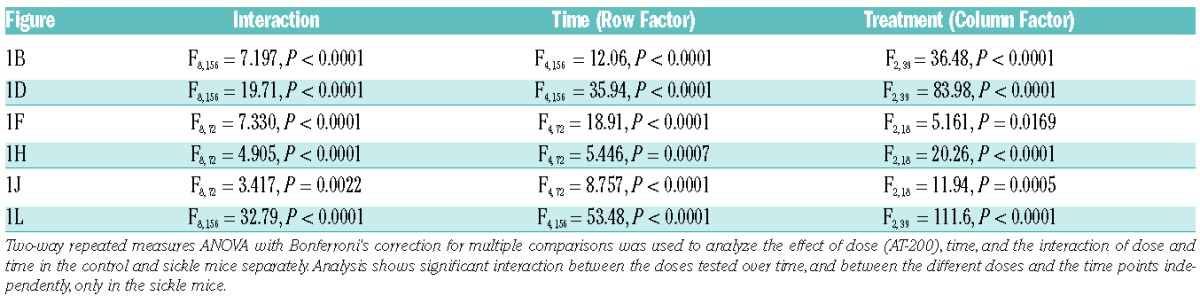
Overall, AT-200 at a dose of 10 mg/kg reduced sensitivity to all stimuli in sickle mice to levels observed in age-matched control mice and the effect of a single dose lasted up to 24 h (Figure 1). A 5 mg/kg dose, on the other hand, was effective in reducing hyperalgesia significantly for only up to 30 min, whereas, the highest dose of 20 mg/kg led to lethality in sickle mice. Therefore, AT-200 demonstrates dose-dependence. Response to 10 mg/kg AT-200 appears to be optimum, and was, therefore, used in subsequent experiments.
Mechanical hyperalgesia: AT-200 treatment had no effect on mechanical sensitivity in control mice (Figure 1A and C). Mechanical withdrawal threshold in sickle mice was lower compared to control. AT-200 treatment increased withdrawal thresholds in sickle mice from approximately 0.4 g at baseline to 2.1 g over a 3 h period, which remained elevated to 1.1 g even after 24 h (sickle vehicle vs. AT-200; P=0.0108) (last period of observation) (Figure 1B). Similarly, mechanical PWF in sickle mice is higher compared to control mice but decreased after AT-200 treatment, and remained decreased at 24 h (sickle vehicle vs. AT-200, P<0.0001) (Figure 1D).
Deep/musculoskeletal pain: AT-200 showed no significant increase in grip force in control mice after treatment (Figure 1E). Lower grip force in sickle mice reflects musculoskeletal pain in SCA. AT-200 increased grip force exerted by sickle mice by 25 g over a 3 h period (sickle vehicle vs. AT-200; P=0.0021), but its effects were not sustained for 24 h (Figure 1F).
Thermal sensitivity to heat and cold: sickle mice showed a significant increase in thermal sensitivity compared to control mice (Figure 1G–L). AT-200 reduced thermal sensitivity in sickle mice (compared to baseline, F4,156 = 53.48, P<0.0001 (heat), F4,72 = 5.446, P=0.0007 (cold) but had no effect on thermal sensitivity in control mice (Figure 1G–L). Over a 3 h period, AT-200 noticeably doubled paw withdrawal latency (PWL) to heat and to cold in sickle mice: vehicle vs. AT-200, cold (P=0.0001) and heat (P<0.0001) (Figure 1H and L). Further confirming a reduction to cold sensitivity by AT-200, PWF to cold decreased significantly over a 3 h period in sickle mice (vehicle vs. AT-200; P=0.0002) (Figure 1J). Remarkably, 10 mg/kg AT-200 reduced heat sensitivity up to 24 h in sickle mice (sickle vehicle vs. AT-200; P<0.0001) (Figure 1L).
