Abstract
Fenofibrate belongs to hypolipidemic fibrates that act as activators of the peroxisome proliferator-activated receptor-α, a regulator of bile acid synthesis, metabolism and transport. The present study aimed at evaluating the effects of fenofibrate on the circulating bile acid profile in humans. Hundred healthy men and women completed a 3-week intervention with fenofibrate, and 17 bile acid species were measured in serum samples drawn before and after fenofibrate treatment. Fenofibrate caused significant reductions in levels of chenodeoxycholic (−26.4%), ursodeoxycholic (−30.5%), lithocholic (−18.4%), deoxycholic (−22.3%) and hyodeoxycholic (−19.2%) acids. A gender-related difference was observed in the response of various bile acids and the total bile acid concentration was significantly reduced only in men (−18.6%), while remaining almost unchanged in women (+0.36%). This difference detected suggests that fenofibrate should be more efficient at reducing bile acid toxicity in men than in women in cholestatic liver diseases.
Keywords: Non-cholestatic donors, fenofibrate, human serum, liquid chromatography, mass spectrometry
INTRODUCTION
Bile acids are natural detergents involved in cholesterol homeostasis. These acids are formed from cholesterol in the liver and their synthesis represents an important pathway for cholesterol elimination from the body (1). The bile acid biosynthetic pathway requires a number of enzymatic modifications of the cholesterol backbone, followed by β-oxidation of the cholesterol side chain (1). In humans, the bile acid concentration is mainly represented by the primary cholic (CA) and chenodeoxycholic acids (CDCA) (reviewed in Monte et al. (2)). These acids are conjugated with taurine and glycine to form amidated detergents. After their delivery into the intestine, primary bile acids are subjected to dehydroxylation achieved by the bacterial 7α-dehydroxylase from the gastrointestinal tract. This reaction converts CDCA and CA into the secondary lithocholic (LCA) and deoxycholic (DCA) acids, which are highly hydrophobic. About 95% of bile acids are absorbed in the intestine and return to the liver through the portal vein. Bile acids reabsorbed in the intestine can then be further modified back in the liver (2). These modifications involve reconjugation with taurine and glycine, or the conversion of CDCA and LCA to the 6α-hydroxylated acids hyocholic (HCA) and hyodeoxycholic acids (HDCA), respectively (3, 4).
Beyond their major physiological roles in cholesterol homeostasis, their detergent properties render bile acids cytotoxic at high concentrations (5). Alteration in bile acid synthesis, biliary transport and/or metabolism can cause their accumulation in liver cells, thereby leading to oxidative stress, apoptosis and subsequent damage to the liver parenchyma (2). Such features are characteristic of cholestatic phenomena, where a reduction of the bile flow impairs bile acid elimination from hepatocytes (6). Such an accumulation of toxic acids is particularly characteristic of chronic cholestatic liver diseases such as primary biliary cirrhosis (PBC) or primary sclerosing cholangitis (PSC), two autoimmune inflammatory diseases (2). Thus, a reduction in bile acid hepatic levels is an important goal of anti-cholestatic strategies (7).
Fenofibrate belongs to the fibrate group of hypolipidemic agents used for the treatment of hypertriglyceridemia and combined hyperlipidemia (8). These compounds activate the peroxisome proliferator-activated receptor-α (PPARα), a member of the nuclear receptor superfamily. Upon ligand activation, PPARα binds as a heterodimer with the retinoid X receptor (RXR) to a peroxisome proliferator response element (PPRE) located in the promoter region of target genes. PPARα regulates the expression of various genes crucial for triglyceride/cholesterol metabolism and gluconeogenesis (9).
Fibrates exert beneficial effects on liver biochemistry in PBC patients (10–14). These benefits have been primarily associated to the lipoprotein-lowering and antiinflammatory properties of PPARα activators (10, 14). However, PPARα is also an important regulator of genes encoding bile acid-synthesizing and -metabolizing enzymes, such as the cytochrome P450s (CYP)7A1, CYP8B1 and CYP27, UDPglucuronosyltransferase 2B4 (UGT2B4) and sulfotransferase 2A1 (SULT2A1) (6, 8, 15, 16). Various PPARα activators also modulate hepatic and/or intestinal transporters involved in bile acid uptake and/or excretion, such as the Na+-taurocholate cotransporting polypeptide (NTCP, SLC10A1), the organic anion-transporting polypeptide 1B (OATP1B, SLCO1B1), the bile salt export pump (BSEP, ABCB11), the apical sodium-dependent bile acid transporter (ASBT, SLC10A2) and the organic solute transporters-α and - β (OSTα/β) (reviewed in (17)). Overall, these observations suggest that pharmacological activators of PPARα such as fenofibrate may also modulate bile acid homeostasis in humans. Therefore, the present study aimed at testing this hypothesis by examining the profile of circulating bile acids in pre- and post-treatment sera from 200 non-cholestatic volunteers treated with fenofibrate for 3 weeks.
RESULTS
Analytical method
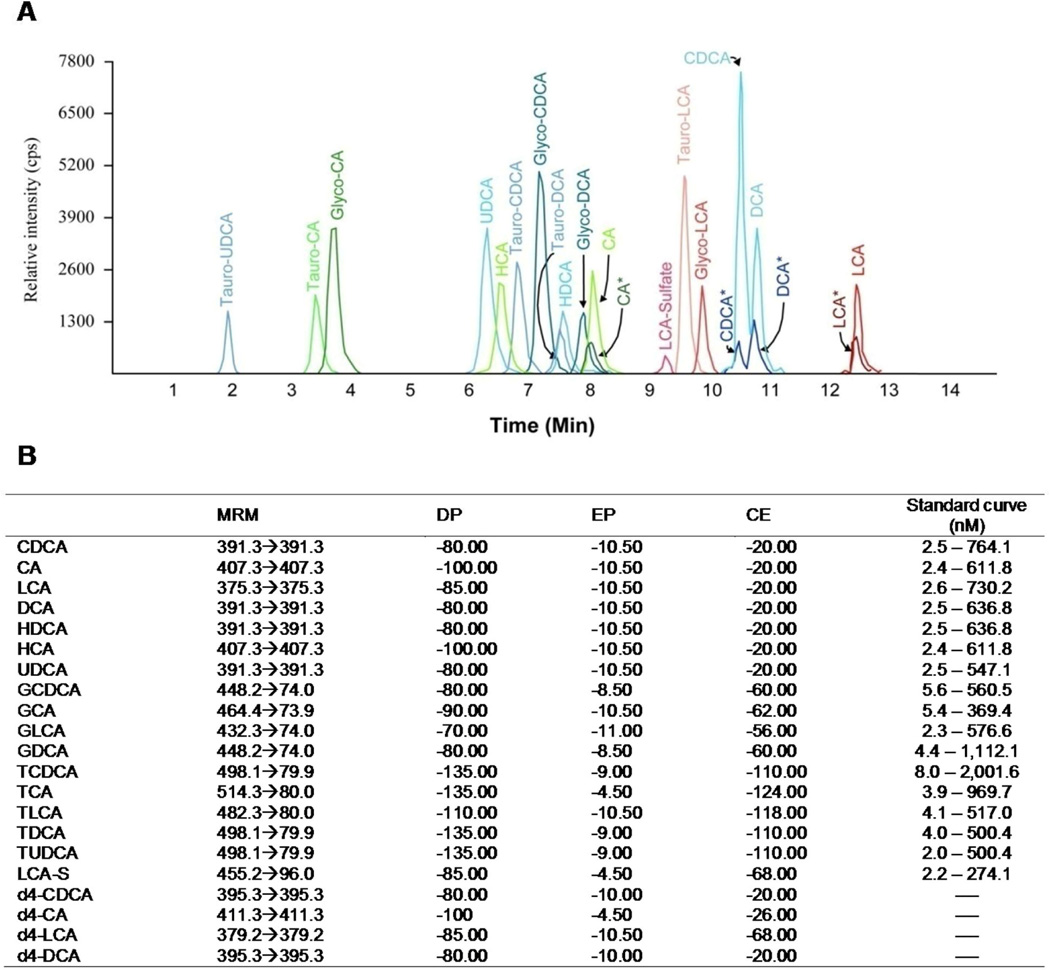
The liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) method allowed the simultaneous determination of 17 bile acid species in a single serum sample. The analytical run required only 15 min (Figure 1A), and the lower limit of detection varied from 2.2 (LCA-S) to 8.0 nM (TCDCA) (Figure 1B).
