Abstract
Importance
Although depression and type 2 diabetes may independently increase dementia risk, no studies have examined whether the risk of dementia among people with both is higher than the sum of each individually.
Objective
To examine risk of all-cause dementia among persons with depression, diabetes or both compared to those with neither.
Design
A population-based cohort study of 2,454,532 adults, including 477,133 (19.4%) with depression, 223,174 (9.1%) with diabetes and 95,691 (3.9%) with both.
Setting
Denmark
Participants
All dementia-free Danish citizens ≥50 years old between January 1, 2007 through 2013.
Main outcome measure
Dementia was ascertained by physician diagnosis from the Danish National Patient Register, the Danish Psychiatric Central Register (DPCR), and/or prescription of a cholinesterase inhibitor or memantine from the Danish National Prescription Registry (DNPR). Depression was ascertained by psychiatrist diagnosis from the DPCR or antidepressant prescription from the DNPR. Diabetes was identified using the Danish National Diabetes Register. The risk of all-cause dementia associated with diabetes, depression or both was estimated using Cox proportional hazards regression models that adjusted for potential confounding factors such as demographics and potential intermediates such as medical comorbidity.
Results
During 13,834,645 million person-years of follow-up, 59,663 (2.4%) developed dementia of whom 6,466 (10.8%) had diabetes, 15,729 (26.4%) had depression and 4,022 (6.7%) had both. The adjusted hazard ratio of developing all-cause dementia was 1.83 (95% confidence interval: 1.80, 1.87) for persons with depression, 1.20 (95% CI: 1.17, 1.23) for persons with diabetes, and 2.17 (95% CI: 2.10, 2.24) for those with both as compared to those with neither. The excess risk of all-cause dementia observed for individuals with comorbid depression and diabetes surpassed the summed risk associated with the two individually, especially for younger persons. The corresponding Attributable Proportion due to the interaction of comorbid depression and diabetes was 0.25 (95% CI = 0.13, 0.36; P<0.001) for those under 65 years old and 0.06 (95% CI = 0.02, 0.10; P=0.001) for those over 65.
Conclusions
Depression and diabetes were independently associated with greater dementia risk and the combined association of the two disorders with risk of all-cause dementia was stronger than additive.
INTRODUCTION
Diabetes and major depression are very common in western populations. Type 2 diabetes occurs in approximately 8–14% of most western populations,1 while approximately 25% of women and 16% of men will have a major depressive episode over their lifetime.2 Up to 20% of persons with type 2 diabetes have comorbid depression.3 Furthermore, a recent meta-analysis has established a bidirectional link between depression and diabetes.4 Patients with comorbid depression and diabetes have poorer adherence to diet, smoking cessation, exercise, and diabetes-controlling medications.5 Depression is also associated with increased cortisol levels,6 autonomic nervous system dysregulation7 and increased inflammation,8 all of which worsen glycemic control. Therefore, it is not surprising that patients with comorbid depression and diabetes have increased risk of microvascular and macrovascular complications as well as mortality.9
An extensive literature has also identified that diabetes and depression are independent risk factors for dementia. A meta-analysis found that persons with diabetes have a 47% increased risk of all-cause dementia.10 Two recent meta-analyses found that depression doubled subsequent all-cause dementia risk.11,12 Two additional studies have shown that comorbid depression and type 2 diabetes was associated with a two-fold greater risk of developing all-cause dementia versus diabetes alone.13,14 A study of over 29,000 patients with type 2 diabetes developed a 10-year risk prediction model for dementia, identifying depression as an important risk factor.15 Another recent study using data from a large randomized trial aimed at optimally controlling glycemic and cardiovascular risk factors in patients with diabetes found that those with comorbid depression and type 2 diabetes versus those with diabetes alone had greater risk of cognitive decline over a 40-month period.16
However, these prior studies have all been limited to diabetes cohorts13–16 and so could not ascertain whether people with both depression and diabetes have an elevated dementia risk due to an additive (or more than additive) interaction between the two. Given the increasing incidence of dementia in aging modern societies,17 understanding the risk associated with potentially modifiable depression and diabetic disease trajectories is essential.
In a population-based cohort of 2.4 million adults, we aimed to study the risk of all-cause dementia among persons with diabetes, depression, and comorbid diabetes and depression versus those with neither illness. Given rapidly increasing diabetes incidence in younger age groups18 and the demographic, clinical, and prognostic differences in patients who develop diabetes in middle versus older age,19,20 we also examined whether age (comparing those younger than 65 versus those older than 65) modified the risk of all-cause dementia in this population.
METHODS
Population
We conducted a population-based cohort study using data from the Danish Civil Registration System.21 This register includes information on gender, month of birth, and continuously updated information on vital status and migration since 1968. In this register, Danish citizens are each assigned a unique personal identification number, providing accurate linkage to person-level data.21 Diagnoses in the registers are classified according to the Danish version of the International Classification of Diseases, 8th Revision [ICD-8]22 before January 1, 1994. Hereafter, diagnoses were classified according to the ICD-10.23 Our cohort included all individuals who were ≥50 years of age between January 1, 2007 through 2013, born in Denmark, free of dementia, and alive as of January 1, 2007. We followed our sample from 2007 through 2013 in order to ensure maximum validity of dementia diagnoses24 and homogenous calendar period.
The study protocol was approved by the Danish Data Protection Agency and the Danish Health and Medicines Authority.
Primary Independent Variables
Our primary independent variables of interest were the presence of either depression, diabetes, or comorbid depression and diabetes. We identified individuals with depression by either a depression diagnosis made by a psychiatrist or redemption of at least one antidepressant prescription using data from the Danish Psychiatric Central Register (DPCR) 25 and the Danish National Prescription Registry (DNPR) (see Appendix 1).26 The DPCR contains diagnostic information on all psychiatric admissions since 1969 and outpatient specialty mental health visits since 1995.25 The DNPR contains information on all prescriptions dispensed at Danish pharmacies since 1995, including day of purchase and classification of drugs according to the Anatomical Therapeutic Chemical (ATC) Classification.27 Individuals with schizophrenia, schizoaffective disorders or bipolar disorder were censored at the date of diagnosis (see Appendix 2). We supplemented our depression definition by identifying all antidepressant prescriptions (i.e. selective serotonin re-uptake inhibitors, monoamine oxidase inhibitors, and other non-tricyclic (TCA) antidepressants) redeemed between 1995 and 2014 (see Appendix 1). Our primary depression definition did not include redemption of TCA prescriptions because of their frequent use for insomnia and/or pain, nor bupropion or trazodone since neither was approved for the treatment of depression in Denmark during the study period.
