Abstract
Recent monkey studies showed that motoneurons of the oculomotor nucleus involved in upward eye movements receive a selective input from afferents containing calretinin (CR). Here, we investigated the sources of these CR-positive afferents. After injections of tract-tracers into the oculomotor nucleus (nIII) of two monkeys, the retrograde labeling was combined with CR-immunofluorescence in frozen brainstem sections. Three sources of CR inputs to nIII were found: the rostral interstitial nucleus of the medial longitudinal fascicle (RIMLF), the interstitial nucleus of Cajal, and the y-group. CR is not present in all premotor upward-moving pathways. The excitatory secondary vestibulo-ocular neurons in the magnocellular part of the medial vestibular nuclei contained nonphosphorylated neurofilaments, but no CR, and they received a strong supply of large CR-positive boutons. In conclusion, the present study presents evidence that only specific premotor pathways for upward eye movements—excitatory upgaze pathways—contain CR, but not the up vestibulo-ocular reflex pathways. This property may help to differentiate between premotor up- and downgaze pathways in correlative clinico-anatomical studies in humans.
Keywords: oculomotor nucleus, superior rectus muscle, inferior oblique muscle, eyelid, monkey
Introduction
Recent studies in the monkey have shown that the motoneurons in the oculomotor nucleus for upward eye movements—for example, superior rectus, inferior oblique, and levator palpebrae muscle—receive a selective set of afferent terminals containing the calcium-binding protein calretinin (CR).1 The functional basis of the CR content is unclear, but it indicates that up- and downgaze pathways differ in their histochemistry and may provide a reason for their selective involvement in certain eye movement disorders—for example, isolated upgaze or downgaze palsy, or selective upbeat or downbeat nystagmus.2 The premotor pathways for vertical eye movements are well known for the vestibular system;3,4 they lie in the magnocellular part of the medial vestibular nucleus, superior vestibular nucleus, and the y-group. Premotor pathways for the saccadic system involve the interstitial nucleus of Cajal (INC) and rostral interstitial nucleus of the medial longitudinal fascicle (RIMLF).5 With combined tract-tracing and CR immunostaining, we studied in the monkey whether the vestibular nuclei, INC, and RIMLF are sources for CR inputs to the upgaze motoneurons.
Materials and methods
All experimental procedures conformed to the state and university regulations on Laboratory Animal Care, including the Principles of Laboratory Animal Care (NIH Publication 85–23, Revised 1985), and were approved by the Animal Care Officers and Institutional Animal Care and Use Committees at Emory University and University of Washington, where all surgical interventions and perfusions were made.
Rhesus monkeys (Macaca mulatta) received central tracer injections either with 1% cholera toxin subunit B (CTB; case 1 and 3) or 5% wheat-germ agglutinin (WGA; EY Lab.; case 2) into the oculomotor nucleus (nIII) as described previously.6 In brief, under aseptic conditions and isoflurane anesthesia, the animals were stereotaxically implanted with a titanium recording chamber (Crist Instruments, Hagerstown, MD). The injection sites were identified with single-unit recording using tungsten microelectrodes. For injection, the recording electrode was replaced by a micropipette equipped with a bevelled glass tip (20–50 μm diameter) and attached by polyethylene tubing to a picoliter pump (WPI 830). Short-duration (50 ms) pressure pulses delivered over several minutes ejected small tracer volumes at each site. The pipette was left in place for 5–10 minutes after injection and then gradually removed. After three days survival time, the animals were sacrificed with an overdose of sodium pentobarbital (>90 mg/kg, I.V.) and then transcardially perfused with 0.9% saline followed by 4% paraformaldehyde in 0.1 M phosphate buffer (PBS; pH 7.4).
Brainstem sections were immunocytochemically treated free-floating with polyclonal goat antibodies against CTB (103; List Biological Laboratories, Campbell, CA; 1:20,000) or WGA (lot V0128; AS-2024; Vector Laboratories, Burlingame, CA) as described previously.6 The antigenic sites were visualized with an immunoperoxidase protocol using secondary biotinylated anti-goat (1:200; Vector Lab) and extravidin peroxidase (1:1,000; Sigma, St. Louis, MO) and a final reaction in 0.025% diaminobenzidine (DAB) and 0.015% H2O2 in 0.1M TBS (pH 7.6) for 10 minutes. For the simultaneous detection of tracer and CR, double-immunofluorescence sections were incubated in a cocktail of either goat anti-CTB (1:5,000) or goat anti-WGA (1:1,000; Axxora) and rabbit anti-CR (1:1,000; SWant) in 5% normal donkey serum in 0.3% Triton X-100 in 0.1M TBS, pH 7,4 for 48 h at 4° C. After several buffer washes, the sections were treated with a mixture of alexa-488–tagged donkey anti-goat (1:200; A-11055, Molecular Probes) and carbocyanine-3 (Cy3)–tagged donkey anti-rabbit (1: 200; 711–165–152, Dianova). Selected sections containing the vestibular nuclei were stained for the simultaneous detection of CTB and nonphosphorylated neurofilaments (NP-NF) with a mixture of goat anti-CTB (1:5,000) and mouse anti-NP-NF (1:1,000; SMI32; Stern-berger monoclonals). After rinsing, the sections were treated with a cocktail of alexa-488–tagged donkey anti-goat (1:200) and C3-tagged donkey anti-mouse (1:200).
