Abstract
Synthetic cannabinoids (SCs) are herbal blends that use plant material with varying concentrations of synthetic analogues of cannabinoids. These products are sold as incense or potpourri and are labeled “Not for human use.” Even so, rates of abuse are rapidly increasing worldwide, especially in the young adult population. An extensive network of users exists, and the products can easily be ordered on the Internet under various brand names, including the most popular ones, “K2” and “Spice.” Not much is known about their spectrum of toxicity and no specific antidote is available at present. Renal failure is a rare complication associated with SC abuse.
We describe a case of acute kidney injury associated with use of SCs and present a review of the current literature, including the history and some key pharmacologic and epidemiologic findings related to synthetic cannabinoid compounds.
Keywords: synthetic cannabionoid, Spice, acute kidney injury

V. S. Gudsoorkar, M.D.

J. A. Perez, Jr., M.D.
Introduction
Cannabis sativa, more popularly known as marijuana, has been used for its medicinal and recreational properties for several thousand years. Cannabis was regarded as a treatment for arthritis, gastrointestinal disorders, snake bites, malaria, female reproductive disorders and much more, but without scientific evidence. The first introduction of these compounds is credited to Sir William O'Shaughnessy, an Irish physician working in Calcutta, India, in the early 19th century.1 This research ultimately led the way to the present-day understanding of various active chemical ingredients of cannabis including Δ9 tetrahydrocannabinol (THC), largely responsible for the psychotropic effects of cannabis. THC acts through cannabinoid receptors (CB) CB1 and CB2. With increased information about analgesic, antiemetic, antispasmodic, and psychotropic properties attributed to the endogenous cannabinoid system, newer research has focused on synthesizing the compounds to mimic the effects of endogenous and natural cannabinoids.1
Professor John William Huffman of Clemson University is widely credited with advancements in synthetic cannabinoid (SC) research. The analogues of THC that he developed were called “JWH compounds,” named after their inventor. These compounds were not tested in humans, and no specific knowledge about their adverse effects, toxicities, and antidotes is currently available.
In 2004, the brand “Spice” containing SCs appeared on the European market, released by the now defunct company “The Psyche Deli.” It quickly gained popularity, and according to the Financial Times, The Psyche Deli assets rose from £65,000 in 2006 to £899,000 in 2007.2 Soon after, similar products were released all over the world under different brand names, each containing different formulations and nonstandardized amounts of SCs as well as different herbal compounds. Following early reports from Sweden of seizures associated with SC use, the European Monitoring Centre for Drugs and Drug Addiction began to formally monitor these products. Forensic analysis showed that the main active ingredient of the Spice products was JWH-018, a novel CB agonist whose chemical name is 1-pentyl-3-(1-naphthoyl) indole. Since then, several JWH compounds and other SCs have been identified in “Spice”-like products or their components.3 While adverse clinical effects of SCs are variable, the association between renal failure and SC use is becoming increasingly recognized.4–6
Case Report
A 26-year-old male patient with a history of bipolar disorder was brought to the emergency department (ED) by his family immediately after noticing seizure-like activity lasting 2 minutes followed by an altered level of consciousness. He was found to be extremely agitated and combative upon arrival to the ED and was administered sedatives and one dose of intravenous valproic acid. In view of his increasing need for sedation and suspected seizures, he was intubated for airway protection and admitted to the hospital. Upon initial interview, his family reported that he had been in his usual state of health until the onset of this seizure-like episode and had been smoking “synthetic weed” for the previous 2 days. His last inhalation was on the night prior to admission.
Physical examination revealed a well-nourished male who was sedated, intubated, and on mechanical ventilation. His vital signs were remarkable for sinus tachycardia with a heart rate of 120/min and pupils sluggishly reactive to light bilaterally. His blood pressure was 130/85 mm Hg. The remainder of his physical examination was normal.
