Abstract
Background
Gonorrhea (GC) and chlamydia (CT) are the most commonly reported notifiable diseases in the United States. The Centers for Disease Control and Prevention recommends that men who have sex with men (MSM) be screened for urogenital GC/CT, rectal GC/CT, and pharyngeal GC. We describe extragenital GC/CT testing and infections among MSM attending sexually transmitted disease (STD) clinics.
Methods
The STD Surveillance Network collects patient data from 42 STD clinics. We assessed the proportion of MSM attending these clinics during July 2011–June 2012 who were tested and positive for extragenital GC/CT at their most recent visit or in the preceding 12 months and the number of extragenital infections that would have remained undetected with urethral screening alone.
Results
Of 21 994 MSM, 83.9% were tested for urogenital GC, 65.9% for pharyngeal GC, 50.4% for rectal GC, 81.4% for urogenital CT, 31.7% for pharyngeal CT, and 45.9% for rectal CT. Of MSM tested, 11.1% tested positive for urogenital GC, 7.9% for pharyngeal GC, 10.2% for rectal GC, 8.4% for urogenital CT, 2.9% for pharyngeal CT, and 14.1% for rectal CT. More than 70% of extragenital GC infections and 85% of extragenital CT infections were associated with negative urethral tests at the same visit and would not have been detected with urethral screening alone.
Conclusions
Extragenital GC/CT was common among MSM attending STD clinics, but many MSM were not tested. Most extragenital infections would not have been identified, and likely would have remained untreated, with urethral screening alone. Efforts are needed to facilitate implementation of extragenital GC/CT screening recommendations for MSM.
Keywords: extragenital gonorrhea, extragenital chlamydia, men who have sex with men (MSM), STD
Gonorrhea (GC) and chlamydia (CT) are the 2 most commonly reported notifiable diseases in the United States, with >149 000 cases of GC and >380 000 cases of CT among men reported to the Centers for Disease Control and Prevention (CDC) in 2011 [1]. Surveillance data suggest that men who have sex with men (MSM) are disproportionately affected by sexually transmitted diseases (STDs) [1].
GC and CT, and the behaviors associated with acquiring them, may increase the likelihood of acquiring and transmitting human immunodeficiency virus (HIV) infection [2]. In particular, rectal GC is an independent risk factor for HIV acquisition among MSM after adjustment for sexual behavior and other concurrent infections [3, 4]. However, because the majority of pharyngeal and rectal GC and CT infections are asymptomatic [5, 6], these infections are unlikely to be detected without routine screening.
The CDC recommends annual screening for urogenital GC and CT in MSM who have had insertive sex during the preceding year, for rectal GC and CT in MSM who have had receptive anal sex in the preceding year, and for pharyngeal GC in MSM who have had receptive oral sex during the preceding year, regardless of history of condom use during exposure [7]. More frequent screening at 3–6-month intervals may be indicated for MSM if they or their sex partners are at higher risk of acquiring STDs, including having multiple or anonymous sex partners or sex in conjunction with illicit drug use.
Screening for urogenital GC and CT among men has been facilitated in the last decade by the increased availability of nucleic acid amplification tests (NAATs), which can be used on both urine specimens and urethral swabs. When compared with culture, NAATs are less labor intensive, have less-stringent handling and transport requirements, and have improved sensitivity [8]. However, commercially available NAATs have not been cleared by the Food and Drug Administration for use on specimens collected from the rectum or pharynx. Recently, some public and private laboratories have conducted validation tests to meet Clinical Laboratory Improvement Amendment requirements that allow them to perform NAATs on specimens collected from pharyngeal and rectal sites, but culture is the only diagnostic test available for extragenital GC and CT at many clinical sites [9].
Data regarding extragenital GC and CT testing and infections among MSM are limited. To better understand extragenital GC and CT screening among MSM, we assessed extragenital GC and CT testing and infections among MSM attending STD clinics and determined the number of extragenital infections that would have remained undetected, and might have gone untreated, if only urethral screening were being performed.
METHODS
We examined data from the STD Surveillance Network (SSuN), a sentinel surveillance system comprising 42 STD clinics within 12 collaborating jurisdictions (7 state and 5 local health departments) that follow common protocols for data collection and management [10]. Each jurisdiction has 1–12 STD clinics, which are either operated directly by the jurisdiction or are operated by a separate authority. Each jurisdiction has its own GC/CT screening protocol. The SSuN obtains demographic, behavioral (number and sex of sex partners), clinical, and laboratory data from the medical records of all clients at participating clinics.
