The prognosis and treatment of chronic lymphocytic leukemia (CLL) have improved significantly over the last years; however, CLL is still an incurable disease and infections are the major cause of morbidity and mortality, contributing to 25–50% of deaths.1
Susceptibility to infections in CLL patients can be related to immunological defects associated with the disease (including hypogammaglobulinemia, T-cell, natural killer-cell and innate immunity dysfunctions)2 and secondary to chemo-immunotherapy. Hypogammaglobulinemia and T-cell defects are quite common in these patients and become more pronounced with advanced-stage disease.2 Interestingly, it has been described that even patients with monoclonal B-cell lymphocytosis have a higher risk of serious infection than do the general population.3
Strategies to prevent bacterial infections in patients with symptomatic hypogammaglobulinemia include prophylactic antibiotics or immunoglobulin replacement therapy (IgRT). Although passive immunotherapy with immunoglobulins (Ig) can lower the risk of minor and major bacterial infections, several data suggest that IgRT does not result in a decrease of mortality,4 and there are currently no clear indications for this treatment. As a consequence, IgRT is started in subjects with hypogammaglobulinemia complaining of serious or recurrent bacterial infections.5,6
The aim of this study was to identify the clinical and biochemical characteristics of subjects at higher risk of developing major infections; in particular, we focused on the role of hypogammaglobulinemia and the impact of IgRT. We retrospectively reviewed data from 706 patients with CLL referred to our Unit from 1983 to 2013. Major infections were defined as infective events that required inpatient management or intravenous antibiotics. Major infections associated with a concomitant neutropenia (white cell count <1.0×109/L) were excluded. Furthermore, the exclusion of non-serious events from our analysis lowered the risk of including other biases. We collected the closest clinical data preceding the onset of each major infection (mean time between measurement of Ig levels and major infections 2.5±1.3 months); Ig levels measured during major infections were not included in the analysis, because they could have been influenced by the infection. For patients who did not suffer any major infections, we considered the last available Ig level. Detailed information on prognostic markers, IgRT strategies and statistical methods are reported in the Online Supplementary Material. The study was approved by the local research ethics committee and informed consent was obtained from all patients.
The characteristics of our CLL patients are summarized in Table 1. Ninety-eight major infections were detected in 79 patients (11% of the cohort): 67 had pneumonia (85% of patients), including five with pulmonary aspergillosis, 27 (34%) had septic shock, three (3%) had central nervous system infections and one (1%) had endocarditis. Patients with a history of major infection had a shorter overall survival than patients without major infections (Online Supplementary Figure S1, P<0.0001), with the 10-year overall survival rates being 65% versus 83%, respectively. In multivariate analysis age over 65 years, 17p deletion, unmutated IGHV and a history of major infections were the most important markers of survival. In particular, patients with major infections had a 2.25 (95% CI, 1.47–3.44) higher risk of death from any cause compared to subjects who did not experience major infections (Online Supplementary Figure S1, P<0.0002).
Table 1.
Clinical and biological characteristics of the whole population, patients with and without a history of major infections (PwI and PwoI, respectively).
