Abstract
Sleep has been demonstrated to improve consolidation of many types of new memories. However, few prior studies have examined how sleep impacts learning of face-name associations. The recognition of a new face along with the associated name is an important human cognitive skill. Here we investigated whether post-presentation sleep impacts recognition memory of new face-name associations in healthy adults.
Fourteen participants were tested twice. Each time, they were presented 20 photos of faces with a corresponding name. Twelve hours later, they were shown each face twice, once with the correct and once with an incorrect name, and asked if each face-name combination was correct and to rate their confidence. In one condition the 12-hour interval between presentation and recall included an 8-hour nighttime sleep opportunity (“Sleep”), while in the other condition they remained awake (“Wake”).
There were more correct and highly confident correct responses when the interval between presentation and recall included a sleep opportunity, although improvement between the “Wake” and “Sleep” conditions was not related to duration of sleep or any sleep stage.
These data suggest that a nighttime sleep opportunity improves the ability to correctly recognize face-name associations. Further studies investigating the mechanism of this improvement are important, as this finding has implications for individuals with sleep disturbances and/or memory impairments.
Keywords: sleep, recognition memory, declarative memory, hippocampal-dependent memory
1. Introduction
Remembering the name of someone we have recently met is a task that is encountered regularly (Allison et al., 1994; Farah, 1996). However, remembering the correct name that is associated with a face is more difficult, especially when it has been learned recently. Findings from a functional magnetic resonance imaging (fMRI) study suggest that successful encoding of face-name pairs requires the coordination of neural activity in hippocampal, prefrontal, and parietal regions (Miller et al., 2008) and that a stronger connectivity between the hippocampal and posteromedial regions during rest predicts better performance on a face-name task (Wang et al., 2010)
While the benefit of sleep after learning is established [for review see (Ackermann & Rasch, 2014; Diekelmann, 2014; Genzel et al., 2014; Inostroza & Born, 2013; Prince & Abel, 2013; Stickgold & Walker, 2007; Tononi & Cirelli, 2014)] it has been suggested that sleep preferentially enhances the types of memory that rely on the hippocampus (Maquet, 2001; Marshall & Born, 2007), and that the positive effect of sleep on declarative memory stems from an active role of sleep in memory consolidation (Drosopoulos et al., 2007; Inostroza & Born, 2013; Schönauer et al., 2014; Tononi & Cirelli, 2014), rather than from reduced interference (Talamini et al., 2008).
The strength of sleep dependent memory consolidation can be mediated by the “macrostructure”, the amount of time spent in a specific sleep stage (Aeschbach et al., 2008; Gais et al., 2000; Huber et al., 2004; Wagner et al., 2007), as well as by the “microstructure”, which refers to sleep related mechanisms like sleep spindles and synaptic plasticity (Gais et al., 2002; Inostroza & Born, 2013; Schönauer et al., 2014; Tononi & Cirelli, 2014). Recent studies using hippocampal-dependent episodic associative memory-encoding tasks such as the contextual learning paradigm (van der Helm et al., 2011) and the face-name task (Mander et al., 2011) found that performance correlates positively with stage-2 NREM sleep from a nap. Those same studies have also found that fast spindles associate with successful encoding (Mander et al., 2014; Mander et al., 2011; van der Helm et al., 2011).
However, the effects of sleep macrostructure in associative recognition tasks are not consistent. Two studies using word-based recognition tasks found that sleep early in the night enhanced recognition memory (Drosopoulos et al., 2005), especially if that interval was rich in SWS (Daurat et al., 2007). In contrast, a nap study by Schönauer et al. (Schönauer et al., 2014) found that while subjects performed significantly better in the nap condition (two hours of sleep in the morning or afternoon) than in the wake condition in an associative recognition task for phone numbers and names, the time spent in specific sleep stages did not correlate with memory performance.
The duration of sleep required for memory enhancement has also been investigated. Enhancement of declarative memory has been observed after an 8-hour overnight sleep opportunity (Benson & Feinberg, 1977; Drosopoulos et al., 2007; Gais et al., 2007; Gais et al., 2006), as well as after the first half of nocturnal sleep (Barrett & Ekstrand, 1972; Fowler et al., 1973; Plihal & Born, 1997; Yaroush et al., 1971) and after shorter 1–2 hour naps in the afternoon (Gorfine et al., 2007; Tucker & Fishbein, 2008). However, the optimal amount of sleep needed to benefit memory is unclear, and comparing results from different studies is complicated by the fact that retention intervals (between presentation and recall) of equal length but containing different amounts of sleep will contain different amounts of wakefulness, which itself may influence memory. Two main ideas for how memory consolidation might be affected by the duration of sleep have been suggested: a minimum duration of sleep that improves memory in an “all-or-none” way, with longer sleep durations producing no additional enhancement; or a dose-dependent impact of sleep duration on memory consolidation, where more sleep produces a greater benefit (Diekelmann et al., 2009). In the case of recognition memory, there is evidence to support both hypotheses (Daurat et al., 2007; Drosopoulos et al., 2005; Hu et al., 2006; Wagner et al., 2007). Furthermore, one experiment even found that recognition performance was not better for a sleep group than for a sleep deprivation group, suggesting that the effect of sleep on recognition memory may be partially due to interactions between sleep and circadian rhythmicity (Nesca & Koulack, 1994).
These studies have demonstrated that sleep can improve associative recognition memory but do not answer the question as to whether sleep dependent memory consolidation for face-name pairs is mediated by the duration of sleep or the amount of time spent in a specific sleep stage during typical sleep at night. We designed the present study to explore the role of a full night of sleep on recognition memory for face-name associations. We aimed to determine whether sleep improves memory consolidation resulting in more correct face-name recognitions after sleep than after a similar interval of wakefulness, and to determine if there is an association between the duration of sleep or any sleep stage and improvement in the number of correct face-name recognitions (compared with the waking condition).
