Widespread neuroanatomic changes in patients with stimulant dependence were observed in women but not in men after prolonged abstinence; sexual dimorphism in drug-related brain morphometry and brain-behavior relationships may be mechanisms underlying the different clinical profiles of addiction in women and men.
Abstract
Purpose
To investigate whether sex modulates the effects of stimulant dependence on gray matter volume (GMV) in patients who have achieved long-term abstinence and to characterize how sex modulates GMV according to specific behavioral measures, such as dependence symptom count, behavioral approach, and impulsivity.
Materials and Methods
Colorado Multiple Institutional Review Board approval and informed consent were obtained. In this prospective parallel group study, 127 age- and sex-matched participants (68 control subjects [28 women, 40 men] and 59 patients with stimulant dependence [28 women, 31 men]) underwent T1-weighted spoiled gradient-echo inversion recovery magnetic resonance imaging of the brain at 3 T. Images were segmented by using voxel-based morphometric software. After adjustment for age, education, and head size, the effects of group according to sex on GMV and main effects were analyzed throughout the whole brain by using an analysis of covariance family-wise cluster corrected for multiple comparisons, with a threshold P value of less than .05. Dependence symptom count and behavioral measurements were correlated with GMV in the whole brain and in five a priori regions of interest.
Results
The effects of group according to sex on GMV were significant in numerous regions (P < .001). Compared with female control subjects, women with stimulant dependence had significantly lower GMV in widespread brain regions (P < .001). There were no significant differences in GMV between male control subjects and men with stimulant dependence (P = .625). Dependence symptom count negatively correlated with GMV in the nucleus accumbens in women (left: r = −0.364, P = .047; right: r = −0.407, P = .031) but not in men (left: r = −0.063, P = .737; right: r = −0.174, P = .349). Behavioral approach (P = .002) and impulsivity (P = .013) correlated negatively with frontal and temporal GMV changes in women with stimulant dependence but not in the other groups.
Conclusion
Vast changes in GMV were observed in women with stimulant dependence after prolonged abstinence, but were not observed in men. Sexual dimorphism in drug-related neuroanatomic changes and brain-behavior relationships may be mechanisms underlying the difference in clinical profiles of addiction between women and men.
© RSNA, 2015
Introduction
Substance use disorders are common, with lifetime prevalence estimated to be 10.3% of the U.S. population (1). Understanding the neurobiology of substance dependence is requisite to advancing treatments. Neuroanatomic changes in patients with drug addiction have been studied extensively by using voxel-based morphometry (2). Structural changes have been observed in the orbitofrontal cortex, medial frontal gyrus, anterior cingulate gyrus, insula, and nucleus accumbens in patients with stimulant dependence (2,3). In the largest meta-analysis of stimulant dependence to date, Ersche et al (2) reported significant decreases in gray matter in the insula, ventromedial prefrontal cortex, inferior frontal gyrus, anterior cingulate gyrus, and anterior thalamus. Gray matter changes also have been studied in adult sibling pairs, in which one sibling is dependent on stimulants and the other has no history of dependence, with age- and sex-matched control subjects (4). The results of this study revealed changes in limbic and sensory areas in both members of the sibling pair compared with the control subjects, suggesting that gray matter volume (GMV) changes may predate addiction and could be an endophenotype for substance use disorder.
To our knowledge, authors of few previous studies (5,6) have investigated the role of sex on changes in brain structure in patients with stimulant dependence. This is surprising considering the well-characterized sex differences in clinical presentation and natural history of stimulant addiction. Women exhibit an accelerated clinical course compared with men: Women begin cocaine or amphetamine use at earlier ages (5,7,8), show accelerated escalation of drug use (9–11), report more difficulty quitting (10,12), and report the use of larger quantities of these drugs when they seek treatment than do men (5,13). Neuroendocrine factors have been hypothesized to underlie an accelerated clinical course (5). Another hypothesis is that, compared with men, women respond differently to stress, which influences drug-related behavior (14). However, scant evidence exists for a neuroanatomic correlation of these clinical differences. Authors of some studies (15–17) primarily have recruited men to exclude the confounding effects of sex, and other investigators (2,18,19) have not included sex as a factor in their analyses of gray matter in patients with stimulant dependence. In fact, to our knowledge, only two studies (20,21) have included descriptions of structural differences between the sexes in patients with stimulant dependence. Rando et al (21) reported lower GMV in the left inferior frontal gyrus, insula, superior temporal gyrus, and hippocampus in women with stimulant dependence than in female control subjects and lower GMV in the precentral gyrus and mid cingulate gyrus in men with stimulant dependence compared with male control subjects. However, this study was significantly limited by the potential effects of recent alcohol use (mean number of drinks in the month before imaging, 87) and lack of long-term abstinence (mean length of abstinence before imaging, 3 weeks), allowing acute effects of substances to skew results. Tanabe et al (20) reported differential effects of sex on insular volumes in patients with stimulant dependence: Women with stimulant dependence had smaller insulae, whereas men with stimulant dependence had larger insulae. This study was limited by the small sample size (28 patients with stimulant dependence) and rudimentary methodology (the use of free open-source software [FreeSurfer]) to estimate GMV. In this study we addressed the paucity of large, prospective, well-controlled studies to investigate long-term sex differences associated with abstinent patients with stimulant dependence. We investigated whether sex modulates the effects of stimulant dependence on GMV in patients who have achieved long-term abstinence. We further sought to characterize how sex modulates brain-behavior relationships between GMV and specific behavioral measures, such as dependence symptom count, behavioral approach, and impulsivity.