For this analysis, we examined records of MSM who had at least 1 visit to an SSuN clinic during the period 1 July 2011–30 June 2012. Because annual GC and CT screening is recommended, we reviewed GC and CT testing and positivity of those tests at each patient’s most recent visit during the period 1 July 2011–30 June 2012 and in the 12 months before that visit in the same clinic. MSM were defined as men who self-identified as gay or bisexual or who ever reported having had sex with a male partner.
Urogenital GC/CT infections were diagnosed using urine specimens (87.4%) and patient-collected or clinician-collected urethral swabs (12.6%). Pharyngeal and rectal GC/CT infections were diagnosed using either patient-collected or clinician-collected oropharyngeal and rectal swabs. Both culture and NAAT results were included in the overall analysis. Test positivity was defined as the proportion of MSM who tested positive at least once divided by the total number of MSM who were tested. Indeterminate, inadequate, and contaminated test results were excluded. Because NAATs have been shown to have increased sensitivity compared with culture [11], 2 separate positivity analyses were conducted: first, with all test types included; and second, with only NAATs included. HIV-infected MSM were identified as those with documentation of a positive HIV antibody test or a self-reported history of HIV infection.
Eleven of the 12 SSuN jurisdictions were included in this analysis; one jurisdiction (Alabama) and 5 clinics from Chicago were excluded because anatomic site of test was not recorded. Test type used to diagnose GC and CT varied by jurisdiction. During the study period, 1 jurisdiction used culture to diagnose urogenital GC, whereas the remainder used NAATs. For pharyngeal and rectal GC, 4 jurisdictions used NAATs, 4 used culture, 2 switched from culture to NAATS during the study period, and 1 did not test for extragenital GC. All jurisdictions used NAATs to diagnose urogenital CT. For pharyngeal CT, 5 jurisdictions used NAATs, and 6 jurisdictions did not test for pharyngeal CT. For rectal CT, 5 jurisdictions used NAATs, 1 switched from CT culture to NAATs during the study period, and 5 did not test for rectal CT. For the positivity analysis, only jurisdictions that performed at least 20 tests during the period 1 July 2010–30 June 2012 were included.
Analyses are descriptive and were performed in SAS version 9.3.
RESULTS
Study Population
We reviewed data from 21 994 MSM who had 44 105 visits (median, 1; range, 1–30 visits per MSM) during the period 1 July 2010–30 June 2012. Characteristics of the study population are shown in Table 1. More than 40% of MSM attending SSuN clinics were non-Hispanic white, and 43% were aged 20–29 years. Overall, 17% were infected with HIV. MSM from Chicago, New York City, and San Francisco comprised 59% of all MSM in the analytic sample.
Table 1.
Characteristics and Jurisdictions of Men Who Have Sex With Men Attending STD Surveillance Network Clinics, United States, 2010–2012
| Characteristic | No. | % |
|---|---|---|
| Race/ethnicitya | ||
| White, non-Hispanic | 9600 | 44.4 |
| Black, non-Hispanic | 4984 | 23.0 |
| Hispanic | 4931 | 22.8 |
| Asian/multiracial/other | 2130 | 9.8 |
| Age, y | ||
| <20 | 738 | 3.4 |
| 20–29 | 9440 | 42.9 |
| 30–39 | 5816 | 26.4 |
| 40–49 | 3837 | 17.5 |
| ≥50 | 2163 | 9.8 |
| Documented HIV infection | 3763 | 17.1 |
| Jurisdiction | ||
| Baltimore | 775 | 3.5 |
| Chicago | 2993 | 13.6 |
| Coloradob | 1118 | 5.1 |
| Connecticutb | 185 | 0.8 |
| Los Angeles | 2154 | 9.8 |
| Louisianab | 451 | 2.1 |
| New York City | 5895 | 26.8 |
| Philadelphia | 1133 | 5.2 |
| San Francisco | 4111 | 18.7 |
| Virginiab | 383 | 1.7 |
| Washingtonb | 2796 | 12.7 |
| Total | 21 944 | 100.0 |
Abbreviations: HIV, human immunodeficiency virus; STD, sexually transmitted disease.
Race/ethnicity data are missing for 299 subjects.
Clinic locations: Denver, Colorado; New Haven and Hartford, Connecticut;
New Orleans, Louisiana; Henrico and Chesterfield counties and Richmond, Virginia; Seattle, Washington.
Gonorrhea
Overall, 18 460 (83.9%; range, 55.9%–96.4% by jurisdiction) MSM were tested for urogenital GC either at their most recent visit or in the 12 months before their most recent visit, 14 484 (65.9%; range, 0%–80.7%) were tested for pharyngeal GC, and 11 092 (50.4%; range, 0%–65.2%) were tested for rectal GC (Table 2). At every SSuN jurisdiction except Washington, a higher proportion of MSM were tested at the urethra than at extragenital sites and a higher proportion were tested at the pharynx than at the rectum.