2. Materials and Methods
2.1 Participants and Screening. Main Study
Data were obtained from fourteen healthy participants (six male, eight female; age range 21–28 years; mean age = 23.33). Each participant underwent an extensive screening procedure to ensure they were free from any medical or psychological disorders. The evaluation included biochemical tests on blood and urine, an electrocardiogram, a physical examination, psychological questionnaires (Beck Depression Inventory, MMPI-2), and an interview with a clinical psychologist. Only those participants who reported a usual sleep duration of seven to nine hours, who were not night shift workers, who had not recently crossed time zones, and who denied significant sleep complaints (Buysse et al., 1989) or daytime sleepiness (Johns, 1991) were included. Prior to admission, participants kept a regular eight hour nightly sleep schedule for at least one week, documented this in a written sleep-wake diary, and wore a wrist activity monitor to verify compliance. These criteria were used to ensure that the participant was stably entrained to local environmental time and to their individual sleep-wake schedule, and that they were not chronically sleep deprived. Participants were asked to avoid alcohol, nicotine, caffeine, dietary supplements and drugs (prescription or non-prescription) before and during the study.
2.2 Ethics
The study procedures were approved by the Human Research Committee of Partners Health Care System and conducted in accordance with the Declaration of Helsinki. Before participation, every subject received a written description of the procedures and general purpose of the experiment and provided written informed consent.
2.3 Laboratory Conditions
The data reported here were collected during a 13-day inpatient circadian rhythm study during which participants were scheduled to sleep for eight hours at their habitual times on most nights. The studies took place at the Brigham and Women’s Hospital Center for Clinical Investigation, part of the Harvard Catalyst Clinical and Translational Science Center (CTSC). Throughout the study, each participant lived in a private room in an environment free of time cues, with sleep episodes and test sessions scheduled by the investigators. Other than scheduled tests, meals, electrode placement, and sleep, participants had free time during which they could pursue sedentary activities such as reading, listening to music, or hobbies.
2.4 The Face-Name Task
The Face-Name task (FNT) is a test for memory of the name associated with a face, and is based on the task of Sperling et al. (Sperling et al., 2001). In the present study, the task consisted of a presentation session followed by a recognition session 12 hours later. In the presentation session the participant was shown a series of 20 new face-name pairs on a computer screen, each presented one at a time on a black background for three seconds. Immediately after the presentation of the 20th face-name pair, each of the face-name pairs was presented on the screen again (in a random order) for three seconds. The participant was instructed to memorize the name associated with each face. For the recognition session, the participant was shown a face-name pair and asked “Is this x?”. After responding (yes/no), the participant was asked to rate their confidence in their response on a horizontal scale ranging from ‘Not Confident’ to ‘Confident’, with seven tic marks between those extremes (resulting in nine possible confidence choices). The response time for each of these questions was limited to four seconds. During the recognition session, each of the 20 faces was shown twice, once with the correct name and once with an incorrect name, resulting in 40 responses. Participants used a response box with two buttons to respond to yes/no questions and to move the cursor left or right to respond to the confidence scale. All participants were oriented to the presentation and recognition portions of the test on the first study day by the same investigator (JFD), and each participant took a training session of the test (both presentation and recognition) later on that first study day.
The Face-Name task program used a database of over 600 color photographs of adult neutral expression faces (Minear & Park, 2004), and once a photograph was used (in a practice session or in a test session) it was removed from the database for that participant so it would not be used again. The faces varied in age (18–94) and race/ethnicity, and individual testing sessions presented half male and half female faces. Names were obtained at random from a database of the 200 most common names relative to the sex and age of the person in the photograph.
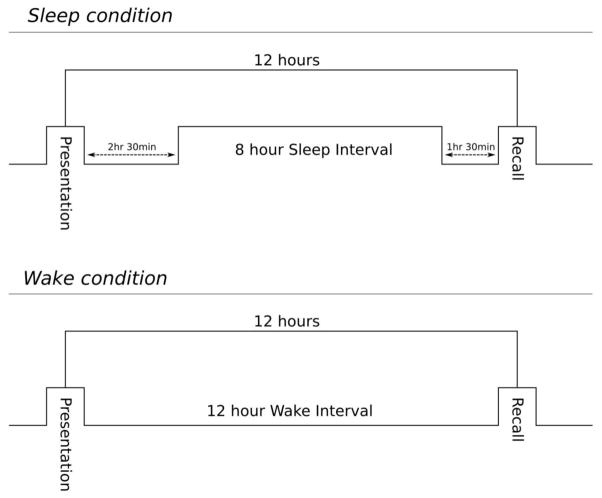
Each participant took the Face-Name task twice as part of a larger neurobehavioral test battery, with presentation and recognition 12 hours apart each time. In the “Sleep” condition, participants received the presentation in the evening, 2.5 hours before an eight hour sleep opportunity, with the recognition occurring 12 hours later the next morning (1.5 hours after scheduled wake time). In the “Wake” condition, they received the presentation in the morning, 1.5 hours after waking from an eight-hour sleep opportunity and had the recognition in the evening, 12 hours later during the same wake episode (see Figure 1). To account for potential order effects, counterbalancing was used, with half of the participants experiencing the Sleep condition first and half experiencing the Wake condition first.
Figure 1.
Schematic of test presentation and recall in the Sleep (upper panel) and Wake (lower panel) conditions. In the Sleep condition (upper), participants received the presentation 2.5 hours before an eight-hour sleep opportunity, with the recognition occurring 12 hours later (1.5 hours after scheduled wake time). In the Wake condition (lower), participants received the presentation 1.5 hours after waking from an 8-hour sleep opportunity, and had the recognition 12 hours later during the same wake episode.
2.5 Subjective Sleepiness
During the waking episodes, participants completed computer-administered sleepiness and mood assessments approximately twice per waking hour. The subjective sleepiness assessments were carried out using the Karolinska Sleepiness Scale (KSS) (Kaida et al., 2006). The task asks the participant to rate their subjective sleepiness on a numeric scale ranging from “Extremely Alert” to “Extremely Sleepy – Fighting Sleep”, with seven tic marks between those extremes (resulting in nine possible choices). The subjective assessments completed by the participants before the FNT presentation and recall sessions were used to examine whether subjective sleepiness influenced the ability to encode or recall the face-name information.