Materials and Methods
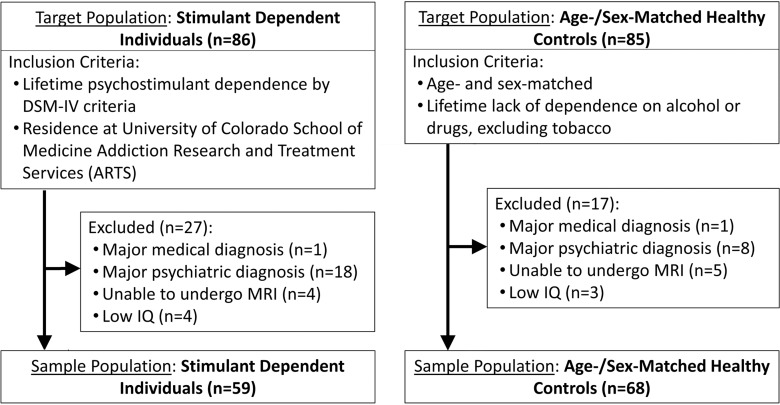
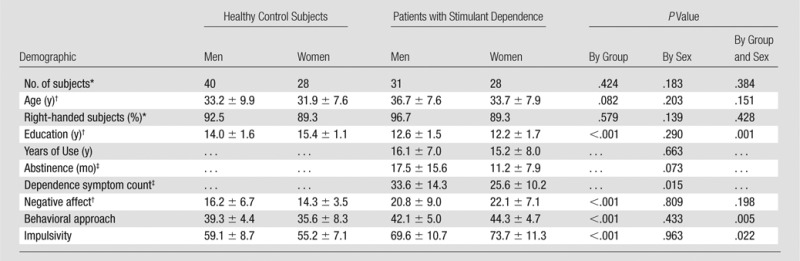
A total of 127 individuals including 68 healthy control subjects (28 women, 40 men) and 59 patients with stimulant dependence (28 women, 31 men) were recruited prospectively (Fig 1). Control subjects were similar in age and sex to those with stimulant dependence. Demographic information is reported in Tables 1 and 2. Patients with stimulant dependence were recruited from a residential treatment program at the University of Colorado School of Medicine Addiction Research and Treatment Services. The inclusion criterion was dependence on stimulants (methamphetamine, cocaine, or amphetamine-class substances) diagnosed according to criteria in the Diagnostic and Statistical Manual of Mental Disorders, fourth edition. Control subjects were recruited from the community and were excluded if they were dependent on alcohol or other drugs of abuse, excluding tobacco. Exclusion criteria for all subjects were depression within the past 2 months, psychosis, neurologic illness, prior head trauma resulting in a loss of consciousness for more than 15 minutes, prior neurosurgery, HIV-positive status, diabetes, hepatitis C, bipolar disorder, other major medical illness, inability to tolerate MR imaging, intelligence quotient of less than 80, urine screen (AccuTestTM, www.accutest.net) or saliva screen (AlcoScreenTM, www.alcoscreen.com) results positive for drugs. All participants provided written informed consent, and the study was approved by the Colorado Multiple Institutional Review Board.
Figure 1:
Flow chart shows sample population selection and inclusion and exclusion criteria. DSM-IV = Diagnostic and Statistical Manual of Mental Disorders, 4th Edition.
Table 1.
Demographic Description of the Sample Population
Note.—Unless otherwise indicated, data are means ± standard deviation.
*χ2 test.
†2 × 2 analysis of covariance.
‡Independent samples t test, equal variances not assumed.
Table 2.
Percentage of Patients Satisfying Criteria for Dependence
*χ2 test.
Structured Interviews
The Composite International Diagnostic Interview Substance Abuse Module is a computerized structured interview that allows assessment of diagnoses of substance dependence and symptoms for 11 different drugs of abuse (22). All subjects were administered the Composite International Diagnostic Interview Substance Abuse Module to verify dependence in the patients with stimulant dependence and to exclude control subjects who were dependent on substances other than tobacco.
The Diagnostic Interview Schedule version IV is a computerized structured interview used to screen for psychiatric disorders (23). All subjects were administered the Diagnostic Interview Schedule version IV to exclude those with a history of psychosis or bipolar disorder or major depressive disorder in the past 2 months. This computerized interview (24) allows computation of four abuse (ie, legal problems due to drugs) and seven dependence (ie, uncontrolled substance use escalation) symptoms for each drug class. Drug use severity was calculated by adding the number of abuse and dependence symptoms. This approach is consistent with the single set of clinically relevant criteria in the Diagnostic and Statistical Manual of Mental Disorders, fifth edition, which was released after we performed data collection for the current study.
The Barratt Impulsiveness Scale (25) is a 30-item self-reported questionnaire that is used to quantify impulsiveness. Participants rate whether phrases and words describing aspects of impulsivity are self-descriptive. The Behavioral Activation System (26,27) scale is a 13-item questionnaire used to measure responsiveness of motivational systems; “behavioral approach” characterizes the level of arousal and response to cues toward favorable outcomes and positive affective states.
MR Imaging Examination and Image Processing
MR imaging of the brain was performed by using a 3-T MR imager (GE, Milwaukee, Wis) and standard quadrature head coil. High-spatial-resolution T1-weighted spoiled gradient-echo inversion-recovery sequences were performed in each subject with the following parameters: repetition time msec/echo time msec, 45/20; flip angle, 45°; matrix, 256 × 256; field of view, 240 × 240 mm2 (0.9 × 0.9 mm2 in plane), section thickness, 1.7 mm; and acquisition plane, coronal. All images were evaluated by a board-certified neuroradiologist (J.T., a neuroradiology professor with 20 years of experience) for structural abnormalities. No examinations were excluded on this basis.
T1-weighted MR images of the brain were processed (M.F.R., a radiology resident with 2 years of experience; and M.D., a graduate student with 8 years of experience studying brain morphometric methods; and J.T.) by using the VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm8/) and SPM8 (http://www.fil.ion.ucl.ac.uk/spm/) software. Images were segmented into gray matter, white matter, and cerebrospinal fluid probability maps anatomically coregistered by using diffeomorphic anatomic registration through exponentiated lie algebra (28). Custom templates were created for our sample population and were used to register the images. Segmented images were nonlinearly modulated after registration to preserve relative regional volume, after correction for different brain sizes. Segmented gray matter probability maps of each subject were visually inspected for quality control (M.F.R.); no images were excluded. Normalized, modulated images were smoothed with an 8-mm3 full-width at half-maximum Gaussian kernel.
