Abstract
Cellular senescence of endothelial cells plays an important role in the development of vascular lesions that ultimately lead to an atherosclerotic plaque. This review focuses on the age-related changes of endothelial and vascular smooth muscle cells that contribute to vascular disease and discusses potential new targets that could rejuvenate the vascular system and thereby prevent or delay atherosclerosis.
Keywords: telomerase, cellular senescence, endothelium, atherosclerosis, prevention, modified MRNA, aging

T. Z. Nazari-Shafti, M.D.

J. P. Cooke, M.D. Ph.D.
Introduction
Aging of the vascular system is considered a major contributor in the development of atherosclerotic lesions.1,2 The structural and functional integrity of the arterial wall progressively declines with aging, as manifested by endothelial and vascular smooth muscle cell dysfunction, reduced regenerative capacity, and a decline in circulating and tissue resident progenitor cells.3–5 The addition of systemic and local complications associated with insulin resistance, dyslipidemia, tobacco exposure, hypertension, and genetic predisposition engender atherogenic processes in the vessel wall that lead to the clinical manifestation of atherosclerosis. This review will focus on the age-related changes of the endothelial and vascular smooth muscle cells that contribute to vascular disease and examines potential new targets that could prevent or delay atherosclerosis through rejuvenation of the vascular system.
Cellular Aging and Senescence: Role in Development of Atherosclerotic Plaques
Cellular aging and associated cellular dysfunction is caused by multiple factors, such as accumulation of DNA damage, misfolded proteins, and telomere attrition.6 Telomere shortening plays a prominent role in cellular age-related dysfunction.6 Telomeres are a series of nucleotide repeats (TTAGGG/AATCCC) at the ends of chromosomes. The telomere is protected by a tightly controlled complex of DNA binding proteins and transcription factors called the shelterin complex (Figure 1a).7 Nevertheless, in somatic cells, telomeres shorten with each cell division due to a phenomenon called the “end-replication problem” (Figure 1b). After 40 to 60 population doublings, telomere length is reduced to the extent that a DNA injury response is induced. This response is associated with increased cellular oxidative stress and many of the phenotypic and functional changes associated with senescence. Researcher Leonard Hayflick determined the number of population doublings that somatic cells can undergo before they become senescent and incapable of cellular replication, a term referred to as the “Hayflick limit.”8
Figure 1.
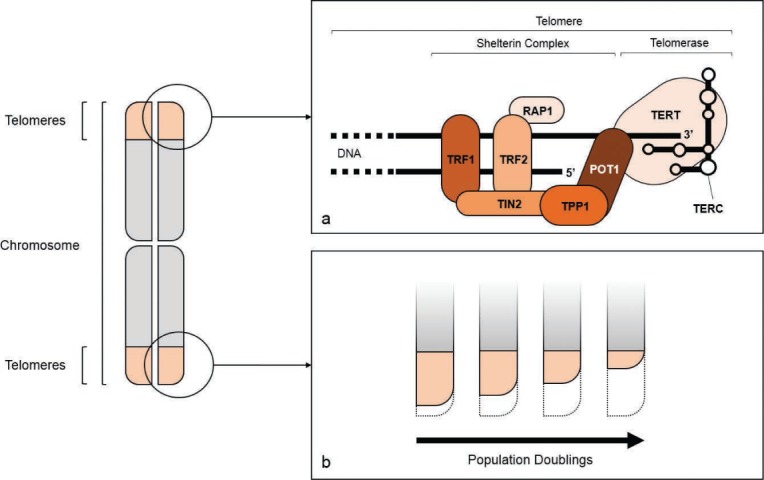
Telomere structure. The repetitive nucleotide sequences at the end of each chromatid are called telomeres. The “end-replication problem” during DNA replication results in a 3-prime overhang of the DNA in the telomere region (a). The shelterin complex protects the telomeres from degradation. It consists of six subunits, namely telomeric repeat-binding proteins 1 and 2 (TRF1 and 2), TRF1-interacting nuclear factor 2 (TIN2), tripeptidyl peptidase 1 (TPP1), protection of telomeres protein 1 (POT1), and ras-related protein 1 (RAP1) (a). Each DNA replication cycle is associated with the loss of nucleotides at the telomeres (b) and may be compensated in some cell types by activation of telomerase, a polymerase complex consisting of a reverse transcriptase (TERT, telomerase reverse transcriptase) and a guide RNA (TERC, telomerase RNA component) (a). Telomerase is tightly regulated by the shelterin complex and expression and activation is detectable in embryonic or induced pluripotent stem cells as well as in most malignant cell types.