Individuals diagnosed with diabetes between 1990 and 2014 were identified in the Danish National Diabetes Register using a validated algorithm28 (see Appendix 3). The Register’s registration of diabetes is considered complete from 1995 onwards with a sensitivity of 86% and a positive predictive value of 89%.28
Outcome of Interest
We identified incident all-cause dementia using data from the Danish National Patient Register,29 the DPCR, and the DNPR (see Appendix 4). The Danish National Patient Register contains information on all Danish medical hospitalizations since 1977 as well as all outpatient contacts since 1995.29 Approximately two-thirds of all dementia cases in Denmark are diagnosed within the secondary health care system.24 While the diagnosis of all-cause dementia in either the Danish National Patient Register or the DPCR has a positive predictive value of 86%,30 the validity is lower among individuals under age 65 for dementia sub-types.31 We identified all inpatient or outpatient contacts with a diagnosis of dementia made between 1969 and 2014 based on a validated algorithm.24 In addition, we supplemented our all-cause dementia definition with redemption of at least one prescription for a cholinesterase inhibitor or memantine between 1995 and 2014. We excluded all cases of dementia prevalent before January 1, 2007 to identify all incident cases of dementia.
Covariates of Interest
Covariates were chosen a priori based on their availability and prior research identifying their potential associations with depression, diabetes and dementia risk.32
We obtained marital status information (defined as married/living in a registered partnership or single) from the Danish Civil Registration System.
We used the Danish National Patient Register to obtain data on all hospital contacts between 1977 and 2014 for one or more of the following chronic diseases: ischemic heart disease, congestive heart failure (CHF), peripheral vascular disease, atrial fibrillation/flutter, cerebrovascular disease, traumatic brain injury (TBI), chronic pulmonary disease, renal disease, retinopathy and neuropathy (see Appendix 6).
Statistical Analysis
We used Cox proportional hazards regression models to estimate hazard ratios (HRs) and 95% Confidence Intervals (95%CIs) for the associations between depression, diabetes and risk of all-cause dementia. Age was chosen as the underlying time scale and was thus intrinsically corrected for. Individuals contributed at-risk time from January 1, 2007 or from their 50th birthday, whichever came last (delayed entry). Censoring occurred at day of dementia, day of schizophrenia or bipolar disorder diagnosis, death, immigration from Denmark, at their 100th birthday or January 1, 2014, whichever came first.
Apart from using age as the time scale, our primary regression model was adjusted for gender, marital status and calendar period. Next, we adjusted for potential intermediates on the pathway from depression and diabetes to dementia, including medical comorbidities (i.e. ischemic heart disease, CHF, peripheral vascular disease, atrial fibrillation/flutter, cerebrovascular disease, TBI, and chronic pulmonary disease) and diabetes complications (i.e. renal disease, retinopathy and neuropathy). 10–12 In order to minimize the possibility that any associations between depression and all-cause dementia risk could be confounded by similarities between late-life depressive symptoms and prodromal dementia,33 we added two years to the date of initial depression diagnosis or initial antidepressant prescription.
Furthermore, because guidelines recommend that individuals with suspected dementia have fasting blood glucose or HbA1c levels drawn as part of the medical work-up34 and are therefore likely to be diagnosed with diabetes soon after dementia diagnosis, one year was added to the date of initial diabetes diagnosis. To validate this approach, we performed a sensitivity analysis where we repeated our regression models stratified by time since depression and time since diabetes without postponement of these exposures; these models were adjusted for age, gender and marital status.
We examined whether there was an additive interaction by testing the hypothesis of no excess hazard due to the interaction.35 We performed interaction analyses between diabetes and depression using the entire sample as well as stratifying by age (i.e. over/under 65 years) and calculated the attributable proportion (AP) due to interaction as a measure of the excess HR for individuals with both conditions not explained by the independent effects of either. In this setting, the attributable proportion is given by the following formula: APinteraction = (HRdep+dm-HRdep-HRdm+1)/HRdep+dm.35 All interaction analyses were adjusted for age, gender, marital status and calendar period.
We conducted two secondary analyses. First, we created a categorical variable denoting early-versus late-onset diabetes using the median age of onset, 63, as the cut-point. To facilitate this categorization based on the National Diabetes Register, this analysis was restricted to individuals born after 1932. Next, we ascertained the associations of our independent variables of interest with risk of Alzheimer’s disease or vascular dementia diagnoses individually in regression models adjusted for age, gender, calendar period and marital status.
In a sensitivity analysis, we examined whether our findings were impacted by expanding our definition of depression to include TCA prescription(s).
We used two-sided significance tests for all analyses with statistical significance set at P < 0.05. The proportional hazards assumption was assessed graphically for all variables using the log-minus-log plots, finding no violations. All statistical analyses were performed using Stata 13 (StataCorp, College Station, Texas, USA).
RESULTS
We followed a cohort of 2,454,532 individuals for a total of 13,834,645 million person-years including 477,133 (19.4%) with a diagnosis of depression, 223,174 (9.1%) with diabetes and 95,691 (3.9%) with comorbid depression and diabetes. The mean age of initial diabetes diagnosis was 63.1 (Standard Deviation [SD]: 12.0) and 58.5 (SD: 13.5) for depression.
During the study period, 59,663 (2.4%) persons developed dementia. The mean age at first dementia diagnosis was 80.9 (SD: 8.7). Of those who developed dementia, 15,729 (26.4%) had depression alone, 6,466 (10.8%) had diabetes alone, and 4,022 (6.7%) had comorbid depression and diabetes (See Table 1).
Table 1.