Figure 1. Chromatogram of the 17 bile acids analyzed (A) and parameters for LC-MS/MS quantification (B).
MRM: representing ion transitions as precursor ion [M-H]− → product ion [M-H]−; DP: declustering potential; EP: entrance potential; CE: collision energy.
Baseline profile of serum bile acids
The baseline composition of bile acids is illustrated in Table 3. The serum bile acids mainly consisted of conjugated and unconjugated primary acids (62%), while secondary acid species were only half as abundant (30%) and the 6α-hydroxylated acids HDCA and HCA, together represented only 2.3% of total bile acids. Unconjugated bile acids (1009.1 ± 74.3 nM) and glycine conjugates (1238.1 ± 84.4 nM) represented 40 and 49% of the species, respectively. Taurine-conjugated acids (266.7 ± 25.7 nM) were less abundant (10%). When considering each species, the serum bile acid composition was: GCDCA>>DCA>GDCA≥CDCA>GCA≥CA>UDCA=TCA. All other species were present at concentrations < 100 nM (Table 2).
Table 3.
Gender differences in serum bile acid profile before fenofibrate treatment.
| Baseline | ||
|---|---|---|
| Bile acids | Male | Female |
| CDCA | 314.6 ± 49.4 | 199.3 ± 31.5b |
| TCDCA | 108.6 ± 14.8 | 77.1 ± 9.2 |
| GCDCA | 805.3 ± 80.7 | 686.5 ± 72.3 |
| CA | 217.4 ± 42.1 | 135.0 ± 33.9b |
| TCA | 140.2 ± 28.6 | 87.1 ± 13.1 |
| GCA | 221.2 ± 26.0 | 175.5 ± 21.5 |
| UDCA | 108.9 ± 12.0 | 103.5 ± 10.3 |
| TUDCA | 6.6 ± 2.2 | 5.5 ± 0.9 |
| LCA | 18.3 ± 1.6 | 16.9 ± 2.0 |
| TLCA | 19.4 ± 1.6 | 18.7 ± 1.6 |
| GLCA | 19.3 ± 2.9 | 17.4 ± 2.0 |
| LCA-S | 7.3 ± 0.9 | 6.7 ± 0.9 |
| DCA | 414.6 ± 32.5 | 371.6 ± 37.0 |
| TDCA | 35.2 ± 3.9 | 35.0 ± 5.1 |
| GDCA | 291.2 ± 28.3 | 259.7 ± 26.4 |
| HDCA | 53.5 ± 3.4 | 53.5 ± 4.9 |
| HCA | 6.2 ± 0.6 | 4.9 ± 0.8b |
| Total CDCA | 1,228.5 ± 122.3 | 962.9 ± 90.8 |
| Total CA | 578.8 ± 65.8 | 397.7 ± 45.5a |
| Total DCA | 741.0 ± 57.3 | 666.3 ± 57.2 |
| Total LCA | 64.2 ± 4.9 | 59.8 ± 4.2 |
| Total primary | 1,807.3 ± 178.6 | 1,360.6 ± 123.3a |
| Total secondary | 805.2 ± 60.5 | 726.2 ± 60.0 |
| Total 6α-hydroxy | 59.7 ± 3.5 | 58.3 ± 5.0 |
| Total free | 1,133.4 ± 117.1 | 884.7 ± 90.5a |
| Total glyco | 1,337.0 ± 129.1 | 1,139.1 ± 108.6 |
| Total tauro | 309.9 ± 44.9 | 223.5 ± 24.4 |
| TOTAL | 2,787.7 ± 231.1 | 2,254.0 ± 163.6 |
Bile acid concentrations are expressed in nM (mean ± SEM). Statistically significant differences in bile acid concentrations and in the response to fenofibrate treatment between men and women were determined by the Wilcoxon/Mann-Whitney rank-sum test: a, p<0.05; b, p<0.01; c, p<0.001.
CA, cholic acid; CDCA, chenodeoxycholic acid; DCA, deoxycholic acid; GCDCA, glycochenodeoxycholic acid; GCA, glycocholic acid; GDCA, glycodeoxycholic acid; GLCA, glycolithocholic acid; HCA, hyocholic acid; HDCA, hyodeoxycholic acid; LCA, lithocholic acid; LCA-S, LCA-sulfate; TCDCA, taurochenodeoxycholic acid; TCA, taurocholic acid; TDCA, taurodeoxycholic acid; TLCA, taurolithocholic acid; TUDCA, tauroursodeoxycholic acid; UDCA, ursodeoxycholic acid; TOTAL, sum of all bile acids.
Table 2.
Changes in serum bile acid profiles after a 3-week treatment period with fenofibrate.