In some cells, telomere length may be restored by activity of the enzyme telomerase reverse transcriptase (TERT) together with its RNA component (TERC), which are regulated by the shelterin complex of the telomere (Figure 1a).9 The ability of embryonic or induced pluripotent stem cells to replicate indefinitely is due to the expression by these cells of functional TERT and TERC. Notably, TERT and TERC are reactivated in about 90% of malignancies, accounting for their transformation into essentially immortalized cells. Accordingly, one potential therapeutic approach to treating some malignancies would be to antagonize the activity of telomerase in cancer stem cells.10,11
On the other hand, a transient restoration of telomerase activity to somatic cells could have therapeutic effects. Evidence suggests that inducing telomerase activity in somatic cells and thereby restoring telomere length may reverse cell senescence and restore a functional phenotype.12–14 Multiple observational and interventional studies have shown a strong correlation between telomere attrition in cells of the cardiovascular system and the development of atherosclerosis in animal models and humans.3,15,16 Furthermore, shortened telomere length of circulating lymphocytes, used as an indirect marker of a declining pool of circulating progenitors, has been identified as a predictor for early onset of cardiovascular disease.17
Cellular senescence of endothelial cells, vascular smooth muscle cells, tissue resident cells, and circulating progenitor cells plays an important role in the early stages of a developing vascular lesion that ultimately leads to an atherosclerotic plaque. Aged endothelial cells manifest increased expression of proinflammatory surface markers, a decrease in nitric oxide (NO) production, and a change of structural phenotype that compromises the barrier function of the endothelial monolayer of arterial vessel walls (Figure 2).18–20 Aged vascular smooth muscle cells, on the other hand, undergo a switch from a differentiated to a secretory phenotype that leads to medial thickening, loss of elasticity, and sclerosis of the media.4 Additionally, the decrease in circulating endothelial progenitors fails to compensate for micro-injuries of the arterial vessel wall, in turn exposing the subendothelial vessel structures to circulating factors that further promote lesion formation.5,21 Preclinical studies suggest that activation of telomerase can delay or even reverse the senescent phenotype of aged vascular cells.13,14,22
Figure 2.
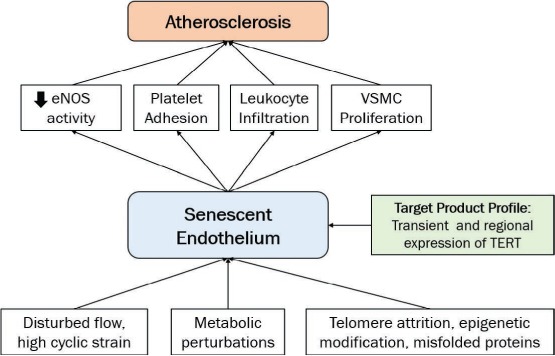
Role of the senescent endothelium in the development of atherosclerosis. Various factors such as disturbed flow, metabolic perturbations, telomere attrition, epigenetic modification and accumulation of misfolded proteins accelerate the aging process in endothelial cells. Cellular senescence of endothelial cells plays an important role in the early stages of vascular lesion development. This ultimately leads to growth of atherosclerotic plaque by increased expression of proinflammatory surface markers, a decrease in nitric oxide (NO) production, and change of structural phenotype that compromises the barrier function of the endothelial monolayer of arterial vessel walls. The proposed target product profile described an agent that reverses senescence in endothelial cells by transient expression of TERT. eNOS: endothelial nitric oxygen synthase; VSMC: vascular smooth muscle cells; TERT: telomerase reverse transcriptase.
The importance of telomere integrity for a healthy cardiovascular system is further highlighted by diseases of premature aging that are caused or accompanied by accelerated telomere decay. One of the best-studied syndromes, Hutchinson-Gilford Progeria Syndrome (HGPS), is accompanied by severe and early onset atherosclerosis. Indeed, patients die in the second decade from coronary or carotid disease due to severe atherosclerosis. Notably, the lamin A mutation (that produces the abnormal protein, progerin) particularly affects tissues of mesodermal origin including the vascular system.23,24 Notwithstanding the fact that treatment of this disease cannot solely depend on restoring telomere length and integrity, development of a reliable therapeutic strategy to rejuvenate the vascular system may play an important role in ameliorating this devastating syndrome.