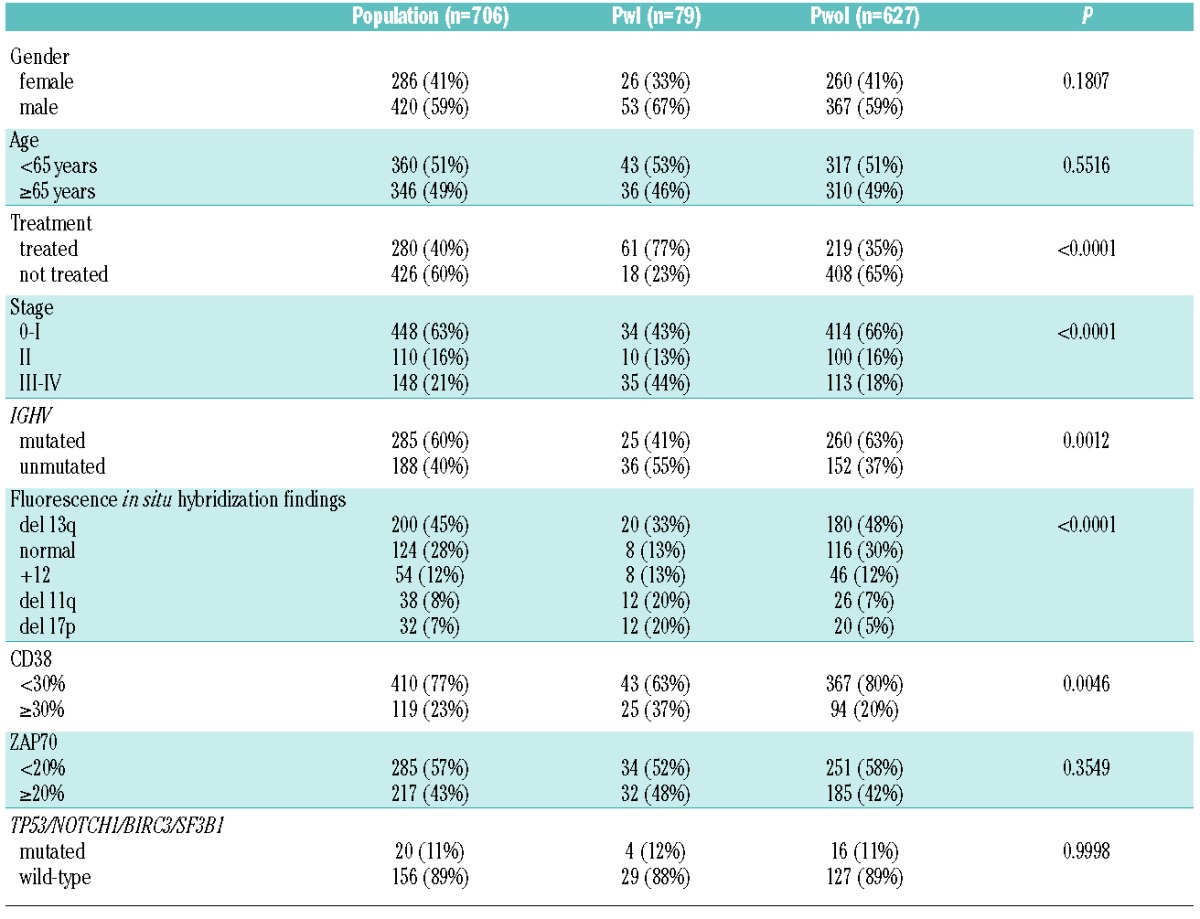
In accordance with previous works,7–9 factors associated with the occurrence of major infections were previous treatment, advanced Rai stage, high-risk cytogenetics determined by fluorescence in situ hybridization analysis (i.e. 11q or 17p deletion), unmutated IGHV and CD38 positivity (Table 1). By contrast, we did not find any statistical association between the risk of major infections and gender, age over 65 years, median age, ZAP70 and TP53, NOTCH1, SF3B1 mutations and BIRC3 abnormalities (Table 1).
Focusing on hypogammaglobulinemia, Ig levels were significantly lower in patients with a history of major infections than in patients who did not experience infections (Figure 1A–C). Using receiver operating characteristic curve analysis we identified the best protective cut-off for each Ig isotype: 744 mg/dL for IgG [sensitivity 73%, specificity 65%, area under the curve (AUC) 0.73], 79 mg/dL for IgA (sensitivity 70%, specificity 73%, AUC 0.76) and 21 mg/dL for IgM (sensitivity 47%, specificity 80%, AUC 0.62). Using these cut-offs we detected low levels of IgG associated with low levels of either IgA or IgM (further referred to as combined antibody deficiency, CAD) in 65% of patients with major infections while a similar defect was observed in only 20% of patients who never experienced a major infection (Figure 1D).
Figure 1.