The slides were examined with a Zeiss Axioplan microscope (Carl Zeiss MicroImaging, Germany) equipped with appropriate filters for red fluorescent Cy3 (Zeiss: excitation filter BP 546 nm, dichromatic beam splitter FT 580 nm, barrier filter LP 590 nm) and green fluorescent Alexa 488 (Zeiss: excitation filter BP 475 nm; dichromatic beam splitter FT 500 nm, barrier filter LP 530 nm). Photographs were taken with a digital camera (MicroFire 2.3A, Optronics, Goleta, CA), captured on a computer with PictureFrame, 2.3 (Optronics), and processed in Photoshop 7.0 (Adobe Systems, Mountain View, CA). The sharpness, contrast, and brightness were adjusted to reflect the appearance of the labeling seen through the microscope. The pictures were arranged and labeled in CorelDraw 11.0 (Corel Corp.). The graphical illustration of the distribution of tracer-positive in relation to double-labeled cells (plots) was performed using Neurolucida software (MicroBrightField, Inc., VT; Version 6).
Results
In all cases, tracer injections hit the center of nIII on one side (Fig. 1) and led to retrogradely labeled neurons in known premotor areas—for example, the RIMLF, the INC, the internuclear neurons in the abducens nucleus (nVI), the nucleus prepositus hypoglossi, and the vestibular nuclei including the y-group, as described earlier.7–9 For a detailed analysis of retrogradely labeled neurons that express CR immunoreactivity, we chose three areas that are known to control vertical eye movements: the RIMLF, INC, and the vestibular nuclei including the y-group.10
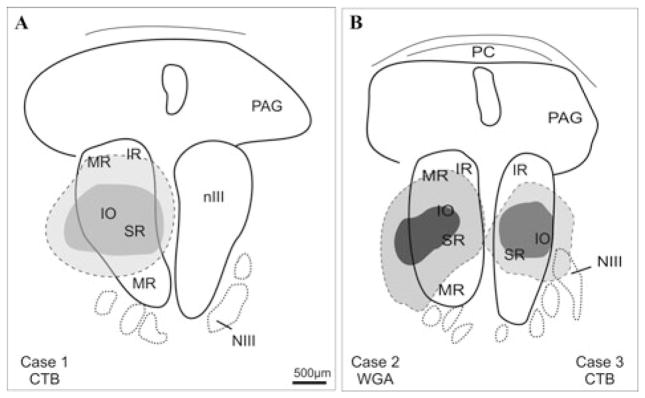
Figure 1.
Schematic drawings of transverse sections through the monkey oculomotor nucleus (nIII) to show the location of tracer injection sites (dark) and the extent of the uptake area (light gray) in three cases. NIII, oculomotor nerve; PAG, periaqueductal gray; PC, posterior commissure.
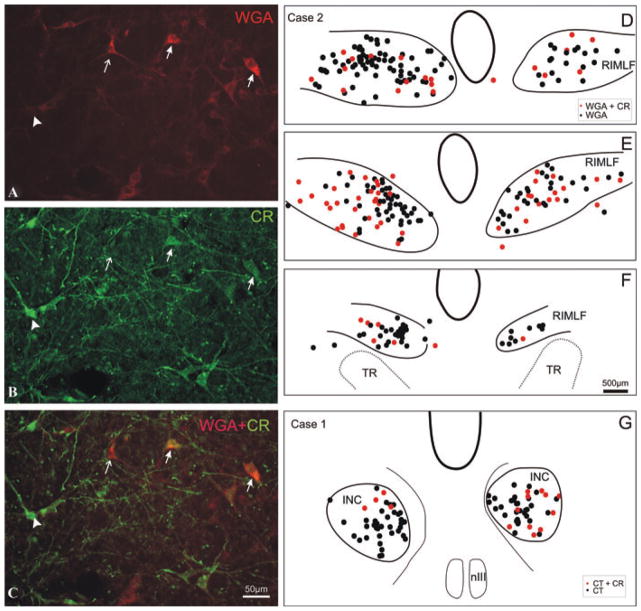
Combined immunofluorescence labeling in all studied areas allowed the analysis of tracer-labeled premotor neurons for the presence of CR, as shown for the RIMLF as an example (Fig. 2A–C). In the RIMLF, the tracer-labeled neurons expressing CR immunoreactivity made up approximately 20% (Fig. 2C; solid arrows). These double-labeled neurons were distributed across the whole extent of the RIMLF with no preference for caudal or rostral, medial, or lateral locations (Fig. 2D–F). In the INC on both sides, only a small portion of small- to medium-sized tracer-labeled neurons expressed CR immunoreactivity (Fig. 2G). As in the RIMLF, no preference for a location of double-labeled neurons was noted in the INC.