Senescent Endothelial Cells as a Primary Target for Cardiovascular Rejuvenation
Cardiovascular health requires a healthy endothelium since this diaphanous film of tissue produces a panoply of factors that maintain cardiovascular homeostasis. In the arterial circulation, a normal endothelium is resistant to platelet and leukocyte adherence, maintains the vascular smooth muscle in a quiescent state, and plays an important role in regulating vascular tone. In response to the tractive force of fluid flow, the endothelium secretes vasodilator factors. Paradigmatic of these is endotheliumderived nitric oxide, which, in addition to inducing potent vasodilation, inhibits platelet and leukocyte interaction with the vessel wall and is demonstrably antiatherogenic (Figure 2).25
With aging, phenotypic changes occur within endothelial cells as they switch to an activated state, expressing inflammatory surface markers such as VCAM-1 and ICAM-1 and secreting proinflammatory cytokines.26,6 The chronic activation of the immune system and leukocyte recruitment to the dysfunctional regions of endothelial cell layers further accelerates the aging process.19 Aging of the endothelium is accelerated at sites of disturbed flow such as the iliac artery bifurcation, where the telomeres of human endothelial cells are demonstrably shorter15,27 and histological analysis reveals an increased number of senescent endothelial cells.16,28 This accelerated aging at vascular bifurcations may be due in part to the hemodynamic activation of an inflammatory phenotype by low and oscillating shear stress at these sites.29,30 Thus, a pathologic cycle of inflammation and aging occurs at the very sites (bends, branches, and bifurcations) where the most severe atherosclerotic lesions typically occur.
This pathologic cycle could potentially be reversed by therapeutic extension of telomeres.9,13 Previously, we have shown that aged human aortic endothelial cells manifest many attributes of a senescent vasculature, including reduced ability to proliferate and respond normally to shear stress, to generate nitric oxide, and to resist adhesion of leukocytes. When we transfected these endothelial cells using a lentiviral vector to overexpress telomerase, these senescent properties were reversed. Telomerasetransfected endothelial cells made more nitric oxide, manifested fewer adhesion molecules, were less adhesive for mononuclear cells, and had greater replicative capacity. Such changes would be expected to reduce the progression of atherosclerosis if vascular regeneration by telomere extension could be achieved in patients (Figure 2).
However, use of a viral vector that integrates the gene for telomerase in a human cell is unsafe since it could cause a malignant transformation of the vasculature. Another approach is to use therapeutic RNA to transiently express proteins of interest for a defined period of time.31 Indeed, we recently assessed the effect of modified mRNA encoding telomerase in senescent human cells and found that it can increase telomere length and replicative capacity.32 However, such an approach would require a method for delivering the therapeutic RNA to the vasculature. Because of the ubiquitous nature of RNases, therapeutic RNA would have a very short half-life without some protection. Furthermore, RNA provided systemically would be expected to activate innate immunity, potentially causing severe side effects. These problems may be overcome by modifications of the RNA (e.g., modified nucleotides that reduce innate immune activation) as well as novel delivery devices. The differential expression by senescent endothelial cells of inflammatory surface markers such as ICAM-1 and VCAM-1 offers a potential target for enhancing delivery. Tasciotti's group has shown that functionalized nano-and microparticles are capable of releasing therapeutic molecules to sites of inflamed endothelium.33 In these studies, anti-inflammatory drugs or other therapeutics may be packaged into nanoparticles that are partially composed of autologous leucocyte membranes. The interaction of the leucocyte surface markers with the inflammatory surface markers on the inflamed endothelium allow region-specific release of the therapeutic agent.
Summary and Conclusions
Cardiovascular disease remains the greatest cause of morbidity and mortality worldwide, and cardiovascular senescence is a major contributor to the progression of atherosclerosis and other degenerative vascular diseases. A strategy of cardiovascular regeneration could attenuate or reverse vascular senescence and reduce the progression of vascular disease. Targeting the endothelium using nanoparticles that preferentially deliver RNA encoding telomerase is one such strategy. Endothelial regeneration would be expected to slow or reverse vascular diseases and may provide a higher quality of life to the aging population.
Conflict of Interest Disclosure: This work was supported in part by National Institutes of Health grants U01 HL100397, U01 HL099997, R01 AR063963, and R21 AG044815.
Footnotes
Editor's Note: We are pleased to offer 1 credit of Continuing Medical Education for successfully completing an online quiz about this article. You may access the quiz at www.houstonmethodist.org/cme-online.