2.6 Polysomnographic Recordings
During selected sleep episodes, polysomnograms (F3-A2, F4-A1, C3-A2, C4-A1, O1-A2, O2-A1, EOG, EMG) were recorded using a Vitaport-3 digital sleep recorder. The electrodes were placed on the participant in the evening, and they got into bed about 20 minutes prior to scheduled lights out time so that the recording could be started. From the time of scheduled lights out until scheduled lights on eight hours later, the participant was required to remain in bed in the dark, attempting to sleep. If requested, a staff member entered the room briefly during the scheduled sleep episode to bring the participant a bedpan or urinal. Polysomnograms from the 6th and 9th sleep episode of the study were recorded and then scored manually in 30s epochs according to established criteria (Iber et al., 2007). Results of the analysis of the sleep structure are given in minutes or percentages.
2.7 Study 2
Data from an additional eight healthy participants (five male, three female; age range = 22–30 years; mean age = 24.75+/− 3.37) who took part in an earlier version of the same experiment, but whose FNT presentation and recognition sessions were scheduled differently, were also analyzed. These eight participants performed the face-name task four times. The “Sleep” condition was identical to that of the main study, with presentation and recognition 12h apart and an 8h sleep opportunity provided. The other three FNT occurred later in the study, and in some cases involved sleep deprivation, so only the first FNT which involved a Sleep condition are reported here.
2.8 Data Processing and Analysis
Responses on the Face-Name task were classified as correct or incorrect, and that result, along with the confidence rating (on a scale of 1– 9), and reaction time, were automatically collected by the program and stored in a database. Because the responses were yes/no responses and therefore subject to guessing, in addition to analyzing all correct responses we also separately analyzed only high confidence (HC) correct responses. HC answers were assumed to be those in which the participant knew the answer with no element of guessing. We defined the high confidence responses as those in which the participant rated their confidence between seven and nine on the nine-point scale.
Condition (Sleep vs. Wake) differences on the Face-Name task within participants were analyzed by paired two sided t-tests or by mixed model ANOVA. To explore the association between sleep measures and performance on the Face-Name task between participants, we used a Pearson correlation. Results are presented as mean ± standard deviation unless otherwise noted. We compared the amount of time spent in each sleep stage as well as total sleep time (TST) for the control night and the night between the FNT with paired t-tests. Normality of every data set was tested with the Shapiro-Wilk Test, and if the test failed the groups were compared using a Wilcoxon Signed Rank Test on that data set. We compared the ratings of the KSS in the morning with the ratings in the evening with a one-way repeated measures analysis of variance (RM ANOVA). Pairwise Multiple Comparison Procedures (Holm-Sidak method) were used to test which groups differed from each other.
3. Results
3.1 Main Study
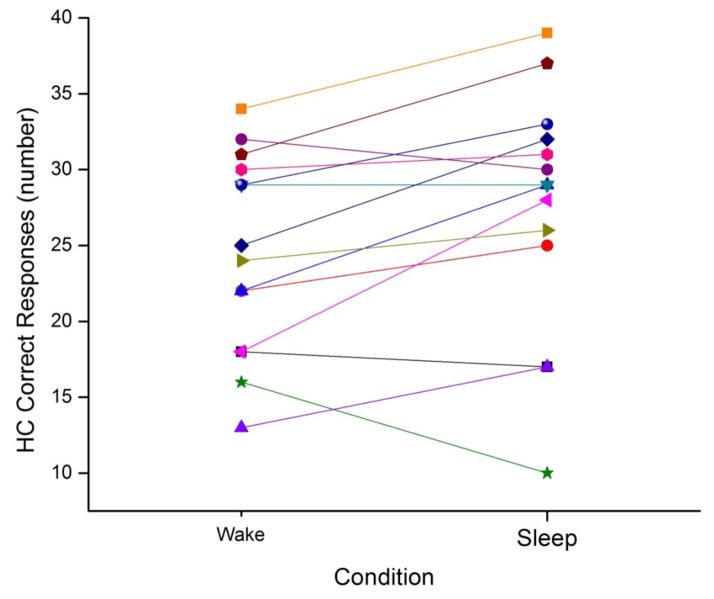
The average number of correct responses was significantly greater after a 12-hour interval between presentation and recognition that included sleep than after a similar interval that consisted of all wake, and this was true for all correct responses (Sleep=34.14 ± 3.34; Wake=31.57 ± 4.18; t(13) = 3.8, p=0.002) as well as for the subset of responses where the participant rated their response as highly confident (Sleep=27.36 ± 8.0; Wake=24.57 ± 6.44; t(13) = 2.48, p=0.028; see Table 1). An interval between presentation and recognition that included sleep resulted in about 2.9 more highly confident correct responses, an improvement of approximately 12% (see Figure 2). In addition to an increase in the number of highly confident correct responses, when the interval between presentation and recognition included sleep the proportion of incorrect responses decreased by about 30% (see Table 1).
Table 1.
| Correct Responses | Incorrect Responses | ||
|---|---|---|---|
| Condition | All | HC | All |
| Sleep | 34.1(3.4) | 27.4(8.0) | 5.9(3.4) |
| Wake | 31.6(4.2) | 24.5(6.6) | 8.4(4.2) |
| P-value | 0.002 | 0.025 | 0.002 |
Correct and incorrect responses on the FNT in the Sleep and Wake conditions. Correct responses (mean ± standard deviation) are separated into high confidence correct (HC) and all correct responses.
Figure 2.
Highly confident correct responses in the Sleep and Wake conditions. Each participant’s number of highly confident correct face-name recognitions for the Wake (left symbol) and Sleep (right symbol) conditions is presented, connected by a line so that the change between conditions can be visualized.
To account for possible order effects, we conducted a two-way repeated measures ANOVA on the highly confident correct responses with factors “condition” (sleep vs. wake), “order”, and an interaction “condition x order”. We found that there was a significant effect for the factor “condition” (F(1, 12) =6.43, p=0.026), but no significant effect for the factor “order” (F(1,12)=0.5, p=0.491), and no significant interaction between the factors “condition” and “order” (F(1,12)=1.03, p=0.331). Overall confidence ratings were not significantly different between the Sleep and Wake conditions (Sleep= 7.13 ± 0.93; Wake= 6.92 ± 0.89; p=0.172).