His initial laboratory studies were remarkable for a creatinine of 2.3 mg/dL, blood urea nitrogen (BUN) of 25 mg/dL, and a mildly elevated creatinine phosphokinase (CPK) of 2337 units/L. His urinalysis showed more than 200 red blood cells (RBCs) per high-power field (HPF) that reduced to 5 RBC/HPF the next day and was assumed to be related to Foley catheter placement. The patient was thought to have acute kidney injury (AKI), although urine myoglobin levels were normal, thus ruling out significant rhabdomyolysis as a potential etiology. He had 1+ proteinuria by dipstick method, and no white blood cells or casts were seen. His fractional excretion of sodium was 5.09, which was consistent with intrinsic renal failure. Renal ultrasound revealed a normal echotexture and size, and no other abnormality of the urinary tract was seen. Blood and urine cultures were negative. No other hemodynamic, infectious, pharmacologic, or autoimmune cause was found to explain the patient's acute renal failure. His urine output remained normal (above 0.5 ml/kg/h) throughout his hospitalization. Other laboratory studies were significant for metabolic acidosis that resolved with conservative management.
Over the next few days, the patient's serum creatinine continued to rise and peaked at 8.1 mg/dL on the fourth day of hospitalization. His BUN on the same day was 53 mg/dL. The patient underwent one session of hemodialysis for volume overload. His renal function continued to improve with supportive management, and his creatinine was 2 mg/dL upon discharge with complete resolution of electrolyte abnormalities and metabolic acidosis.
Discussion
Renal involvement with SC intoxication is very rare and has only been reported in two case series, one with 16 patients reported by the Centers for Disease Control and Prevention4 and the other of four patients by Bhanushali et al.5 Additionally, Thornton reported a single case of AKI after SC use.6 Among reported cases, the youngest patient was 15 years old while the oldest was 33 years old. None of these 21 patients had a prior history of kidney disease. Twelve patients underwent biopsy, and acute tubular injury was demonstrated in nine of those. The remaining three had pathology showing acute interstitial nephritis, with two of those also showing calcium oxalate crystals. One patient's biopsy showed glomerular sclerosis without evidence of acute glomerular disease. No immune complex deposition or proliferative changes were noted in any of the biopsies. All patients had improvement in renal function, although the time course was variable. The exact pathogenesis of renal involvement remains unclear, but the putative mechanism may involve the metabolites XLR-11 or UR-144 found on toxicological analysis in the SC products as well as in the patients' urine and serum samples.
The magnitude of problems associated with SC use is troubling. The Drug Abuse Warning Network (DAWN) is a public health surveillance and warning system that monitors drug-related visits to the ED. According to their report, SCs were implicated in 11,400 visits in 2010, 75% of which were in the age group of 12 to 29 year olds (Figure 1).7 Unfortunately, 76% of them did not appear to have any kind of follow-up.7 The 2012 Monitoring the Future survey of youth drug use showed that one in nine 12th graders in America reported using SCs in the past year (Figure 2).8 In 2012, 51 new synthetic cannabinoid compounds were identified compared to just two in 2009.8 The assays to detect SCs and their metabolites in blood and urine using gas or liquid chromatography-mass spectrometry9 are expensive, time consuming, and not easily available.
Figure 1.
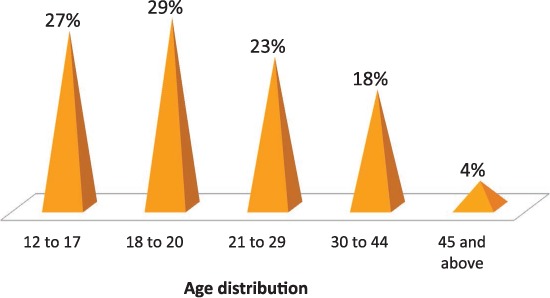
Emergency department visits in 2010 due to synthetic cannabinoid use, stratified by patient age.7
Figure 2.