Table 2.
Number and Percentage of Men Who Have Sex With Men Who Were Tested for Gonorrhea and Chlamydia and Who Tested Positive Either at Last Visit or in 12 Months Prior by Anatomic Site and Jurisdiction
| MSM | Urogenital Gonorrhea
|
Pharyngeal Gonorrhea
|
Rectal Gonorrhea
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tested | Tested Positive | Tested | Tested Positive | Tested | Tested Positive | ||||||||
| Jurisdiction | Total No. | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % |
|
| |||||||||||||
| Baltimore | 775 | 534a | 68.9 | 60 | 11.2 | 310 | 40.0 | 39 | 12.6 | 223 | 28.8 | 47 | 21.1 |
|
| |||||||||||||
| Chicago | 2993 | 1673 | 55.9 | 208 | 12.4 | 439b | 14.7 | 41 | 9.3 | 237b | 7.9 | 28 | 11.8 |
|
| |||||||||||||
| Coloradoc | 1118 | 1022 | 91.4 | 105 | 10.3 | 792a | 70.8 | 45 | 5.7 | 551a | 49.3 | 43 | 7.8 |
|
| |||||||||||||
| Connecticutc | 185 | 149 | 80.5 | 10 | 6.7 | 56a | 30.3 | 0 | 0.0 | 38a | 20.5 | 0 | 0.0 |
|
| |||||||||||||
| Los Angeles | 2154 | 2076 | 96.4 | 160 | 7.7 | 1716 | 79.7 | 183 | 10.7 | 1305 | 60.6 | 154 | 11.8 |
|
| |||||||||||||
| Louisianac | 451 | 401 | 88.9 | 63 | 15.7 | 0 | 0.0 | . . . | . . . | 0 | 0.0 | . . . | . . . |
|
| |||||||||||||
| New York City | 5895 | 5678 | 96.3 | 831 | 14.6 | 4725a | 80.2 | 58 | 1.2 | 3609a | 61.2 | 194 | 5.4 |
|
| |||||||||||||
| Philadelphia | 1133 | 1037 | 91.5 | 155 | 15.0 | 904 | 79.8 | 174 | 19.3 | 666 | 58.8 | 136 | 20.4 |
|
| |||||||||||||
| San Francisco | 4111 | 3620 | 88.1 | 280 | 7.7 | 3282 | 79.8 | 358 | 10.9 | 2636 | 64.1 | 298 | 11.3 |
|
| |||||||||||||
| Virginiac | 383 | 285 | 74.4 | 36 | 12.6 | 3a | 0.8 | 3 | . . . | 3a | 0.8 | 3 | . . . |
|
| |||||||||||||
| Washingtonc | 2796 | 1985 | 71.0 | 148 | 7.5 | 2257b | 80.7 | 243 | 10.8 | 1824b | 65.2 | 233 | 12.8 |
|
| |||||||||||||
| Overall | 21 994 | 18 460 | 83.9 | 2056 | 11.1 | 14 484 | 65.9 | 1144 | 7.9 | 11 092 | 50.4 | 1136 | 10.2 |
| MSM | Urogenital Chlamydia
|
Pharyngeal Chlamydia
|
Rectal Chlamydia
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tested | Tested Positive | Tested | Tested Positive | Tested | Tested Positive | ||||||||
| Jurisdiction | Total No. | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % |
|
| |||||||||||||
| Baltimore | 775 | 192 | 24.8 | 5 | 2.6 | 249 | 32.1 | 7 | 2.8 | 217 | 28.0 | 43 | 19.8 |
|
| |||||||||||||
| Chicago | 2993 | 1680 | 56.1 | 158 | 9.4 | 329 | 11.0 | 12 | 3.7 | 175 | 5.9 | 20 | 11.4 |
|
| |||||||||||||
| Coloradoc | 1118 | 1014 | 90.7 | 90 | 8.9 | 0 | 0.0 | . . . | . . . | 0 | 0.0 | . . . | . . . |
|
| |||||||||||||
| Connecticutc | 185 | 44 | 23.8 | 6 | 13.6 | 1 | 0.5 | 1 | . . . | 0 | 0.0 | . . . | . . . |
|
| |||||||||||||
| Los Angeles | 2154 | 2076 | 96.4 | 146 | 7.0 | 0 | 0.0 | . . . | . . . | 1303 | 60.5 | 217 | 16.7 |
|
| |||||||||||||
| Louisianac | 451 | 402 | 89.1 | 49 | 12.2 | 0 | 0.0 | . . . | . . . | 0 | 0.0 | . . . | . . . |
|
| |||||||||||||
| New York City | 5895 | 5675 | 96.3 | 577 | 10.2 | 0 | 0.0 | . . . | . . . | 3291 | 55.8 | 385 | 11.7 |
|
| |||||||||||||
| Philadelphia | 1133 | 913 | 80.6 | 67 | 7.3 | 892 | 78.7 | 37 | 4.2 | 657 | 58.0 | 112 | 17.1 |
|
| |||||||||||||
| San Francisco | 4111 | 3642 | 88.6 | 239 | 6.6 | 3286 | 79.9 | 78 | 2.4 | 2635 | 64.1 | 336 | 12.8 |
|
| |||||||||||||
| Virginiac | 383 | 285 | 74.4 | 23 | 8.1 | 0 | 0.0 | . . . | . . . | 0 | 0.0 | . . . | . . . |
|
| |||||||||||||
| Washingtonc | 2796 | 1975 | 70.6 | 135 | 6.8 | 2204 | 78.8 | 65 | 3.0 | 1813b | 64.8 | 314 | 17.3 |
|
| |||||||||||||
| Overall | 21 994 | 17 898 | 81.4 | 1495 | 8.4 | 6961 | 31.7 | 199 | 2.9 | 10 091 | 45.9 | 1427 | 14.1 |
Abbreviation: MSM, men who have sex with men.