Patient Characteristics from a Population-Based Danish Cohort (All Years)
| NO. WITH DEMENTIA |
NO. WITHOUT DEMENTIA* |
PERSON YEARS AT RISK |
|
|---|---|---|---|
| Total | 59,663 | 2,394,869 | 13,834,645 |
| Age | |||
| <65 years | 3,269 | 1,147,053 | 7,604,829 |
| ≥65 years | 56,394 | 1,247,816 | 6,229,815 |
| Gender | |||
| Women | 35,843 | 1,232,159 | 7,214,160 |
| Men | 23,820 | 1,162,710 | 6,620,485 |
| Calendar period | |||
| 2007 | 8,545 | 50,572 | 1,920,880 |
| 2008 | 8,669 | 48,940 | 1,938,299 |
| 2009 | 9,109 | 49,185 | 1,954,875 |
| 2010 | 8,924 | 49,152 | 1,973,373 |
| 2011 | 8,296 | 47,250 | 1,994,020 |
| 2012 | 8,089 | 47,271 | 2,016,872 |
| 2013 | 8,031 | 2,102,499 | 2,036,325 |
| Exposure diseases | |||
| None | 33,446 | 1,625,088 | 10,116,443 |
| Diabetes | 6,466 | 216,708 | 1,048,696 |
| Depression | 15,729 | 461,404 | 2,310,165 |
| Depression and diabetes | 4,022 | 91,669 | 359,341 |
| Marital status | |||
| Married | 22,360 | 1,393,722 | 8,589,713 |
| Single | 35,329 | 968,408 | 5,084,545 |
| Missing | 1,974 | 32,739 | 160,386 |
| Chronic diseases | |||
| Ischemic heart disease | 12,622 | 308,389 | 1,444,251 |
| Congestive heart failure | 5,909 | 120,500 | 410,542 |
| Peripheral vascular disease | 5,097 | 125,448 | 508,264 |
| Atrial fibrillation or flutter | 9,725 | 185,422 | 698,446 |
| Cerebrovascular disease | 14,713 | 231,213 | 971,917 |
| Traumatic brain injury | 5,601 | 155,803 | 744,497 |
| Chronic pulmonary disease | 7,024 | 221,382 | 927,227 |
| Renal disease/Nephropathy | 2,603 | 80,353 | 254,381 |
| Retinopathy | 1,526 | 42,222 | 208,846 |
| Neuropathy | 1,592 | 37,854 | 161,895 |
| Duration of diabetes | |||
| No diabetes | 49,175 | 2,086,492 | 12,426,608 |
| 0–2 years | 9,322 | 223,292 | 1,213,969 |
| 2–4 years | 763 | 39,848 | 130,709 |
| 4–6 years | 372 | 32,469 | 56,967 |
| > 6 years | 31 | 12,768 | 6,392 |
| Anti-diabetic treatment | |||
| No insulin | 57,386 | 2,327,123 | 13,526,400 |
| Insulin | 2,277 | 67,746 | 308,245 |
Persons are assigned the category in which they are last observed in the study.
Compared with persons without depression or diabetes, diabetes alone was associated with 20% greater risk of all-cause dementia (HR = 1.20, 95% CI = 1.17, 1.23), depression alone with 83% greater risk, (HR = 1.83, 95% CI = 1.80, 1.87) and comorbid depression and diabetes with 117% higher risk (HR = 2.17, 95% CI = 2.10, 2.24) after adjustment for age, gender, calendar period and marital status. The estimates decreased slightly after adjustment for chronic diseases (Table 2).
Table 2.
Adjusted hazard ratios (HRs) for the risk of All-Cause of dementia among persons with depression alone, diabetes alone, and both diseases, compared to persons with neither disease
| Neither Depression Nor Diabetes |
Diabetes | Depression | Depression and diabetes |
|
|---|---|---|---|---|
| HR(95%CI)a | 1(ref) | 1.20 (1.17;1.23) | 1.83 (1.80;1.87) | 2.17 (2.10;2.24) |
| HR(95%CI) b | 1(ref) | 1.14 (1.11;1.17) | 1.68 (1.65;1.71) | 1.88 (1.82;1.94) |