Figure 2.
High-power magnification of a transverse section through the rostral interstitial nucleus of the medial longitudinal fascicle (RIMLF) showing the tracer-labeled (WGA) premotor neurons (red; A) and calretinin (CR)-positive neurons (green; B). In the superposition of A and B, double-labeled neurons appear in orange (solid arrows), clearly distinguished from neurons containing either only CR (green, arrow head) or the tracer WGA (red, arrow; C). Plots of transverse sections through the RIMLF (D–F) and interstitial nucleus of Cajal (INC; G) of a monkey with a CTB-injection into the left oculomotor nucleus (nIII) in rostrocaudal order (D–F): tracer-labeled CR-positive putative upburst neurons (red dots) are intermingled with tracer-labeled CR-negative putative down-burst neurons (black dots). TR, tractus retroflexus.
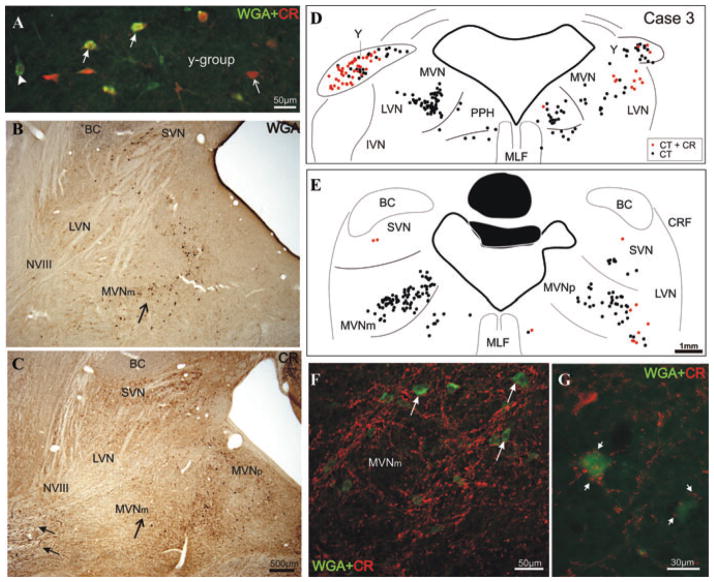
In all three cases numerous, tracer-labeled neurons were found in the vestibular nuclear complex, as described earlier.11,12 The largest population of CR-positive tracer-labeled neurons was identified in the dorsal part of the y-group of both sides with a predominance contralateral, where CR-positive neurons made up more than 50% of all tracer-labeled neurons (Fig. 3A, D, and E). Although the medial (MVN) and superior vestibular nuclei (SVN) contain numerous CR-positive and tracer-labeled neurons (Fig. 3B and C), only few scattered double-labeled “CR-positive projection neurons” were detected in the MVN and SVN, mainly ipsilateral (Fig. 3D and E). These tracer-labeled CR-positive neurons did not represent the secondary vestibulo-ocular neurons in the magnocellular part of the MVN (MVNm) (Fig. 3F).13 Instead, tracer-labeled secondary vestibulo-ocular neurons in the contralateral MVNm were embedded in a densely stained network of CR-positive fibers (Fig. 3C and F). Close inspection revealed that the tracer-labeled neurons were outlined by large CR-positive terminals (Fig. 3F and G). The vestibular nerve (NVIII) entering the vestibular nuclei at this level contains numerous CR-positive nerve fibers (Fig. 3C, arrows). The presence of many medium- and large-sized neurons immunoreactive for NP-NF in the MVNm encouraged us to investigate the tracer-labeled neurons for this marker. A considerable portion of tracer-labeled medium-sized neurons in the MVNm at the border to the lateral vestibular nucleus show NP-NF–immunoreactivity (Fig. 4A–C; solid arrows), but not all of them (Fig. 4A–C; arrow head).
Figure 3.