AT-200 attenuates hypoxia/reoxygenation-evoked pain
Hypoxia/re-oxygenation injury leads to increased sensitivity in all pain measures in sickle mice and recapitulates VOC in SCA.25 Mechanical threshold (t9 = 3.4, P=0.0078) and PWL to heat (t9 = 5.7, P=0.0003) markedly decreased and mechanical PWF (t9 = 6.2, P=0.0002) increased after incitement of H/R in sickle mice (Figure 2A, B and D). Pain evoked by H/R was responsive to AT-200 and to a high dose of morphine in sickle mice. Morphine attenuated mechanical threshold and heat sensitivity up to 4 h and PWF up to 90 min. Both threshold and heat sensitivity returned to pre-H/R levels after 24 h and PWF after 4 h in sickle mice. AT-200, on the other hand, provided sustained mechanical anti-nociceptive response for up to 24 h (t11 = 2.4, P=0.035) (Figure 2A and B). AT-200 treatment showed a remarkable increase in PWL, more than 2-fold that of morphine at 30 min and sustained anti-nociception up to 24 h: baseline vs. 24 h: t9 = 1.08 (P=0.307) for morphine and t11 = 5.1 (P=0.0003) for AT-200) (Figure 2D). AT-200 was notably superior to morphine and showed a sustained effect for up to 24 h.
Deep tissue pain (compared to baseline: F5,24 = 9.12 (P<0.0001) for morphine, F5,30 = 16.15, (P<0.0001 for AT-200 and cold sensitivity increased following H/R, but both AT-200 and morphine similarly attenuated these features of pain in sickle mice (Figures 2C, E and F). Morphine and AT-200 increased grip force up to 30 min, but returned to base-line levels by 90 min (Figure 2C). Cold sensitivity returned to base-line levels by 30 min following morphine treatment, but AT-200 markedly reduced cold sensitivity up to 90 min (t5 = 7.6, P=0.0006) before returning to baseline levels by 240 min (Figure 2E and F). Importantly, sickle mice, but not control mice, exhibited sustained hyperalgesia up to 24 h with vehicle treatment following H/R.
Chronic treatment shows improved antinociceptive response over time without causing tolerance
Sickle mice were treated with AT-200, morphine and vehicle daily, and behavioral measures were obtained at baseline and 30 min after injection of drugs every other day (Figure 3A–C). A significant and consistent decrease in PWF response to mechanical stimuli, an increase in grip force and in PWL to heat occurred with both morphine and AT-200 treatment up to seven days (last period of drug injections) of testing compared to vehicle-treated sickle mice: vehicle vs. morphine vs. AT-200 F2,14 = 87.6 (P<0.0001) (PWF), F2,14 = 16.02 (P=0.0002 (grip force) and F2,14 = 129.1, P<0.0001 (PWL heat) (Figure 3A–C). The anti-nociceptive effect of AT-200 was significantly higher than morphine on each day of testing. A significant decrease in hyperalgesia was also observed with AT-200 on day 8, 24 h after the last injection on day 7 (vehicle vs. AT-200 t9 = 6.2, P=0.0002 (PWF), t9 = 4.4, P=0.0016 (grip force) and t9 = 8.3, P<0.0001 (PWL heat)) (Figure 3A–C). Morphine did not show a significant reduction in hyperalgesia compared to baseline on day 8. On the contrary, 24 h after the last injection, morphine-treated mice showed an increase in PWF in response to von Frey fiber application, suggestive of an increase in mechanical hyperalgesia compared to baseline (Figure 3A–C). Together these data demonstrate that daily treatment with AT-200 maintains its antinociceptive potency and shows a sustained effect even 24 h following the last injection.
Figure 3.