| HR(95%CI) c | 1(ref) | 1.15 (1.12;1.18) | 1.83 (1.79;1.86) | 2.07 (2.00;2.14) |
| HR(95%CI) d | 1(ref) | 1.11 (1.08;1.14) | 1.68 (1.64;1.71) | 1.82 (1.76;1.89) |
Adjusted for age, gender, calendar period and marital status
As a but also adjusted for ischemic heart disease, congestive heart failure, peripheral vascular disease, atrial fibrillation or flutter, cerebrovascular disease, traumatic brain injury and chronic pulmonary disease
As a but also adjusted for retinopathy, renal disease and neuropathy
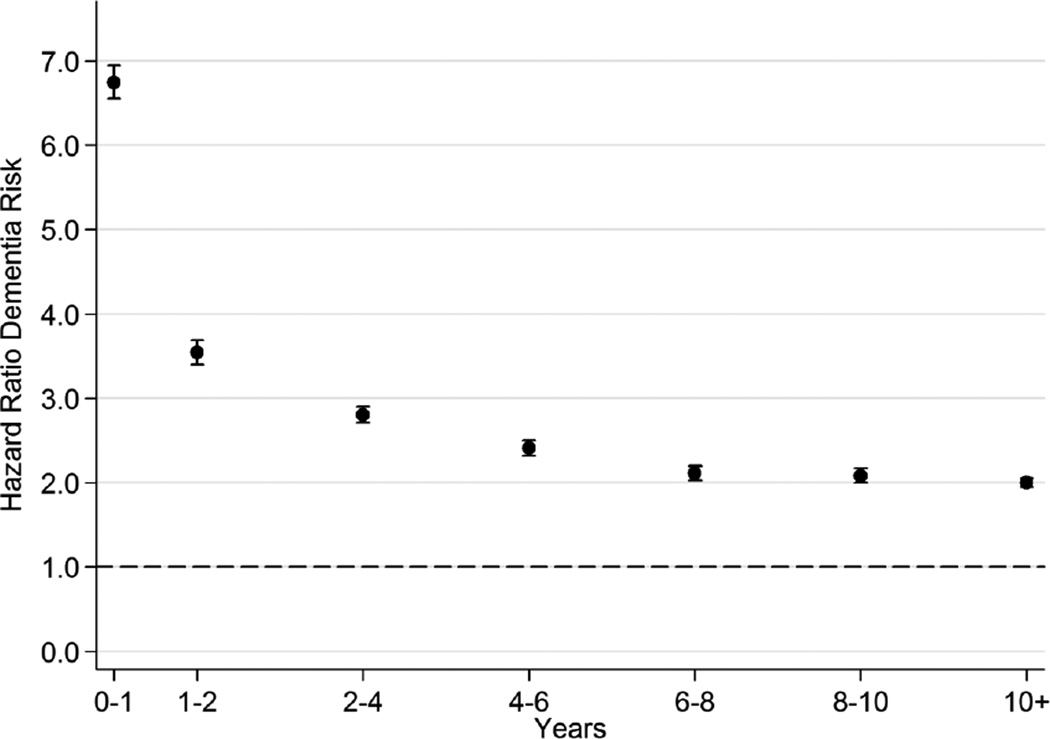
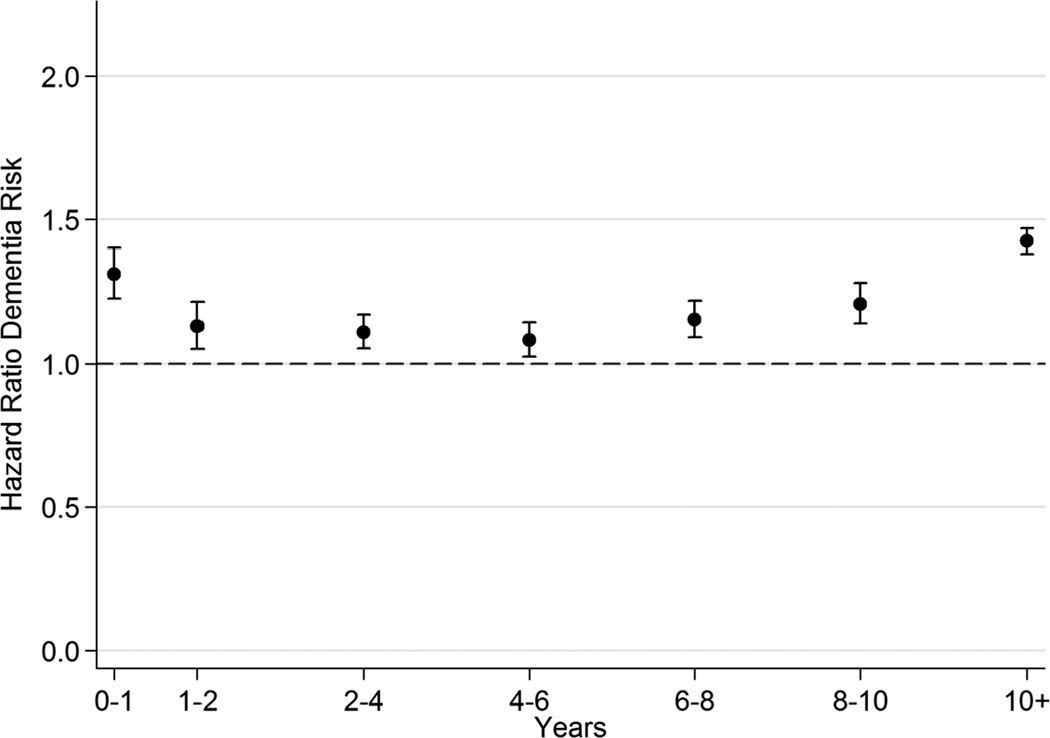
As shown in Figure 1, during the first year after depression, the associated hazard of all-cause dementia was elevated by nearly seven-fold (HR=6.75, 95% CI = 6.55, 6.95), but thereafter it decreased consistently to a constant hazard of approximately two (as compared to those without depression). As shown in Figure 2, during the first year after diabetes diagnosis, the associated hazard of all-cause dementia was 31% higher (HR=1.31, 95% CI = 1.22, 1.40), with a decrease in subsequent years. The long-term HR rose to 42% higher at 10 years post-diabetes diagnosis (HR=1.42, 95% CI = 1.38, 1.47).
Figure 1.
Time Since Depression Diagnosis and All-Cause Dementia Risk*
* Adjusted for age, gender, calendar year, and marital status.
Figure 2.
Time Since Diabetes Diagnosis and All-Cause Dementia Risk*
* Adjusted for age, gender, calendar year, and marital status.
Among participants under age 65, the HRs for all-cause dementia were 2.93 (95% CI = 2.71, 3.16) for depression alone, 1.71 (95% CI = 1.49, 1.97) for diabetes alone and 4.84 (95% CI = 4.21, 5.55) for those with both (See Table 3). The combined effect of the two illnesses on all-cause dementia risk was larger than the sum of the two individual diseases; i.e., the AP due to the interaction was 0.25 (95% CI = 0.13, 0.36; P < 0.001) for persons younger than 65 and 0.06, 95% CI = 0.02, 0.100; P = 0.001) for those older than 65.
Table 3.
Interaction analyses: Hazard ratios (HRs) for the risk of All-Cause dementia among persons with depression alone, diabetes alone, and depression and diabetes, compared to persons with neither disease
| Neither Depression Nor Diabetes |
Depression HR(95% CI) |
Diabetes HR(95% CI) |
Depression and diabetes HR(95% CI) |
Attributable proportion due to interaction (95% CI) |
P-value for H:AP=0 |
|
|---|---|---|---|---|---|---|
| Total | 1 (ref) | 1.83 (1.80;1.87) |
1.20 (1.17;1.23) |
2.17 (2.10;2.24) |
0.06 (0.03;0.10) | 0.001 |
| Age-stratified: | ||||||
| <65 years | 1 (ref) | 2.93 (2.71;3.16) |
1.71 (1.49;1.97) |
4.84 (4.21;5.55) |
0.25 (0.13;0.36) | <0.001 |
| >65 years | 1 (ref) | 1.78 (1.75;1.82) |
1.18 (1.15;1.21) |
2.08 (2.01;2.16) |
0.06 (0.02;0.10) | 0.001 |
When we examined the impact of age at diabetes-onset, the HR for the association between early onset-diabetes and all-cause dementia risk was significantly higher than that for late-onset diabetes (early-onset: HR = 1.82; 95% CI = 1.73, 1.91; late-onset: HR = 1.30, 95%CI = 1.24, 1.36; P < 0.001).