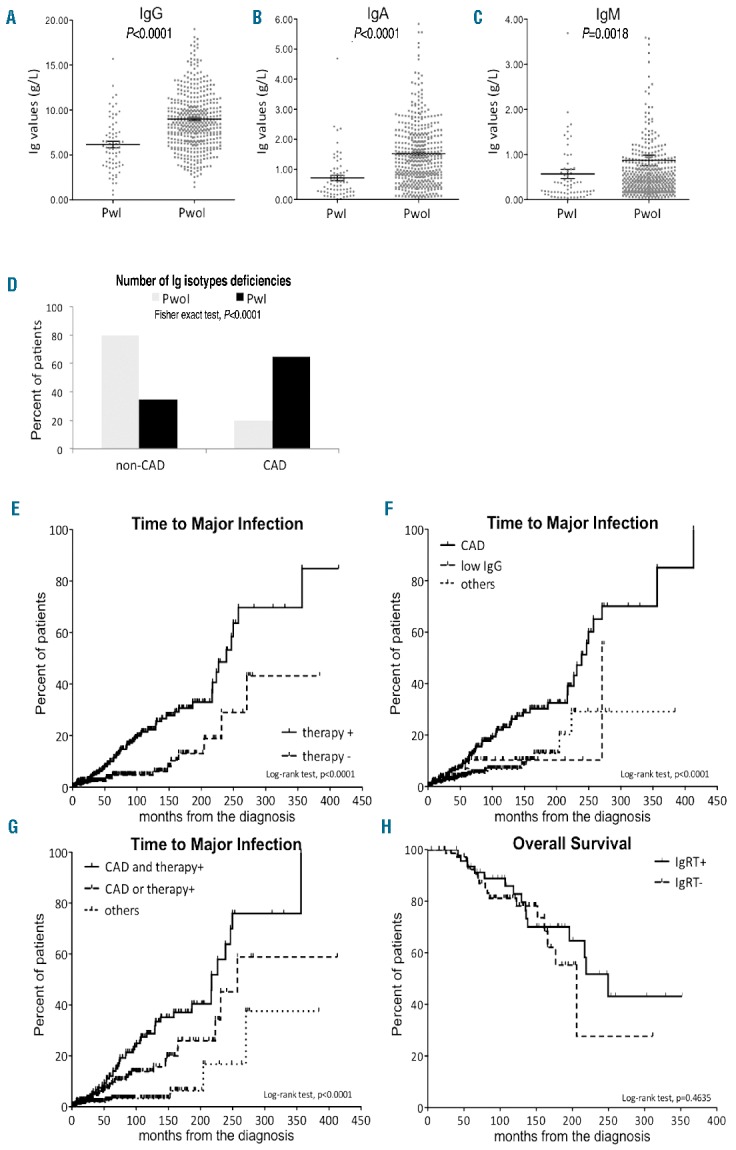
Comparison of Ig levels between patients with and without a history of infection and Kaplan-Meier curves estimate of time to major infection and overall survival. The upper panels compare (A) IgG, (B) IgA and (C) IgM between patients with (PwI) and without a history of major infection (PwoI) by the Mann-Whitney test. Panel (D) shows a histogram of the percentage of patients with and without combined antibody deficiency (CAD and non-CAD, respectively). Times to infection were estimated according to (E) previous need of treatment, (F) immunoglobulin deficiency and (G) combined analysis. (H) Overall survival was estimated according to immunoglobulin replacement therapy.
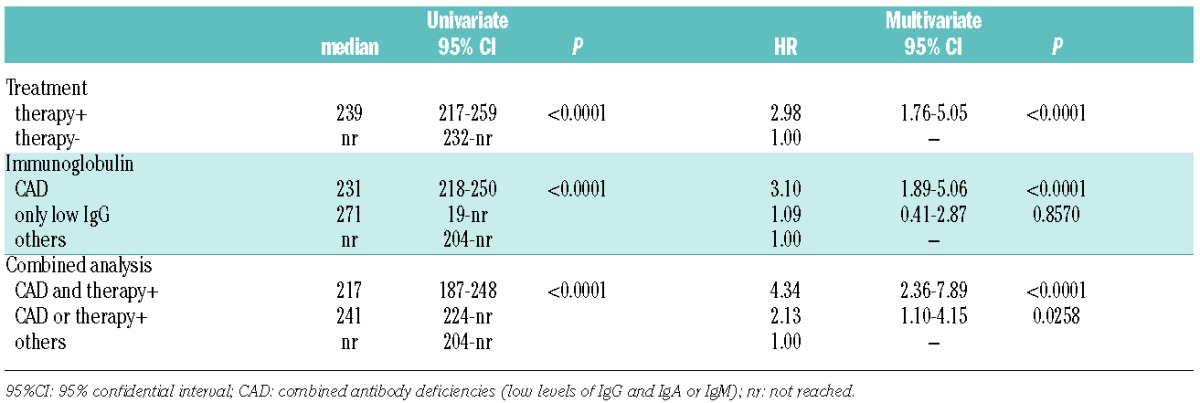
In the univariate analysis, previously treated patients and those with combined antibody deficiency developed major infections in a significantly shorter time than patients who did not need CLL-specific therapy (239 months versus not reached, Figure 1E, Table 2) or those without CAD (239 months versus 270 versus not reached, Figure 1F, Table 2). By multivariate analysis the hazard ratios for treatment and CAD were 2.98 and 3.10, respectively (Table 2). Taken together, these data suggest that previous chemo-immunotherapy and CAD induced similar risks for the development of major infections. Furthermore, using a unique Cox regression model, we showed that the presence of a combination of these two markers identified the subset of patients with the highest risk of major infections. In fact, the median time to major infections was significantly shorter in patients who had both a history of treatment and CAD than in subjects with only one or none of these markers (217 months versus 241 months versus not reached, Figure 1G, Table 2). This model was also internally validated (detailed information is provided in the Online Supplementary Material). The impact of all other clinical and biological prognostic markers on time to major infections is reported in Online Supplementary Table SI.
