Ponatinib is a tyrosine kinase inhibitor (TKI) designed to overcome resistance-inducing mutations, including the T315I mutation, in the ABL kinase domain. Initial clinical trials of ponatinib reported major cytogenetic response (MCyR) rates over 60% in heavily treated patients with chronic myeloid leukemia in chronic phase (CML-CP) and approval for patients who had received prior treatment with a TKI was granted by regulatory authorities in 2012.1–3 Due to concerns of thrombotic vascular events, ponatinib was transiently withdrawn from the US market in October 2013 on the recommendation of the FDA and later re-introduced in January 2014. Ponatinib is currently indicated for: 1) CML patients with the T315I mutation or in cases where no other TKI is indicated (US label); or 2) those resistant or intolerant to dasatinib or nilotinib, or for those in whom imatinib therapy is not clinically appropriate or who have the T315I mutation (European label).
Over 90% of patients enrolled in earlier clinical trials of ponatinib had received at least 2 prior TKI. Among patients who have received just one TKI, data are only available for imatinib failure. Less than 50% of patients achieve a MCyR with dasatinib, nilotinib or bosutinib after imatinib failure, and deeper (i.e. molecular) responses are even less common. There are no prospective data on the use of ponatinib after failure of a 2nd generation TKI, making this sequence of therapy empirical.
We designed a single center phase II clinical trial (clinicaltrials.gov identifier: 01746836) of ponatinib in patients with CML-CP and resistance or intolerance to only one FDA-approved TKI (imatinib, nilotinib or dasatinib). Failure of previous TKI therapy was defined according of European LeukemiaNet (ELN) recommendations.4 Exclusion criteria included: exposure to other non-FDA approved TKI, New York Heart Association (NYHA) cardiac class 3–4 heart disease, history of acute coronary syndrome, history of stroke or transient ischemic attack, history of peripheral arterial occlusive disease, history or venous thromboembolism, history of clinically significant ventricular arrhythmia, uncontrolled hypertension, history of pancreatitis, pregnancy or breastfeeding, and previous use of more than one FDA-approved TKI. The primary objective of the study was to estimate the rate of MCyR (CCyR + PCyR) at six months. Secondary outcomes included cytogenetic response at other time points, molecular response and toxicity profile. Hematologic, cytogenetic and molecular responses were determined according to ELN 2009 guidelines.4 The severity of adverse events (AEs) were graded according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE).5 Patients were initially treated at a starting dose of 45 mg/day; in October 2013 the starting dose was amended to 30 mg/day.
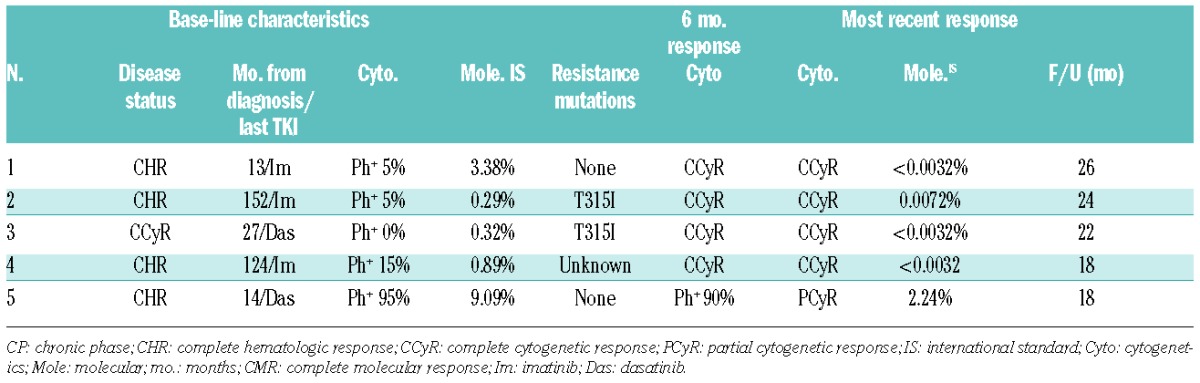
Recruitment onto the trial took place between March 2013 and September 2013. Five patients were enrolled before the study was closed to new patient enrollment at the recommendation of the FDA due to safety concerns. All patients enrolled onto the trial continued ponatinib upon closure of the study, and the dose was reduced to 15–30 mg/day to minimize the risk of thrombotic vascular events. All patients were eligible for the trial due to failure of previous TKI therapy with specific reasons including: loss of CCyR (n=1), no CCyR after 18 months (n=1), no CCyR after 18 months + T315I mutant (n=1), T315I mutant (n=1) and no PCyR after 12 months (n=1). No other resistance mutations were identified at the time of enrollment. Median age was 50 years (range 42–74 years) and the previous TKI exposure included imatinib (n=3) and dasatinib (n=2). Only one patient had significant base-line cardiovascular (CV) risk factors (hypertension).
