In their recent study, Griewank and colleagues proposed that new therapies effective in cutaneous melanoma could hold promise in the management of conjunctival melanoma due to oncogenic BRAF and NRAS mutations and similarities in chromosomal gains and losses (1). Specifically, 27% of 78 primary or locally recurrent conjunctival melanoma samples harbored a BRAF V600E mutation. Another study also demonstrated BRAF V600E mutations in 58% of conjunctival melanomas (primary and metastatic) (2). The incidence of this mutation approximates that reported in cutaneous melanoma (3). Now that two phase III studies have demonstrated marked antitumor activity of the BRAF inhibitors vemurafenib and dabrafenib in patients with BRAF V600E–mutant cutaneous melanoma (3), Griewank and colleagues suggest that patients with BRAF V600E–mutant conjunctival melanoma might derive similar benefit. However, there are no published data on the use of BRAF inhibitors in patients with conjunctival melanoma.
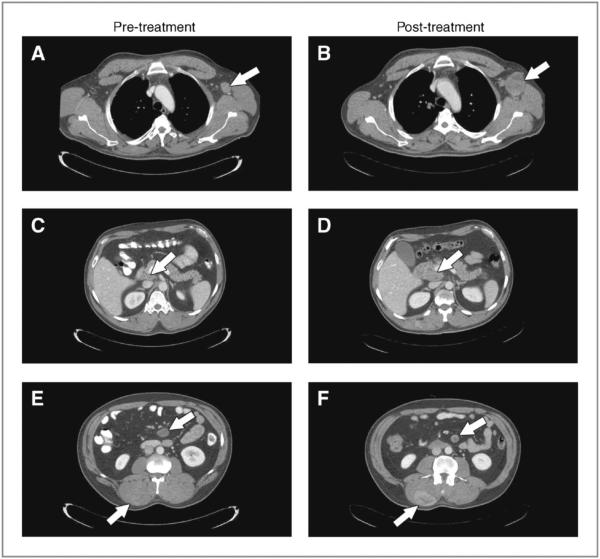
We recently treated a BRAF V600E–mutant metastatic conjunctival melanoma patient with vemurafenib. This 45-year-old male developed metastatic disease 14 months after resection of a primary conjunctival melanoma on the right cornea. On evaluation, he had nodal, subcutaneous, pulmonary, and osseous metastases. Pyrosequencing demonstrated that his melanoma harbored the BRAF V600E mutation. He was initiated on vemurafenib 960 mg twice daily. After 1 month on therapy, he experienced clinical benefit with improvement in pain and subjective tumor regression. However, restaging scans after 2 months of treatment showed a mixed response, predominantly with disease progression (Fig. 1). This included progressive adenopathy in the left axilla and retroperitoneum, as well as an enlarging paraspinal mass, while regression occurred at a single mesenteric mass. Vemurafenib was discontinued and he pursued further systemic therapy options.
Figure 1.
Staging scans at baseline and after 2 months of vemurafenib therapy in a patient with metastatic BRAF V600E–mutant conjunctival melanoma. A and B, marked growth in a left axillary lymph node mass. C and D, progression of a peripancreatic lymph node mass. E and F, modest regression in a mesenteric mass along with significant growth in a right paraspinal mass.
Although this is only one case example, the activity of selective BRAF inhibitors in BRAF-mutant conjunctival melanoma may not be identical to that seen with cutaneous melanoma. Of note, the Griewank and colleagues’ study found frequent copy number loss of the PTEN 10q23 locus, indicating probable loss of PTEN expression and concurrent activation of the phosphoinositide 3-kinase (PI3K) pathway (1). This is supported by another study of conjunctival melanoma, in which 55% of cells analyzed demonstrated weak to no PTEN expression by immunohistochemistry (4). Because concurrent PTEN loss has been described as an intrinsic resistance mechanism and is associated with shorter progression-free survival in patients with cutaneous BRAF-mutant melanoma treated with dabrafenib (5), perhaps a similar mechanism of resistance is common in conjunctival melanoma. Further prospective study of BRAF inhibitors, possibly in combination with a PI3K or AKT inhibitor, is warranted in BRAF V600E–mutant conjunctival melanoma.
Footnotes
Disclosure of Potential Conflicts of Interest
V.K. Sondak is a consultant to GSK. G.T. Gibney is a consultant/advisory board member to Genentech/Roche. No potential conflicts of interest were disclosed by the other authors.
References
- 1.Griewank KG, Westekemper H, Murali R, Mach M, Schilling B, Wiesner T, et al. Conjunctival melanomas harbor BRAF and NRAS mutations and copy number changes similar to cutaneous and mucosal melanomas. Clin Cancer Res. 2013;19:3143–52. doi: 10.1158/1078-0432.CCR-13-0163. [DOI] [PubMed] [Google Scholar]
- 2.Lake SL, Jmor F, Dopierala J, Taktak AF, Coupland SE, Damato BE. Multiplex ligation-dependent probe amplification of conjunctival melanoma reveals common BRAF V600E gene mutation and gene copy number changes. Invest Ophthalmol Vis Sci. 2011;52:5598–604. doi: 10.1167/iovs.10-6934. [DOI] [PubMed] [Google Scholar]
- 3.Jang S, Atkins MB. Which drug, and when, for patients with BRAF-mutant melanoma? Lancet Oncol. 2013;14:e60–9. doi: 10.1016/S1470-2045(12)70539-9. [DOI] [PubMed] [Google Scholar]
- 4.Westekemper H, Karimi S, Susskind D, Anastassiou G, Freistuhler M, Steuhl KP, et al. Expression of HSP 90, PTEN and Bcl-2 in conjunctival melanoma. Br J Ophthalmol. 2011;95:853–8. doi: 10.1136/bjo.2010.183939. [DOI] [PubMed] [Google Scholar]
- 5.Nathanson KL, Martin AM, Wubbenhorst B, Greshock J, Letrero R, D'Andrea K, et al. Tumor genetic analyses of patients with metastatic melanoma treated with the BRAF inhibitor dabrafenib (GSK2118436) Clin Cancer Res. 2013;19:4868–78. doi: 10.1158/1078-0432.CCR-13-0827. [DOI] [PMC free article] [PubMed] [Google Scholar]