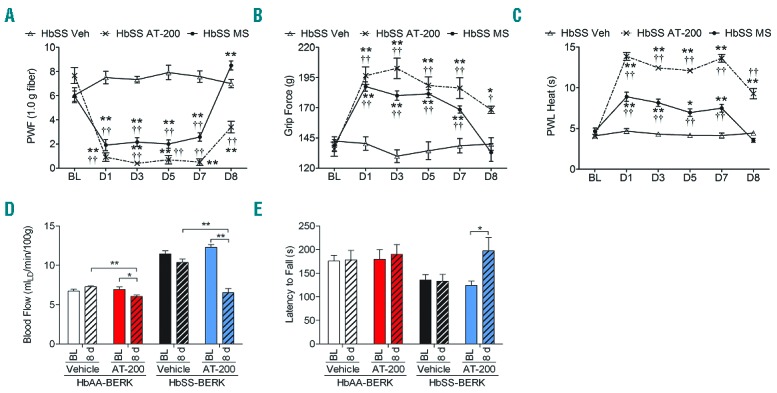
Sustained anti-nociceptive effect and lack of tolerance with chronic AT-200 treatment. Mice were treated daily with vehicle, morphine 20 mg/kg/day or AT-200 10 mg/kg/day for seven days. Sensory testing was performed at baseline before drug treatment and 30 minutes following the drug treatment on days 1, 3, 5 and 7 and on d8 (24 h following the last injection). Responses of sickle mice with each pain stimulus are shown (A–C). A separate set of control and sickle mice were treated daily for seven days with AT-200 or vehicle and tested before (baseline) and after eight days of starting the treatments, i.e. 24 h after the last daily drug injection (given on day 7, for dorsal cutaneous blood flow measured by laser Doppler velocimetry (D) and performance on an accelerating rotarod (0–72 rpm) over a period of 5 min to measure balance and motor co-ordination (E). Statistical significance was calculated by comparing each value to BL (*), and with vehicle (†) for each time point. *P<0.05 and **P<0.005 compared to BL; and ††P<0.005 compared to vehicle for that time point. Mean age of mice ± SEM in months were, HbSS-BERK Vehicle, 4.53 ± 0.22 (n=6); HbSS-BERK Morphine, 4.6± 0.25 and HbSS-BERK AT-200 (n=6), 4.6 ± 0.20. For the analysis of physiological parameters 5 HbAA-BERK mice and 8 HbSS-BERK mice were used in each group. Veh: vehicle; PWF: paw withdrawal frequency; PWL: paw withdrawal latency; BL: baseline; MS: morphine sulphate.
Chronic AT-200 treatment reduces cutaneous blood flow and enhances motor co-ordination
The dorsal cutaneous blood flow is drastically reduced in sickle mice chronically treated with AT-200 compared to control mice (vehicle vs. AT-200 t14 = 5.7, P<0.0001 and t8 = 4.2, P=0.0028; sickle and control, respectively) (Figure 3D). In addition, the latency to fall increased in sickle mice treated with AT-200, suggesting that motor co-ordination and balance were significantly enhanced on day 8 (baseline vs. day 8 t7 = 2.4, P=0.04) (Figure 3E). Overall, vehicle-treated mice showed no changes in any parameters tested.
AT-200 exerts anti-nociceptive effect via NOP/R in sickle mice
To determine whether the anti-nociceptive effect of AT-200 is via NOP/R and/or MOP/R, mice were pre-treated with SB-612111, a NOP/R antagonist, or naloxone, an opioid receptor antagonist. Pre-treatment of sickle mice with SB-612111 inhibited the anti-nociceptive effects of AT-200 on mechanical PWF, musculoskeletal pain, and heat sensitivity, whereas pre-treatment with naloxone did not influence the anti-nociceptive effect of AT-200 (Figure 4A–C). Importantly, the anti-nociceptive effect of AT-200 was sustained up to 24 h in sickle mice pre-treated with naloxone (vehicle vs. Naloxone + AT-200 t28 = 3.7, P=0.0009, t13 = 5.18, P=0.0002 and t28 = 6.8, P<0.0001; PWF, grip force and PWL (heat), respectively). Naloxone alone had no effect compared to baseline.
AT-200 inhibits cutaneous mast cell degranulation
Mast cell density/mm2 and percent degranulating mast cells were nearly 2- and 1.7-fold higher respectively in sickle mouse skin, compared to control mice (control and sickle vehicle vs. AT-200 F3, 22 = 12.39, P<0.0001 for density and F3, 22 = 26.02, P<0.0001 for degranulating cells; Figures 5A–F). Interestingly, AT-200 showed a robust 1.6-fold decrease in the percentage of degranulating mast cells in skin (vehicle versus AT-200 t14 = 7.4, P<0.0001) (Figure 5F) but did not decrease mast cell density significantly (Figure 5E). Importantly, AT-200 treatment significantly reduced plasma tryptase in sickle mice (vehicle versus AT-200 t6 = 3.3, P = 0.015) (Figure 5G), suggesting reduced mast cell degranulation. In addition, laser scanning confocal microscopy (LSCM) revealed a strong immunoreactivity of mast cell activation markers FcεRI (green), CD117 (red), and tryptase (turquoise) in the skin of sickle mice, which was reduced appreciably upon AT-200 treatment (Online Supplementary Figure S2).