Statistical Analysis
Whole-brain analyses of GMV were performed (M.F.R. and J.T.) by using two-way analysis of covariance to determine the effects of group (healthy control subjects and patients with stimulant dependence) according to sex and the main effects of group and sex. All analyses were adjusted for age, education, and head size measured as total intracranial volume. Age did not differ between the groups but is an important covariate, because it directly affects global GMV (29). The level of patient education differed between the groups (Table 1). A P value of less than .05 was considered to indicate a significant difference, corrected for multiple comparisons by using family-wise error (FWE) correction with simulation software (AlphaSim, http://afni.nimh.nih.gov/afni/) and Monte Carlo simulations (10 000 simulations), with a voxel-wise threshold P value of less than .005. The cluster threshold corresponded to 1202 voxels (each voxel, 3.375 mm3) or 4056.8 mm3. Whole-brain analysis interpretation was restricted to the supratentorial space, because of the reported methodologic difficulties of infratentorial space segmentation (30).
In patients with stimulant dependence, whole-brain regression analyses allowed examination of the association of GMV with the severity of drug use. In exploratory regression analysis, we compared behavior according to sex and group with whole-brain GMV (M.F.R. and J.T). Significance was determined by using the aforementioned cluster-based FWE-corrected P value of less than .05 and threshold-free cluster enhancement with correction for multiple comparisons by using an FWE-corrected P value of less than .05 to indicate a significant difference (31). Threshold-free cluster enhancement was used to evaluate small structures measuring less than 4.05 cm3 such as the nucleus accumbens, the results of which, otherwise, would have been precluded mathematically from reaching significance (31,32).
Although whole-brain analysis offers statistical robustness, cross-validation with predefined regions of interest (ROIs) by using prior knowledge improves classification (33–35). Whole-brain voxel-level analysis is data driven, without regard to specific anatomy, while predefined ROI analysis is hypothesis driven for specific neuroanatomic structures on the basis of prior knowledge. To confirm results from whole-brain analyses, five a priori neuroanatomic structures were hypothesized to differ in patients with stimulant dependence compared with those in control subjects on the basis of their involvement in reward, learning, executive control, and affective processing, which are altered in patients with stimulant dependence (2,3,36): the orbitofrontal cortex, medial frontal gyrus, anterior cingulate gyrus, insula, and nucleus accumbens. Masks for these structures were created by using the Automated Anatomical Labeling atlas toolbox (37). Total GMV of each structure was calculated by summing the volume of the voxels. Individual voxel volumes were calculated by multiplying each voxel by its respective voxel modulation. GMV in each ROI was analyzed by using a two-way analysis of covariance for group, sex, and group according to sex, after adjusting for age, education, and head size (M.F.R. and J.T.). To correct for multiple comparisons, results were considered to indicate a significant difference at an FWE-corrected P value of less than .05 with Bonferroni correction for five ROIs (pairwise comparison, P < .01). In exploratory regression analyses, GMV in ROIs was compared with dependence symptom count. Scores for behavior according sex and group were compared with GMV in ROIs, and the differences were considered significant with a Bonferroni correction for FWE throughout all structures (P < .05; pairwise comparison, P < .01).
Results
Demographics, Drug Severity, and Behavioral Comparisons
Patients with stimulant dependence and control subjects were similar in age and sex (Table 1). There was no difference in age between the sexes or by group. There was a difference in education between the sexes and according to group (F1,127 = 10.936, P < .001) and in the main effects according to group (F1,127 = 74.914, P < .001). Female control subjects had the most years of education, followed by male control subjects, men with stimulant dependence, and finally, women with stimulant dependence had the fewest years of education. Patients with stimulant dependence had a mean of 2.2 fewer years of education than did control subjects.
In patients with stimulant dependence, there were differences between the sexes in the severity of drug use (P = .015), with men having greater dependence symptom counts. There were no differences between the sexes in drug exposure, abstinence duration, or years of drug abuse. There were significant differences between the sexes according to group in behavioral approach and impulsivity: Women with stimulant dependence had the highest approach and impulsivity scores, followed by men with stimulant dependence, male control subjects, and finally, female control subjects, with the lowest approach and impulsivity scores.
Whole Brain Analysis
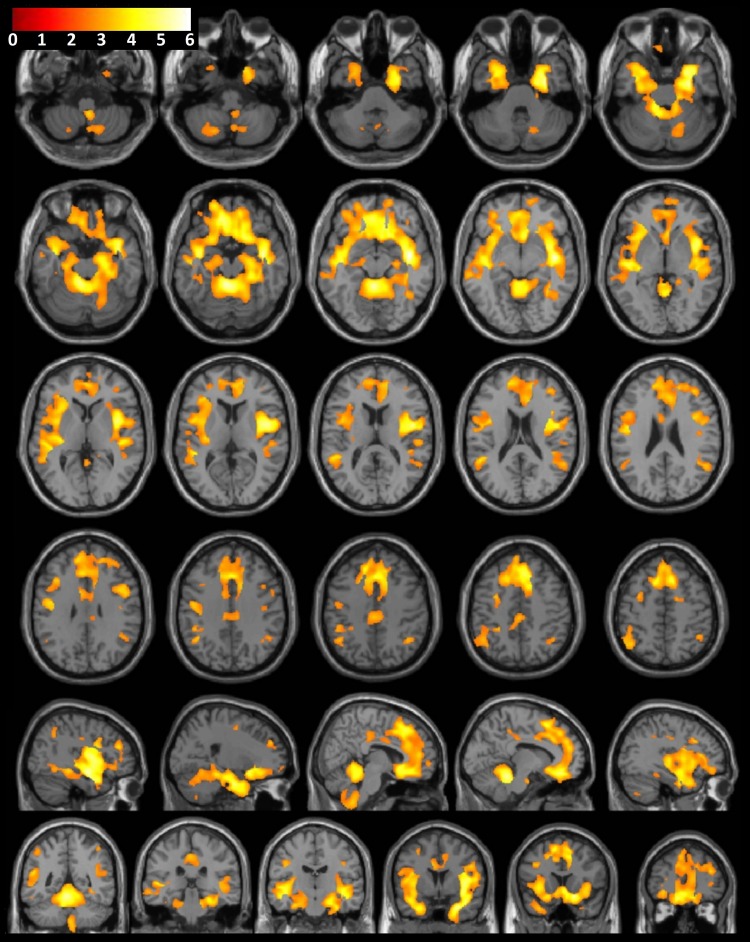
A significant difference in GMV between the sexes according to group was found in widespread areas of the cerebral cortex, thalamus, and basal ganglia. To further characterize the interaction, the effect of the group was investigated in each sex separately. No differences in GMV were observed between male control subjects and men with stimulant dependence. However, large, widespread differences in GMV were observed between female control subjects and women with stimulant dependence (Fig 2). Compared with female control subjects, women with stimulant dependence had a significantly lower GMV in the frontal lobe (orbitofrontal cortex, medial frontal gyrus, superior frontal gyrus), limbic regions (insula, amygdala, cingulate gyrus), temporal lobe (temporal pole, uncus, parahippocampal gyrus, hippocampus, occipitotemporal gyri, superior temporal gyrus, middle temporal gyrus), and inferior parietal lobule. Greater GMV observed in female control subjects than in women with stimulant dependence showed anatomic congruence with the comparisons between the sexes according to group.