Table 2.
Median months to infection and hazard ratio by univariate and multivariate analysis, respectively.
In 126 patients with such high-risk features, the incidence of major infections was 0.044 major infections/people-years; this incidence was almost three times higher than that in our patients with monoclonal B cell lymphocytosis (0.016 major infections/people-years). IgRT significantly decreased the cumulative incidence of major infections from 0.044 to 0.019 major infections/people-years. We also observed a slight improvement of overall survival with IgRT (250 months versus 206 months, respectively) (Figure 1H); however, this difference was not statistically significant. Given the small size of this subset, the possible role of IgRT in modifying survival of CLL patients should be studied in a larger group.
In this study we confirmed the well-known disease-related risk factors for major infections, which are indeed major causes of morbidity and mortality. Although the association between symptomatic hypogammaglobulinemia and CLL is well-recognized,9 in two recent studies, significant associations were not found between Ig levels and infections.7–9 In both studies, Ig levels were recorded independently of infectious events; thus, Ig levels could have been different when determined at diagnosis or during the infectious event. Of note, our study was designed to collect the clinical data closest to the major infections, in order to consider the actual Ig level at the time of the infection. This approach is more appropriate for evaluating the role of a dynamic and gradually worsening risk factor such as hypogammaglobulinemia.
Randomized controlled studies on prophylactic IgRT in patients with CLL have been summarized recently:4 they suggest the use of IgRT in patients with symptomatic hypogammaglobulinemia, in particular when IgG levels are below 500 mg/dL, since this therapy could significantly decrease the number of infections, the use of antibiotics, hospitalizations and loss of working days.10–12 Attempts have been made to define the risk factors for infections in CLL in order to select patients who could benefit most from IgRT, even with a pre-emptive approach. Dhalla et al.1 suggested that immunization responses could be used to stratify infection risk and select patients for IgRT. Freeman et al.13 proposed that screening patients with CLL for IgG subclass deficiency could be a useful adjunct in stratifying the patients’ risk for infection.
Herein, we identified the clinical profile of patients at high-risk of major infection characterized by aggressive disease needing chemo-immunotherapy and CAD; in these patients IgRT significantly decreased the incidence of major infections. We have described and quantified the importance of CAD, rather than isolated IgG deficit, in determining the risk of major infections in CLL patients. These results, combined with the improvement in the quality of life obtained with IgRT described in our previous paper,6 suggest that IgRT is useful in selected patients to prevent life-threatening major infections.
Recently, novel small molecule inhibitors (ibrutinib, idelalisb, ABT199) have been shown in clinical trials to induce a lower risk of grade 3–4 infections, if compared to conventional chemo-immunotherapy,14 and lenalidomide seems to increase Ig levels.15 We hope that optimal use of pre-emptive IgRT together with new treatment strategies could actually reduce the risk of major infections and morbidity, improving patients’ survival as well as their quality of life.
Acknowledgments
We thank Mrs. Luisa De Rossi and Mrs. Cristina Balsamo, our archivists, for their precious work done during all these years.