AT-200 inhibits neuropeptides and inflammatory cytokines
Plasma of sickle mice showed a nearly 1.4-fold and a 1.8-fold increase in SP and CGRP respectively, compared to control mice (t12 = 4.0, P = 0.0016 and t11 = 6.7, P<0.0001 respectively) (Figure 5H and I). Treatment with AT-200 significantly reduced plasma SP and CGRP in sickle mice. Plasma SAP, an acute-phase protein and a marker of inflammation, was elevated 3-fold in sickle mice compared to control mice. AT-200 reduced plasma SAP in sickle mice by 2.25-fold (vehicle vs. AT-200 t6 = 7.5, P=0.0003) (Figure 5J). Complementary to the increase in SAP, plasma IL-6, TNF-α, and RANTES were significantly higher in sickle mice compared to control mice (Figure 5K–M). Two-way ANOVA suggests that there is interaction between the treatment and genotype in almost all of the parameters tested. AT-200 treatment led to a significant decrease in these inflammatory cytokines in sickle mice. Together, AT-200 reduces neuroinflammation and the inflammatory response in sickle mice.
Discussion
Pain in SCA is unique, often spanning the entire lifespan of an individual, and requiring long-term treatment with opioids. Due to the liabilities of dependence, tolerance, and side-effects such as constipation and hyperalgesia, opioid analgesia remains a sub-optimal approach. The observed effectiveness of AT-200, a NOP/R agonist, to treat hyperalgesia and VOC-associated nociception in sickle mice suggests a promising new approach for treating pain in SCA. Since chronic AT-200 treatment does not result in diminished analgesic efficacy but reduces mast cell activation and neuroinflammation, it may have distinct advantages over opioids for a long-term use in ameliorating pain in SCA.
Pain in SCA displays enormous phenotypic variability characterized by deep tissue pain and increased sensitivity to mechanical, heat and cold stimuli.1,24 Sickle mice show these features of pain, which are further enhanced with increasing age and upon H/R, recapitulating VOC.16,25 A relatively small dose (10 mg/kg) of AT-200 was able to ameliorate characteristic features of sickle pain in sickle mice. AT-200 induced dose-dependent anti-nociception, while a starting high dose above the therapeutic range caused lethality, which could be at least in part due to the fragility of sickle mice. The 10 mg/kg dose of AT-200 was determined to be the optimum dose showing a potent anti-nociceptive effect which was sustained for 24 h. Chronic pain is reported in about 50% of patients at all times, and increases with age.32 A significant number of these patients do not respond to opioid analgesia.1 AT-200 was effective in reducing pain measures in older mice, which could be similar to chronic pain in patients with SCA. Small-molecule NOP/R agonists as well as bifunctional NOP-MOP/R agonists show anti-nociceptive effects in several rodent models of chronic and neuropathic pain.12,14,33 Therefore, AT-200 may be an alternative to opioids when pain is chronic and/or non-responsive to opioids.
Acute pain due to VOC can be recurrent, lasting several days and requiring hospitalization.1 Treatment at the primordial stage of pain is suggested to be more effective in SCA. Opioid treatment poses a major challenge due to its dependence liability and Schedule II classification leading to delays in treatment. Given the lack of rewarding effects of AT-200,34 availability of NOP/R-based analgesics such as AT-200 may provide an advantage in treating SCA pain at an early stage, possibly without hospitalization. Indeed, our data show that AT-200 remained moderately effective for up to 24 h following the H/R-evoked nociception in sickle mice. Therefore, NOP/R agonists represent an innovative class of non-addicting analgesics effective in several sickle pain modalities.12,34
AT-200 binds to both NOP/R and MOP/R, but its affinity for NOP/R is approximately 20-fold higher than for the MOP/R receptor.26,34 Opioid antagonist naloxone did not inhibit the anti-nociceptive effect of AT-200, but NOP/R antagonist SB-612111 completely attenuated the anti-nociceptive effect of AT-200 on mechanical and heat stimuli and deep tissue/musculoskeletal pain in sickle mice. Thus, AT-200 likely exhibits its anti-nociceptive effect predominantly via NOP/R. Studies with NOP/R-targeted drugs in rodents and primates suggest that the anti-nociceptive effects of NOP/R agonists may be devoid of abuse liability, tolerance, respiratory depression, constipation and pruritis.33,35,36 Moreover, NOP/R agonists also show anxiolytic-like effects.37–39 Pain in SCA is also associated with depression and anxiety.40 NOP/R agonists may, therefore, provide a therapeutic profile suitable for the management of pain in SCA.