Figure 2:
Consecutive axial, sagittal, and coronal sections of a canonical T1-weighted MR imaging examination of the brain with superimposed population-level T-value map show significantly greater GMV in female healthy control subjects than in women with substance dependence, after correction for age, brain size, and years of education (P < .001).
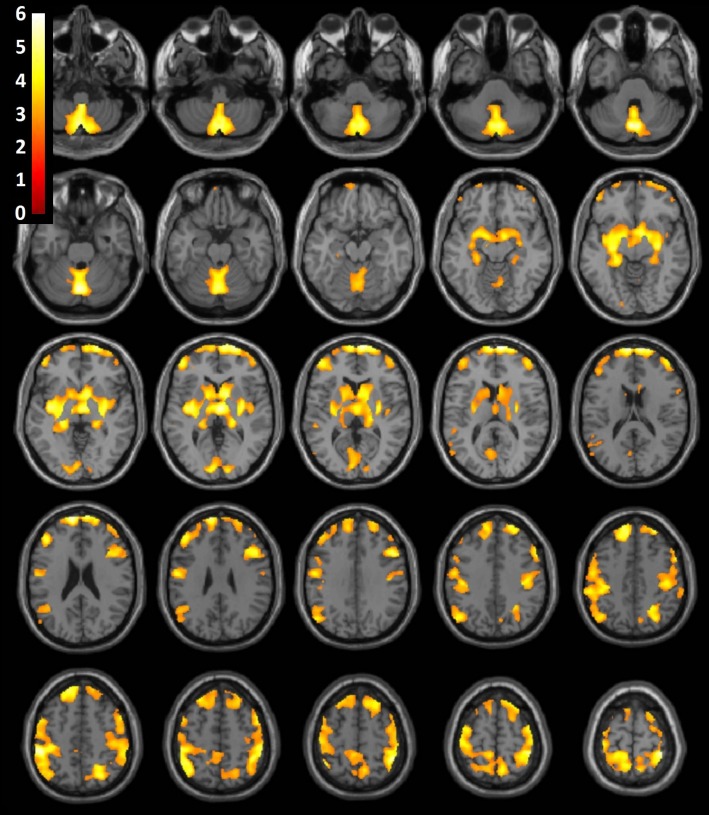
A significant main effect of sex was found, with women exhibiting comparatively greater regional GMV than men throughout the cerebral cortex, thalamus, and basal ganglia (P < .001). Subgroup analysis demonstrated anatomically similar significant differences between male and female control subjects (Fig 3). A significant main effect of the group was found throughout frontal, temporal, insular, and parietal regions (P < .001). Control subjects had significantly greater GMV than patients with stimulant dependence.
Figure 3:
Consecutive axial, sagittal, and coronal sections of a canonical T1-weighted MR imaging examination of the brain with superimposed population-level T-value map shows significantly greater regional GMV in female control subjects compared with male control subjects, after correction for age, brain size, and years of education (P < .001).