| Before fenofibrate | After fenofibrate | |||||||
|---|---|---|---|---|---|---|---|---|
| Bile acids | Mean | SEM | 10th | 90th | Mean | SEM | 10th | 90th |
| CDCA | 256.9 | 29.5 | 16.4 | 610.9 | 189.2c | 20.8 | 14.2 | 570.1 |
| TCDCA | 92.9 | 8.8 | 12.7 | 268.5 | 103.1 | 9.5 | 15.8 | 242.3 |
| GCDCA | 745.9 | 54.2 | 169.8 | 1,694.2 | 703.8 | 46.4 | 130.5 | 1,656.3 |
| CA | 176.2 | 27.1 | 2.6 | 403.2 | 159.7 | 23.0 | 2.4 | 389.7 |
| TCA | 113.7 | 15.8 | 8.1 | 269.5 | 112.0 | 16.1 | 12.4 | 203.3 |
| GCA | 198.4 | 16.9 | 29.8 | 461.5 | 227.3 | 18.5 | 39.2 | 531.2 |
| UDCA | 106.2 | 7.9 | 13.6 | 269.1 | 73.9c | 5.0 | 10.2 | 166.8 |
| TUDCA | 6.1 | 1.2 | 0.00 | 13.3 | 9.2c | 2.6 | BLQ | 15.1 |
| LCA | 17.6 | 1.3 | 4.4 | 34.3 | 14.3c | 1.3 | 3.2 | 27.7 |
| TLCA | 19.1 | 1.1 | BLQ | 32.3 | 19.9 | 1.1 | BLQ | 32.3 |
| GLCA | 18.4 | 1.7 | 4.6 | 35.0 | 16.9a | 1.9 | 4.6 | 35.9 |
| LCA-S | 7.0 | 0.6 | BLQ | 14.4 | 5.4b | 0.5 | BLQ | 11.0 |
| DCA | 393.1 | 24.6 | 102.9 | 782.4 | 305.6c | 19.4 | 64.6 | 627.0 |
| TDCA | 35.1 | 3.2 | 4.5 | 81.6 | 44.8b | 3.9 | 5.5 | 104.0 |
| GDCA | 275.5 | 19.3 | 62.5 | 625.2 | 252.2 | 18.9 | 45.8 | 539.0 |
| HDCA | 53.5 | 3.0 | 17.4 | 104.5 | 43.2c | 2.3 | 11.8 | 81.8 |
| HCA | 5.5 | 0.5 | BLQ | 13.6 | 7.4c | 0.6 | BLQ | 17.5 |
| Total CDCA | 1,095.7 | 76.5 | 257.3 | 2,414.3 | 996.1 | 59.6 | 213.0 | 2,179.1 |
| Total CA | 488.2 | 40.4 | 74.6 | 1,245.4 | 499.0 | 38.5 | 82.3 | 1,197.3 |
| Total DCA | 703.7 | 40.4 | 201.8 | 1,465.3 | 602.6c | 36.2 | 140.5 | 1,217.1 |
| Total LCA | 62.0 | 3.2 | 14.6 | 106.2 | 56.5a | 3.2 | 12.6 | 98.8 |
| Total primary | 1,583.9 | 109.4 | 354.1 | 3,504.5 | 1,495.1 | 89.4 | 310.8 | 3,312.4 |
| Total secondary | 765.7 | 42.6 | 241.6 | 1,566.6 | 659.2c | 38.1 | 197.3 | 1,313.4 |
| Total 6α-hydroxy | 59.0 | 3.1 | 21.6 | 110.1 | 50.6c | 2.4 | 16.0 | 91.4 |
| Total free | 1,009.1 | 74.3 | 252.8 | 2,056.6 | 793.3c | 55.2 | 168.0 | 1,685.2 |
| Total glyco | 1,238.1 | 84.4 | 318.1 | 2,536.7 | 1,200.3 | 77.1 | 264.6 | 2,551.5 |
| Total tauro | 266.7 | 25.7 | 35.7 | 747.9 | 288.9 | 27.5 | 50.0 | 606.1 |
| TOTAL | 2,520.9 | 142.5 | 795.1 | 5,015.2 | 2,287.9 | 116.9 | 774.3 | 4,434.6 |
Bile acid concentrations are expressed in nM. Statistically significant differences in bile acid concentrations were determined by the Wilcoxon matched-pairs signed-ranks test: a, p<0.05; b, p<0.01; c, p<0.001.
BLQ, below the limit of quantification. CA, cholic acid; CDCA, chenodeoxycholic acid; DCA, deoxycholic acid; GCDCA, glycochenodeoxycholic acid; GCA, glycocholic acid; GDCA, glycodeoxycholic acid; GLCA, glycolithocholic acid; HCA, hyocholic acid; HDCA, hyodeoxycholic acid; LCA, lithocholic acid; LCA-S, LCA-sulfate; TCDCA, taurochenodeoxycholic acid; TCA, taurocholic acid; TDCA, taurodeoxycholic acid; TLCA, taurolithocholic acid; TUDCA, tauroursodeoxycholic acid; UDCA, ursodeoxycholic acid; TOTAL, sum of all bile acids.
Changes in bile acid levels after fenofibrate treatment
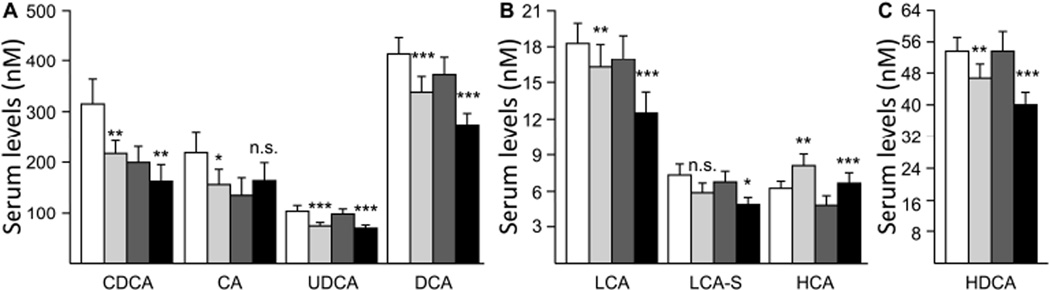
Treatment with fenofibrate resulted in significant reductions in the serum concentration of CDCA (−26.4%, p<0.001), UDCA (−30.4%, p<0.001), LCA (−18.4%, p<0.001), GLCA (−7.8%, p<0.05), LCA-S (−23.1%, p<0.01), DCA (−22.3%, p<0.001), TDCA (−27.6%, p<0.01) and HDCA (−19.2%, p<0.001), whereas TUDCA (+50.5%, p<0.001) and HCA (+33.5%, p<0.001) levels were significantly increased and other species were not significantly altered (Table 2). Since 6 out of the 10 affected bile acids were unconjugated, the total of free acids was also significantly reduced (−21.4%, Table 2). Furthermore, the −22.3% reduction in DCA concentration (from 393.0 to 305.6 nM, p<0.001) entailed both a reduction in the total (conjugated and unconjugated) level of this species (i.e. DCA+TDCA+GDCA: −14.4%, p<0.001) and total secondary acids (unconjugated and conjugated forms of LCA and DCA: −13.9%, p<0.001, Table 2).
Gender differences in baseline and fenofibrate profiles of serum bile acids
Comparison of the baseline bile acid profiles in men and women revealed a series of gender differences (Table 3). Indeed, sera from women contained significantly less of the primary acids CDCA (p<0.01) and CA (p<0.01), and of the 6α-hydroxylated acid, HCA (p<0.01). Furthermore, the total levels of CA (p<0.05), primary (p<0.05) and unconjugated acids (p<0.05) were also significantly lower in women.