Reaction times of correct vs. incorrect responses were not normally distributed and therefore were compared using a Wilcoxon Signed Rank Test. Participants were significantly faster when their response was correct (1759.83 ± 235.11 msec) than when their response was incorrect (2162 ± 552.74 msec; p= 0.017), and their highly confident correct response times were significantly faster than all of their correct response times (1598.12 ± 226.85 msec; p=0.01). We also compared reaction times for responses when the face-name pairs were correctly matched (“correct associations”) with those where the face was presented with an incorrect name (“incorrect associations”), and those did not differ significantly (correct associations=1841.62 ± 259 msec; incorrect associations=1839.96 ± 251.02 msec; p=0.971). Overall reaction times between the Sleep (with recognition in the morning) and Wake (with recognition in the evening) conditions were not significantly different (Sleep=1828.19 ± 319.36 msec; Wake=1832.71 ± 205.28 msec; p=0.939). When we examined only the reaction times from the correct responses, those also did not differ significantly between the Sleep and Wake conditions (Sleep=1764.14 ± 313.53 msec; Wake=1755.52 ± 207.26 msec; p=0.898), and the reaction times between the Sleep and Wake conditions also did not differ in the subset of highly confident responses (Sleep=1621.73 ± 298.77 msec; Wake=1575.15 ± 214 msec; p=0.51).
Polysomnographic recordings showed an average sleep efficiency of 84.28% (range 59.75–96.26%) on the night of the FNT and 91.44% (range 80.85–98.13%) on the control night. Total sleep time was not significantly different on the two nights (FNT night=404.54 ± 65.88; Control night=437.57 ± 23.25), and there was no significant difference in the percentage of sleep stages between the two nights (see Table 2).
Table 2.
| FNT Night | Control Night | Comparison (min) | ||||
|---|---|---|---|---|---|---|
| Sleep Stage | Mean (min) | SD | Mean (min) | SD | t(13) | p |
| N1 | 13.2 | 7.4 | 17.7 | 6.00 | −2.03 | 0.06 |
| N2 | 179.6 | 41.2 | 199.7 | 20.20 | −2.04 | 0.06 |
| N3 | 104.9 | 25.0 | 98.5 | 19.50 | 1.02 | 0.33 |
| NREM | 297.8 | 50.1 | 315.9 | 20.80 | −1.35 | 0.20 |
| REM | 107.0 | 30.2 | 121.7 | 23.30 | −1.73 | 0.11 |
Descriptive statistics for sleep variables from the main study. The percentage and minutes of each sleep stage (N1–N3, REM) and of NREM sleep (mean ± standard deviation) for the Control night (when no FNT was given) and for the FNT night (when the presentation was given before sleep and the recognition occurred after wake) are provided. The difference (in minutes) between the two nights, along p values calculated using a two-tailed paired t-test, are also presented.
When we examined the relationship between improvement in overall correct responses (or improvement in highly confident correct responses) between the Wake and Sleep conditions for each subject, we found no significant correlation between overall improvement in correct responses and any sleep stage, and no significant correlation between improvement in highly confident correct responses and any sleep stage (see Table 3).
Table 3.
| Sleep Stage (minutes) | ||||||
|---|---|---|---|---|---|---|
| Correct responses | N1 | N2 | N3 | NREM | REM | TST |
| HC Improvement | ||||||
| r | 0.129 | −0.159 | −0.461 | −0.342 | −0.123 | −0.319 |
| p | 0.660 | 0.586 | 0.097 | 0.231 | 0.675 | 0.266 |
| Total Improvement (all) | ||||||
| r | 0.165 | −0.205 | 0.070 | −0.108 | 0.282 | 0.048 |
| p | 0.566 | 0.482 | 0.811 | 0.712 | 0.328 | 0.868 |
| HC Performance after Sleep | ||||||
| r | 0.356 | 0.212 | −0.731 | −0.138 | −0.336 | −0.263 |
| p | 0.212 | 0.466 | 0.003 | 0.637 | 0.241 | 0.363 |
| Total Performance after Sleep (all) | ||||||
| r | 0.386 | 0.310 | −0.724 | −0.050 | −0.421 | −0.236 |
| p | 0.172 | 0.281 | 0.003 | 0.864 | 0.133 | 0.416 |
Correlation matrix between duration of sleep stages and performance on the face-name task. Pearson correlation coefficients (r) and significance (p) for the association between duration (minutes) of sleep stages N1, N2, N3, REM, NREM (N1+N2+N3), and TST (total sleep time, REM+NREM), and performance on the Face-Name task are presented. HC improvement refers to the improvement in Face-Name test performance for participants between the Wake and Sleep conditions when only high confidence correct responses are considered. Total improvement refers to the improvement in Face-Name test performance for participants between the Wake and Sleep conditions when all correct responses are considered. HC performance after sleep and total performance after sleep refers to the number of high confidence correct responses (or all correct responses) in the Sleep condition between participants. Bold text indicates those correlations that were statistically significant.