Four patients (80%) achieved a CCyR at both the 3-month and 6-month assessment. Molecular responses at 12 months were: 1 patient MR4.5, 2 patients MMR, one patient with a significant reduction in transcript (0.11%), and one patient with no significant molecular response. After a median follow up of 22 months (range 18–26 months), cumulative best response for all patients was PCyR in one (previously treated with dasatinib) and MR4.5 in 4 patients. Responses were durable with all 4 patients who achieved a CCyR at six months maintaining an MR4.0 (n=1) or MR4.5 (n=3) at last follow up. The base-line disease status and response data are summarized in Table 1.
Table 1.
Base-line characteristics and responses.
Grade 3–4 AEs included: hypertension (n=2), development of a neoplasm (superficially invasive skin squamous cell carcinoma) (n=1), increased lipase (n=2), increased amylase (n=1), increased ALT (n=2), increased AST (n=2), thrombocytopenia (n=1), and neutropenia (n=1). Four patients developed hypertension (Grade 2–3), one of them with base-line hypertension. Three of these 4 patients with hypertension were successfully treated with antihypertensive medication, while the other was monitored and was normotensive at subsequent visits without intervention. No venous or arterial thrombotic events were observed in any of the patients. Neither of the 2 patients who had increased lipase/amylase developed clinical pancreatitis. In both patients, a rise in pancreatic enzymes was observed within two months of starting ponatinib. In both instances, ponatinib was held for 1–2 weeks and re-started without significant rise in lipase or amylase. Similarly, neither patient experiencing grade 3 increases in AST/ALT had clinical symptoms of liver dysfunction or abnormal liver function testing. These patients were managed with dose interruption and ponatinib was successfully re-introduced at a reduced dose in both patients (15 mg/day). Recurrent grade 3–4 neutropenia and thrombocytopenia occurred in the patient who did not achieve a CCyR. These episodes were managed with treatment interruption and dose reduction with improvement in blood counts. All patients had a dose reduction; 2 patients continue on 30 mg/day and 3 patients on 15 mg/day. The median time to first dose reduction was three months (range 1–8 months) and this occurred either empirically to reduce CV risk in 3 patients or due to an AE in 2 patients.
In summary, 80% of patients with exposure to one prior TKI achieved a CCyR and MR4.0 or better, with sustained responses over a relatively long follow-up period. Although interpretation of the findings of our trial is limited by the small number of patients, our results reinforce the efficacy of ponatinib in patients failing only one TKI. Our findings are consistent with the results of the phase I and phase II trials of ponatinib in CML-CP patients with TKI-resistance/intolerance, in which the rate of CCyR was 63% and 46%, respectively.2,3 Response rates in the phase II clinical trial were highest in CML-CP patients treated with only one previous TKI with CCyR and MMR rates of 74% and 47%, respectively.3 However, only 19 such patients were treated. The rate of venous and arterial thrombotic events with ponatinib in earlier studies was 37% and 24% in the phase I and II clinical trials, respectively, reported over longer follow-up periods.6,7 No patients in our trial experienced a thrombotic event, which could be explained by a random null rate due to the small number of patients and/or by the relative absence of significant CV risk factors in these patients. Most patients experiencing thrombotic events in previous clinical trials had pre-existing risk factors for CV events.8,9 In our series, the only CV event observed was hypertension, which was manageable with therapy in all patients concerned.
It is also possible that, as patients in our trial received ponatinib at lower doses for most of the time they were on treatment due to early dose reductions, the risk of vascular thrombotic events might have been reduced. Currently, the approved starting dose of ponatinib is 45 mg/day, although it is acknowledged that the optimal starting dose of ponatinib is not known.1 In the phase I clinical trial, a peak serum concentration of 40 nM was achieved with a dose of 15 mg/day, which is the concentration required to suppress the development of resistance mutations in vitro.2 Furthermore, a post hoc study of previous clinical trials using ponatinib projected that dose reductions of 15 mg/day were associated with a 40% relative risk reduction of arterial thrombosis.10 Importantly, it is apparent from our results that patients can have sustained responses on lower doses of ponatinib, and the toxicity profile of ponatinib was manageable at these doses in our group.