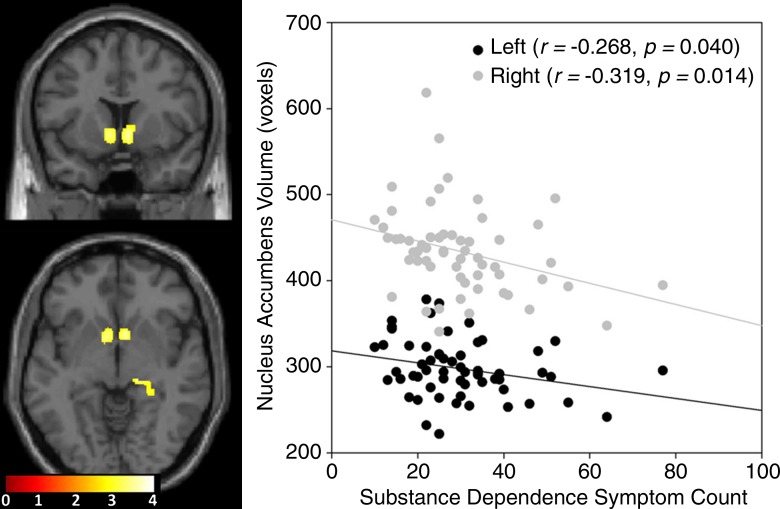
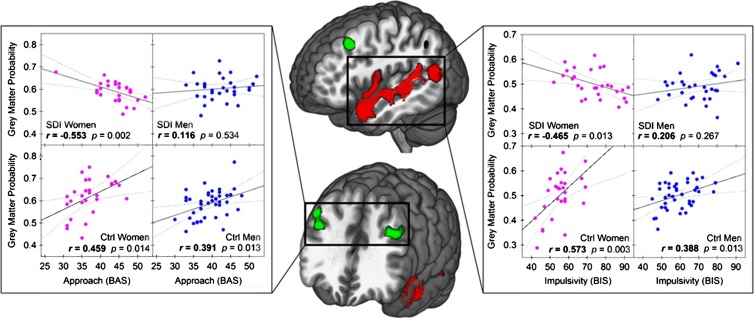
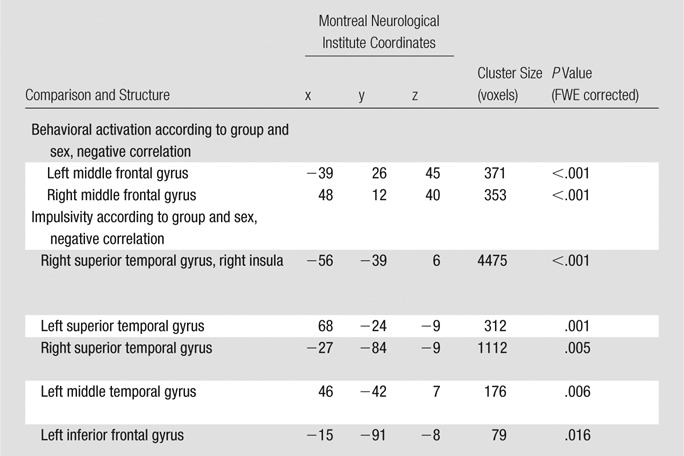
Significant negative correlations were found between the severity of drug use and the GMV in the bilateral nucleus accumbens (Fig 4; left, 290 voxels; Montreal Neurological Institute coordinates, −9, 4, −2; P < .0001, FWE corrected; right, 271 voxels; Montreal Neurological Institute coordinates, 8, 10, −6; P < .0001, FWE corrected). Years of substance use did not correlate with GMV. Abstinence positively correlated with a small area of the left superior frontal gyrus (171 voxels, Montreal Neurological Institute coordinates −18, 38, 33; P = .006, FWE corrected). Years of substance use, abstinence, and drug use severity were not found to have significant sex-related effects on GMV. Significant three-way interactions among behavioral approach, group, and sex were seen in the bilateral middle frontal gyri (Fig 5, green; Table 3) In these areas, behavioral approach in women with stimulant dependence correlated negatively with GMV, whereas positive correlation coefficients were seen in female control subjects and both groups of men. Significant three-way interactions among impulsivity, group, and sex were seen in the bilateral superior and middle temporal gyri, right insula, right superior temporal sulcus, and right inferior temporal gyrus (Fig 5, red; Table 3). In these areas in women with stimulant dependence, impulsivity correlated negatively with GMV, whereas female control subjects and both groups of men showed positive correlation coefficients.
Figure 4:
T1-weighted MR imaging brain map (left) including coronal (top) and axial (bottom) section images shows significantly negative correlations at whole-brain level between stimulant dependence symptom count and GMV in the bilateral nucleus accumbens in a patient with stimulant dependence. Scatterplot (right) shows negative correlation between substance dependence symptom count and total volume of nucleus accumbens defined according to ROI.
Figure 5:
Effects of sex on GMV according to group and behavior. Left, scatterplots show effect of group according to sex on correlation between approach and GMV in bilateral middle frontal gyri (dorsolateral prefrontal cortex). Right, scatterplots show effect of group according to sex on correlation between impulsivity and GMV in left superior temporal gyrus and left insula. Middle, illustrations show clusters of whole-brain significant differences in group according to sex and impulsivity (red) and group according to sex and approach (green). BAS = Behavioral Activation System, BIS = Barratt Impulsiveness Scale, Ctrl = control subject, SDI = substance dependent individual.
Table 3.
Significant Correlations between Behavior and GMV According to Group and Sex
ROI Analysis
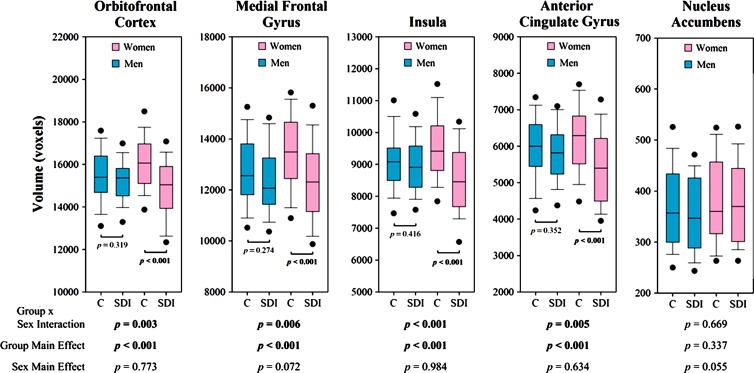
Two-way analysis of covariance revealed statistically significant differences between the sexes according to group and main effects of group for all structures except the nucleus accumbens (Fig 6). Post hoc pairwise comparisons revealed greater GMV in female control subjects than in women with stimulant dependence in total volumes of each significant structure (P < .001) but not between male control subjects and men with stimulant dependence, which was consistent with the whole-brain results. Drug use severity correlated negatively with GMV in the nucleus accumbens (Fig 4, right). This correlation was driven by women with stimulant dependence; a steeper and significant negative correlation was seen in women (left: r = −0.364, P = .047; right: r = −0.407, P = .031) compared with men (left: r = −0.063, P = .737; right: r = −0.174, P = .349), in whom the correlations were not significant. No other correlations between drug characteristics or behavioral metrics were significant. No effects of behavior according to sex and group or behavior according to group were significant in ROI structures.
Figure 6:
Boxplots show total GMV in each subpopulation for each ROI. Top and bottom edges of boxes indicate third and first quartiles, respectively. Lines in middle of boxes indicate medians. Whiskers above and below boxes indicate 90th and 10th percentiles, respectively. Points above and below whiskers indicate 95th and 5th percentiles, respectively. P values were determined by using two-way analysis of covariance. C = healthy control subjects, SDI = stimulant-dependent individuals.