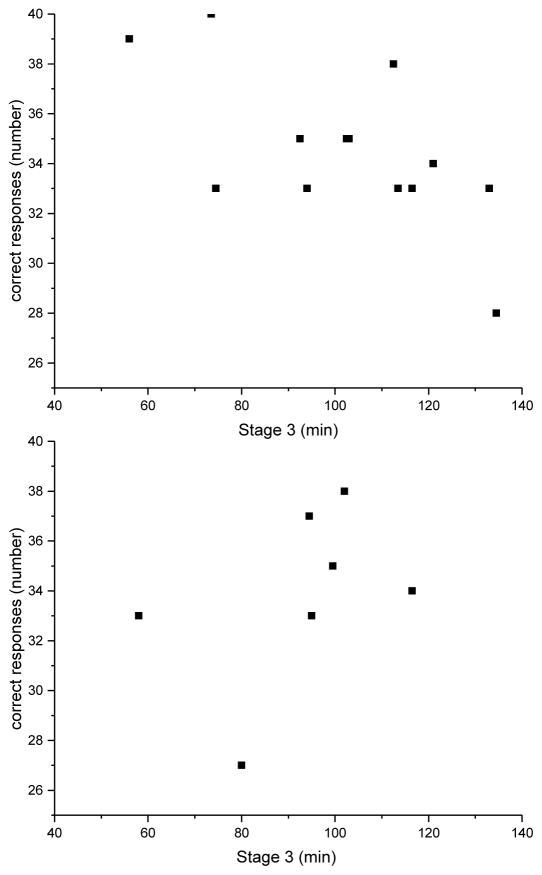
We also conducted a between-subject analysis of the relationship between number of correct responses (or highly confident correct responses) and duration of sleep stages. In this analysis, we found a significant negative correlation between the duration of N3 sleep and the number of correct responses (r=−0.72, p=0.003; see Figure 3, upper panel), and the number of highly confident correct responses (r=−0.73, p=0.003). To explore this further, we also carried out the same correlation analysis on data from Study 2. When we examined whether there was a correlation between correct recall on the FNT and sleep between subjects in Study 2, we did not find any significant association between sleep and correct recall for any stage of sleep, including N3 (r=0.02, p= 0.95; see Figure 3, lower panel). However, when we pooled the data from Study 2 with those from the main study (n=22), the correlation between correct recall and N3 remained significant (r= −0.43, p=0.05).
Figure 3.
Between-subjects association of SWS (N3) sleep duration and number of correct responses. Upper panel: Results from the twelve participants in the main study. Lower panel: Results from the eight participants in study two.
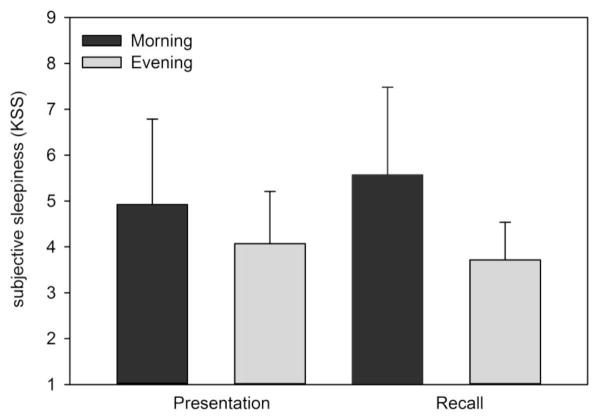
In our main study design, the time of day at which the presentation (and recognition) occurred in the two conditions was 12 hours apart. Given evidence from prior studies that there may be a circadian rhythm in memory performance (Folkard & Monk, 1980; Johnson et al., 1992; Wright Jr. et al., 2002; Wyatt et al., 1999), we conducted an additional analysis to explore whether time of day might have an impact on encoding, thereby contributing to our finding. First, we examined subjective sleepiness ratings from the KSS conducted before each FNT presentation and recognition to control for a possible impact of differences in subjective sleepiness between morning and evening FNT tests. We compared the KSS ratings between presentation sessions in the morning (the Wake condition) and presentation sessions in the evening (the Sleep condition) with a one way analysis of variance with repeated measures (RM ANOVA) and found no significant difference in subjective sleepiness (KSS before Morning presentation=4.93 ± 1.86 vs. KSS before Evening presentation=4.07 ± 1.14; F (1,13)=0.377, p=0.55). When we compared the subjective sleepiness between the recognition sessions in the evening (the Wake condition) and recognition sessions in the morning (the Sleep condition), we found that subjective sleepiness was significantly greater in the morning (KSS before Morning recall=5.57 ± 1.9) than in the evening (KSS before Evening recall=3.71 ± 0.8; F (1, 13)=13.73, p=0.003; see Figure 4).
Figure 4.
Subjective sleepiness estimates before presentation and recognition sessions of the FNT in the morning and evening. Each bar shows the mean (± SD) subjective sleepiness rating from the Karolinska Sleepiness Scale taken before morning (black) and evening (grey) presentation and recognition sessions of the FNT.
4. Discussion
The present study investigated the impact of post-learning sleep on recognition memory for face-name associations. In the main study we tested every subject twice, once with an eight-hour nocturnal opportunity to sleep in the 12-hour interval between presentation and recognition and once when the 12-hour interval between presentation and recognition occurred during the regular waking day. We found that recognition memory for face-name associations was enhanced when sleep occurred between presentation and recognition. The improvement in the number of correct responses in the sleep vs. wake condition was found for all correct responses as well as only for the subset of correct responses which the participants rated as highly confident.
The beneficial effect of sleep on face-name recognition memory is unlikely to be due to time-of-day effects on encoding of new associations, although we cannot rule out that possibility. We examined subjective sleepiness data to determine whether differences in sleepiness may have contributed to better encoding in the evening (sleep condition) vs. the morning (wake condition) and found no significant difference in subjective sleepiness between the two conditions. Mander and colleagues examined the role of subjective sleepiness on encoding of face-name pairs and found that while sleepiness was greater during evening encoding than during morning encoding sessions, subjective sleepiness was not associated with successful encoding, hippocampal activation during encoding, or fast sleep spindles after encoding (Mander et al., 2014). We found difference in subjective sleepiness between the morning and evening recognition sessions, although the participants rated themselves as significantly sleepier in the morning recognition sessions (sleep condition) than in the evening recognition sessions (wake condition), suggesting that this could not have contributed to the better FNT recognition we observed in the morning. Thus, our data do not support the idea that circadian rhythm effects contribute to the differences we observed between the sleep and wake conditions (Koulack, 1997; Nesca & Koulack, 1994), consistent with the findings of Talamini and colleagues (Talamini et al., 2008), although we did not design our study to test that possibility explicitly.
We also found that reaction time and confidence ratings did not differ between the sleep and wake conditions, suggesting that those response features did not contribute to the difference in correct responses between conditions. While we found that an overnight interval that included sleep was associated with an improvement in the number of correct responses, this sleep-dependent improvement did not appear to be related to sleep duration or duration of any sleep stages. Our finding that sleep is associated with improvement on this recognition memory task is consistent with prior studies indicating a critical function of sleep for the consolidation of associations (Drosopoulos et al., 2005; Schönauer et al., 2014).