Diabetes, depression and their comorbid combination were all associated with increased Alzheimer’s disease risk (diabetes alone: HR = 1.06, 95%CI = 1.01, 1.11; depression alone: HR = 1.39, 95%CI = 1.35, 1.44; comorbid depression and diabetes: HR = 1.46, 95%CI = 1.37, 1.55). However, the magnitude of the associations of diabetes, depression and their comorbid combination with vascular dementia risk were more pronounced (diabetes alone: HR = 1.55, 95%CI = 1.44, 1.66; depression alone: HR = 2.42, 95%CI = 2.29, 2.55; comorbid depression and diabetes: HR = 3.56, 95%CI = 3.28, 3.86).
Finally, our results were unaffected by expanding our depression definition to include TCA prescription(s) redemption (diabetes alone: HR = 1.20, 95%CI = 1.17, 1.24; depression alone: HR = 1.79, 95%CI = 1.75, 1.82; comorbid depression and diabetes: HR = 2.07, 95%CI = 2.01, 2.14).
DISCUSSION
In a nationwide, population-based cohort study of over 2.4 million persons ≥50 years old, diabetes and depression were associated with increased risk of all-cause dementia, and the combined effect of both disorders appeared more than additive, especially among younger persons. Among those with depression and diabetes in our cohort, six percent of incident dementia may be accounted for by the interaction between depression and diabetes overall, and 25% among those under age 65. Although the underlying risk of dementia is low in this younger age group, the marked increase in incidence of diabetes in younger age groups18 makes this finding quite worrisome.
Our study extends beyond prior studies by identifying that compared to a population with neither depression nor diabetes, depression alone is associated with the highest relative risk of all-cause dementia. Further, we found similar results when examining associations with risk of Alzheimer’s disease or vascular dementia, though the magnitude of the associations of depression as well as comorbid depression and diabetes with vascular dementia risk was more pronounced, in line with the results of a recent meta-analysis.36 Additionally, we found that having both depression and diabetes is associated with a level of risk greater than the sum of the two illnesses. Although underlying causal mechanisms are unclear, one explanation could be depression and diabetes having many shared risk factors for dementia including increased inflammation, decreased insulin sensitivity, autonomic nervous system dysregulation, obesity and vascular disease.37
Prior studies have found that patients with depression and diabetes are younger than those with diabetes alone and were diagnosed with diabetes approximately five years earlier.38 Also, depression earlier in life may be a risk factor for developing type 2 diabetes.4 Given that depression in patients with diabetes is associated with poor self-care, treatment non-adherence and adverse psychobiologic changes,5–8 this younger age group with comorbid depression and diabetes may be vulnerable to developing dementia later in life.
From a public health perspective, developing screening and interventions improving both quality of depression and diabetes treatment in this subgroup of patients could be important in reducing dementia risk. A recent prospective cohort study of 1,433 older adults with diabetes found that both effective treatment of diabetes and depression and improving diet could lead to as much as a 20% decrease in incident dementia.39 Primary care-based collaborative care models have been developed and shown to reduce depressive symptoms in patients with comorbid depression and chronic medical illnesses such as diabetes and heart disease.40–42 Although adequately powered trials of these interventions to test their effects on preventing dementia may require very large sample sizes (e.g., > 50,000 subjects) and long durations (e.g., 15–20 years), they are warranted given dementia’s societal costs.
Our study has several strengths and limitations. We followed a nationwide cohort virtually without loss to follow-up making non-response bias an unlikely explanation for our findings. Also, information on diabetes, depression and dementia was collected prospectively and did not rely on patient or proxy recall. An important limitation of our study was that the population was from a single country with a well-developed national health care system and relatively homogenous population, therefore limiting generalizability. However, this factor should improve internal validity since the role of socioeconomic factors in health care-seeking behavior is expected to be minimal. Further, our definition of depression was based on a combination of psychiatric diagnoses and antidepressant prescription records, thereby introducing selection bias since patients with more severe depression are more likely to be prescribed antidepressants and/or referred to psychiatrists.43,44 This issue is further complicated by inability to capture depressed individuals who have not sought treatment.45 Similarly, the use of ICD-8 and ICD-10 codes to identify dementia and diabetes cases could miss patients with initial symptoms of these illnesses until symptoms or functional impairments become more prominent, as well as limit ability to accurately differentiate dementia subtypes.46,47 A further limitation was lack of data on possible confounders such as health-risk behaviors including smoking, obesity and sedentary lifestyle. However, these lifestyle factors may be mediators of the associations presented here, and previous studies did not find attenuation of the association of comorbid depression and diabetes with dementia risk after adjusting for health-risk behaviors.13,14 Finally, residual confounding remains a possibility, as in any observational study.
In conclusion, we found that depression and diabetes were both associated with greater risk of all-cause dementia, as well as both Alzheimer’s disease and vascular dementia. These associations appeared to be stronger among those with depression alone compared to those with diabetes alone. Persons with co-existing diabetes and depression appeared to have the highest relative risk of dementia, and this association tended to be stronger than additive. The interaction between diabetes and depression tended to be particularly strong for individuals under 65. In light of the increasing societal burden of chronic diseases, further research is needed to elucidate the pathophysiologic mechanisms linking depression, diabetes, and adverse outcomes such as dementia, as well as to develop interventions aimed at preventing these dreaded complications.
ACKNOWLEDGEMENTS
Funding: The study has been supported by an unrestricted grant from the Lundbeck Foundation. Dr. Davydow is supported by grant KL2 TR000421 from the National Institutes of Health.
Role of Sponsor: The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
APPENDICES
APPENDIX 1: Information on depression obtained from the Danish Psychiatric Central Register and the Danish National Prescription Registry
EXPOSURES (Appendices 1-3)
A diagnosis of depression was identified if at least one of the following criteria applied
-
Registration of a diagnosis of depression in the Danish Psychiatric Central Register.