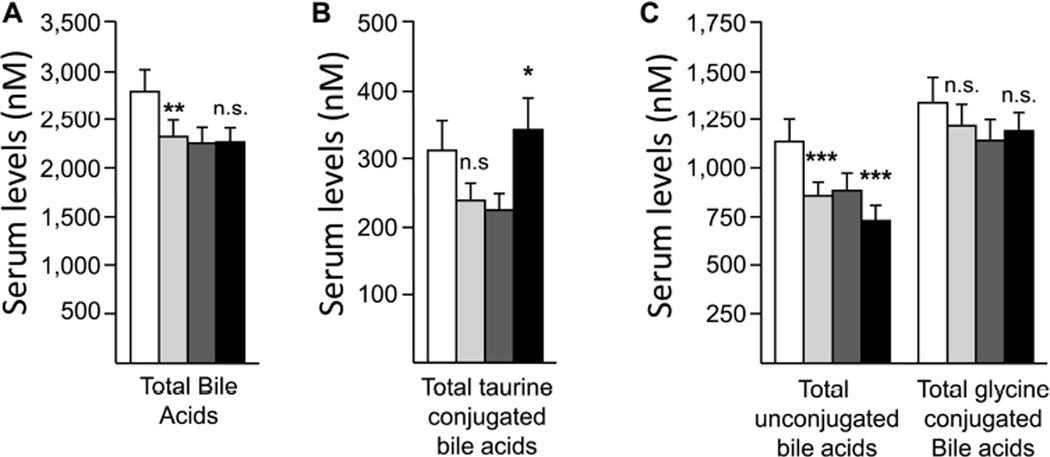
When changes after fenofibrate were analyzed separately in men and women, significant differences were also observed in the bile acid profile (Figures 2–4). The total bile acid concentration was significantly reduced in men (−18.6%, p<0.01), while remaining almost unchanged in women (+0.36%) (Figure 2A). The effects of fenofibrate on total bile acid concentration were indeed significantly stronger in male than in female (p<0.05). However, the statistical significance of this difference was reduced when the response to treatment was adjusted for body mass index (BMI; p=0.051) and body weight (p=0.151).
Figure 2. Differential modulation of total serum bile acids (A), total taurineconjugated (B), total glycine-conjugated and total unconjugated bile acids (C) by fenofibrate in men and women.
 : Men pre-treatment; : Men pre-treatment; |
 : Men post-treatment : Men post-treatment |
 : Women pre-treatment; : Women pre-treatment; |
 : Women post-treatment : Women post-treatment |
Statistically significant differences in pre- versus post-fenofibrate samples from men or women were determined by the Wilcoxon matched-pairs signed-ranks test *, p<0.05; **, p<0.01; ***, p<0.001; n.s., not significant.
Figure 4. Differential modulation of serum unconjugated bile acids by fenofibrate in men and women.
 : Men pre-treatment; : Men pre-treatment; |
 : Men post-treatment : Men post-treatment |
 : Women pre-treatment; : Women pre-treatment; |
 : Women post-treatment : Women post-treatment |
Statistically significant differences in pre- versus post-fenofibrate samples from men or women were determined by the Wilcoxon matched-pairs signed-ranks test *, p<0.05; **, p<0.01; ***, p<0.001; n.s., not significant. CA, cholic acid; CDCA, chenodeoxycholic acid; DCA, deoxycholic acid; HCA, hyocholic acid; HDCA, hyodeoxycholic acid; LCA, lithocholic acid; LCA-S, LCA-sulfate; UDCA, ursodeoxycholic acid.
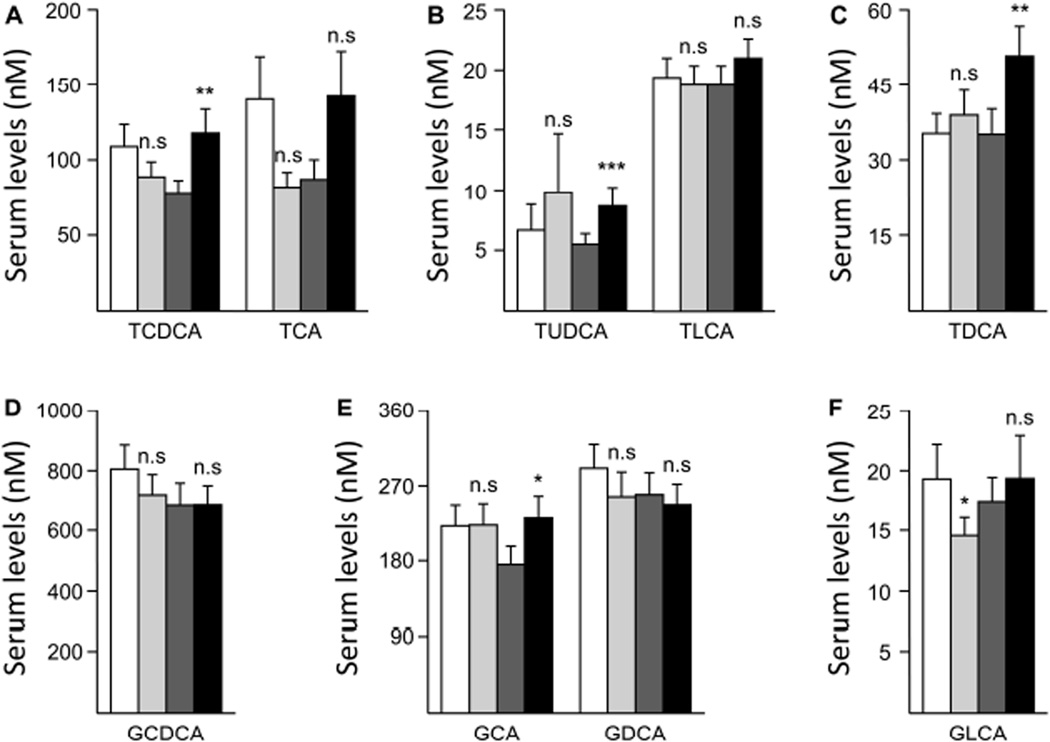
Due to statistically non-significant changes in TCA levels (−41.7% in men and +63.3% in women, Figure 3A), the total of taurine conjugates showed a genderdependent variation with a −23.2% reduction in men and a +52.2% increase in women (Figure 2B). However, the reduction in men failed to reach statistical significance. TLCA was not significantly changed in either sex, while TUDCA and TDCA concentrations were increased in both genders, but reached statistical significance only in sera from women (Figures 3B&C).
Figure 3. Differential modulation of serum taurine-conjugated (A–C) and glycine-conjugated (D–F) bile acid species by fenofibrate in men and women.
 : Men pre-treatment; : Men pre-treatment; |
 : Men post-treatment : Men post-treatment |
 : Women pre-treatment; : Women pre-treatment; |
 : Women post-treatment : Women post-treatment |
Statistically significant differences in pre- versus post-fenofibrate samples from men or women were determined by the Wilcoxon matched-pairs signed-ranks test *, p<0.05; **, p<0.01; ***, p<0.001; n.s., not significant. GCDCA, glycochenodeoxycholic acid; GCA, glycocholic acid; GDCA, glycodeoxycholic acid; GLCA, glycolithocholic acid; TCDCA, taurochenodeoxycholic acid; TCA, taurocholic acid; TDCA, taurodeoxycholic acid; TLCA, taurolithocholic acid; TUDCA, tauroursodeoxycholic acid.
As shown in Figure 2C, the level of total glycine conjugates was not significantly affected by fenofibrate in either men and women. This situation reflected the absence of significant changes for GCDCA and GDCA levels in samples from both man and woman volunteers (Figure 3D&E). Nevertheless, GLCA was significantly reduced in men, while GCA levels were significantly increased only in samples from women (Figure 3E&F).
The unique gender-associated difference observed for unconjugated species specifically affected CA levels, which were significantly reduced in men (−28.4%, p<0.05) while being not significantly increased (+22.2%) in women (Figure 4A). With the notable exception of serum HCA, which was significantly more abundant in treated individuals of both sexes, other unconjugated species (i.e. CDCA, UDCA, LCA, DCA and HDCA) were reduced in both sexes (Figure 4). Likewise, serum levels of LCA-S were also reduced in men and women, but the reduction reached statistical significance only in women (Figure 4B).