Our finding in the main study that the number of correct responses was negatively associated with the time spent in slow wave (N3) sleep was found between subjects, and thus is difficult to interpret. In order to better understand this finding, we extended our analysis and examined sleep data from another night in each of the same participants. Depending on the group (wake condition first vs. sleep condition first), this additional night of PSG was either three days before or after the test night. We did not find any significant differences in sleep between the two nights, suggesting that the difference in duration of N3 between participants was a characteristic of the individuals’ normal sleep. This further suggests that the negative correlation between N3 and correct recognition after sleep that we observed between subjects may be due to chance rather than to a direct impact of N3 sleep on recall, as postulated by Schönauer et al. (2014). Further supporting this interpretation, we examined data from eight additional participants whose study began in an identical way to that of main study participants in the sleep condition. When we examined whether there was a correlation between correct recognition on the FNT and N3 sleep (or any other stage), we did not find any significant association between sleep architecture and correct recognition between the participants in Study 2. Genzel et al. (2014) have proposed that light sleep is of greater importance than slow wave sleep to memory consolidation. While we did not find an association between N1 or N2 sleep and memory and found a negative association between N3 sleep and memory (which may be an artifact), our results are more consistent with Genzel’s interpretation that light sleep is important in consolidating some types of memory, rather than the idea that slow wave sleep or REM sleep are most important. Additional studies of the face-name task or other tests of recognition memory in which the same individuals are studied in both sleep and wake conditions multiple times will be needed to interpret this finding from our study.
The face-name task has been used extensively to study memory and neural degeneration in older adults, although typically with a shorter presentation-recall interval than the 12 hours used in our study. Our finding in young adults that sleep has an impact on the number of correctly recognized face-name pairs thus has potential implications for age-related memory changes. A recent study by Mander and colleagues (2014) found that encoding ability on the face-name task was associated with pre-frontal sleep spindles, and that older adults showed both reduced spindle activity at night and a corresponding reduced hippocampal activation during encoding on the face-name task the following day. This raises the possibility that treatments or interventions that improve sleep in older adults may improve memory functions, and that diagnosis and treatment of sleep problems and sleep disorders in older adults may be an important step in reducing memory deficits (Cooke et al., 2009; Mander et al., 2013; Yaffe et al., 2011). Given the role of sleep in many different aspects of memory, future studies should explore the contribution of age-related changes in sleep to age-related changes in memory.
While our findings contribute to evidence supporting an effect for sleep on recognition memory, they leave open the question of what aspect of sleep produces the improvement. There have been different hypotheses regarding how sleep improves declarative memories. In one, sleep is proposed to enable the ideal circumstances for the consolidation of the memory by eliminating interference of the memory consolidation by new sensory information and experiences that typically occur during wake (Jenkins & Dallenbach, 1924). However, several recent studies have concluded that reduced interference is insufficient to explain the role of sleep on memory (Schönauer et al., 2014; Talamini et al., 2008). Schönauer and colleagues (2014) compared declarative memory performance between sleep, wake (external interference), and meditation (which the authors hypothesize reduced both external and internal interference), and found that only sleep but not the reduced interference provided by meditation, improved declarative memory. Another hypothesis proposes a more active role of sleep, whereby some unique activity that occurs during sleep, such as slow waves, directly contributes to the memory consolidation (Barrett & Ekstrand, 1972). In our study, we did not find that the macrostructure of sleep was significantly associated with improvement on the task. However, we did not conduct a more quantitative analysis of the sleep EEG, and thus could not determine whether other features of sleep (such as spindle activity or density, slow wave activity, or fragmentation) were associated with memory improvement.
In conclusion, we found that an 8-hour overnight sleep opportunity improved recognition of face-name pairs learned 12 hours earlier. While the duration of N3 sleep was negatively correlated with performance on the task between individuals, our study design did not allow us to test whether the duration of sleep or the duration of sleep stages was associated with performance within individuals. Furthermore, we were unable to examine the EEG in detail in this study, and thus could not determine whether sleep features such as sleep spindles, slow wave activity, or other aspects of sleep were associated with performance on the face-name task. Future studies in which participants are given the face-name test multiple times with a night of sleep between presentation and recall should be carried out in order to determine whether there are any aspects of sleep.
Highlights.
We tested whether sleep influences the accuracy of remembering face-name associations
Presentation and recall were 12h apart, one time with 8h sleep and once without
More correct face-name pairs were recalled when there was a sleep opportunity
Sleep duration or sleep stage was not associated with improvement between conditions
Acknowledgments
We wish to thank the study participants; Ms. K. Ward for assistance with subject recruitment and data processing; the staff of the BWH CCI for assistance with carrying out the studies; Drs. R.A. Sperling, S.W. Cain, and M. Pomplun for advice and assistance in developing and implementing the face-name task in our laboratory. Many of the photographs in the Face-Name Task were used with permission from the Center for Vital Longevity Face Database at the University of Michigan. Support for the development of the Face-Name task and analysis was provided by NIH grants P01 AG09975 and R01 AG044416. KMZ is supported by the Finnish Cultural Foundation, the Emil Aaltonen Foundation, The Gyllenberg Foundation, and the Finnish Work Environment Fund; LM was supported in part by the Department of Psychology at the University of Konstanz. The studies were supported by NIH grant R01 HL094654, and were carried out in the Intensive Physiological Monitoring Unit of the Brigham and Women’s Hospital Center for Clinical Investigation, part of Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award UL1 TR0001102 and financial contributions from Harvard University and its affiliated academic health care centers).
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, or the National Institutes of Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Leonie Maurer, Email: leonie.maurer@gmx.de.
Kirsi-Marja Zitting, Email: kzitting@partners.org.
Kieran Elliott, Email: elliottkj1@gmail.com.
Charles A. Czeisler, Email: cacadmin@partners.org.
Joseph M. Ronda, Email: jronda@research.bwh.harvard.edu.
Jeanne F. Duffy, Email: jduffy@research.bwh.harvard.edu.