And/or
Registration of at least one prescription of antidepressants redeemed in the Danish National Prescription Registry
Diagnosis according to a record of depression in the Danish Psychiatric Central Register
| ICD-8 | ICD-10 |
|---|---|
| 296.09, 296.29, 296.99, 298.09, 300.49, and 300.19 |
F32, F33 |
Diagnosis according to a record of prescriptions for antidepressants in the Danish National Prescription Registry
| Name | Drug | ATC-codes |
|---|---|---|
| SSRI (Selective serotonin re-uptake inhibitors) |
Fluoxetine, citalopram, paroxetine, sertraline, fluvoxamine, and escitalopram |
N06AB |
| MAOIs (Monoamine oxidase inhibitors) | Isocarboxazid and moclobemide | N06AF, N06AG |
| Other antidepressants | Mianserin, nefazodone, mirtazapine, venlafaxine, reboxetine, duloxetine, and agomelatine |
N06AX |
| Tricyclic antidepressants (TCAs) | Desipramine, imipramine, imipramine oxide, clomipramine, opipramol, trimipramine, Iofepramine, dibenzepin, amitriptyline, nortriptyline, protriptyline, doxepin, iprindole, melitracen, butriptyline, dosulepin, amoxapine, dimetacrine, amineptine, maprotiline, quinupramine |
N06AA |
APPENDIX 2: Information on severe mental illness obtained from the Danish Psychiatric Central Register
| ICD-8 | ICD-10 | |
|---|---|---|
| Schizophrenia | 295 (excluding 295.79) | F20 |
| Schizoaffective disorders | 295.79, 296.8 | F25 |
| Bipolar affective disorders | 296.19, 296.39 | F30, F31 |
APPENDIX 3: Information on diabetes obtained from the Danish National Diabetes Register
Algorithm: Individuals were classified as having diabetes on the day where at least one of the following six criteria was met
A diagnosis of diabetes made at any Danish hospital as registered in the Danish National Patient Register (ICD-8:249, 250; ICD-10:E10-14, H36.0, O24, excluding O24.4).
A referral to chiropody of diabetic patients as registered in the Danish National Health Service Register.
Five blood glucose measurements within one year as registered in the Danish National Health Service Register.
Two blood glucose measurements per year for five consecutive years as registered in the Danish National Health Service Register.
Two redemptions of oral anti-diabetic drugs within six months as registered in the Danish National Prescription Registry.
Two redemptions of prescribed insulin as registered in the Danish National Prescription Registry.
APPENDIX 4: Information on dementia obtained from the Danish Psychiatric Central Register, the Danish National Patient Register, and the Danish National Prescription Registry
OUTCOME
A diagnosis of dementia was identified if at least one of the following criteria applied
-
Registration of a diagnosis of dementia in the Danish Psychiatric Central Register or the Danish National Patient Register
And/or
Registration of at least one prescription of anti-dementia drug redeemed in the Danish National Prescription Registry
Diagnosis according to a record of dementia in the Danish Psychiatric Central Register or the Danish National Patient Register
| ICD-8 | ICD-10 | |
|---|---|---|
| AD (Alzheimer’s disease) | 290.10 | F00.0, F00.1, F00.2, F00.9, G30.0, G30.1, G30.8, G30.9 |
| VaD (Vascular dementia) | 293.09–19 | F01.0, F01.1, F01.2, F01.3, F01.8, F01.9 |
| FTD (Frontotemporal dementia) | 290.11 | F02.0 |
| Dementia without specification | 290.09–19 | F03.9 |
Diagnosis according to a record of prescriptions for anti-dementia drugs in the Danish National Prescription Registry
| Name | Drug | ATC-codes |
|---|---|---|
| Anticholinesterases | ||
| Tacrine | N06DA01 | |
| Donepezil | N06DA02 | |
| Rivastigmine | N06DA03 | |
| Galantamine | N06DA04 | |
| Donepezil and Memantine | N06DA52 | |
| Other anti-dementia drugs | ||
| Memantine | N06DX1 |
APPENDIX 5: Information on marital status obtained from the Danish Civil Registration System
COVARIATES
| Family type/marital status |
|---|
| Single |
| Partners/married |
APPENDIX 6: Information on chronic diseases and diabetic complications obtained from the Danish National Patient Register
| ICD-8 | ICD-10 | |
|---|---|---|
| Ischemic heart disease | 410–414 | I20–I25 |
| Congestive heart failure | 427.09, 427.10, 427.11, 427.19, 428.99, 782.49 |
I50; I11.0; I13.0; I13.2 |
| Peripheral vascular disease | 440, 441, 442, 443, 444, 445, | I70; I71; I72; I73; I74; I77 |
| Atrial fibrillation or flutter | 427.93, 427.94 | I48 |
| Cerebrovascular disease | 430–438 | I60–I69; G45; G46 |
| Traumatic Brain Injury | 850.99, 851.29–854.99, 800.99–801.09, 803.99 |
S06.0, S06.1-S06.9, S02– S02.1, S02.7, S02.9 |
| Chronic pulmonary disease | 490–493, 515–518 | J40–J47; J60–J67; J68.4; J70.1; J70.3; J84.1; J92.0; J96.1; J98.2; J98.3 |
| Renal Disease | 249.02, 250.02, 403,404,580- 583, 584, 590.09, 593.19, 753.10–753.19, 792 |
I12; I13; N00-N05; N07; N08.3, N11; N14; N17- N19; Q61 E10.2, E11.2, E14.2 |
| Retinopathy | 249.01, 250.01, 377.00 |
H33.4, H36.0, H43.1, H45.0, E10.3, E11.3, E14.3 |
| Neuropathy | 249.03, 250.03 | G62.9, G63.2, E10.4, E11.4 |
Footnotes
Conflicts of Interest: The authors have no relevant conflicts of interest to disclose.