The results in this small cohort of patients suggest that ponatinib can be of clinical benefit for patients with CML-CP who have received only one prior TKI. Despite the availability of multiple TKIs, the results in this patient population could be improved, as the rate of CCyR in previous trials does not reach 50%, and EFS at 4–5 years is only approximately 50%.11–13 Further studies are required to help define the efficacy, safety and optimal dosing of ponatinib in this patient population.
Acknowledgments
We would like to thank Ariad Pharmaceuticals for providing ponatinib and M.D. Anderson Cancer Center for financial support of this study.
Footnotes
Funding: the University of Texas M. D. Anderson Cancer Center is supported in part by the National Institutes of Health through a Cancer Center Support Grant (P30 CA16672).
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.FDA Approval for Ponatinib Hydrochloride 2014 March 12, 2014. [cited 2015 June 15, 2015]; Available from: http://www.cancer.gov/about-cancer/treatment/drugs/fda-ponatinibhydrochloride
- 2.Cortes JE, Kantarjian H, Shah NP, et al. Ponatinib in refractory Philadelphia chromosome-positive leukemias. N Engl J Med. 2012; 367(22):2075–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cortes JE, Kim DW, Pinilla-Ibarz J, et al. A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias. N Engl J Med. 2013; 369(19):1783–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baccarani M, Cortes J, Pane F, et al. Chronic myeloid leukemia: an update of concepts and management recommendations of European LeukemiaNet. J Clin Oncol. 2009;27(35):6041–6051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The Revised National Cancer Institute Cancer Therapy Evaluation Program Common Terminology Criteria for Adverse Events, Version 3.0. Available from: http://ctep.cancer.gov/reporting/ctc.html
- 6.Talpaz M, Cortes JE, Kantarjian HM, et al. Longer-term follow up of a phase 1 study of ponatinib in patients (pts) with Philadelphia chromosome-positive (Ph+) leukemias. J Clin Oncol. 2014; 32(5s):(Abstract)7078. [Google Scholar]
- 7.Cortes JE, Kim DW, Pinilla-Ibarz J, et al. Long-Term Follow-up of Ponatinib Efficacy and Safety in the Phase 2 PACE Trial. Blood. 2014; 124(21):(Abstract)3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lipton JH, Chuah C, Guerci-Bresler A, et al. A Phase 3 Trial of Ponatinib Compared with Imatinib in Patients with Newly Diagnosed Chronic Myeloid Leukemia in Chronic Phase (CP-CML). Blood. 2014;124(21):519.24740813 [Google Scholar]
- 9.Khoury HJ, Cortes JE, Kim DW, et al. Analysis of the cardiovascular risk profile of Ph+ leukemia patients treated with ponatinib. J Clin Oncol. 2013;31:(Abstract)7048. [Google Scholar]
- 10.Hochhaus A, Pinilla-Ibarz J, Kim DW, et al. Clinical impact of dose modification and dose intensity on response to ponatinib (PON) in patients (pts) with Philadelphia chromosome-positive (Ph+) leukemias. J Clin Oncol. 2014;32(5s):(Abstract)7084. [Google Scholar]
- 11.Shah NP, Guilhot F, Cortes JE, et al. Long-term outcome with dasatinib after imatinib failure in chronic-phase chronic myeloid leukemia: follow-up of a phase 3 study. Blood. 2014;123(15):2317–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giles FJ, le Coutre PD, Pinilla-Ibarz J, et al. Nilotinib in imatinib-resistant or imatinib-intolerant patients with chronic myeloid leukemia in chronic phase: 48-month follow-up results of a phase II study. Leukemia. 2013;27(1):107–112. [DOI] [PubMed] [Google Scholar]
- 13.Gambacorti-Passerini C, Brummendorf TH, Kim DW, et al. Bosutinib efficacy and safety in chronic phase chronic myeloid leukemia after imatinib resistance or intolerance: Minimum 24-month follow-up. Am J Hematol. 2014;89(7):732–742. [DOI] [PMC free article] [PubMed] [Google Scholar]


