Figure 1.
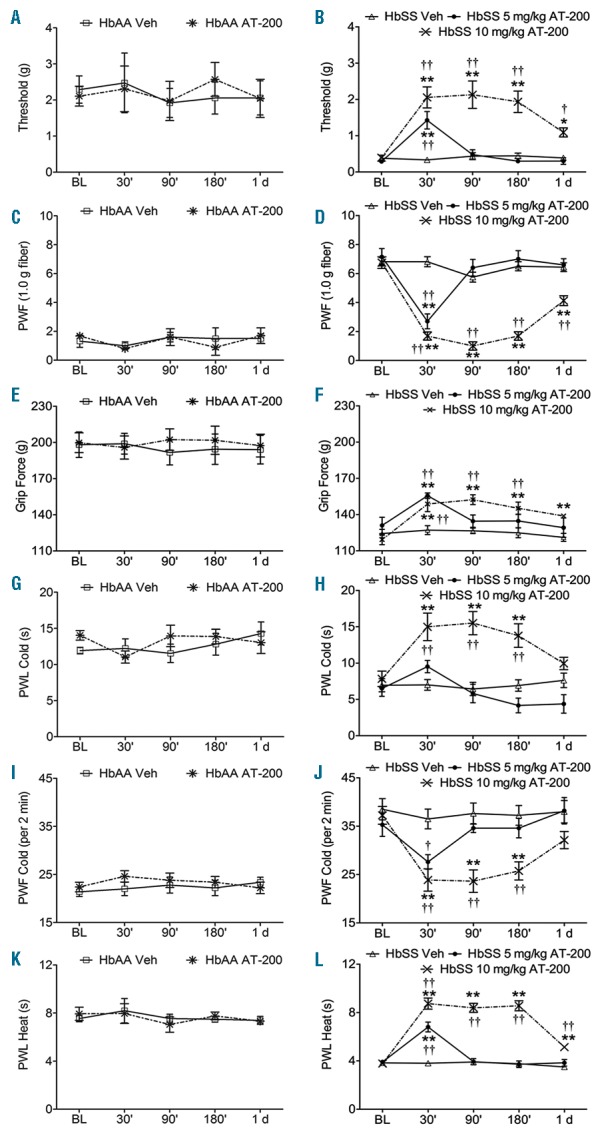
AT-200 ameliorates chronic mechanical, deep tissue and thermal hyperalgesia. Sickle mice were injected subcutaneously with 10 mg/kg AT-200, 5 mg/kg AT-200 or vehicle, while control mice were injected subcutaneously with 10 mg/kg AT-200 or vehicle. The sensitivity to mechanical stimuli (A–D), deep tissue hyperalgesia (E and F), and thermal hyperalgesia (G–L) were measured over the indicated time period. (A and B) Mechanical withdrawal threshold using von Frey monofilaments, a measure of cutaneous nociception is shown. Lower thresholds are indicative of increased sensitivity to cutaneous nociception. (C and D) PWF in response to 10 applications of a 9.8 mN (1.0 g) von Frey monofilament suggestive of mechanical hyperalgesia. A higher PWF indicates increased nociception. (E and F) Grip force measurements indicating deep tissue/musculoskeletal pain are shown. Lower grip force suggests increased deep tissue hyperalgesia. (G and H) PWL and (I and J) PWF on a cold plate maintained at 4 ± 1°C. A lower PWL and higher PWF in a 2-min period on a cold plate are indicative of increased sensitivity to cold-induced nociception. (K and L) PWL to a heat stimulus. Shorter PWL (in seconds) in response to heat stimulus is indicative of increased heat sensitivity. Sickle mice treated with 10 mg/kg of AT-200 showed significant analgesic response at all the time points tested compared to control mice. However, sickle mice treated with 5 mg/kg of AT-200 did not show sustained anti-nociceptive effect. Statistical significance was calculated by comparing each value to BL (*) and between vehicle and AT-200 (†). *P<0.05 and **P<0.005 compared to BL; and †P<0.05 and ††P<0.005 compared to vehicle for that time point. Mean age of mice ± SEM in months were, HbAA-BERK vehicle (n=12) and HbAA-BERK AT-200 (n=12), 17.2 ± 1.6; HbSS-BERK vehicle (n=16), 21.2 ± 1.2, and HbSS-BERK AT-200, 20.7 ± 1.4 (10 mg/kg; n=16); 10.8 ± 1.9 (5 mg/kg; n=10). BL: baseline; PWF: paw withdrawal frequency; PWL: paw withdrawal frequency; Veh: vehicle.