Pain was consistently ameliorated over a period of time by daily treatment of mice with AT-200 without the decrease in efficacy that normally accompanies tolerance development. Morphine at 20 mg/kg dose was significantly less effective than AT-200 upon daily treatment throughout the 7-day period. Moreover, 24 h after the last injection, morphine appeared to lose its anti-nociceptive efficacy, whereas AT-200-treated mice still showed a significant anti-hyperalgesic effect. In another study, we found that daily subcutaneous administration of AT-200 (10 mg/kg) to ICR (normal) mice over nine days showed no decrease in its anti-nociceptive efficacy in the tail-flick assay, unlike with morphine (3 mg/kg) (Zaveri et al., personal communication). Furthermore, we observed a similar lack of tolerance development with another bifunctional NOP/mu agonist compound AT-201 (SR16435) in an acute tail-flick assay41 and after repeated intrathecal administration of SR16435 (3 mg/kg) in mice with neuropathic pain.14 Together, these findings suggest that AT-200 maintains its anti-nociceptive efficacy upon repeated administration over an extended period of time.
Reduced cutaneous blood flow following seven days of AT-200 treatement in sickle mice is suggestive of reduced inflammation. Indeed, AT-200 reduced cutaneous mast cell degranulation and decreased circulating inflammatory cytokines, neuropeptides, mast cell tryptase and SAP. We have previously shown that treatment of sickle mice with mast cell inhibitors imatinib or Cromolyn sodium for five days reduced plasma CGRP and SP, inflammation marker SAP, and markers of mast cell activation, namely tryptase and b-hexosaminidase.7 Release of inflammatory cytokines TNF-α, IL6 and RANTES from the skin of sickle mice was also significantly reduced upon treatment with mast cell inhibitors, suggesting that activated mast cells contribute to inflammation in SCA.7 Mast cell activation has also been strongly implicated in the development of pain.42–45 Therefore, inhibition of mast cell-associated tryptase, inflammatory cytokines and neuropeptides with corresponding decrease in hyperalgesia in sickle mice treated with AT-200 is suggestive of an anti-inflammatory effect of AT-200. Moreover, the inhibitory effect of AT-200 on peripheral mast cell activity observed herein is consistent with dose-dependent inhibition of mucosal mast cell density for up to 52 h upon s.c. infusion of N/OFQ into the distal colon.46
Increased SP and CGRP in sickle mouse skin and plasma are accompanied by neurogenic inflammation and TRPV1 activation in the peripheral nerve terminals.7,28 N/OFQ reduces local axon reflex-mediated neurogenic inflammation by reducing the release of vasoactive neuropeptides CGRP and SP from sensory afferent nerve terminals in the periphery.18 In the chronically denervated rat model, an intraperitoneal injection of N/OFQ inhibited the release of neuropeptides SP, CGRP and somatostatin from isolated rat trachea in response to capsaicin or bradykinin.19 The concomitant decrease in mast cell degranulation and serum SP, SAP and cytokines in sickle mice following AT-200 treatment is suggestive of reduced nociceptor excitation and decreased inflammation. Since SP is elevated in SCA patients ranging from 2–18 years of age compared to age-matched normal subjects,17 mast cell activation may start early in age and contribute to elevated SP and pain observed in SCA. Our results suggest that AT-200 may have direct peripheral anti-inflammatory effects, possibly by inhibiting the release of chemical mediators from mast cells and capsaicin-sensitive afferent nerve terminals.
NOP/R agonist AT-200 significantly ameliorates tonic and H/R evoked hyperalgesia and inhibits peripheral neuroinflammation in sickle mice. The antinociceptive effect of AT-200 lasts for more than 24 h when administered immediately following the incitement of H/R. Small-molecule NOP/R agonists such as AT-200 are a novel class of analgesics, which directly target mechanisms underlying pain in SCA. Thus, NOP/R agonists are promising analgesics for sickle pain, without the liabilities associated with opioids.