Superposition of high-power photographs of a transverse section through the y-group showing premotor tracer-labeled neurons (green, arrowhead), some of which contain CR (orange, solid arrow). Not all CR-positive neurons are tracer labeled (red, thin arrow; A). Photographs of corresponding sections through the vestibular nuclei of monkey stained for retrograde tracer WGA (black cells; B) or CR (brown cells; C). Note that MVNm contains numerous tracer-labeled neurons (arrow; B), but few CR-positive neurons (arrow; C). The vestibular nerve (NVIII) contains numerous CR-positive fibers (solid arrows). Transverse sections through the vestibular nuclei of a monkey with a tracer (CTB) injection into the right oculomotor nucleus with the retrogradely labeled neurons plotted (black dots; D, E). Tracer-labeled CR-positive neurons (red dots) are mainly found in the y-group of both sides (D). Putative excitatory secondary vestibulo-ocular neurons in the left MVm lack CR (E), but they are contacted by numerous large CR-positive terminals (red, arrows; F, G).
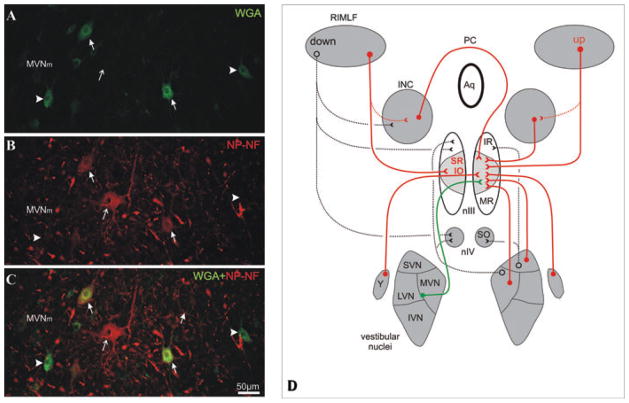
Figure 4.
High-power magnification of a transverse section through the magnocellular part of the medial vestibular nucleus (MVNm) showing the WGA-labeled secondary vestibulo-ocular neurons (A) and neurons stained for nonphosphorylated neurofilaments (NP-NF; B). The superposition reveals that many, but not all, vestibulo-ocular neurons (green, arrowhead) are NP-NF–positive (solid arrow) and not all NP-NF–positive neurons are tracer labeled (red, thin arrow; C). Simplified diagram summarizing the premotor pathways for vertical eye movements. The projections found after a tracer injection into the right oculomotor nucleus (nIII) and associated with CR found are drawn in red, and those associated with NP-NF are drawn in green (D). IVN, inferior vestibular nucleus; LVN, lateral vestibular nucleus; PC, posterior commissure.
Discussion
With combined tract-tracing and immunostaining methods, three main sources of CR containing afferents to motoneurons of upward-pulling eye muscles were identified in the monkey—for example, the RIMLF, the INC, and the y-group.
The expression of CR in one subpopulation of premotor neurons in the RIMLF was already indicated by a previous observation, that approximately 40% of the neurons expressing parvalbumin (PV)—a calcium-binding protein present in all premotor neurons in the RIMLF—contain CR as well.14,15 Based on the finding that only the motoneurons of upward-pulling eye muscles receive CR-positive afferents,1 the tracer-labeled CR-positive neurons in the RIMLF are likely to represent premotor saccadic up-burst neurons. Taking into account that in the monkey, the RIMLF does not contain GABAergic premotor neurons16—a potential source of the strong GABAergic input to SR and IO motoneurons17—and the lack of glycinergic input to SR and IO motoneurons, the tracer-labeled CR-positive neurons in the RIMLF must be considered as excitatory premotor up-burst neurons. Combined tract-tracing and immunocytochemical staining in the cat revealed glutamate and/or aspartate as the transmitter of excitatory burst neuron.17 Moreover, the lack of a topographical organization of CR-positive up-burst neurons seen in this study confirms earlier findings from recording experiments and anatomical studies in the monkey that found saccadic up- and down-burst neurons intermingled.15,18–20 It may now be possible to use CR in humans to resolve this question.
In contrast to the RIMLF, the INC contains GABAergic premotor neurons projecting to motoneurons of vertical moving eye muscles, which were mainly shown for motoneurons subserving downgaze.16 Because there is some evidence that CR is not present in GABAergic neurons in the INC (Ahlfeld, personal observation), the CR-positive tracer-labeled neurons in the INC are considered as excitatory premotor neurons, presumably up-burst tonic or tonic neurons.21
In accordance with previous reports, CR-positive neurons are present in the vestibular nuclear complex in the monkey including the y-group.22,23 The y-group was identified as the main source of CR-positive premotor neurons. In the monkey, the dorsal y-group—also known as infracerebellar nucleus in the cat24—does not receive saccular afferents25 but receives a strong inhibitory input from the flocculus and the ventral paraflocculus26,27 and is involved in the adaptation and modulation of the vertical vestibulo-ocular reflex (VOR).28,29 Furthermore, electrical stimulation of the y-group results in smooth upward eye movements whose velocity increases with stimulus frequency and therefore are thought to be part of the pathways from the cerebellum for smooth-pursuit eye movements.10,30 Additional hypotheses for these pathways have been suggested.31,32 With anatomical and physiological methods, it was shown that the dorsal y-group projects to SR and IO motoneurons in the contralateral nIII and the inferior rectus (IR) and superior oblique (SO) of the ipsilateral side.33,34 Here, we could show that at least a subpopulation of CR-positive neurons project to nIII of both sides. Electrical stimulation of the y-group produced excitatory postsynaptic potentials in the SR and IO motoneurons of the contralateral nIII,35 which confirms the observation that CR is not present in GABAergic neurons within the y-group (Ahlfeld, personal observations).