Discussion
The current finding of significantly lower GMV in abstinent women with stimulant dependence compared with healthy control women is striking for two reasons: First, no group differences were observed in men, and second, the involved regions in women were anatomically vast and overlapped substantially with pathways implicated in reward, learning, executive control, and affective processing (3). To our knowledge, the extent of the widespread anatomic differences between abstinent men and women with substance dependence has not been reported. These differences between the sexes could reflect a greater neuroanatomic endophenotype in women that predisposes them to stimulant dependence or a vulnerability to morphologic changes that result from stimulant dependence. Decreased GMV in women with stimulant dependence compared with female control subjects was most striking in the limbic regions, particularly the insula, further suggesting a functional role of these structures in mediating the clinical phenotype.
We expanded on these structural results with brain-behavioral correlations. Nucleus accumbens volume was negatively correlated with the severity of drug use, consistent with its role in reward and salience. Authors of previous studies have shown that ventral striatal activity, including that in the nucleus accumbens, correlates with the intensity of received rewards (38). Persons who are dependent on stimulants undergo pathologic overstimulation of this nucleus and may exhibit compensatory downregulation of neuronal synapses with reduced dendritic branching, number of axonal boutons, and degree of axon myelination leading to reduced GMV (39,40). The negative relationship observed in this study between severity of drug use and the GMV of the nucleus accumbens was significant in women but not in men, despite men exhibiting greater severity of drug use. This result suggests that women may demonstrate greater susceptibility to changes in the severity of drug use, possibly through neuroendocrine mechanisms. Behavioral approach and impulsivity scores between the sexes and according to group correlated significantly with GMV. Higher behavioral approach and impulsivity scores were associated with lower GMV in women with stimulant dependence. Behavioral approach scores quantify an individual’s positive affective and approach response to appetitive stimuli. Higher approach motivates behavior. Impulsivity describes decreased inhibitory control over potential actions leading to reward. Authors of a previous study (6) have reported significant sex differences in approach and impulsivity characteristics in persons with stimulant dependence; however, our study is the first to report structural neuroanatomic correlates of these findings. Higher approach in women with stimulant dependence was correlated with lower GMV in the bilateral dorsolateral perfrontal cortex and may reflect a deficit in top-down control over approach behaviors toward drug cues. The current structural and brain-behavioral relationship differences between the sexes may result from neuroendocrine factors. For example, sex and ovarian hormones affect the number, density, and firing rate of dopaminergic neurons, and women show enhanced engagement of the dopaminergic system during initial exposure to drugs and exacerbated negative affective states during drug withdrawal (5).
Few previous studies have been focused on the investigation of sexual dimorphism in GMV in patients with stimulant dependence; in fact, authors of only two studies reported structural sex differences in persons with stimulant dependence: Rando et al (21) and Tanabe et al (20). Consistent with our results, Rando et al’s (21) findings showed greater GMV in healthy subjects than in women with cocaine dependence in the left inferior frontal gyrus, left insula, left superior temporal gyrus, right temporo-occipital cortex, and left hippocampus. Tanabe et al (20) observed a differential effect of sex on small regions of the insula. They reported that women with stimulant dependence exhibited smaller insulae compared with those of control subjects, which was consistent with our results. However, our results showed that the differences span nearly the entire insulae bilaterally. Both of these studies had modest patient sample sizes, with 36 patients in Rando and 28 in Tanabe. In a meta-analysis, Ersche et al (2) studied 494 subjects with stimulant dependence (79% men) and 428 healthy control subjects (69% men) and reported smaller GMV in those with stimulant dependence than in control subjects in the insulae, inferior frontal gyrus, anterior cingulate gyrus, and anterior thalamus; however, the authors did not comment on the effect of sex on the results. Authors of other studies of drug effects on brain morphometry excluded women (15–17), which shows the need for prospective studies to investigate the effects of sex. Here we report significant neuroanatomical sexual dimorphism in the largest prospective sample of patients with stimulant dependence evaluated according to sex and group to date.
The lack of group differences in men was unexpected. Unlike the results of our study, Rando et al found small differences in men, with lower GMV in a small portion of the precentral and midcingulate gyrus in men with cocaine dependence compared with male control subjects. There are several possible explanations for this difference, such as recent large alcohol intake (mean number of drinks in prior month, 87), short length of abstinence (mean, 3 weeks), and the inclusion of significantly older men with cocaine dependence than control subjects in the Rando population. Our sample had much longer abstinence, with a mean of 13.5 months. GMV recovery has been reported to be associated with sustained abstinence (41). For example, in the Ersche et al (2) meta-analysis of 494 subjects with stimulant dependence, only four of the 13 studies included subjects who were abstinent for more than 1 month, with the majority of studies being investigations of active users. Thus, authors of most studies examined acute drug effects. Because our study included subjects who were abstinent for at least 60 days, there may have been a “ceiling” effect. This hypothesis is consistent with results from Connolly et al (41), who found that in men, GMV positively correlated with early abstinence but tapered at 35 weeks to become equivalent to those of drug-naïve controls. Given the average 13.5 months of abstinence in our study, GMV recovery may have already reached a steady-state in men by the time of recruitment.
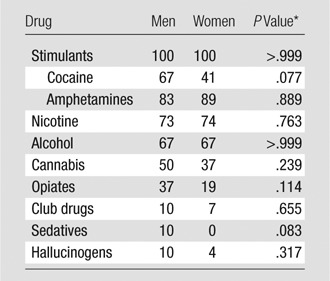
Our current study had several limitations. The first limitation was polysubstance use (Table 2) in the population with stimulant dependence. Although this precluded us from relating structural changes to a single drug, our sample had biologic and ecologic validity because it reflected an important, real-world, clinical population of persons with stimulant dependence. Epidemiologic data show that stimulant dependence does not often occur in isolation; instead, most patients meet dependence criteria for other substances (42,43). The differences in GMV observed were not due to differences in drug exposure or symptom severity. Second, our sample was referred from the justice system, and we cannot exclude the possibility that antisocial personality traits contributed to the findings. Third, the patients with stimulant dependence and control subjects differed in years of education. Although we performed an analysis of covariance for this confounding variable, education could have influenced the observed differences.