Overall, these observations reveal the presence of gender differences in both circulating levels of bile acids and in individual responses to fenofibrate.
DISCUSSION
This study provides the first comprehensive analysis of the effects of fenofibrate on the circulating bile acid profile. This investigation was enabled by the development and implementation of a novel protocol for the simultaneous analysis of 17 bile acid species in a single serum sample. This approach was efficient at quantifying low levels of serum bile acids, and exhibited limits of detection comparable to those obtained with other recently reported methods (18–20). Furthermore, the concentrations of the various species detected in baseline samples were remarkably similar to those reported in healthy populations, as was the relative abundance of each acid (18, 19, 21). Baseline levels of almost all bile acid species tended to be greater in men than in women, and even CDCA, CA and HCA concentrations reached higher levels in samples from males. The reason why concentrations in men are higher than in women cannot be ascertained from the present study but might be related to the larger body weight of men, which could result in a higher bile acid concentration. The primary purpose of the current study was to examine how fenofibrate affects circulating bile acid levels. As illustrated in table 2, many bile acid species (such as CDCA, UDCA, LCA, GLCA, LCA-S, DCA, GDCA and HDCA) underwent significant reductions in concentrations. These reductions are in keeping with the total cholesterol-lowering effect of fenofibrate (9, 22). Indeed, since bile acids are synthesized from cholesterol, a reduction of in the precursor concentration would be expected to lead to a decrease in total bile acid synthesis. However, in addition to a decrease in cholesterol levels, most of the present observations are also in agreement with the regulatory effects that fibrate drugs exert on bile acid synthesizing and metabolizing enzymes. These drugs activate the PPARα nuclear receptor which, in turn, reduces expression of the human CYP7A1 and rodent CYP27 enzymes, two proteins critical for bile acid biosynthesis (6). In parallel, PPARα activators also stimulate hepatic expression of bile acid-metabolizing enzymes such as UGT2B4 (15). Such effects are consistent with the reduction of circulating levels of bile acids such as CDCA, a primary acid that reflects CYP7A1 activity, and HDCA, which is efficiently metabolized by UGT2B4 (15, 23). By contrast, the reduction in LCA-S levels was unexpected based on the previously reported, PPARα-dependent, upregulation of immunoreactive levels of SULT2A1, a bile acid sulfotransferase (24). However, it remains possible that the reduction of LCA-S in post-treatment sera only reflects the reduction of LCA levels, and that the PPARα-mediated accumulation of SULT2A1 protein in the liver cannot compensate for the decreased availability of its substrate. Another interesting observation is the differential response of CDCA and CA levels. PPARα was previously identified as a positive regulator of the CYP8B1 gene in the human and murine liver (16). This gene encodes 12α-hydroxylase, a key branch in the bile acid biosynthetic pathway which favors CA instead of CDCA formation, and thus determines the CA/CDCA ratio (16). It is thus of interest that in the current study, fenofibrate causes a greater reduction in CDCA than CA levels (Table 2). Such a difference results in an increase of the CA/CDCA ratio from 0.68 in pre-treatment samples to 0.84 in postfenofibrate sera. Actually, this increase depends on gender, since CA levels were reduced in men while undergoing a significant increase in women. In both sexes, CDCA was reduced whereas the CA/CDCA ratio remained unchanged in men (0.69 and 0.71 pre- and post-fenofibrate, respectively) but increased from 0.67 to 1.01 in women. These observations might indicate that the PPARα-dependent up-regulation of CYP8B1 is stronger in women than in men.
The response to fenofibrate almost erases the sex-related difference of baseline bile acid levels discussed above, fenofibrate entailing a “feminization” of the bile acid profile in men. Indeed, the total bile acid concentration in women before drug exposure is closer to that found in fenofibrate-treated men when compared to pre-treatment male samples. The same remark also applies to individual bile acid species, such as CDCA, CA, LCA, TCDCA, TCA, GCDCA and GDCA. Overall, these observations indicate that fenofibrate is more efficient at reducing circulating bile acids in men as compared to women. Interestingly, previous studies reported similar gender differences in the response to fenofibrate. For example, in C57BL/6J mice fed with a high-fat diet, fenofibrate reduces body weight gain and total cholesterol only in males (25). Similarly, Liu et al. recently reported from the GOLDN study that fenofibrate differentially affected the concentration of large LDL particles in men versus women (26). These observations further support the existence of a gender specificity in the response to fenofibrate for selected parameters such as bile acid levels.
Nevertheless, a significant number of bile acid parameters are affected similarly in men and women. These include the total secondary acids, as well as total DCA and total LCA levels, which are reduced in all post-treatment samples, even if specific acids (i.e. TLCA and GLCA) may be differentially affected (Table 3). The secondary acids DCA and LCA are hydrophobic molecules and among the most toxic bile acids (27, 28), and their reduction suggests that fenofibrate might contribute to decrease the toxicity of specific bile acids. Accumulation of toxic bile acids is a hallmark of chronic cholestatic liver diseases such as PBC and PSC (2). Interestingly, recent pilot studies have reported a positive effect of fenofibrate on the liver functions of PBC patients resistant to the classical anticholestatic drug, UDCA (10–14, 29). These benefits were primarily associated with the lipoprotein-lowering and anti-inflammatory properties of PPARα activators. However, results from the present study support that an additional favorable effect of fenofibrate in these patients might be a reduction of toxic secondary bile acid levels.
On the other hand, the fact that fenofibrate preferentially reduces the bile acid concentration in men also suggests that the drug may be more efficient at reducing the cytotoxic properties of these detergents in men than in women. Cholestatic diseases such as PBC and PSC are sex-related pathologies since middle-aged women constitute the major part of PBC patients, whereas PSC affects children, teenagers and adults, with a predominance of male subjects (7). Consequently, data herein presented suggest that fenofibrate may be more efficient at correcting bile acid toxicity in male PSC patients than in women with PBC. Accordingly, Chazouillères O. and collaborators recently reported that combination therapy of fenofibrate and UDCA induces significant biochemical improvement in patients with PSC and incomplete response to UDCA (30). However, further studies are needed to evaluate the extent of bile acid reduction reached with fenofibrate in these patients, and to determine whether such a reduction might participate in the improvement of their liver functions.
In conclusion, the present study provides evidence that fenofibrate alters the profile of circulating bile acids in humans, an effect which is thought to participate in the reported improvement of liver functions observed in fenofibrate-treated PBC patients. Furthermore, the gender difference detected suggests that fenofibrate could be more efficient at reducing bile acid toxicity in men than in women.
METHODS
Materials
Unconjugated and taurine- and glycine-conjugated bile acids were purchased from Steraloids (Newport, RI). Deuterated bile acids (d4-CA, d4-CDCA, d4-LCA and d4-DCA) were purchased from C/D/N Isotopes (Montréal, QC, Canada). Protein assay reagents were obtained from Bio-Rad Laboratories Inc. (Marnes-la-Coquette, France). HPLC-grade solvents were from VWR Canlab (Montréal, QC, Canada). Strata-X 33-µm polymeric reversed-phase solid-phase extraction (SPE) columns (60 mg/3 mL) were from Phenomenex (Torrance, CA).