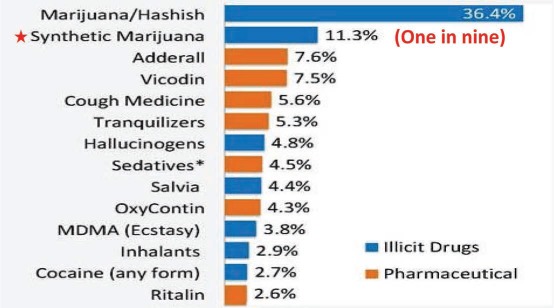
Survey of illicit drug use among high school seniors in 2012, with one in nine using synthetic cannabinoids.8
With the relatively recent discovery and varying chemical composition of these compounds and unavailability of tests to screen for their abuse in routine clinical settings, the data on SC-associated toxicity are sparse. As expected, psychiatric manifestations dominate the spectrum of toxicities, ranging from anxiety and paranoia to psychosis and seizures. Other symptoms may include tachycardia, hypertension, nausea, and vomiting. No specific pharmacologic antidote is available to treat these toxicities, and symptom control with supportive treatment remains the cornerstone of their management.9,10
Conclusion
Synthetic cannabinoid toxicity should be considered as an important cause of AKI in the young population. SC-associated AKI is potentially reversible with supportive treatment, although the long-term effects are unknown. It is crucial that these patients have regular outpatient follow-up with frequent monitoring of renal function. While public health efforts are underway to control these substances, it is likely difficult to eliminate their abuse. Thus, the physicians caring for adolescents with unexplained AKI should be educated about the potential of SC-induced AKI and the importance of appropriate reporting of these cases.
Acknowledgment
The authors thank Dr. Peter T. Nguyen for his expert comments and consultation on this case.
Conflict of Interest Disclosure: The authors have completed and submitted the Methodist DeBakey Cardiovascular Journal Conflict of Interest Statement and none were reported.
References
- 1.Di Marzo V. A brief history of cannabinoid and endocannabinoid pharmacology as inspired by the work of British scientists. Trends Pharmacol Sci. 2006 Mar;27(3):134–40. doi: 10.1016/j.tips.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 2.Jack A. “The story of Spice.” The Financial Times [Internet] 2009 Feb 13 [cited 2015 May 1]. Available from: http://www.ft.com/intl/cms/s/0/1721e2da-f8a0-11dd-aae8-000077b07658.html#axzz39wJl8VdR.
- 3.Lisbon, Portugal: The European Monitoring Centre for Drugs and Drug Addiction; c2009. European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) [Internet] Understanding the ‘Spice’ phenomenon; 2009 Nov [cited 2015 May 1]. Available from: http://www.emcdda.europa.eu/publications/thematic-papers/spice. [Google Scholar]
- 4.Centers for Disease Control and Prevention (CDC) Acute kidney injury associated with synthetic cannabinoid use — multiple states, 2012. MMWR Morb Mortal Wkly Rep. 2013 Feb 15;62(6):93–8. [PMC free article] [PubMed] [Google Scholar]
- 5.Bhanushali GK, Jain G, Fatima H, Leisch LJ, Thornley-Brown D. AKI associated with synthetic cannabinoids: a case series. Clin J Am Soc Nephrol. 2013 Apr;8(4):523–6. doi: 10.2215/CJN.05690612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thornton SL, Wood C, Friesen MW, Gerona RR. Synthetic cannabinoid use associated with acute kidney injury. Clin Toxicol (Phila) 2013 Mar;51(3):189–90. doi: 10.3109/15563650.2013.770870. [DOI] [PubMed] [Google Scholar]
- 7.Rockville, MD: Substance Abuse and Mental Health Services Administration; c2105. Substance Abuse and Mental Health Services Administration [Internet] The DAWN Report: Drug-related emergency department visits involving synthetic cannabinoids; 2012 Dec 4 [cited 2015 May 1]. Available from: http://www.samhsa.gov/data/sites/default/files/DAWN105/DAWN105/SR105-synthetic-marijuana.pdf. [Google Scholar]
- 8.Washington, D.C.: The White House; c2015. The White House [Internet] Office of National Drug Control Policy: Synthetic Drugs (a.k.a. K2, Spice, Bath Salts, etc.); 2015 [cited 2015 May 1]. Available from: http://www.whitehouse.gov/ondcp/ondcp-fact-sheets/synthetic-drugs-k2-spice-bath-salts. [Google Scholar]
- 9.Rosenbaum CD, Carreiro SP, Babu KM. Here today, gone tomorrow…and back again? A review of herbal marijuana alternatives (K2, Spice), synthetic cathinones (bath salts), kratom, Salvia divinorum, methoxetamine, and piperazines. J Med Toxicol. 2012 Mar;8(1):15–32. doi: 10.1007/s13181-011-0202-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alexandria, PA: American Association of Poison Control Centers; c2015. American Association of Poison Control Centers [Internet] Synthetic Marijuana; 2015 [cited 2015 May 1]. Available from: http://www.aapcc.org/alerts/synthetic-marijuana. [Google Scholar]