Used culture throughout study period.
Switched from culture to nucleic acid amplification tests during study period.
Clinic locations: Denver, Colorado; New Haven and Hartford, Connecticut; New Orleans, Louisiana; Henrico and Chesterfield counties and Richmond, Virginia; Seattle, Washington.
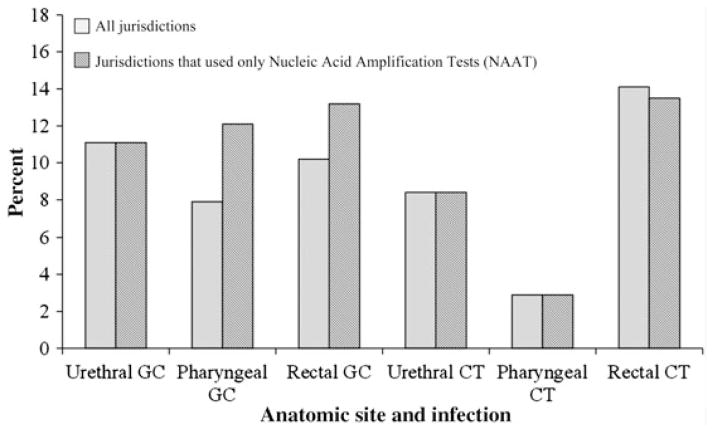
Of MSM tested, 2056 (11.1%) tested positive for urogenital GC at least once, either at their most recent visit or in the 12 months prior, 1144 (7.9%) tested positive for pharyngeal GC, and 1136 (10.2%) tested positive for rectal GC (Table 2). In total, extragenital GC infections made up 53% of all GC infections found among our sample of MSM. During the study period, every jurisdiction except Baltimore used NAATs exclusively for urogenital GC tests, and 4 jurisdictions (Baltimore, Los Angeles, Philadelphia, and San Francisco) used NAATs exclusively for extragenital GC tests. When restricting the analysis to those sites that used only NAATs, the proportion of MSM who were positive for urogenital GC remained the same at 11.1%, whereas the proportion of MSM who were positive for pharyngeal GC increased to 12.1% and the proportion of MSM who were positive for rectal GC increased to 13.2% (Figure 1).
Figure 1.
Proportion of men who have sex with men who tested positive for gonorrhea and chlamydia by anatomic site and test type. Abbreviations: CT, chlamydia; GC, gonorrhea.
To examine the proportion of men who were positive for GC at >1 site at the same visit, we reviewed 2042 visits where MSM were positive for GC at any anatomic site and were tested for GC at all 3 anatomic sites. Of these, 1426 (69.8%) were positive for GC at a single anatomic site, 508 (24.9%) were positive at 2 anatomic sites, and 108 (5.3%) were positive at all 3 anatomic sites.
Chlamydia
Overall, 17 898 (81.4%; range, 23.8%–96.4% by jurisdiction) MSM were tested for urogenital CT, 6961 (31.7%; range, 0%–79.9%) were tested for pharyngeal CT, and 10 091 (45.9%; range, 0%–64.8%) were tested for rectal CT either at their most recent visit or in the 12 months before their most recent visit (Table 2). Of MSM tested, 1495 (8.4%) tested positive for urogenital CT at least once, 199 (2.9%) tested positive for pharyngeal CT, and 1427 (14.1%) tested positive for rectal CT (Table 2). Extragenital CT infections comprised 52% of all CT infections found among our sample of MSM. Every jurisdiction that tested for urogenital and pharyngeal CT used NAATs at these sites. Six jurisdictions (Baltimore, Chicago, Los Angeles, New York City, Philadelphia, and San Francisco) of 7 that tested for rectal CT used only NAATs during the study period; the proportion of MSM who tested positive for rectal CT decreased slightly to 13.5% when restricting to these 6 jurisdictions (Figure 1).