Donors
The population for this study consisted of 200 Caucasian participants (100 men and 100 women) from the Genetics of Lipid Lowering Drugs and Diet Network (GOLDN) study who were recruited exclusively at the Minnesota fields center (Minneapolis, MN). Demographic data and laboratory characteristics of donors are shown in Table 1.
Table 1.
Clinical and laboratory characteristics of the volunteers
| Male (n=100) |
Female (n=100) |
Total (n=200) |
|
|---|---|---|---|
| Age (years) | 52.42 ± 1.45 | 52.73 ± 1.53 | 52.6 ± 14.9 |
| Alanine aminotransferase (U/L) | 30.5 ± 1.20 | 19.6 ± 0.65 | 25.05 ± 11.05 |
| Aspartate aminotransferase (U/L) | 31.82 ± 0.74 | 26.83 ± 0.56 | 29.33 ± 7.01 |
| Creatinine (mg/dL) | 0.915 ± 0.015 | 0.736 ± 0.016 | 0.826 ± 0.179 |
| Weight (lb) | 204.2 ± 2.7 | 165.9 ± 3.6 | 185.1 ± 3.7 |
| BMI (kg/m2) | 29.3 ± 0.5 | 28.1 ± 0.6 | 28.7 ± 0.5 |
| Waist (cm) | 101.9 ± 1.1 | 90.3 ± 1.7 | 96.1 ± 1.5 |
GOLDN is a single-arm, uncontrolled, nonrandomized intervention aimed at identifying genetic factors associated with interindividual variability of the triglyceride response to high-fat meals and fenofibrate (31–34). This study is part of the PRogram for GENetic Interaction (PROGENI) and is funded by the NIH (22, 31–34). Only participants who had not taken lipid-lowering agents for at least 4 weeks before the initial visit were included. As extensively described in Lai et al. (33), participants took part in five visits. However, sera used in the present study were drawn at visits 1 and 3. Between the two visits, participants were given 160 mg of fenofibrate/day (TriCor®, Abbott Laboratories, Chicago, IL), and were instructed to take one tablet with a breakfast meal once daily for 3 weeks (33, 34). Adherence to fenofibrate therapy was primarily assessed using tablet counts. Generally, medication adherence was considered acceptable using an arbitrary threshold of compliance when study participants took >75% of their expected doses prior to their study day. This threshold was achieved by 99.0% (99/100) of males and 97.0% (97/100) of female subjects participating in this cohort.
At both visits, blood was drawn after a 12-h fast, serum was isolated and frozen at −80°C until subsequent analyses. As this was a study of ambulatory outpatients who were generally healthy, no attempt was made to standardize diet within or between subjects prior to the requisite 12 hour fast preceding baseline and post-fenofibrate assessments. Of course we cannot exclude the possibility of dietary changes within a patient’s enrolment period within the study, but we would expect that any significant changes in diet would be rare and, in the context of generally healthy individuals, have minimal if any impact on the findings of this study.
Exclusion criteria included the following: fasting triglycerides ≥ 1500 mg/dL; recent history (six months) of myocardial infarction, coronary bypass surgery, coronary angioplasty or PTCA; self report of a positive history of liver, kidney, pancreas, or gall bladder disease, or a history of malabsorption of nutrients; current use of insulin or warfarin; serum concentrations of aspartate aminotransferase (AST) exceeding 52 U/L in males or 42 U/L in females; serum concentrations of alanine transaminase (ALT) exceeding 66 U/L in males or 44 U/L in females; glomerular filtration rate <30 l/min/1.73 m2 estimated from the MDRD equation; pregnant women or women of childbearing potential not using contraception; and women nursing a child. Individuals who reported current use of prescription and/or over-the-counter (OTC) hypolipidemic drugs or dietary supplements known to influence lipid values (e.g., fish oil, flaxseed oil, niacin, etc.) were required to consult with their physician for approval to discontinue these lipid-lowering agents for 4 weeks prior to study participation. Even if known history of liver disease was an exclusion criterion, we cannot exclude the possibility of undiagnosed underlying liver disease. This may be considered as a possible limitation of the current study.
A description of the co-morbidities of subjects included in this cohort resemble that of the overall GOLDN study (33) with limited relevance related to liver disease or function. Specifically; 8% of our population had T2DM (evenly split between male and females), 5% had some form of cardiovascular disease (predominantly males), 27% had a diagnosis of hypertension and 44% meet the definition of metabolic syndrome (both of which were evenly distributed between the sexes). Given the exclusion criteria described above, specifically as it pertains to measures of liver function tests (AST/ALT), we feel that there is no obvious systematic basis for presumed effect of co-morbidities on our major findings from this study. With respect to co-administered drugs, few subjects were taking any chronic drug therapy (including nutraceuticals and over the counter medications) known to affect bile acids. One possible exception may be those who reported receiving chronically administered estrogens or progestin’s (or their combination). The number of subjects taking any form of systemic estrogen or progestin (or their combination), was limited to 30 of the 100 females participating in this cohort. Based on their declared indication or presumed indication, 15 individuals were using them for hormonal replacement therapy (n=15) and 15 individuals were using them for the purpose of oral contraception use. As these medications are usually taken on a chronic basis, their impact on the observations of fenofibrate’s effect on bile acid disposition would be expected to be modest.
The protocol was approved by the institutional review boards at the University of Minnesota (Minneapolis, MN), Laval University and the CHUQ Research Center (Québec, Canada). Written informed consent was obtained from all participants.
Bile acid measurements
Bile acid concentrations were determined using a HPLC-MS/MS system with an electrospray interface, using a novel method adapted from Ye et al. (20). The modified method allows the simultaneous evaluation of 17 species in the same sample (Figure 1A). Solid-phase extraction (SPE) was initiated by adding 2 mL of a 0.1% (w/v) formic acid solution and 30 µL of internal standards (i.e: the deuterated bile acids d4-CDCA, d4-CA, d4-LCA and d4-DCA) to 100 µL of serum. The same treatment was applied to analytical standards which were diluted (1:1) with 100 µL of adsorbed serum, and subsequently used to generate calibration equations. SPE columns were conditioned with 1 mL MeOH and 2 mL of 0.1% formic acid. Columns were successively washed with 2 mL of H2O and 2 mL of H2O:MeOH (80:20) containing 0.1% formic acid under negative pressure. Bile acids were eluted with 2 mL of MeOH. Eluates were completely evaporated at 45°C under N2 and reconstituted in 100 µL of H2O:MeOH (50:50) containing 5 mM ammonium acetate and 0.01% formic acid. Fifteen µL of sample or calibration standards were then injected into the LC-MS/MS system.