The tract-tracing experiments back-labeled the well-known projections of secondary vestibulo-ocular connections, which are organized in a consistent pattern in all animals. The secondary vestibulo-ocular neurons receive primary vestibular afferents from the canals and, in turn, send excitatory fibers to the respective motoneurons on the contralateral side and inhibitory projections to the motoneurons of antagonistic eye muscles on the ipsilateral side.4,12 The fact that the contralaterally tracer-labeled secondary vestibulo-ocular neurons in the MVNm do not express CR indicates that not all premotor inputs to motoneurons for upward eye movements contain CR. On the other hand, these neurons colocalize NP-NF and thereby extend the findings of Baizer, who described the presence of NP-NF–positive neurons in the MVNm area in the monkey.36 We could demonstrate baskets of large CR-positive terminals around CR-negative tracer-labeled secondary vestibulo-ocular neurons (Fig. 3G); the terminals most probably derive from the vestibular nerve, where a subfraction of fibers and vestibular ganglion cells express CR immunoreactivity (Fig. 3C).37,38 This hypothesis is confirmed by the elimination of CR-labeled fibers and terminals in the MVNm after a vestibular nerve lesion in the chinchilla and guinea pig.39 Immunocytochemical studies in rodents have shown that CR is confined to primary vestibular afferents that terminate as single or multiple calyces around type I hair cells in the apex of the crista in the canals or the striola of the maculae.40,41 These calyx afferents exhibit an irregular discharge with a high sensitivity to stimulation.41,42 The large CR-positive terminals around tracer-labeled vestibulo-ocular neurons in the present study resemble the morphology of intracellularly tracer-injected irregular-type afferents in the cat, which terminate as large boutons on so-mata and proximal dendrites of large neurons in the ventral MVN.43,44
The association of CR with specific premotor pathways in the oculomotor system differs between species. In contrast to the monkey, the medial rectus subgroup in the cat nIII receives a strong projection from CR-positive afferents, which arise from internuclear neurons in the abducens nucleus, where 80% were found to contain CR.45 In correlation with the lack of CR afferents in the MR subgroup, no CR-positive neurons are present in the abducens nucleus of the monkey.23
The function of CR in specific neurons is not yet clear. Both PV and CR belong to the EF-hand family of low molecular weight calcium-binding proteins that are highly conserved throughout evolution.46,47 In general, they regulate the intracellular calcium level and may participate in many different intra-cellular functions, for example, neuron survival, pathological degeneration, excitability, firing rate, but also in second messenger pathways controlled by calcium-sensitive enzymes.48,49 Whereas PV is found in many fast-firing or highly active neurons—also in the oculomotor system5,15,50—CR does not appear to be correlated with a certain functional cell type. Recent reports suggest that CR plays an important role in the regulation of neuronal excitability,51 a concept supported by the selective association of CR in the irregular-type calyx afferents of vestibular primary afferents.39 It is unclear why the calcium-binding protein CR is present in specific premotor pathways of the oculomotor system, here the upgaze pathways. Other examples of histochemical specification of premotor neurons are seen in the inhibitory pathways of the oculomotor system: the inhibition for horizontal saccades and VOR is mediated via glycine, whereas that for vertical eye movements, saccades, and VOR is mediated via GABA.17,52
In conclusion, the present work shows that upgaze and downgaze premotor pathways differ in their histochemistry: the excitatory direct premotor pathways for upgaze can be selectively labeled using CR. Despite possible species differences, these histochemical characteristics may open the possibility of identifying the corresponding premotor upgaze pathways in humans.