Access to Data and Data Analysis: Dr. Vestergaard had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
REFERENCE LIST
- 1.Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metr. 2010;8:29. doi: 10.1186/1478-7954-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kessler RC, Petukhova M, Sampson NA, Zaslavsky AM, Wittchen HU. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res. 2012 Sep;21(3):169–184. doi: 10.1002/mpr.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ali S, Stone MA, Peters JL, Davies MJ, Khunti K. The prevalence of co-morbid depression in adults with Type 2 diabetes: a systematic review and meta-analysis. Diabet. Med. 2006 Nov;23(11):1165–1173. doi: 10.1111/j.1464-5491.2006.01943.x. [DOI] [PubMed] [Google Scholar]
- 4.Mezuk B, Eaton WW, Albrecht S, Golden SH. Depression and type 2 diabetes over the lifespan: a meta-analysis. Diabetes Care. 2008 Dec;31(12):2383–2390. doi: 10.2337/dc08-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin EH, Katon W, Von Korff M, et al. Relationship of depression and diabetes self-care, medication adherence, and preventive care. Diabetes Care. 2004 Sep;27(9):2154–2160. doi: 10.2337/diacare.27.9.2154. [DOI] [PubMed] [Google Scholar]
- 6.Vreeburg SA, Hoogendijk WJ, van Pelt J, et al. Major depressive disorder and hypothalamic-pituitary-adrenal axis activity: results from a large cohort study. Arch. Gen. Psychiatry. 2009 Jun;66(6):617–626. doi: 10.1001/archgenpsychiatry.2009.50. [DOI] [PubMed] [Google Scholar]
- 7.Kemp AH, Quintana DS, Gray MA, Felmingham KL, Brown K, Gatt JM. Impact of depression and antidepressant treatment on heart rate variability: a review and meta-analysis. Biol. Psychiatry. 2010 Jun 1;67(11):1067–1074. doi: 10.1016/j.biopsych.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 8.Dowlati Y, Herrmann N, Swardfager W, et al. A meta-analysis of cytokines in major depression. Biol. Psychiatry. 2010 Mar 1;67(5):446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 9.Lin EH, Rutter CM, Katon W, et al. Depression and advanced complications of diabetes: a prospective cohort study. Diabetes Care. 2010 Feb;33(2):264–269. doi: 10.2337/dc09-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu FP, Lin KP, Kuo HK. Diabetes and the risk of multi-system aging phenotypes: a systematic review and meta-analysis. PLoS One. 2009;4(1):e4144. doi: 10.1371/journal.pone.0004144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jorm AF. Is depression a risk factor for dementia or cognitive decline? A review. Gerontology. 2000 Jul-Aug;46(4):219–227. doi: 10.1159/000022163. [DOI] [PubMed] [Google Scholar]
- 12.Ownby RL, Crocco E, Acevedo A, John V, Loewenstein D. Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Arch. Gen. Psychiatry. 2006 May;63(5):530–538. doi: 10.1001/archpsyc.63.5.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katon WJ, Lin EH, Williams LH, et al. Comorbid depression is associated with an increased risk of dementia diagnosis in patients with diabetes: a prospective cohort study. J. Gen. Intern. Med. 2010 May;25(5):423–429. doi: 10.1007/s11606-009-1248-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katon W, Lyles CR, Parker MM, Karter AJ, Huang ES, Whitmer RA. Association of depression with increased risk of dementia in patients with type 2 diabetes: the Diabetes and Aging Study. Arch. Gen. Psychiatry. 2012 Apr;69(4):1410–417. doi: 10.1001/archgenpsychiatry.2011.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Exalto LG, Biessels GJ, Karter AJ, et al. Risk score for prediction of 10 year dementia risk in individuals with type 2 diabetes: a cohort study. Lancet Diabetes Endocrinol. 2013 Nov;1(3):183–190. doi: 10.1016/S2213-8587(13)70048-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sullivan MD, Katon WJ, Lovato LC, et al. Association of depression with accelerated cognitive decline among patients with type 2 diabetes in the ACCORD-MIND trial. JAMA Psychiatry. 2013 Oct;70(10):1041–1047. doi: 10.1001/jamapsychiatry.2013.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reitz C, Brayne C, Mayeux R. Epidemiology of Alzheimer disease. Nat Rev Neurol. 2011 Mar;7(3):137–152. doi: 10.1038/nrneurol.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.May AL, Kuklina EV, Yoon PW. Prevalence of cardiovascular disease risk factors among US adolescents, 1999–2008. Pediatrics. 2012 Jun;129(6):1035–1041. doi: 10.1542/peds.2011-1082. [DOI] [PubMed] [Google Scholar]
- 19.Song SH, Hardisty CA. Early onset type 2 diabetes mellitus: a harbinger for complications in later years--clinical observation from a secondary care cohort. QJM. 2009 Nov;102(11):799–806. doi: 10.1093/qjmed/hcp121. [DOI] [PubMed] [Google Scholar]
- 20.Chan JC, Lau ES, Luk AO, et al. Premature mortality and comorbidities in young-onset diabetes: a 7-year prospective analysis. Am. J. Med. 2014 Jul;127(7):616–624. doi: 10.1016/j.amjmed.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 21.Pedersen CB. The Danish Civil Registration System. Scand J Public Health. 2011 Jul;39(7 Suppl):22–25. doi: 10.1177/1403494810387965. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization. Manual of the International Classification of diseases (ICD-8) Geneva: 1967. [Google Scholar]
- 23.World Health Organization. International Statistical Classification of Diseases and Related Health Problems (ICD10) 1992 [Google Scholar]
- 24.Phung TK, Waltoft BL, Kessing LV, Mortensen PB, Waldemar G. Time trend in diagnosing dementia in secondary care. Dement. Geriatr. Cogn. Disord. 2010;29(2):146–153. doi: 10.1159/000269933. [DOI] [PubMed] [Google Scholar]