A single LC method was used for the separation of the free (CDCA, CA, UDCA, LCA, DCA, HDCA, and HCA), taurine (TCDCA, TCA, TUDCA, TLCA and TDCA), glycine (GCDCA, GCA, GLCA and GDCA), and sulfate (LCA-S) conjugates of bile acids (Figure 1). The chromatographic system consisted of an Alliance 2690 Separations Module (Waters, Milford, MA). Analytes were separated using a 50 × 3 mm Synergi Hydro-RP column (2.5-µm particles) (Phenomenex, Torrance, CA). The chromatographic conditions used were: 5 mM ammonium acetate−0.01% formic acid in water (solvent A), 5 mM ammonium acetate−0.01% formic acid in MeOH (solvent B), and acetonitrile (solvent C) at a flow rate of 800 µL/min. The chromatographic program was as follows: (i) initial conditions: 40% A: 55% B: 5% C for 4 min; (ii) a linear gradient to 80% B was applied over the next 8 min; (iii) the column was flushed with 90% B for the next 2 min; and (iv) re-equilibration to the initial conditions over the next 4 min. All analytes were quantified by tandem mass spectrometry (MS/MS) using an API3200 LC/MS/MS instrument (Applied Biosystems, Concord, ON, Canada). The temperature of the source was set at 350°C and the parameter settings used are shown in Figure 1B.
Data analysis
Baseline characteristics were calculated as means and ranges (10th – 90th percentile). The response to treatment was calculated as the difference of bile acid concentrations post- minus pre-treatment. The total bile acid concentration corresponds to the sum of the 17 bile acid concentrations. The sums of glyco- and tauro-conjugates were calculated by adding the concentrations of conjugated CDCA, CA, DCA and LCA. The sum of free (i.e. unconjugated) bile acids also included HDCA and HCA levels. The total of primary, secondary and 6α-hydroxylated species was determined by adding all unconjugated and conjugated species of CDCA + CA, LCA + DCA or HDCA + HCA, respectively. As bile acid concentrations did not follow a normal distribution according to the Shapiro-Wilk test, the Wilcoxon matched-pairs signed-ranks test was used for statistical analysis of the response to treatment in men, women and both sexes. For comparison of baseline bile acid profiles and the response to treatment between men and women, the Wilcoxon/Mann-Whitney rank-sum test was used. Statistical analyses were performed using the JMP Statistical Discovery V7.0.2 program (SAS Institute, Cary, NC). Finally, analyses of covariance (ANCOVA) was used to compare the means of each log10-tranformed bile acid variable between 2 or more independent groups adjusted for body weight and BMI (SAS version 9.2, SAS Institute Inc., Cary, NC).
ACKNOWLEDGEMENTS
We wish to thank Dr. Virginie Bocher for critical reading of the manuscript and helpful discussions on this work. This manuscript has been edited by the “Service de révision et de rédaction scientifiques” from the CHUQ research center. This study was supported by grants from the Canadian Institutes of Health Research (CIHR; grant #MOP-84338) and the Canadian Foundation for Innovation (CFI, grant #10469). J. Trottier is holder of a Scholarship from CIHR. O. Barbier is holder of a salary grant from the CIHR (New Investigator Award #MSH95330) and the Fonds pour la Recherche en Santé du Québec (Junior 2 Scholarship). The Genetics of Lipid Lowering Drugs and Diet Network (GOLDN) study was supported by the National Heart, Lung and Blood Institute (NIH) Grant U 01 HL72524 Genetic and Environmental Determinants of Triglycerides.
Footnotes
Disclosure: Authors disclose no conflicts of interest.
Contributor Information
J. Trottier, Email: jocelyn.trottier@crchul.ulaval.ca.
P. Caron, Email: patrick.caron@crchul.ulaval.ca.
R. J. Straka, Email: strak001@umn.edu.
O. Barbier, Email: olivier.barbier@crchul.ulaval.ca.
REFERENCES
- 1.Russell DW. Fifty years of advances in bile acid synthesis and metabolism. J Lipid Res. 2009 Apr;50(Suppl):S120–S125. doi: 10.1194/jlr.R800026-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Monte MJ, Marin JJ, Antelo A, Vazquez-Tato J. Bile acids: chemistry, physiology, and pathophysiology. World J Gastroenterol. 2009 Feb 21;15(7):804–816. doi: 10.3748/wjg.15.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Araya Z, Wikvall K. 6alpha-hydroxylation of taurochenodeoxycholic acid and lithocholic acid by CYP3A4 in human liver microsomes. Biochim Biophys Acta. 1999;1438(1):47–54. doi: 10.1016/s1388-1981(99)00031-1. [DOI] [PubMed] [Google Scholar]
- 4.Bodin K, Lindbom U, Diczfalusy U. Novel pathways of bile acid metabolism involving CYP3A4. Biochim Biophys Acta. 2005;1687(1–3):84–93. doi: 10.1016/j.bbalip.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Pauli-Magnus C, Meier PJ. Hepatocellular transporters and cholestasis. J Clin Gastroenterol. 2005 Apr;39(4) Suppl 2:S103–S110. doi: 10.1097/01.mcg.0000155550.29643.7b. [DOI] [PubMed] [Google Scholar]
- 6.Wagner M, Zollner G, Trauner M. Nuclear receptor regulation of the adaptive response of bile acid transporters in cholestasis. Semin Liver Dis. 2010 May;30(2):160–177. doi: 10.1055/s-0030-1253225. [DOI] [PubMed] [Google Scholar]
- 7.Paumgartner G. Pharmacotherapy of cholestatic liver diseases. J Dig Dis. 2010;11(3):119–125. doi: 10.1111/j.1751-2980.2010.00427.x. [DOI] [PubMed] [Google Scholar]
- 8.Barbier O, Trottier J, Kaeding J, Caron P, Verreault M. Lipid-activated transcription factors control bile acid glucuronidation. Mol Cell Biochem. 2009 Jun;326(1–2):3–8. doi: 10.1007/s11010-008-0001-5. [DOI] [PubMed] [Google Scholar]
- 9.Brown JD, Plutzky J. Peroxisome proliferator-activated receptors as transcriptional nodal points and therapeutic targets. Circulation. 2007;115(4):518–533. doi: 10.1161/CIRCULATIONAHA.104.475673. [DOI] [PubMed] [Google Scholar]
- 10.Dohmen K, Mizuta T, Nakamuta M, Shimohashi N, Ishibashi H, Yamamoto K. Fenofibrate for patients with asymptomatic primary biliary cirrhosis. World J Gastroenterol. 2004 Mar 15;10(6):894–898. doi: 10.3748/wjg.v10.i6.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iwasaki S, Akisawa N, Saibara T, Onishi S. Fibrate for treatment of primary biliary cirrhosis. Hepatol Res. 2007 Oct;37(Suppl 3):S515–S517. doi: 10.1111/j.1872-034X.2007.00232.x. [DOI] [PubMed] [Google Scholar]
- 12.Iwasaki S, Ohira H, Nishiguchi S, Zeniya M, Kaneko S, Onji M, et al. The efficacy of ursodeoxycholic acid and bezafibrate combination therapy for primary biliary cirrhosis: A prospective, multicenter study. Hepatol Res. 2008;38(6):557–564. doi: 10.1111/j.1872-034X.2007.00305.x. [DOI] [PubMed] [Google Scholar]