Acknowledgments
We deeply thank Prof. John Connett for his extensive advice and support with statistical analysis. The authors thank Katie NH Johnson, Stefan Kren, Barbara Benson and Toan Chu for breeding, genotyping and phenotyping transgenic mice and Susan Thompson for preparation of graphics. We are also thankful to Drs. Robert P. Hebbel and David Archer from the University of Minnesota and Emory University respectively, for providing the breeder sickle mice and Dr. Donald A. Simone for the use of the behavioral testing facility.
Footnotes
The online version of this article has a Supplementary Appendix.
Funding
This work was supported by NIH grants R01DA014026 and R43HL115984 (to NZ) and R01HL68802, R01HL103773 and U01 HL117664-01 and Institute for Engineering in Medicine grants to KG.
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Ballas SK, Gupta K, Adams-Graves P. Sickle cell pain: a critical reappraisal. Blood. 2012; 120(18):3647–3656. [DOI] [PubMed] [Google Scholar]
- 2.Platt OS, Thorington BD, Brambilla DJ, et al. Pain in sickle cell disease. Rates and risk factors. N Engl J Med. 1991;325(1):11–16. [DOI] [PubMed] [Google Scholar]
- 3.Mellbye A, Karlstad O, Skurtveit S, Borchgrevink PC, Fredheim OM. Co-morbidity in persistent opioid users with chronic non-malignant pain in Norway. Eur J Pain. 2014;18(8):1083–1093. [DOI] [PubMed] [Google Scholar]
- 4.Chu LF, Angst MS, Clark D. Opioid-induced hyperalgesia in humans: molecular mechanisms and clinical considerations. Clin J Pain. 2008;24(6):479–496. [DOI] [PubMed] [Google Scholar]
- 5.Degenhardt L, Whiteford HA, Ferrari AJ, et al. Global burden of disease attributable to illicit drug use and dependence: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382(9904):1564–1574. [DOI] [PubMed] [Google Scholar]
- 6.Wang X, Loram LC, Ramos K, et al. Morphine activates neuroinflammation in a manner parallel to endotoxin. Proc Natl Acad Sci USA. 2012;109(16):6325–6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vincent L, Vang D, Nguyen J, et al. Mast cell activation contributes to sickle cell pathobiology and pain in mice. Blood. 2013;122(11):1853–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mogil JS, Pasternak GW. The molecular and behavioral pharmacology of the orphanin FQ/nociceptin peptide and receptor family. Pharmacol Rev. 2001;53(3):381–415. [PubMed] [Google Scholar]
- 9.Mollereau C, Parmentier M, Mailleux P, et al. ORL1, a novel member of the opioid receptor family. Cloning, functional expression and localization. FEBS Lett. 1994;341(1):33–38. [DOI] [PubMed] [Google Scholar]
- 10.Neal CR, Jr, Mansour A, Reinscheid R, Nothacker HP, Civelli O, Watson SJ., Jr Localization of orphanin FQ (nociceptin) peptide and messenger RNA in the central nervous system of the rat. J Comp Neurol. 1999;406(4):503–547. [PubMed] [Google Scholar]
- 11.Neal CR, Jr, Akil H, Watson SJ., Jr Expression of orphanin FQ and the opioid receptor-like (ORL1) receptor in the developing human and rat brain. J Chem Neuroanat. 2001;22(4):219–249. [DOI] [PubMed] [Google Scholar]
- 12.Khroyan TV, Polgar WE, Orduna J, et al. Differential effects of nociceptin/orphanin FQ (NOP) receptor agonists in acute versus chronic pain: studies with bifunctional NOP/mu receptor agonists in the sciatic nerve ligation chronic pain model in mice. J Pharmacol Exp Ther. 2011;339(2):687–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toll L, Khroyan TV, Polgar W, Husbands SM, Zaveri NT. Pharmacology of mixed NOP/Mu ligands. In: Ko MC, Husbands SM, eds. Research and Development of Opioid-Related Ligands. Washington DC: American Chemical Society, 2013:369–391 (ACS Symposium Series; 1131). [Google Scholar]