To examine the number of men who were positive for CT at >1 site, we reviewed 966 visits where MSM were positive for CT and were tested for CT at all 3 anatomic sites. Of these, 809 (83.7%) were positive for CT at a single anatomic site, 152 (15.7%) were positive at 2 anatomic sites, and 5 (0.5%) were positive at all 3 anatomic sites.
Co-Infections
Dual GC and CT infections were found in 799 (20.4%) of 3910 visits where MSM had a positive GC test and in 799 (25.6%) of 3124 visits where MSM had a positive CT test. Of 1043 positive GC tests and 875 positive CT tests diagnosed in those 799 visits, 672 (64.4% of positive GC tests and 76.8% of CT tests) were positive for both GC and CT at the same anatomic site. The remaining GC/CT co-infections were located at discordant anatomic sites.
Prevalent HIV infection was reported in 478 (23.3%) MSM with urogenital GC, 266 (23.3%) MSM with pharyngeal GC, and 406 (35.7%) MSM with rectal GC. Similarly, among MSM with CT, 263 (17.6%) with urogenital CT, 63 (31.7%) with pharyngeal CT, and 489 (34.3%) with rectal CT were infected with HIV.
Infections That May Have Been Missed
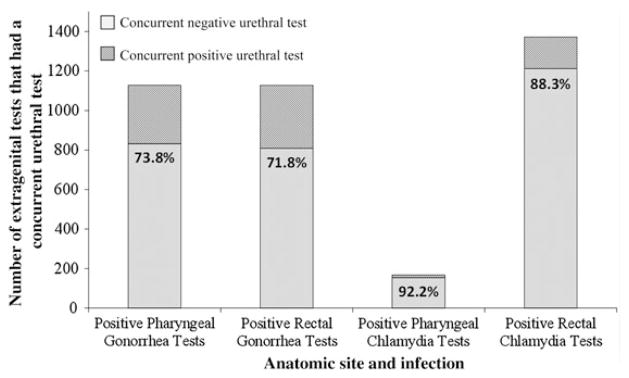
Among the 1136 MSM who tested positive for rectal GC, there were 1234 positive rectal GC tests; during the same visits when the 1234 positive rectal GC tests were performed, 1127 (91.3%) urethral GC tests were also performed. Of those urethral tests, 809 (71.8%) were negative, indicating that 71.8% of positive rectal GC tests in our sample would not have been detected, and presumably would have remained untreated, if only urethral screening had been performed (Figure 2). Associated urethral tests were negative for 73.8% of pharyngeal GC infections, 92.2% of pharyngeal CT infections, and 88.3% of rectal CT infections.
Figure 2.
Proportion of extragenital gonorrhea and chlamydia infections associated with concurrent negative urethral tests.
DISCUSSION
This multisite study demonstrates that extragenital GC and CT infections are common among MSM tested at STD clinics in the United States and highlights a number of critical issues. First, more than half of the GC/CT infections identified among this sample of MSM seen at STD clinics were not in the urethra. Second, in many jurisdictions, a larger proportion of MSM were positive for GC/CT at extragenital sites than were positive for urogenital GC/CT, although the proportion of positive MSM may have been affected by clinic testing practices because MSM with certain risk behaviors, such as multiple or anonymous partners, may be more likely to be tested at extragenital sites. Additionally, as has been shown in earlier single-site studies [12, 13], most patients with extragenital GC/CT infections did not have concurrent urethral infections (Figure 2). Among MSM who had tests performed at both urogenital and extragenital sites, >70% of extragenital GC infections and >85% of extragenital CT infections were associated with a negative urethral test and would not have been identified, and perhaps would have remained untreated, if only urethral screening had been performed. Importantly, across the diverse SSuN jurisdictions, the high prevalence of pharyngeal GC, rectal GC, and rectal CT and the low prevalence of pharyngeal CT found in this study support the current CDC recommendations to screen MSM attending STD clinics at least annually for pharyngeal and rectal GC and rectal CT.
Although many MSM in this population were screened for extragenital GC/CT at least annually according to CDC recommendations, a number of MSM were not screened for extragenital GC/CT at all. Population-based studies investigating sexual behaviors of MSM in the United States indicate that 62%–90% of MSM participated in unprotected receptive oral sex in the past 6 months and that 57%–83% of MSM participated in receptive anal sex in the past 6 months–1 year [14–17]. If these numbers are extrapolated to our sample of MSM, it seems likely that many MSM at risk of extragenital infection were not screened, possibly resulting in many infections being missed because MSM who seek care at STD clinics may be at higher risk for STDs than MSM seeking care in other healthcare settings.