References
- Ackermann S, Rasch B. Differential effects of non-REM and REM sleep on memory consolidation? Curr Neurol Neurosci Rep. 2014;14:430. doi: 10.1007/s11910-013-0430-8. [DOI] [PubMed] [Google Scholar]
- Aeschbach D, Cutler AJ, Ronda JM. A role for non-rapid-eye-movement sleep homeostasis in perceptual learning. J Neurosci. 2008;28:2766–2772. doi: 10.1523/JNEUROSCI.5548-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison T, Ginter H, McCarthy G, Nobre AC, Puce A, Luby M, Spencer DD. Face recognition in human extrastriate cortex. J Neurophysiol. 1994;71:821–825. doi: 10.1152/jn.1994.71.2.821. [DOI] [PubMed] [Google Scholar]
- Barrett TR, Ekstrand BR. Effect of sleep on memory: III. Controlling for time-of-day effects. J Exp Psychol. 1972;96:321–327. doi: 10.1037/h0033625. [DOI] [PubMed] [Google Scholar]
- Benson K, Feinberg I. The beneficial effect of sleep in an extended Jenkins and Dallenbach paradigm. Psychophysiol. 1977;14:375–384. doi: 10.1111/j.1469-8986.1977.tb02967.x. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Cooke JR, Ayalon L, Palmer BW, Loredo JS, Corey-Bloom J, Natarajan L, Liu L, Ancoli-Israel S. Sustained use of CPAP slows deterioration of cognition, sleep, and mood in patients with Alzheimer’s disease and obstructive sleep apnea: A preliminary study. J Clin Sleep Med. 2009;5:305–309. [PMC free article] [PubMed] [Google Scholar]
- Daurat A, Terrier P, Foret J, Tiberge M. Slow wave sleep and recollection in recognition memory. Conscious Cogn. 2007;16:445–455. doi: 10.1016/j.concog.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Diekelmann S. Sleep for cognitive enhancement. Front Syst Neurosci. 2014;8:46. doi: 10.3389/fnsys.2014.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekelmann S, Wilhelm I, Born J. The whats and whens of sleep-dependent memory consolidation. Sleep Med Rev. 2009;13:309–321. doi: 10.1016/j.smrv.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Drosopoulos S, Schulze C, Fischer S, Born J. Sleep’s function in the spontaneous recovery and consolidation of memories. J Exp Psychol. 2007;136:169–183. doi: 10.1037/0096-3445.136.2.169. [DOI] [PubMed] [Google Scholar]
- Drosopoulos S, Wagner U, Born J. Sleep enhances explicit recollection in recognition memory. Learn Mem. 2005;12:44–51. doi: 10.1101/lm.83805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farah MJ. Is face recognition ‘special’? Evidence from neuropsychology. Behav Brain Res. 1996;76:181–189. doi: 10.1016/0166-4328(95)00198-0. [DOI] [PubMed] [Google Scholar]
- Folkard S, Monk TH. Circadian rhythms in human memory. Br J Psychol. 1980;71:295–307. [Google Scholar]
- Fowler MJ, Sullivan MJ, Ekstrand BR. Sleep and memory. Science. 1973;179:302–304. doi: 10.1126/science.179.4070.302. [DOI] [PubMed] [Google Scholar]
- Gais S, Albouy G, Boly M, Dang-Vu TT, Darsaud A, Desseilles M, Rauchs G, Sterpenich V, Vandewalle G, Maguet P, et al. Sleep transforms the cerebral trace of declarative memories. Proc Natl Acad Sci USA. 2007;104:18778–18783. doi: 10.1073/pnas.0705454104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gais S, Lucas B, Born J. Sleep after learning aids memory recall. Learn Mem. 2006;13:259–262. doi: 10.1101/lm.132106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gais S, Mölle M, Helms K, Born J. Learning-dependent increases in sleep spindle density. J Neurosci. 2002;22:6830–6834. doi: 10.1523/JNEUROSCI.22-15-06830.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gais S, Plihal W, Wagner U, Born J. Early sleep triggers memory for early visual discrimination skills. Nat Neurosci. 2000;3:1335–1339. doi: 10.1038/81881. [DOI] [PubMed] [Google Scholar]
- Genzel L, Kroes MC, Dresler M, Battaglia FP. Light sleep versus slow wave sleep in memory consolidation: A question of global versus local processes? Trends Neurosci. 2014;37:10–19. doi: 10.1016/j.tins.2013.10.002. [DOI] [PubMed] [Google Scholar]
- Gorfine T, Yeshurun Y, Zisapel N. Nap and melatonin-induced changes in hippocampal activation and their role in verbal memory consolidation. J Pineal Res. 2007;43:336–342. doi: 10.1111/j.1600-079X.2007.00482.x. [DOI] [PubMed] [Google Scholar]
- Hu P, Stylos-Allan M, Walker MP. Sleep facilitates consolidation of emotional declarative memory. Psychol Sci. 2006;17:891–898. doi: 10.1111/j.1467-9280.2006.01799.x. [DOI] [PubMed] [Google Scholar]
- Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430:78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- Iber C, Ancoli-Israel S, Chesson A, Quan SF. The AASM manual for the scoring of sleep and associated events: Rules, terminology and technical specifications. American Academy of Sleep Medicine; Westchester, Illinois: 2007. [Google Scholar]
- Inostroza M, Born J. Sleep for preserving and transforming episodic memory. Annu Rev Neurosci. 2013;36:79–102. doi: 10.1146/annurev-neuro-062012-170429. [DOI] [PubMed] [Google Scholar]
- Jenkins JG, Dallenbach KM. Obliviscence during sleep and waking. Am J Psychol. 1924;35:605–612. [Google Scholar]
- Johns MW. A new method for measuring daytime sleepiness: The Epworth Sleepiness Scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- Johnson MP, Duffy JF, Dijk DJ, Ronda JM, Dyal CM, Czeisler CA. Short-term memory, alertness and performance: A reappraisal of their relationship to body temperature. J Sleep Res. 1992;1:24–29. doi: 10.1111/j.1365-2869.1992.tb00004.x. [DOI] [PubMed] [Google Scholar]