- 14.Sukhtankar DD, Zaveri NT, Husbands SM, Ko MC. Effects of spinally administered bifunctional nociceptin/orphanin FQ peptide receptor/mu-opioid receptor ligands in mouse models of neuropathic and inflammatory pain. J Pharmacol Exp Ther. 2013; 346(1):11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inoue M, Kawashima T, Takeshima H, et al. In vivo pain-inhibitory role of noci-ceptin/orphanin FQ in spinal cord. J Pharmacol Exp Ther. 2003;305(2):495–501. [DOI] [PubMed] [Google Scholar]
- 16.Kohli DR, Li Y, Khasabov SG, et al. Pain-related behaviors and neurochemical alterations in mice expressing sickle hemoglobin: modulation by cannabinoids. Blood. 2010;116(3):456–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Michaels LA, Ohene-Frempong K, Zhao H, Douglas SD. Serum levels of substance P are elevated in patients with sickle cell disease and increase further during vaso-occlusive crisis. Blood. 1998;92(9):3148–3151. [PubMed] [Google Scholar]
- 18.Helyes Z, Nemeth J, Pinter E, Szolcsanyi J. Inhibition by nociceptin of neurogenic inflammation and the release of SP and CGRP from sensory nerve terminals. Br J Pharmacol. 1997;121(4):613–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nemeth J, Helyes Z, Oroszi G, Than M, Pinter E, Szolcsanyi J. Inhibition of nociceptin on sensory neuropeptide release and mast cell-mediated plasma extravasation in rats. Eur J Pharmacol. 1998;347(1):101–104. [DOI] [PubMed] [Google Scholar]
- 20.Galli SJ, Tsai M. IgE and mast cells in allergic disease. Nat Med. 2012;18(5):693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abraham SN, St John AL. Mast cell-orches trated immunity to pathogens. Nat Rev Immunol. 2010;10(6):440–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zuo Y, Perkins NM, Tracey DJ, Geczy CL. Inflammation and hyperalgesia induced by nerve injury in the rat: a key role of mast cells. Pain. 2003;105(3):467–479. [DOI] [PubMed] [Google Scholar]
- 23.Amadesi S, Nie J, Vergnolle N, et al. Protease-activated receptor 2 sensitizes the capsaicin receptor transient receptor potential vanilloid receptor 1 to induce hyperalgesia. J Neurosci. 2004;24(18):4300–4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brandow AM, Stucky CL, Hillery CA, Hoffmann RG, Panepinto JA. Patients with sickle cell disease have increased sensitivity to cold and heat. Am J Hematol. 2013;88(1):37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cain DM, Vang D, Simone DA, Hebbel RP, Gupta K. Mouse models for studying pain in sickle disease: effects of strain, age, and acuteness. Br J Haematol. 2012;156(4):535–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zaveri NT, Jiang F, Olsen Cris M, et al. A novel series of piperidin-4-yl-1,3-dihy-droindol-2-ones as agonist and antagonist ligands at the nociceptin receptor. J Med Chem. 2004;47(2973–2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paszty C, Brion CM, Manci E, et al. Transgenic knockout mice with exclusively human sickle hemoglobin and sickle cell disease. Science. 1997;278(5339):876–878. [DOI] [PubMed] [Google Scholar]
- 28.Hillery CA, Kerstein PC, Vilceanu D, et al. Transient receptor potential vanilloid 1 mediates pain in mice with severe sickle cell disease. Blood. 2011;118(12):3376–3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cataldo G, Rajput S, Gupta K, Simone DA. Sensitization of nociceptive spinal neurons contributes to pain in a transgenic model of sickle cell disease. Pain. 2015;156(4):722–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Belcher JD, Bryant CJ, Nguyen J, et al. Transgenic sickle mice have vascular inflammation. Blood. 2003;101(10):3953–3959. [DOI] [PubMed] [Google Scholar]
- 31.Barlocco D, Cignarella G, Giardina G, Grugni M, Ronzoni S, inventors. (2006) inventors; GaxoSmithKline S.p.A., assignee. Benzosuberonylpiperidine compounds as analgesics. U.S. Patent 7,115,633. 2006. October 3. [Google Scholar]