The proportion of MSM screened at extragenital sites varied widely by SSuN jurisdiction, and ongoing evaluations of barriers to extragenital screening will help elucidate the reasons for this variability. Most jurisdictions that used NAATs had higher screening rates than those that used culture. However, this is not true for all jurisdictions—for example, New York City used culture-based diagnostics for extragenital GC detection throughout the entire study period and had screening rates similar to jurisdictions that used NAATs—so laboratory capabilities cannot solely account for the differences in screening rates. There may be a number of other factors influencing the rates of extragenital screening in jurisdictions, including presence or absence of extragenital screening protocols, the length of time such protocols have been in place, whether the jurisdictions directly operate the STD clinics themselves or the clinics are operated by a separate authority, and whether clinics that currently use NAATs are asking patients to use self-collected specimens, which may be easier for clinics and more acceptable to patients [18, 19].
GC and CT co-infection was common. Dual GC and CT infections were found in 20% of visits where MSM had a positive GC test and 26% of visits where MSM had a positive CT test. However, these are likely an underestimate because 13% of rectal GC tests and 50% of pharyngeal GC tests were not performed with concurrent extragenital CT tests at the same visit. Additionally, HIV infection was common among MSM diagnosed with extragenital GC or CT, although the true proportion of MSM infected with HIV may be larger because HIV status was not collected routinely in all jurisdictions. More than 1 in 3 patients with rectal GC, rectal CT, and pharyngeal CT had a documented HIV infection, and almost 1 in 4 patients with urogenital or pharyngeal GC had a documented HIV infection, which suggests that routine STD screening may serve to identify MSM at risk of acquiring or transmitting HIV.
Given that most MSM receive primary healthcare from a private provider [20] and that extragenital GC/CT screening rates of high-risk MSM in healthcare settings other than STD clinics has been shown to be much lower than in our study [9, 21–23], efforts are needed to facilitate implementation of CDC screening recommendations for MSM in both public and private sectors. Because extragenital testing depends on both providers eliciting exposure histories and patients disclosing exposure, a multifaceted approach should be used, including both provider training and direct patient outreach. The comfort level of health providers in evaluating same-sex partner sexual behaviors can be a barrier to STD screening, and disclosure of sexual behaviors in a healthcare setting remains difficult for many MSM [24–27].
The fact that extragenital infections are typically asymptomatic highlights the importance of screening based on a comprehensive history that includes sites of sexual exposure. However, a recent study found that 49%–60% of rectal GC/CT infections were missed with symptom- and sexual history–based testing among MSM when compared with universal testing for GC/CT at the rectum [28]. Although screening based on a comprehensive history that includes sites of sexual exposure is an important component in identifying and treating extragenital GC/CT infections, certain settings may want to consider adopting universal screening as a means to increase the identification and treatment of extragenital GC/CT infections among MSM.
Recent studies have shown that self-collected rectal and pharyngeal specimens are of equal quality to, and sometimes better quality, than those taken by clinicians and are highly acceptable among MSM [18, 19, 29–31]. Efforts to increase the use of self-collected extragenital specimens may be another method of facilitating appropriate screening in this population.
There are a number of limitations to this study. First, we were unable to determine the true number of MSM that should have been screened. The CDC recommends that MSM be screened at anatomic sites of exposure, but for this analysis, history of exposure at each anatomic site was not available. Additionally, we were unable to assess why patients were not screened at these clinic visits. It is possible, for instance, that patients were offered screening but refused or that patients were recently screened at another healthcare facility. However, given that extragenital screening rates at non–STD clinics are much lower than those found in our study, this was unlikely to have a large impact on our results. Our results may not be generalizable to MSM in other healthcare settings. Finally, although these data are the most recent data available, clinic screening practices are rapidly changing. For instance, an increasing number of jurisdictions are validating the use of NAATs, which may facilitate extragenital testing because NAATs are less labor-intensive and more sensitive than culture specimens [8].
In summary, this evaluation shows that a substantial burden of GC and CT infection among MSM would not have been identified, and presumptively would not have been treated, if screening and treatment were based on urethral screening alone. To control the ongoing spread of GC and CT infections and potentially reduce the risk of HIV transmission among MSM, efforts are needed to expand the availability and use of rectal and pharyngeal NAATs for this population and to facilitate implementation of appropriate screening practices.
Acknowledgments
Financial support. This work was supported by the Centers for Disease Control and Prevention, Division of STD Prevention, STD Surveillance Network (CDC RFA PS08-865).