Vast neuroanatomic changes observed in abstinent patients with stimulant dependence were present in women but not in men. In particular, structures involved in reward, learning, executive control, and affective processing pathways were affected: the insula, orbitofrontal cortex, anterior cingulate cortex, medial frontal gyrus, and nucleus accumbens. These changes correlated with drug use and behavioral measures and may help to explain differences in the clinical course of stimulant dependence in women compared with that in men.
Advances in Knowledge
■ After long-term abstinence, women with stimulant dependence showed cortical and subcortical differences in gray matter volume (P < .001) but men did not show these differences (P = .625); women with stimulant dependence exhibited smaller neuroanatomic volumes in vastly distributed regions of the frontal, parietal, temporal, insular, and subcortical areas.
■ Reward, learning, executive control, and affective processing areas including the insula (P < .001), orbitofrontal cortex (P = .003), cingulate cortex (P = .005), medial frontal gyrus (P = .006), and nucleus accumbens were significantly different between women with substance dependence and female control subjects after long-term abstinence, but no differences were found between the corresponding groups of men.
■ Gray matter volume in the nucleus accumbens, or “reward center” of the brain negatively correlated with the severity of drug use in women (left: r = −0.364, P = .047; right: r = −0.407, P = .031) but not in men (left: r = −0.063, P = .737; right: r = −0.174, P = .349).
■ Behavioral approach and impulsivity scores correlated with gray matter deficits in women with stimulant dependence (P = .002, P = .013), but not in men (P = .534, P = .267).
Implications for Patient Care
■ Neuroimaging results showed that men and women with stimulant dependence exhibit different neuroanatomic changes that may underlie known sex differences in the clinical natural history of this disease.
■ Sex-based gray matter changes in patients with stimulant dependence correlated with sex-based behavioral differences in behavioral approach and impulsivity, showing that the different psychologic profiles in men and women with substance dependence are associated with specific neuroanatomic loci.
■ Understanding sex differences in both the neuroimaging and clinical course of substance dependence could lead to improved sex-specific or individualized medical treatment and recovery plans.
Received November 1, 2014; revision requested January 6, 2015; revision received March 12; accepted April 2; final version accepted April 17.
Funding: This research was supported by the National Institutes of Health (grants DA024104, DA027748, and DA031761).
Disclosures of Conflicts of Interest: M.F.R. disclosed no relevant relationships. M.D. disclosed no relevant relationships. D.Y. disclosed no relevant relationships. R.I.P. disclosed no relevant relationships. J.T.S. Activities related to the present article: reimbursement for travel expenses from the National Institute on Drug Abuse. Activities not related to the present article: disclosed no relevant relationships. Other relationships: disclosed no relevant relationships. J.M.H. disclosed no relevant relationships. J.T. disclosed no relevant relationships.
Abbreviations:
- GMV
- gray matter volume
- FWE
- family-wise error
- ROI
- region of interest
References
- 1.Miller TR, Hendrie D. Substance abuse prevention dollars and cents: A cost-benefit analysis. Rockville, Md: U.S. Department of Health and Human Services, Substance Abuse and Mental Health Services Administration, Center for Substance Abuse Prevention, 2009. [Google Scholar]
- 2.Ersche KD, Williams GB, Robbins TW, Bullmore ET. Meta-analysis of structural brain abnormalities associated with stimulant drug dependence and neuroimaging of addiction vulnerability and resilience. Curr Opin Neurobiol 2013;23(4):615–624. [DOI] [PubMed] [Google Scholar]
- 3.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology 2010;35(1):217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ersche KD, Jones PS, Williams GB, Turton AJ, Robbins TW, Bullmore ET. Abnormal brain structure implicated in stimulant drug addiction. Science 2012;335(6068):601–604. [DOI] [PubMed] [Google Scholar]
- 5.Becker JB, Perry AN, Westenbroek C. Sex differences in the neural mechanisms mediating addiction: a new synthesis and hypothesis. Biol Sex Differ 2012;3(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perry RI, Krmpotich T, Thompson LL, Mikulich-Gilbertson SK, Banich MT, Tanabe J. Sex modulates approach systems and impulsivity in substance dependence. Drug Alcohol Depend 2013;133(1):222–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Griffin ML, Weiss RD, Mirin SM, Lange U. A comparison of male and female cocaine abusers. Arch Gen Psychiatry 1989;46(2):122–126. [DOI] [PubMed] [Google Scholar]
- 8.Mendelson JH, Weiss R, Griffin M, et al. Some special considerations for treatment of drug abuse and dependence in women. NIDA Res Monogr 1991;106:313–327. [PubMed] [Google Scholar]
- 9.Greenfield SF, Back SE, Lawson K, Brady KT. Substance abuse in women. Psychiatr Clin North Am 2010;33(2):339–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lynch WJ. Sex differences in vulnerability to drug self-administration. Exp Clin Psychopharmacol 2006;14(1):34–41. [DOI] [PubMed] [Google Scholar]
- 11.Brady KT, Randall CL. Gender differences in substance use disorders. Psychiatr Clin North Am 1999;22(2):241–252. [DOI] [PubMed] [Google Scholar]
- 12.Back SE, Brady KT, Jackson JL, Salstrom S, Zinzow H. Gender differences in stress reactivity among cocaine-dependent individuals. Psychopharmacology (Berl) 2005;180(1):169–176. [DOI] [PubMed] [Google Scholar]
- 13.Kosten TA, Gawin FH, Kosten TR, Rounsaville BJ. Gender differences in cocaine use and treatment response. J Subst Abuse Treat 1993;10(1):63–66. [DOI] [PubMed] [Google Scholar]