- 25.Mors O, Perto GP, Mortensen PB. The Danish Psychiatric Central Research Register. Scand J Public Health. 2011 Jul;39(7 Suppl):54–57. doi: 10.1177/1403494810395825. [DOI] [PubMed] [Google Scholar]
- 26.Kildemoes HW, Sorensen HT, Hallas J. The Danish National Prescription Registry. Scand J Public Health. 2011 Jul;39(7 Suppl):38–41. doi: 10.1177/1403494810394717. [DOI] [PubMed] [Google Scholar]
- 27.Skrbo A, Zulic I, Hadzic S, Gaon ID. [Anatomic-therapeutic-chemical classification of drugs] Med. Arh. 1999;53(3 Suppl 3):57–60. [PubMed] [Google Scholar]
- 28.Carstensen B, Kristensen JK, Marcussen MM, Borch-Johnsen K. The National Diabetes Register. Scand J Public Health. 2011 Jul;39(7 Suppl):58–61. doi: 10.1177/1403494811404278. [DOI] [PubMed] [Google Scholar]
- 29.Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scand J Public Health. 2011 Jul;39(7 Suppl):30–33. doi: 10.1177/1403494811401482. [DOI] [PubMed] [Google Scholar]
- 30.Phung TK, Andersen BB, Hogh P, Kessing LV, Mortensen PB, Waldemar G. Validity of dementia diagnoses in the Danish hospital registers. Dement. Geriatr. Cogn. Disord. 2007;24(3):220–228. doi: 10.1159/000107084. [DOI] [PubMed] [Google Scholar]
- 31.Salem LC, Andersen BB, Nielsen TR, et al. Overdiagnosis of dementia in young patients - a nationwide register-based study. Dement. Geriatr. Cogn. Disord. 2012;34(5–6):292–299. doi: 10.1159/000345485. [DOI] [PubMed] [Google Scholar]
- 32.de Toledo Ferraz Alves TC, Ferreira LK, Wajngarten M, Busatto GF. Cardiac disorders as risk factors for Alzheimer’s disease. J Alzheimers Dis. 2010;20(3):749–763. doi: 10.3233/JAD-2010-091561. [DOI] [PubMed] [Google Scholar]
- 33.Butters MA, Young JB, Lopez O, et al. Pathways linking late-life depression to persistent cognitive impairment and dementia. Dialogues Clin Neurosci. 2008;10(3):345–357. doi: 10.31887/DCNS.2008.10.3/mabutters. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feldman HH, Jacova C, Robillard A, et al. Diagnosis, treatment of dementia: 2. Diagnosis. CMAJ. 2008 Mar 25;178(7):825–836. doi: 10.1503/cmaj.070798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andersson T, Alfredsson L, Kallberg H, Zdravkovic S, Ahlbom A. Calculating measures of biological interaction. Eur. J. Epidemiol. 2005;20(7):575–579. doi: 10.1007/s10654-005-7835-x. [DOI] [PubMed] [Google Scholar]
- 36.Diniz BS, Butters MA, Albert SM, Dew MA, Reynolds CF., 3rd Late-life depression and risk of vascular dementia and Alzheimer’s disease: systematic review and meta-analysis of community-based cohort studies. The British journal of psychiatry : the journal of mental science. 2013 May;202(5):329–335. doi: 10.1192/bjp.bp.112.118307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ismail K, Winkley K, Stahl D, Chalder T, Edmonds M. A cohort study of people with diabetes and their first foot ulcer: the role of depression on mortality. Diabetes Care. 2007 Jun;30(6):1473–1479. doi: 10.2337/dc06-2313. [DOI] [PubMed] [Google Scholar]
- 38.Katon W, von Korff M, Ciechanowski P, et al. Behavioral and clinical factors associated with depression among individuals with diabetes. Diabetes Care. 2004 Apr;27(4):914–920. doi: 10.2337/diacare.27.4.914. [DOI] [PubMed] [Google Scholar]
- 39.Ritchie K, Carriere I, Ritchie CW, Berr C, Artero S, Ancelin ML. Designing prevention programmes to reduce incidence of dementia: prospective cohort study of modifiable risk factors. BMJ. 2010;341:c3885. doi: 10.1136/bmj.c3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Katon WJ, Von Korff M, Lin EH, et al. The Pathways Study: a randomized trial of collaborative care in patients with diabetes and depression. Arch. Gen. Psychiatry. 2004 Oct;61(10):1042–1049. doi: 10.1001/archpsyc.61.10.1042. [DOI] [PubMed] [Google Scholar]
- 41.Williams JW, Jr, Katon W, Lin EH, et al. The effectiveness of depression care management on diabetes-related outcomes in older patients. Ann. Intern. Med. 2004 Jun 15;140(12):1015–1024. doi: 10.7326/0003-4819-140-12-200406150-00012. [DOI] [PubMed] [Google Scholar]
- 42.Katon WJ, Lin EH, Von Korff M, et al. Collaborative care for patients with depression and chronic illnesses. N. Engl. J. Med. 2010 Dec 30;363(27):2611–2620. doi: 10.1056/NEJMoa1003955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gaynes BN, Rush AJ, Trivedi M, et al. A direct comparison of presenting characteristics of depressed outpatients from primary vs. specialty care settings: preliminary findings from the STAR*D clinical trial. Gen. Hosp. Psychiatry. 2005 Mar-Apr;27(2):87–96. doi: 10.1016/j.genhosppsych.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 44.Cooper-Patrick L, Crum RM, Ford DE. Characteristics of patients with major depression who received care in general medical and specialty mental health settings. Med. Care. 1994 Jan;32(1):15–24. doi: 10.1097/00005650-199401000-00002. [DOI] [PubMed] [Google Scholar]
- 45.Bet PM, Hugtenburg JG, Penninx BW, Balkom A, Nolen WA, Hoogendijk WJ. Treatment inadequacy in primary and specialized care patients with depressive and/or anxiety disorders. Psychiatry Res. 2013 Dec 15;210(2):594–600. doi: 10.1016/j.psychres.2013.06.023. [DOI] [PubMed] [Google Scholar]
- 46.Linn RT, Wolf PA, Bachman DL, et al. The ‘preclinical phase’ of probable Alzheimer’s disease. A 13-year prospective study of the Framingham cohort. Arch. Neurol. 1995 May;52(5):485–490. doi: 10.1001/archneur.1995.00540290075020. [DOI] [PubMed] [Google Scholar]
- 47.Pratley RE. The early treatment of type 2 diabetes. Am. J. Med. 2013 Sep;126(9 Suppl 1):S2–S9. doi: 10.1016/j.amjmed.2013.06.007. [DOI] [PubMed] [Google Scholar]