Acknowledgments
We thank A. Messoudi, M. Phil. for his excellent technical support. Grant support comes from Deutsche Forschungsgemeinschaft; Grant number: HO 1639/4–3; National Institutes of Health EY020744; RR00166.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Schulze C, Mustari MJ, Holstein GR, Horn AKE. Transmitter inputs to different motoneuron subgroups in the oculomotor and trochlear nucleus in monkey. Soc Neurosci Abstr. 2009;35:356–359. doi: 10.3389/fnana.2015.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leigh RJ, Zee DS. The Neurology of Eye Movements. 4. Oxford Univ. Press; New York: 2006. [Google Scholar]
- 3.Büttner-Ennever JA, Gerrits NM. Vestibular System. In: Paxinos G, Mai JK, editors. The Human Nervous System. Elsevier Academic Press; Amsterdam: 2004. pp. 1212–1240. [Google Scholar]
- 4.Highstein SM, Holstein GR. The anatomy of the vestibular nuclei. Prog Brain Res. 2006;151:157–203. doi: 10.1016/S0079-6123(05)51006-9. [DOI] [PubMed] [Google Scholar]
- 5.Horn AKE. The reticular formation. Prog Brain Res. 2006;151:127–155. doi: 10.1016/S0079-6123(05)51005-7. [DOI] [PubMed] [Google Scholar]
- 6.Lienbacher K, Mustari M, Ying HS, et al. Do palisade endings in extraocular muscles arise from neurons in the motor nuclei? Invest Ophthal Vis Sci. 2011;52:2510–2519. doi: 10.1167/iovs.10-6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Büttner-Ennever JA. The extraocular motor nuclei: organization and functional neuroanatomy. Prog Brain Res. 2006;151:95–125. doi: 10.1016/S0079-6123(05)51004-5. [DOI] [PubMed] [Google Scholar]
- 8.Büttner-Ennever JA, Büttner U. A cell group associated with vertical eye movements in the rostral mesencephalic reticular formation of the monkey. Brain Res. 1978;151:31–47. doi: 10.1016/0006-8993(78)90948-4. [DOI] [PubMed] [Google Scholar]
- 9.Steiger HJ, Büttner-Ennever JA. Relationship between motoneurons and internuclear neurons in the abducens nucleus: a double retrograde tracer study in the cat. Brain Res. 1978;148:181–188. doi: 10.1016/0006-8993(78)90387-6. [DOI] [PubMed] [Google Scholar]
- 10.Büttner U, Büuttner-Ennever JA. Present concepts of oculomotor organization. Prog Brain Res. 2006;151:1–42. doi: 10.1016/S0079-6123(05)51001-X. [DOI] [PubMed] [Google Scholar]
- 11.Büttner-Ennever JA, Grob P, Akert K, Bizzini B. Transsynaptic retrograde labeling in the oculomotor system of the monkey with [125I] tetanus toxin BIIb fragment. Neurosci Lett. 1981;26:233–238. doi: 10.1016/0304-3940(81)90138-5. [DOI] [PubMed] [Google Scholar]
- 12.Büttner-Ennever JA. Patterns of connectivity in the vestibular nuclei. Ann N Y Acad Sci. 1992;656:363–378. doi: 10.1111/j.1749-6632.1992.tb25222.x. [DOI] [PubMed] [Google Scholar]
- 13.Büttner-Ennever JA. In: Overview of the vestibular system. Beitz AJ, Anderson JH, editors. CRC Press; Boca Raton London; New York; Washington, DC: 2000. pp. 3–24. [Google Scholar]
- 14.Horn AKE, Brückner G, Härtig W, Messoudi A. Saccadic omnipause and burst neurons in monkey and human are ensheathed by perineuronal nets but differ in their expression of calcium-binding proteins. J Comp Neurol. 2003;455:341–352. doi: 10.1002/cne.10495. [DOI] [PubMed] [Google Scholar]
- 15.Horn AKE, Büttner-Ennever JA. Premotor neurons for vertical eye-movements in the rostral mesencephalon of monkey and man: the histological identification by parvalbumin immunostaining. J Comp Neurol. 1998;392:413–427. [PubMed] [Google Scholar]
- 16.Horn AKE, Helmchen C, Wahle P. GABAergic neurons in the rostral mesencephalon of the Macaque monkey that control vertical eye movements. Ann N Y Acad Sci. 2003;1004:19–28. doi: 10.1196/annals.1303.003. [DOI] [PubMed] [Google Scholar]
- 17.Spencer RF, Wang SF. Immunohistochemical localization of neurotransmitters utilized by neurons in the rostral interstitial nucleus of the medial longitudinal fasciculus (riMLF) that project to the oculomotor and trochlear nuclei in the cat. J Comp Neurol. 1996;366:134–148. doi: 10.1002/(SICI)1096-9861(19960226)366:1<134::AID-CNE9>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 18.Büttner U, Büttner-Ennever JA, Henn V. Vertical eye movement related activity in the rostral mesencephalic reticular formation of the alert monkey. Brain Res. 1977;130:239–252. doi: 10.1016/0006-8993(77)90273-6. [DOI] [PubMed] [Google Scholar]