- 13.Nakai S, Masaki T, Kurokohchi K, Deguchi A, Nishioka M. Combination therapy of bezafibrate and ursodeoxycholic acid in primary biliary cirrhosis: a preliminary study. Am J Gastroenterol. 2000 Jan;95(1):326–327. doi: 10.1111/j.1572-0241.2000.01667.x. [DOI] [PubMed] [Google Scholar]
- 14.Ohira H, Sato Y, Ueno T, Sata M. Fenofibrate treatment in patients with primary biliary cirrhosis. Am J Gastroenterol. 2002;97(8):2147–2149. doi: 10.1111/j.1572-0241.2002.05944.x. [DOI] [PubMed] [Google Scholar]
- 15.Barbier O, Duran-Sandoval D, Pineda-Torra I, Kosykh V, Fruchart JC, Staels B. Peroxisome proliferator-activated receptor alpha induces hepatic expression of the human bile acid glucuronidating UDP-glucuronosyltransferase 2B4 enzyme. J Biol Chem. 2003 Aug 29;278(35):32852–32860. doi: 10.1074/jbc.M305361200. [DOI] [PubMed] [Google Scholar]
- 16.Hunt MC, Yang YZ, Eggertsen G, Carneheim CM, Gafvels M, Einarsson C, et al. The peroxisome proliferator-activated receptor alpha (PPARalpha) regulates bile acid biosynthesis. J Biol Chem. 2000 Sep 15;275(37):28947–28953. doi: 10.1074/jbc.M002782200. [DOI] [PubMed] [Google Scholar]
- 17.Li T, Chiang JY. Regulation of Bile Acid and Cholesterol Metabolism by PPARs. PPAR Res. 2009;2009:501739. doi: 10.1155/2009/501739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gnewuch C, Liebisch G, Langmann T, Dieplinger B, Mueller T, Haltmayer M, et al. Serum bile acid profiling reflects enterohepatic detoxification state and intestinal barrier function in inflammatory bowel disease. World J Gastroenterol. 2009 Jul 7;15(25):3134–3141. doi: 10.3748/wjg.15.3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scherer M, Gnewuch C, Schmitz G, Liebisch G. Rapid quantification of bile acids and their conjugates in serum by liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877(30):3920–3925. doi: 10.1016/j.jchromb.2009.09.038. [DOI] [PubMed] [Google Scholar]
- 20.Ye L, Liu S, Wang M, Shao Y, Ding M. High-performance liquid chromatography-tandem mass spectrometry for the analysis of bile acid profiles in serum of women with intrahepatic cholestasis of pregnancy. J Chromatogr B Analyt Technol Biomed Life Sci. 2007 Dec 1;860(1):10–17. doi: 10.1016/j.jchromb.2007.09.031. [DOI] [PubMed] [Google Scholar]
- 21.Xiang X, Han Y, Neuvonen M, Laitila J, Neuvonen PJ, Niemi M. High performance liquid chromatography-tandem mass spectrometry for the determination of bile acid concentrations in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2010 Jan 1;878(1):51–60. doi: 10.1016/j.jchromb.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 22.Tsai MY, Ordovas JM, Li N, Straka RJ, Hanson NQ, Arends VL, et al. Effect of fenofibrate therapy and ABCA1 polymorphisms on high-density lipoprotein subclasses in the Genetics of Lipid Lowering Drugs and Diet Network. Mol Genet Metab. 2010;100(2):118–122. doi: 10.1016/j.ymgme.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marrapodi M, Chiang JY. Peroxisome proliferator-activated receptor alpha (PPARalpha) and agonist inhibit cholesterol 7alpha-hydroxylase gene (CYP7A1) transcription. J Lipid Res. 2000 Apr;41(4):514–520. [PubMed] [Google Scholar]
- 24.Fang HL, Strom SC, Cai H, Falany CN, Kocarek TA, Runge-Morris M. Regulation of human hepatic hydroxysteroid sulfotransferase gene expression by the peroxisome proliferator-activated receptor alpha transcription factor. Mol Pharmacol. 2005 Apr;67(4):1257–1267. doi: 10.1124/mol.104.005389. [DOI] [PubMed] [Google Scholar]
- 25.Yoon M, Jeong S, Nicol CJ, Lee H, Han M, Kim JJ, et al. Fenofibrate regulates obesity and lipid metabolism with sexual dimorphism. Exp Mol Med. 2002;34(6):481–488. doi: 10.1038/emm.2002.67. [DOI] [PubMed] [Google Scholar]
- 26.Liu Y, Ordovas JM, Gao G, Province M, Straka RJ, Tsai MY, et al. The SCARB1 gene is associated with lipid response to dietary and pharmacological interventions. J Hum Genet. 2008;53(8):709–717. doi: 10.1007/s10038-008-0302-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hofmann AF. Detoxification of lithocholic acid, a toxic bile acid: relevance to drug hepatotoxicity. Drug Metab Rev. 2004;36(3–4):703–722. doi: 10.1081/dmr-200033475. [DOI] [PubMed] [Google Scholar]
- 28.Sharma R, Majer F, Peta VK, Wang J, Keaveney R, Kelleher D, et al. Bile acid toxicity structure-activity relationships: correlations between cell viability and lipophilicity in a panel of new and known bile acids using an oesophageal cell line (HET-1A) Bioorg Med Chem. 2010;18(18):6886–6895. doi: 10.1016/j.bmc.2010.07.030. [DOI] [PubMed] [Google Scholar]
- 29.Levy C, Peter JA, Nelson DR, Keach J, Petz J, Cabrera R, et al. Pilot study: fenofibrate for patients with primary biliary cirrhosis and an incomplete response to ursodeoxycholic acid. Aliment Pharmacol Ther. 2011 Jan;33(2):235–242. doi: 10.1111/j.1365-2036.2010.04512.x. [DOI] [PubMed] [Google Scholar]
- 30.Chazouilleres O, Corpechot C, Gaouar F, Poupon R. Fenofibrate improves liver tests in Primary Sclerosing Cholangitis with incomplete biochemical response to ursodeoxycholic acid. In: AASLD, editor. Hepatology; 61st Annual Meeting of the American-Association-for-the-Study-of-Liver-Diseases; Oct 2010; Boston, MA. 2010. [OCT 29–NOV 02, 2010]. p. 488A. [Google Scholar]
- 31.Wojczynski MK, Gao G, Borecki I, Hopkins PN, Parnell L, Lai CQ, et al. ApoB genetic variants modify the response to fenofibrate: a GOLDN study. J Lipid Res. 2010;51(11):3316–3323. doi: 10.1194/jlr.P001834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith JA, Arnett DK, Kelly RJ, Ordovas JM, Sun YV, Hopkins PN, et al. The genetic architecture of fasting plasma triglyceride response to fenofibrate treatment. Eur J Hum Genet. 2008;16(5):603–613. doi: 10.1038/sj.ejhg.5202003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lai CQ, Arnett DK, Corella D, Straka RJ, Tsai MY, Peacock JM, et al. Fenofibrate effect on triglyceride and postprandial response of apolipoprotein A5 variants: the GOLDN study. Arterioscler Thromb Vasc Biol. 2007 Jun;27(6):1417–1425. doi: 10.1161/ATVBAHA.107.140103. [DOI] [PubMed] [Google Scholar]
- 34.Shen J, Arnett DK, Parnell LD, Peacock JM, Lai CQ, Hixson JE, et al. Association of common C-reactive protein (CRP) gene polymorphisms with baseline plasma CRP levels and fenofibrate response: the GOLDN study. Diabetes Care. 2008 May;31(5):910–915. doi: 10.2337/dc07-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]