Footnotes
Funding: This work was supported by funds from Associazione Italiana per la Ricerca sul Cancro (AIRC) to LT (project N. 15397, 2014), Ministero dell’Istruzione dell’Università e della Ricerca (PRIN 2008, 2010–2011 from LT, FIRB 2010 from GS), AIRC Regional Project with Fondazione CARIPARO and CARIVERONA and Regione Veneto on Chronic Lymphocytic Leukemia.
The online version of this letter has a Supplementary Appendix.
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Dhalla F, Lucas M, Schuh A, et al. Antibody deficiency secondary to chronic lymphocytic leukemia: should patients be treated with prophylactic replacement immunoglobulin? J Clin Immunol. 2014;34(3):277–282. [DOI] [PubMed] [Google Scholar]
- 2.Forconi F, Moss P. Perturbation of the normal immune system in patients with CLL. Blood. 2015;126(5):573–581. [DOI] [PubMed] [Google Scholar]
- 3.Moreira J, Rabe KG, Cerhan JR, et al. Infectious complications among individuals with clinical monoclonal B-cell lymphocytosis (MBL): a cohort study of newly diagnosed cases compared to controls. Leukemia. 2013;27(1):136–141. [DOI] [PubMed] [Google Scholar]
- 4.Raanani P, Gafter-Gvili A, Paul M, Ben-Bassat I, Leibovici L, Shpilberg O. Immunoglobulin prophylaxis in hematological malignancies and hematopoietic stem cell transplantation. Cochrane Database Syst Rev. 2008(4):CD006501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Compagno N, Malipiero G, Cinetto F, Agostini C. Immunoglobulin replacement therapy in secondary hypogammaglobulinemia. Front Immunol. 2014;5:626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Compagno N, Cinetto F, Semenzato G, Agostini C. Subcutaneous immunoglobulin in lymphoproliferative disorders and rituximab-related secondary hypogammaglobulinemia: a single-center experience in 61 patients. Haematologica. 2014;99(6):1101–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Francis S, Karanth M, Pratt G, et al. The effect of immunoglobulin VH gene mutation status and other prognostic factors on the incidence of major infections in patients with chronic lymphocytic leukemia. Cancer. 2006;107(5):1023–1033. [DOI] [PubMed] [Google Scholar]
- 8.Hensel M, Kornacker M, Yammeni S, Egerer G, Ho AD. Disease activity and pretreatment, rather than hypogammaglobulinaemia, are major risk factors for infectious complications in patients with chronic lymphocytic leukaemia. Br J Haematol. 2003;122(4):600–606. [DOI] [PubMed] [Google Scholar]
- 9.Parikh SA, Leis JF, Chaffee KG, et al. Hypogammaglobulinemia in newly diagnosed chronic lymphocytic leukemia: Natural history, clinical correlates, and outcomes. Cancer. 2015;21(17):2883–2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oscier D, Dearden C, Eren E, et al. Guidelines on the diagnosis, investigation and management of chronic lymphocytic leukaemia. Br J Haematol. 2012;159(5):541–564. [DOI] [PubMed] [Google Scholar]
- 11.Anderson D, Ali K, Blanchette V, et al. Guidelines on the use of intravenous immune globulin for hematologic conditions. Transfus Med Rev. 2007;21(2 Suppl 1):S9–56. [DOI] [PubMed] [Google Scholar]
- 12.Orange JS, Hossny EM, Weiler CR, et al. Use of intravenous immunoglobulin in human disease: a review of evidence by members of the Primary Immunodeficiency Committee of the American Academy of Allergy, Asthma and Immunology. J Allergy Clin Immunol. 2006;117(4 Suppl):S525–553. [DOI] [PubMed] [Google Scholar]
- 13.Freeman Ja, Crassini KR, Best OG, et al. Immunoglobulin G subclass deficiency and infection risk in 150 patients with chronic lymphocytic leukemia. Leuk Lymphoma. 2013;54(1):99–104. [DOI] [PubMed] [Google Scholar]
- 14.Hallek M. Chronic lymphocytic leukemia: 2015 update on diagnosis, risk stratification, and treatment. Am J Hematol. 2015;90(5):446–460. [DOI] [PubMed] [Google Scholar]
- 15.Strati P, Keating MJ, Wierda WG, et al. Lenalidomide induces long-lasting responses in elderly patients with chronic lymphocytic leukemia. Blood. 2013;122(5):734–737. [DOI] [PMC free article] [PubMed] [Google Scholar]