- 19.King WM, Fuchs AF. Reticular control of vertical saccadic eye movements by mesencephalic burst neurons. J Neurophysiol. 1979;42:861–876. doi: 10.1152/jn.1979.42.3.861. [DOI] [PubMed] [Google Scholar]
- 20.Moschovakis AK, Scudder CA, Highstein SM. The microscopic anatomy and physiology of the mammalian saccadic system. Prog Neurobiol. 1996;50:133–133. doi: 10.1016/s0301-0082(96)00034-2. [DOI] [PubMed] [Google Scholar]
- 21.Helmchen C, Rambold H, Büttner U. Saccade-related burst neurons with torsional and vertical on- directions in the interstitial nucleus of cajal of the alert monkey. Exp Brain Res. 1996;112:63–78. doi: 10.1007/BF00227179. [DOI] [PubMed] [Google Scholar]
- 22.Baizer JS, Baker JF. Immunoreactivity for calretinin and calbindin in the vestibular nuclear complex of the monkey. Exp Brain Res. 2006;172:103–113. doi: 10.1007/s00221-005-0318-1. [DOI] [PubMed] [Google Scholar]
- 23.McCrea RA, Horn AKE. Nucleus prepositus. Prog Brain Res. 2006;151:205–230. doi: 10.1016/S0079-6123(05)51007-0. [DOI] [PubMed] [Google Scholar]
- 24.Gacek RR. Location of brain stem neurons projecting to the oculomotor nucleus in the cat. Exp Neurol. 1977;57:725–749. doi: 10.1016/0014-4886(77)90105-4. [DOI] [PubMed] [Google Scholar]
- 25.Newlands SD, Vrabec JT, Purcell IM, et al. Central projections of the saccular and utricular nerves in macaques. J Comp Neurol. 2003;466:31–47. doi: 10.1002/cne.10876. [DOI] [PubMed] [Google Scholar]
- 26.Langer TP, Fuchs AF, Chubb MC, et al. Floccular efferents in the rhesus macaque as revealed by autoradiography and horseradish peroxidase. J Comp Neurol. 1985;235:26–37. doi: 10.1002/cne.902350103. [DOI] [PubMed] [Google Scholar]
- 27.Partsalis AM, Zhang Y, Highstein SM. Dorsal Y group in the squirrel monkey. II. Contribution of the cerebellar flocculus to neuronal responses in normal and adapted animals. J Neurophysiol. 1995;73:632–650. doi: 10.1152/jn.1995.73.2.632. [DOI] [PubMed] [Google Scholar]
- 28.Partsalis AM, Zhang Y, Highstein SM. Dorsal Y group in the squirrel monkey. I. Neuronal responses during rapid and long-term modifications of the vertical VOR. J Neurophysiol. 1995;73:615–631. doi: 10.1152/jn.1995.73.2.615. [DOI] [PubMed] [Google Scholar]
- 29.Blazquez P, Partsalis A, Gerrits NM, Highstein SM. Input of anterior and posterior semicircular canal interneurons encoding head-velocity to the dorsal Y group of the vestibular nuclei. J Neurophysiol. 2000;83:2891–2904. doi: 10.1152/jn.2000.83.5.2891. [DOI] [PubMed] [Google Scholar]
- 30.Chubb MC, Fuchs AF. Contribution of y group of vestibular nuclei and dentate nucleus of cerebellum to generation of vertical smooth eye movements. J Neurophysiol. 1982;48:75–99. doi: 10.1152/jn.1982.48.1.75. [DOI] [PubMed] [Google Scholar]
- 31.Pierrot-Deseilligny C, Tilikete C. New insights into the upward vestibulo-oculomotor pathways in the human brainstem. Prog Brain Res. 2008;171:509–518. doi: 10.1016/S0079-6123(08)00673-0. [DOI] [PubMed] [Google Scholar]
- 32.Pierrot-Deseilligny C. Effect of gravity on vertical eye position. Ann N Y Acad Sci. 2009;1164:155–165. doi: 10.1111/j.1749-6632.2009.03864.x. [DOI] [PubMed] [Google Scholar]
- 33.Wasicky R, Horn AKE, Büttner-Ennever JA. Twitch and non-twitch motoneuron subgroups of the medial rectus muscle in the oculomotor nucleus of monkeys receive different afferent projections. J Comp Neurol. 2004;479:117–129. doi: 10.1002/cne.20296. [DOI] [PubMed] [Google Scholar]
- 34.Carpenter MB, Cowie RJ. Connections and oculomotor projections of the superior vestibular nucleus and cell group ‘y’. Brain Res. 1985;336:265–287. doi: 10.1016/0006-8993(85)90653-5. [DOI] [PubMed] [Google Scholar]
- 35.Sato Y, Kawasaki T. Target neurons of floccular caudal zone inhibition in Y-group nucleus of vestibular nuclear complex. J Neurophysiol. 1987;57:460–480. doi: 10.1152/jn.1987.57.2.460. [DOI] [PubMed] [Google Scholar]