Footnotes
Clinical Infectious Diseases
Published by Oxford University Press on behalf of the Infectious Diseases Society of America 2014. This work is written by (a) US Government employee(s) and is in the public domain in the US.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance, 2011. Atlanta: US Department of Health and Human Services; 2012. [Google Scholar]
- 2.Fleming DT, Wasserheit JN. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm Infect. 1999;75:3–17. doi: 10.1136/sti.75.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernstein K, Marcus J, Nieri G, Philip S, Klausner J. Rectal gonorrhea and chlamydia reinfection is associated with increased risk of HIV seroconversion. J Acquir Immune Defic Syndr. 2010;53:537–43. doi: 10.1097/QAI.0b013e3181c3ef29. [DOI] [PubMed] [Google Scholar]
- 4.Craib KJ, Meddings DR, Strathdee SA, et al. Rectal gonorrhoea as an independent risk factor for HIV infection in a cohort of homosexual men. Genitourin Med. 1995;71:150–4. doi: 10.1136/sti.71.3.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wiesner PJ, Tronca E, Bonin P, Pedersen AH, Holmes KK. Clinical spectrum of pharyngeal gonococcal infection. New Engl J Med. 1973;288:181–5. doi: 10.1056/NEJM197301252880404. [DOI] [PubMed] [Google Scholar]
- 6.Klein EJ, Fisher LS, Chow AW, Guze LB. Anorectal gonococcal infection. Ann Intern Med. 1977;86:340–6. doi: 10.7326/0003-4819-86-3-340. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Sexually Transmitted Diseases Treatment Guidelines, 2010. MMWR Morbid Mortal Wkly Rep. 2010;59:12. [Google Scholar]
- 8.Ota KV, Tamari IE, Smieja M, et al. Detection of Neisseria gonorrhoeae and Chlamydia trachomatis in pharyngeal and rectal specimens using the BD Probetec ET system, the Gen-Probe Aptima Combo 2 assay and culture. Sex Transm Infect. 2009;85:182–6. doi: 10.1136/sti.2008.034140. [DOI] [PubMed] [Google Scholar]
- 9.Kent KA, Klausner JD, Bernstein KT, et al. Clinic-based testing for rectal and pharyngeal Neisseria gonorrhoeae and Chlamydia trachomatis infections by community-based organizations—five cities, United States, 2007. MMWR Morb Mortal Wkly Rep. 2009;58:716–9. [PubMed] [Google Scholar]
- 10.Rietmeijer CA. Here comes the SSuN: Early experiences with the STD Surveillance Network. Public Health Rep. 2009;124:72–7. doi: 10.1177/00333549091240S211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schachter J, Moncada J, Liska S, Shayevich C, Klausner JD. Nucleic acid amplification tests in the diagnosis of chlamydial and gonococcal infections of the oropharynx and rectum in men who have sex with men. Sex Transm Dis. 2008;35:637–42. doi: 10.1097/OLQ.0b013e31817bdd7e. [DOI] [PubMed] [Google Scholar]
- 12.Kent CK, Chaw JK, Wong W, et al. Prevalence of rectal, urethral, and pharyngeal chlamydia and gonorrhea detected in 2 clinical settings among men who have sex with men: San Francisco, California, 2003. Clin Infect Dis. 2005;41:67–74. doi: 10.1086/430704. [DOI] [PubMed] [Google Scholar]
- 13.Marcus JL, Bernstein KT, Kohn RP, Liska S, Philip SS. Infections missed by urethral-only screening for chlamydia or gonorrhea detection among men who have sex with men. Sex Transm Dis. 2011;38:922–4. doi: 10.1097/OLQ.0b013e31822a2b2e. [DOI] [PubMed] [Google Scholar]
- 14.Osmond DH, Buchbinder S, Cheng A, et al. Prevalence of Kaposi sarcoma–associated herpesvirus infection in homosexual men at beginning of and during the HIV epidemic. JAMA. 2002;287:221–5. doi: 10.1001/jama.287.2.221. [DOI] [PubMed] [Google Scholar]
- 15.Heywood W, Smith AM. Anal sex practices in heterosexual and male homosexual populations: a review of population-based data. Sex Health. 2012;9:517–26. doi: 10.1071/SH12014. [DOI] [PubMed] [Google Scholar]
- 16.Osmond DH, Page K, Wiley J, et al. HIV infection in homosexual and bisexual men 18 to 29 years of age: the San Francisco Young Men’s Health Study. Am J Public Health. 1994;84:1933–7. doi: 10.2105/ajph.84.12.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. HIV risk, prevention, and testing behaviors among men who have sex with men–National HIV Behavioral Surveillance System, 21 U.S cities, United States 2008. MMWR Morbid Mortal Wkly Rep. 2011;60:1–34. [PubMed] [Google Scholar]