References
- 1.Al-Shaer MH, Choueiri NE, Correia ML, Sinkey CA, Barenz TA, Haynes WG. Effects of aging and atherosclerosis on endothelial and vascular smooth muscle function in humans. Int J Cardiol. 2006 May 10;109(2):201–6. doi: 10.1016/j.ijcard.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 2.Brandes RP, Fleming I, Busse R. Endothelial aging. Cardiovasc Res. 2005 May 1;66(2):286–94. doi: 10.1016/j.cardiores.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 3.Minamino T, Miyauchi H, Yoshida T, Ishida Y, Yoshida H, Komuro I. Endothelial cell senescence in human atherosclerosis: role of telomere in endothelial dysfunction. Circulation. 2002 Apr 2;105(13):1541–4. doi: 10.1161/01.cir.0000013836.85741.17. [DOI] [PubMed] [Google Scholar]
- 4.Orlandi A, Bochaton-Piallat ML, Gabbiani G, Spagnoli LG. Aging, smooth muscle cells and vascular pathobiology: implications for atherosclerosis. Atherosclerosis. 2006 Oct;188(2):221–30. doi: 10.1016/j.atherosclerosis.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 5.Satoh M, Ishikawa Y, Takahashi Y, Itoh T, Minami Y, Nakamura M. Association between oxidative DNA damage and telomere shortening in circulating endothelial progenitor cells obtained from metabolic syndrome patients with coronary artery disease. Atherosclerosis. 2008 Jun;198(2):347–53. doi: 10.1016/j.atherosclerosis.2007.09.040. [DOI] [PubMed] [Google Scholar]
- 6.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013 Jun 6;153(6):1194–217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palm W, de Lange T. How shelterin protects mammalian telomeres. Annu Rev Genet. 2008;42:301–34. doi: 10.1146/annurev.genet.41.110306.130350. [DOI] [PubMed] [Google Scholar]
- 8.Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961 Dec;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 9.Bodnar AG, Ouellette M, Frolkis M et al. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998 Jan 16;279(5349):349–52. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- 10.Park YJ, Kim EK, Moon S, Hong DP, Bae JY, Kim J. Human telomerase reverse transcriptase is a promising target for cancer inhibition in squamous cell carcinomas. Anticancer Res. 2014 Nov;34(11):6389–95. [PubMed] [Google Scholar]
- 11.Romaniuk A, Kopczynski P, Ksiazek K, Rubis B. Telomerase modulation in therapeutic approach. Curr Pharm Des. 2014;20(41):6438–51. doi: 10.2174/1381612820666140630092721. [DOI] [PubMed] [Google Scholar]
- 12.Makpol S, Durani LW, Chua KH, Mohd Yusof YA, Ngah WZ. Tocotrienol-rich fraction prevents cell cycle arrest and elongates telomere length in senescent human diploid fibroblasts. J Biomed Biotechnol. 2011;2011:506171. doi: 10.1155/2011/506171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsushita H, Chang E, Glassford AJ, Cooke JP, Chiu CP, Tsao PS. eNOS activity is reduced in senescent human endothelial cells: Preservation by hTERT immortalization. Circ Res. 2001 Oct 26;89(9):793–8. doi: 10.1161/hh2101.098443. [DOI] [PubMed] [Google Scholar]
- 14.Minamino T, Mitsialis SA, Kourembanas S. Hypoxia extends the life span of vascular smooth muscle cells through telomerase activation. Mol Cell Biol. 2001 May;21(10):3336–42. doi: 10.1128/MCB.21.10.3336-3342.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang E, Harley CB. Telomere length and replicative aging in human vascular tissues. Proc Natl Acad Sci U S A. 1995 Nov 21;92(24):11190–4. doi: 10.1073/pnas.92.24.11190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nzietchueng R, Elfarra M, Nloga J et al. Telomere length in vascular tissues from patients with atherosclerotic disease. J Nutr Health Aging. 2011 Feb;15(2):153–6. doi: 10.1007/s12603-011-0029-1. [DOI] [PubMed] [Google Scholar]
- 17.Panayiotou AG, Nicolaides AN, Griffin M et al. Leukocyte telomere length is associated with measures of subclinical atherosclerosis. Atherosclerosis. 2010 Jul;211(1):176–81. doi: 10.1016/j.atherosclerosis.2010.01.037. [DOI] [PubMed] [Google Scholar]