- 36.Baizer JS. Nonphosphorylated neurofilament protein is expressed by scattered neurons in the vestibular and pre-cerebellar brainstem. Brain Res. 2009;1298:46–56. doi: 10.1016/j.brainres.2009.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kevetter GA, Leonard RB. Use of calcium-binding proteins to map inputs in vestibular nuclei of the gerbil. J Comp Neurol. 1997;386:317–327. [PubMed] [Google Scholar]
- 38.Kevetter GA, Leonard RB. Molecular probes of the vestibular nerve: II. Characterization of neurons in Scarpa’s ganglion to determine separate populations within the nerve. Brain Res. 2002;928:18–29. doi: 10.1016/s0006-8993(01)03264-4. [DOI] [PubMed] [Google Scholar]
- 39.Desmadryl G, Dechesne CJ. Calretinin immunoreactivity in chinchilla and guinea pig vestibular end organs characterizes the calyx unit subpopulation. Exp Brain Res. 1992;89:105–108. doi: 10.1007/BF00229006. [DOI] [PubMed] [Google Scholar]
- 40.Desai SS, Ali H, Lysakowski A. Comparative morphology of rodent vestibular periphery. II. Cristae ampullares. J Neurophysiol. 2005;93:267–280. doi: 10.1152/jn.00747.2003. [DOI] [PubMed] [Google Scholar]
- 41.Baird RA, Desmadryl G, Fernandez C, Goldberg JM. The vestibular nerve of the chinchilla. II. Relation between afferent response properties and peripheral innervation patterns in the semicircular canals. J Neurophysiol. 1988;60:182–203. doi: 10.1152/jn.1988.60.1.182. [DOI] [PubMed] [Google Scholar]
- 42.Goldberg JM, Brichta AM, Wackym PA. Efferent vestibular system: Anatomy, physiology, and neurochemistry. In: Beitz AJ, Anderson JH, editors. Neurochemistry of the Vestibular System. CRC Press; Boca Raton London New York Washington DC: 2000. pp. 61–94. [Google Scholar]
- 43.Sato F, Sasaki H, Ishizuka N, et al. Morphology of single primary vestibular afferents originating from the horizontal semicircular canal in the cat. J Comp Neurol. 1989;290:423–439. doi: 10.1002/cne.902900310. [DOI] [PubMed] [Google Scholar]
- 44.Sato F, Sasaki H. Morphological correlations between spontaneously discharging primary vestibular afferents and vestibular nucleus neurons in the cat. J Comp Neurol. 1993;333:554–566. doi: 10.1002/cne.903330408. [DOI] [PubMed] [Google Scholar]
- 45.De la Cruz RR, Pastor AM, Martinez-Guijarro FJ, et al. Localization of parvalbumin, calretinin, and calbindin D-28K in identified extraocular motoneurons and internuclear neurons of the cat. J Comp Neurol. 1998;390:377–391. doi: 10.1002/(sici)1096-9861(19980119)390:3<377::aid-cne6>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 46.Andressen C, Blümcke I, Celio MR. Calcium-binding proteins—selective markers of nerve cells. Cell Tiss Res. 1993;271:181–208. doi: 10.1007/BF00318606. [DOI] [PubMed] [Google Scholar]
- 47.Baimbridge KG, Celio MR, Rogers JH. Calcium-binding proteins in the nervous system. TINS. 1992;15:303–308. doi: 10.1016/0166-2236(92)90081-i. [DOI] [PubMed] [Google Scholar]
- 48.Gall D, Roussel C, Nieus T, et al. Role of calcium binding proteins in the control of cerebellar granule cell neuronal excitability: experimental and modeling studies. Prog Brain Res. 2005;148:321–328. doi: 10.1016/S0079-6123(04)48025-X. [DOI] [PubMed] [Google Scholar]
- 49.Bastianelli E. Distribution of calcium-binding proteins in the cerebellum. Cerebellum. 2003;2:242–262. doi: 10.1080/14734220310022289. [DOI] [PubMed] [Google Scholar]
- 50.Horn AKE, Büttner-Ennever JA, Suzuki Y, Henn V. Histological identification of premotor neurons for horizontal saccades in monkey and man by parvalbumin immunostaining. J Comp Neurol. 1995;359:350–363. doi: 10.1002/cne.903590212. [DOI] [PubMed] [Google Scholar]
- 51.Camp AJ, Wijesinghe R. Calretinin: modulator of neuronal excitability. Int J Biochem Cell Biol. 2009;41:2118–2121. doi: 10.1016/j.biocel.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 52.Spencer RF, Baker R. GABA and glycine as inhibitory neurotransmitters in the vestibulo-ocular reflex. Ann N Y Acad Sci. 1992;656:602–611. doi: 10.1111/j.1749-6632.1992.tb25239.x. [DOI] [PubMed] [Google Scholar]