- 32.Darbari DS, Ballas SK, Clauw DJ. Thinking beyond sickling to better understand pain in sickle cell disease. Eur J Haematol. 2014; 93(2):89–95. [DOI] [PubMed] [Google Scholar]
- 33.Podlesnik CA, Ko MC, Winger G, Wichmann J, Prinssen EP, Woods JH. The effects of nociceptin/orphanin FQ receptor agonist Ro 64–6198 and diazepam on antinociception and remifentanil self-administration in rhesus monkeys. Psychopharmacology. 2011;213(1):53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toll L, Khroyan TV, Polgar WE, Jiang F, Olsen C, Zaveri NT. Comparison of the anti-nociceptive and anti-rewarding profiles of novel bifunctional nociceptin/orphanin FQ receptor (NOPr)-Mu opioid receptor (MOPr) ligands: implications for therapeutic applications. J Pharmacol Exp Ther. 2009;331(3):954–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin AP, Ko MC. The therapeutic potential of nociceptin/orphanin FQ receptor agonists as analgesics without abuse liability. ACS Chem Neurosci. 2013;4(2):214–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dautzenberg FM, Wichmann J, Higelin J, et al. Pharmacological characterization of the novel nonpeptide orphanin FQ/nociceptin receptor agonist Ro 64–6198: rapid and reversible desensitization of the ORL1 receptor in vitro and lack of tolerance in vivo. J Pharmacol Exp Ther. 2001;298(2): 812–819. [PubMed] [Google Scholar]
- 37.Varty GB, Hyde LA, Hodgson RA, et al. Characterization of the nociceptin receptor (ORL-1) agonist, Ro64–6198, in tests of anxiety across multiple species. Psychopharmacology (Berl). 2005;182(1): 132–143. [DOI] [PubMed] [Google Scholar]
- 38.Varty GB, Lu SX, Morgan CA, et al. The anxiolytic-like effects of the novel, orally active nociceptin opioid receptor agonist 8-[bis(2-methylphenyl)methyl]-3-phenyl-8-azabicyclo[3.2.1]octan-3-ol (SCH 221510). J Pharmacol Exp Ther. 2008;326(2):672–682. [DOI] [PubMed] [Google Scholar]
- 39.Jenck F, Wichmann J, Dautzenberg FM, et al. A synthetic agonist at the orphanin FQ/nociceptin receptor ORL1: anxiolytic profile in the rat. Proc Natl Acad Sci USA. 2000;97(9):4938–4943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sogutlu A, Levenson JL, McClish DK, Rosef SD, Smith WR. Somatic symptom burden in adults with sickle cell disease predicts pain, depression, anxiety, health care utilization, and quality of life: the PiSCES project. Psychosomatics. 2011;52(3):272–279. [DOI] [PubMed] [Google Scholar]
- 41.Khroyan TV, Zaveri NT, Polgar WE, et al. SR 16435 [1-(1-(bicyclo[3.3.1]nonan-9-yl)piperidin-4-yl)indolin-2-one], a novel mixed nociceptin/orphanin FQ/mu-opioid receptor partial agonist: analgesic and rewarding properties in mice. J Pharmacol Exp Ther. 2007;320(2):934–943. [DOI] [PubMed] [Google Scholar]
- 42.Oliveira SM, Silva CR, Ferreira J. Critical role of protease-activated receptor 2 activation by mast cell tryptase in the development of postoperative pain. Anesthesiology. 2013;118(3): 679–690. [DOI] [PubMed] [Google Scholar]
- 43.Hesselink JM. New targets in pain, non-neuronal cells, and the role of palmitoylethanolamide. Open Pain J. 2012;5:12–23. [Google Scholar]
- 44.Levy D, Kainz V, Burstein R, Strassman AM. Mast cell degranulation distinctly activates trigemino-cervical and lumbosacral pain pathways and elicits widespread tactile pain hypersensitivity. Brain Behav Immun. 2012;26(2):311–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ren K, Dubner R. Interactions between the immune and nervous systems in pain. Nat Med. 2010;16(11):1267–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grandi D, Massi M, Guerrini R, Calo G, Morini G. Role of nociceptin/orphanin FQ receptors in the decrease of mucosal mast cells caused by acute stress in the rat colon. Life Sci. 2011;89(19–20):735–740. [DOI] [PubMed] [Google Scholar]