- 18.Mun GI, Boo YC. Identification of CD44 as a senescence-induced cell adhesion gene responsible for the enhanced monocyte recruitment to senescent endothelial cells. Am J Physiol Heart Circ Physiol. 2010 Jun;298(6):H2102–11. doi: 10.1152/ajpheart.00835.2009. [DOI] [PubMed] [Google Scholar]
- 19.Rao RM, Yang L, Garcia-Cardena G, Luscinskas FW. Endothelial-dependent mechanisms of leukocyte recruitment to the vascular wall. Circ Res. 2007 Aug 3;101(3):234–47. doi: 10.1161/CIRCRESAHA.107.151860b. [DOI] [PubMed] [Google Scholar]
- 20.Ito TK, Yokoyama M, Yoshida Y et al. A crucial role for CDC42 in senescence-associated inflammation and atherosclerosis. PloS One. 2014 Jul 24;9(7):e102186. doi: 10.1371/journal.pone.0102186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004 Aug 20;95(4):343–53. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 22.Shen XH, Xu SJ, Jin CY, Ding F, Zhou YC, Fu GS. Interleukin-8 prevents oxidative stress-induced human endothelial cell senescence via telomerase activation. Int Immunopharmacol. 2013 Jun;16(2):261–7. doi: 10.1016/j.intimp.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 23.Bonello-Palot N, Simoncini S, Robert S et al. Prelamin A accumulation in endothelial cells induces premature senescence and functional impairment. Atherosclerosis. 2014 Nov;237(1):45–52. doi: 10.1016/j.atherosclerosis.2014.08.036. [DOI] [PubMed] [Google Scholar]
- 24.McClintock D, Gordon LB, Djabali K. Hutchinson-Gilford progeria mutant lamin A primarily targets human vascular cells as detected by an anti-Lamin A G608G antibody. Proc Natl Acad Sci U S A. 2006 Feb 14;103(7):2154–9. doi: 10.1073/pnas.0511133103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cooke JP. Flow, NO, and atherogenesis. Proc Natl Acad Sci U S A. 2003 Feb 4;100(3):768–70. doi: 10.1073/pnas.0430082100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang M, Jiang L, Monticone RE, Lakatta EG. Proinflammation: the key to arterial aging. Trends Endocrinol Metab. 2014 Feb;25(2):72–9. doi: 10.1016/j.tem.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okuda K, Khan MY, Skurnick J, Kimura M, Aviv H, Aviv A. Telomere attrition of the human abdominal aorta: relationships with age and atherosclerosis. Atherosclerosis. 2000 Oct;152(2):391–8. doi: 10.1016/s0021-9150(99)00482-7. [DOI] [PubMed] [Google Scholar]
- 28.Pedersen EM, Oyre S, Agerbaek M, Kristensen IB, Ringgaard S, Boesiger P et al. Distribution of early atherosclerotic lesions in the human abdominal aorta correlates with wall shear stresses measured in vivo. Eur J Vasc Endovasc Surg. 1999 Oct;18(4):328–33. doi: 10.1053/ejvs.1999.0913. [DOI] [PubMed] [Google Scholar]
- 29.Gimbrone MA, Jr, Topper JN, Nagel T, Anderson KR, Garcia-Cardeña G. Endothelial dysfunction, hemodynamic forces, and atherogenesis. Ann N Y Acad Sci. 2000 May;902:230–9. doi: 10.1111/j.1749-6632.2000.tb06318.x. discussion 239–40. [DOI] [PubMed] [Google Scholar]
- 30.Matharu NM, Rainger GE, Vohra R, Nash GB. Effects of disturbed flow on endothelial cell function: Pathogenic implications of modified leukocyte recruitment. Biorheology. 2006;43(1):31–44. [PubMed] [Google Scholar]
- 31.Sahin U, Karikó K, Türeci Ö. mRNA-based therapeutics--developing a new class of drugs. Nat Rev Drug Discov. 2014 Oct;13(10):759–80. doi: 10.1038/nrd4278. [DOI] [PubMed] [Google Scholar]
- 32.Ramunas J, Yakubov E, Brady JJ et al. Transient delivery of modified mRNA encoding TERT rapidly extends telomeres in human cells. FASEB J. 2015 Jan 22 doi: 10.1096/fj.14-259531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parodi A, Quattrocchi N, van de Ven AL et al. Synthetic nanoparticles functionalized with biomimetic leukocyte membranes possess cell-like functions. Nat Nanotechnol. 2013 Jan;8(1):61–8. doi: 10.1038/nnano.2012.212. [DOI] [PMC free article] [PubMed] [Google Scholar]