- 18.van der Helm JJ, Hoebe CJ, van Rooijen MS, et al. High performance and acceptability of self-collected rectal swabs for diagnosis of Chlamydia trachomatis and Neisseria gonorrhoeae in men who have sex with men and women. Sex Transm Dis. 2009;36:493–7. doi: 10.1097/OLQ.0b013e3181a44b8c. [DOI] [PubMed] [Google Scholar]
- 19.Dodge B, Van Der Pol B, Reece M, et al. Rectal self-sampling in non-clinical venues for detection of sexually transmissible infections among behaviourally bisexual men. Sex Health. 2012;9:190–1. doi: 10.1071/SH11068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention. Dear Colleague: Letter on Recommended Sexually Transmitted Diseases (STD) Prevention Services for Men Who Have Sex With Men (MSM) Atlanta: US Department of Health and Human Services; 2004. [Google Scholar]
- 21.Hoover K, Butler M, Workowski K, et al. STD screening of HIV-infected MSM in HIV clinics. Sex Transm Dis. 2010;37:771–6. doi: 10.1097/OLQ.0b013e3181e50058. [DOI] [PubMed] [Google Scholar]
- 22.Tai E, Sanchez T, Lansky A, Mahle K, Heffelfinger J, Workowski K. Self-reported syphilis and gonorrhoea testing among men who have sex with men: national HIV behavioural surveillance system, 2003–5. Sex Transm Infect. 2008;84:478–82. doi: 10.1136/sti.2008.030973. [DOI] [PubMed] [Google Scholar]
- 23.Mark KE, Gunn RA. Gonorrhea surveillance: estimating epidemiologic and clinical characteristics of reported cases using a sample survey methodology. Sex Transm Dis. 2004;31:215–20. doi: 10.1097/01.olq.0000118421.15177.34. [DOI] [PubMed] [Google Scholar]
- 24.Mimiaga M, Goldhammer H, Belanoff C, Tetu A, Mayer K. Men who have sex with men: perceptions about sexual risk, HIV and sexually transmitted disease testing, and provider communication. Sex Transm Dis. 2007;34:113–9. doi: 10.1097/01.olq.0000225327.13214.bf. [DOI] [PubMed] [Google Scholar]
- 25.Henry Reid L, O’Connor K, Klein J, Cooper E, Flynn P, Futterman D. Current pediatrician practices in identifying high-risk behaviors of adolescents. Pediatrics. 2010;125:e741–7. doi: 10.1542/peds.2009-0271. [DOI] [PubMed] [Google Scholar]
- 26.Bernstein K, Liu K-L, Begier E, Koblin B, Karpati A, Murrill C. Same-sex attraction disclosure to health care providers among New York City men who have sex with men: implications for HIV testing approaches. Arch Intern Med. 2008;168:1458–64. doi: 10.1001/archinte.168.13.1458. [DOI] [PubMed] [Google Scholar]
- 27.Dean L, Heyer IH, Robinson K, et al. Lesbian, gay, bisexual, and trans-gender health: findings and concerns. J Gay Lesbian Med Assoc. 2000;4:101–49. [Google Scholar]
- 28.van Liere GAFS, Hoebe CJPA, Niekamp A-M, Koedijk FDH, Dukers-Muijrers NHTM. Standard symptom- and sexual history-based testing misses anorectal Chlamydia trachomatis and Neisseria gonorrhoeae infections in swingers and men who have sex with men. Sex Transm Dis. 2013;40:285–9. doi: 10.1097/OLQ.0b013e31828098f8. [DOI] [PubMed] [Google Scholar]
- 29.Sexton ME, Baker JJ, Nakagawa K, et al. How reliable is self-testing for gonorrhea and chlamydia among men who have sex with men? J Fam Pract. 2013;62:70–8. [PubMed] [Google Scholar]
- 30.Freeman AH, Bernstein KT, Kohn RP, Philip S, Rauch LM, Klausner JD. Evaluation of self-collected versus clinician-collected swabs for the detection of Chlamydia trachomatis and Neisseria gonorrhoeae pharyngeal infection among men who have sex with men. Sex Transm Dis. 2011;38:1036–9. doi: 10.1097/OLQ.0b013e318227713e. [DOI] [PubMed] [Google Scholar]
- 31.Alexander S, Ison C, Parry J, et al. Self-taken pharyngeal and rectal swabs are appropriate for the detection of Chlamydia trachomatis and Neisseria gonorrhoeae in asymptomatic men who have sex with men. Sex Transm Infect. 2008;84:488–92. doi: 10.1136/sti.2008.031443. [DOI] [PubMed] [Google Scholar]