- Kaida K, Takahashi M, Akerstedt T, Nakata A, Otsuka Y, Haratani T, Fukasawa K. Validation of the Karolinska sleepiness scale against performance and EEG variables. Clin Neurophysiol. 2006;117:1574–1581. doi: 10.1016/j.clinph.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Koulack D. Recognition memory, circadian rhythms, and sleep. Percept Mot Skills. 1997;85:99–104. doi: 10.2466/pms.1997.85.1.99. [DOI] [PubMed] [Google Scholar]
- Mander BA, Rao V, Lu B, Saletin JM, Ancoli-Israel S, Jagust WJ, Walker MP. Impaired prefrontal sleep spindle regulation of hippocampal-dependent learning in older adults. Cereb Cortex. 2014;24:3301–3309. doi: 10.1093/cercor/bht188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mander BA, Rao V, Lu B, Saletin JM, Lindquist JR, Ancoli-Israel S, Jagust W, Walker MP. Prefrontal atrophy, disrupted NREM slow waves and impaired hippocampal-dependent memory in aging. Nat Neurosci. 2013;16:357–364. doi: 10.1038/nn.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mander BA, Santhanam S, Saletin JM, Walker MP. Wake deterioration and sleep restoration of human learning. Curr Biol. 2011;21:R183–184. doi: 10.1016/j.cub.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maquet P. The role of sleep in learning and memory. Science. 2001;294:1048–1052. doi: 10.1126/science.1062856. [DOI] [PubMed] [Google Scholar]
- Marshall L, Born J. The contribution of sleep to hippocampus-dependent memory consolidation. Trends Cogn Sci. 2007;11:442–450. doi: 10.1016/j.tics.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Miller SL, Celone K, DePeau K, Diamond E, Dickerson BC, Rentz D, Pihlajamäki M, Sperling RA. Age-related memory impairment associated with loss of parietal deactivation but preserved hippocampal activation. Proc Natl Acad Sci USA. 2008;105:2181–2186. doi: 10.1073/pnas.0706818105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minear M, Park DC. A lifespan database of adult facial stimuli. Behav Res Methods Instrum Comput. 2004;36:630–633. doi: 10.3758/bf03206543. [DOI] [PubMed] [Google Scholar]
- Nesca M, Koulack D. Recognition memory, sleep and circadian rhythms. Can J Expl Psychol. 1994;48:359–379. doi: 10.1037/1196-1961.48.3.359. [DOI] [PubMed] [Google Scholar]
- Plihal W, Born J. Effects of early and late nocturnal sleep on declarative and procedural memory. J Cog Neurosci. 1997;9:534–547. doi: 10.1162/jocn.1997.9.4.534. [DOI] [PubMed] [Google Scholar]
- Prince TM, Abel T. The impact of sleep loss on hippocampal function. Learn Mem. 2013;20:558–569. doi: 10.1101/lm.031674.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönauer M, Pawlizki A, Köck C, Gais S. Exploring the effect of sleep and reduced interference on different forms of declarative memory. Sleep. 2014;37:1995–2007. doi: 10.5665/sleep.4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Bates JF, Cocchiarella AJ, Schacter DL, Rosen BR, Albert MS. Encoding novel face-name associations: A functional MRI study. Hum Brain Mapp. 2001;14:129–139. doi: 10.1002/hbm.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickgold R, Walker MP. Sleep-dependent memory consolidation and reconsolidation. Sleep Med. 2007;8:331–343. doi: 10.1016/j.sleep.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talamini LM, Nieuwenhuis IL, Takashima A, Jensen O. Sleep directly following learning benefits consolidation of spatial associative memory. Learn Mem. 2008;15:233–237. doi: 10.1101/lm.771608. [DOI] [PubMed] [Google Scholar]
- Tononi G, Cirelli C. Sleep and the price of plasticity: From synaptic and cellular homeostasis to memory consolidation and integration. Neuron. 2014;81:12–34. doi: 10.1016/j.neuron.2013.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker MA, Fishbein W. Enhancement of declarative memory performance following a daytime nap is contingent on strength of initial task acquisition. Sleep. 2008;31:197–203. doi: 10.1093/sleep/31.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Helm E, Gujar N, Nishida M, Walker MP. Sleep-dependent facilitation of episodic memory details. PLoS One. 2011;6:e27421. doi: 10.1371/journal.pone.0027421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner U, Kashyap N, Diekelmann S, Born J. The impact of post-learning sleep vs. wakefulness on recognition memory for faces with different facial expressions. Neurobiol Learn Mem. 2007;87:679–687. doi: 10.1016/j.nlm.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Wang L, Laviolette P, O’Keefe K, Putcha D, Bakkour A, Van Dijk KR, Pihlajamäki M, Dickerson BC, Sperling RA. Intrinsic connectivity between the hippocampus and posteromedial cortex predicts memory performance in cognitively intact older individuals. Neuroimage. 2010;51:910–917. doi: 10.1016/j.neuroimage.2010.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright KP, Jr, Hull JT, Czeisler CA. Relationship between alertness, performance, and body temperature in humans. Am J Physiol Regul Integr Comp Physiol. 2002;283:R1370–R1377. doi: 10.1152/ajpregu.00205.2002. [DOI] [PubMed] [Google Scholar]
- Wyatt JK, Ritz-De Cecco A, Czeisler CA, Dijk DJ. Circadian temperature and melatonin rhythms, sleep, and neurobehavioral function in humans living on a 20-h day. Am J Physiol Regul Integr Comp Physiol. 1999;277:R1152–R1163. doi: 10.1152/ajpregu.1999.277.4.r1152. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Laffan AM, Harrison SL, Redline S, Spira AP, Ensrud KE, Ancoli-Israel S, Stone KL. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011;306:613–619. doi: 10.1001/jama.2011.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaroush R, Sullivan MJ, Ekstrand BR. Effect of sleep on memory. II: Differential effect of the first and second half of the night. J Exp Psychol. 1971;88:361–366. doi: 10.1037/h0030914. [DOI] [PubMed] [Google Scholar]