- 14.Potenza MN, Hong KI, Lacadie CM, Fulbright RK, Tuit KL, Sinha R. Neural correlates of stress-induced and cue-induced drug craving: influences of sex and cocaine dependence. Am J Psychiatry 2012;169(4):406–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barrós-Loscertales A, Garavan H, Bustamante JC, et al. Reduced striatal volume in cocaine-dependent patients. Neuroimage 2011;56(3):1021–1026. [DOI] [PubMed] [Google Scholar]
- 16.Fein G, Di Sclafani V, Meyerhoff DJ. Prefrontal cortical volume reduction associated with frontal cortex function deficit in 6-week abstinent crack-cocaine dependent men. Drug Alcohol Depend 2002;68(1):87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Franklin TR, Acton PD, Maldjian JA, et al. Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biol Psychiatry 2002;51(2):134–142. [DOI] [PubMed] [Google Scholar]
- 18.Sim ME, Lyoo IK, Streeter CC, et al. Cerebellar gray matter volume correlates with duration of cocaine use in cocaine-dependent subjects. Neuropsychopharmacology 2007;32(10):2229–2237. [DOI] [PubMed] [Google Scholar]
- 19.Tanabe J, Tregellas JR, Dalwani M, et al. Medial orbitofrontal cortex gray matter is reduced in abstinent substance-dependent individuals. Biol Psychiatry 2009;65(2):160–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanabe J, York P, Krmpotich T, et al. Insula and orbitofrontal cortical morphology in substance dependence is modulated by sex. AJNR Am J Neuroradiol 2013;34(6):1150–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rando K, Tuit K, Hannestad J, Guarnaccia J, Sinha R. Sex differences in decreased limbic and cortical grey matter volume in cocaine dependence: a voxel-based morphometric study. Addict Biol 2013;18(1):147–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cottler LB, Robins LN, Helzer JE. The reliability of the CIDI-SAM: a comprehensive substance abuse interview. Br J Addict 1989;84(7):801–814. [DOI] [PubMed] [Google Scholar]
- 23.Robins L, Cottler L, Bucholz K, Compton W. The diagnostic interview schedule, version IV. St Louis, Mo: Washington University, 1995. [Google Scholar]
- 24.Gelhorn H, Hartman C, Sakai J, et al. Toward DSM-V: an item response theory analysis of the diagnostic process for DSM-IV alcohol abuse and dependence in adolescents. J Am Acad Child Adolesc Psychiatry 2008;47(11):1329–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol 1995;51(6):768–774. [DOI] [PubMed] [Google Scholar]
- 26.Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS Scales. J Pers Soc Psychol 1994;67(2):319–333. [Google Scholar]
- 27.Campbell-Sills L, Liverant GI, Brown TA. Psychometric evaluation of the behavioral inhibition/behavioral activation scales in a large sample of outpatients with anxiety and mood disorders. Psychol Assess 2004;16(3):244–254. [DOI] [PubMed] [Google Scholar]
- 28.Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage 2007;38(1):95–113. [DOI] [PubMed] [Google Scholar]
- 29.Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage 2001;14(1 Pt 1):21–36. [DOI] [PubMed] [Google Scholar]
- 30.Diedrichsen J, Balsters JH, Flavell J, Cussans E, Ramnani N. A probabilistic MR atlas of the human cerebellum. Neuroimage 2009;46(1):39–46. [DOI] [PubMed] [Google Scholar]
- 31.Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 2009;44(1):83–98. [DOI] [PubMed] [Google Scholar]
- 32.Radua J, Canales-Rodríguez EJ, Pomarol-Clotet E, Salvador R. Validity of modulation and optimal settings for advanced voxel-based morphometry. Neuroimage 2014;86:81–90. [DOI] [PubMed] [Google Scholar]
- 33.Kerr WT, Douglas PK, Anderson A, Cohen MS. The utility of data-driven feature selection: re: Chu et al. 2012. Neuroimage 2014;84:1107–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chu C, Hsu AL, Chou KH, Bandettini P, Lin C; Alzheimer’s Disease Neuroimaging Initiative. Does feature selection improve classification accuracy? Impact of sample size and feature selection on classification using anatomical magnetic resonance images. Neuroimage 2012;60(1):59–70. [DOI] [PubMed] [Google Scholar]
- 35.Nieto-Castanon A, Ghosh SS, Tourville JA, Guenther FH. Region of interest based analysis of functional imaging data. Neuroimage 2003;19(4):1303–1316. [DOI] [PubMed] [Google Scholar]
- 36.Li CS, Sinha R. Inhibitory control and emotional stress regulation: neuroimaging evidence for frontal-limbic dysfunction in psycho-stimulant addiction. Neurosci Biobehav Rev 2008;32(3):581–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 2002;15(1):273–289. [DOI] [PubMed] [Google Scholar]
- 38.Sescousse G, Caldú X, Segura B, Dreher JC. Processing of primary and secondary rewards: a quantitative meta-analysis and review of human functional neuroimaging studies. Neurosci Biobehav Rev 2013;37(4):681–696. [DOI] [PubMed] [Google Scholar]
- 39.Draganski B, Kherif F. In vivo assessment of use-dependent brain plasticity—beyond the “one trick pony” imaging strategy. Neuroimage 2013;73:255–259; discussion 265–267. [DOI] [PubMed] [Google Scholar]
- 40.Fields RD. Changes in brain structure during learning: fact or artifact? Reply to Thomas and Baker. Neuroimage 2013;73:260–264; discussion 265–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Connolly CG, Bell RP, Foxe JJ, Garavan H. Dissociated grey matter changes with prolonged addiction and extended abstinence in cocaine users. PLoS ONE 2013;8(3):e59645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sara G, Burgess P, Harris M, Malhi GS, Whiteford H, Hall W. Stimulant use disorders: characteristics and comorbidity in an Australian population sample. Aust N Z J Psychiatry 2012;46(12):1173–1181. [DOI] [PubMed] [Google Scholar]
- 43.Stinson FS, Grant BF, Dawson DA, Ruan WJ, Huang B, Saha T. Comorbidity between DSM-IV alcohol and specific drug use disorders in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug Alcohol Depend 2005;80(1):105–116. [DOI] [PubMed] [Google Scholar]