Abstract
BACKGROUND
This study evaluated the tolerability and antitumor activity of AMG 386, a peptibody (a peptide Fc fusion) that neutralizes the interaction of angiopoietin-1 and angiopoietin-2 with Tie2 (tyrosine kinase with immunoglobulin-like and EGF-like domains 2), plus sorafenib in patients with clear cell metastatic renal cell carcinoma (mRCC) in a randomized controlled study.
METHODS
Previously untreated patients with mRCC were randomized 1:1:1 to receive sorafenib 400 mg orally twice daily plus intravenous AMG 386 at 10 mg/kg (arm A) or 3 mg/kg (arm B) or placebo (arm C) once weekly (qw). Patients in arm C could receive open-label AMG 386 at 10 mg/kg qw plus sorafenib following disease progression. The primary endpoint was progression-free survival (PFS).
RESULTS
A total of 152 patients were randomized. Median PFS was 9.0, 8.5, and 9.0 months in arms A, B, and C, respectively (hazard ratio for arms A and B vs arm C, 0.88; 95% confidence interval [CI], 0.60–1.30; P = .523). The objective response rate (95% CI) for arms A, B, and C, respectively, was 38% (25%–53%), 37% (24%–52%), and 25% (14%–40%). Among 30 patients in arm C who had disease progression and subsequently received open-label AMG 386 at 10 mg/kg qw, the objective response rate was 3% (95% CI, 0%–17%). Frequently occurring adverse events (AEs) included diarrhea (arms A/B/C, 70%/67%/56%), palmar-plantar erythrodysesthesia syndrome (52%/47%/54%), alopecia (50%/45%/50%), and hypertension (42%/49%/46%). Fifteen patients had grade 4 AEs (arms A/B/C, n = 3/7/5); 4 had fatal AEs (n = 2/1/1), with 1 (abdominal pain, arm B) considered possibly related to AMG 386.
CONCLUSIONS
In patients with mRCC, AMG 386 plus sorafenib was tolerable but did not significantly improve PFS compared with placebo plus sorafenib.
Keywords: sorafenib, AMG 386, clear cell renal cell carcinoma, randomized controlled trial, phase 2 clinical trial
Upregulation of proangiogenic factors in response to inactivation of the von Hippel-Lindau (VHL) gene is a critical component in the development and progression of clear cell renal cell carcinoma (RCC).1 Several inhibitors of the vascular endothelial growth factor (VEGF) signaling pathway have been shown to improve outcomes in patients with metastatic RCC (mRCC).1 However, because almost all patients ultimately develop resistance to therapy, combination treatment strategies that may result in more complete angiogenesis inhibition are of interest.2
The angiopoietin-1/angiopoietin-2 and Tie2 (tyrosine kinase with immunoglobulin-like and EGF-like domains 2) receptor axis may be a legitimate target for inhibiting angiogenesis in mRCC. Preclinical studies have demonstrated that its components are regulated by VHL and are dysregulated in RCC cell lines.3 Plasma angiopoietin-2 concentrations are significantly elevated in patients with mRCC (compared with localized disease or healthy controls), and increase at the time of disease progression.4 Concurrent blockade of the angiopoietin and VEGF pathways augments inhibition of angiogenesis and tumor growth in tumor xenograft models.5 Hence, combinations of angiopoietin/Tie2 inhibitors and VEGF inhibitors might induce clinically meaningful activity.
AMG 386 is an investigational recombinant peptide-Fc fusion protein that neutralizes the receptor-ligand interaction between Tie2 and angiopoietin-1/2.5 In Colo205 xenograft models, simultaneous antagonism of angiopoietin-1/2 with AMG 386 suppressed tumor growth more effectively than did selective inhibition of angiopoietin-1 or angiopoietin-2 alone.5 Interim results of a phase 1b study suggested that treatment of patients who have mRCC with sorafenib or sunitinib plus AMG 386 had an acceptable toxicity profile, distinct from that of VEGF inhibitors, and may have antitumor activity.6 We evaluated in a phase 2 study the tolerability and anti-tumor activity of AMG 386 plus sorafenib in previously untreated patients who have clear cell mRCC.
MATERIALS AND METHODS
Patients
Eligible patients (≥18 years) had previously untreated, histologically confirmed mRCC with a clear-cell component; good/intermediate risk per Memorial Sloan-Kettering Cancer Center (MSKCC) prognostic classification; ≥1 unidimensionally measurable lesion per Response Evaluation Criteria in Solid Tumors (RECIST), version 1.0; complete radiologic assessment and tumor measurement ≤28 days before randomization; Eastern Cooperative Oncology Group performance status ≤1; had not received systemic therapy for mRCC; and had adequate hematologic, renal, and hepatic function. Key exclusion criteria were unresected primary tumor; history of brain metastases; arterial or venous thrombosis within 6 months; bleeding diathesis or significant bleeding within 14 days; uncontrolled hypertension (>90/>150 mm Hg); focal radiation within 14 days or radiation-induced toxicity; and ongoing pancreatitis. Patients provided written, informed consent. Study procedures were approved by an institutional review board or independent ethics committee.
Study Design and Treatment
This randomized, double-blind, placebo-controlled, phase 2 study was conducted at 41 centers in North America and Europe. Patients received sorafenib 400 mg orally twice daily (bid) and were randomly assigned 1:1:1 using an interactive voice response system to also receive AMG 386 at 10 mg/kg qw (arm A) or 3 mg/kg qw (arm B), or placebo qw (arm C), by intravenous infusion over 30 to 60 minutes. Randomization was stratified by MSKCC risk (good versus intermediate). Treatment continued until disease progression, clinical progression, or unacceptable toxicity. Investigators and patients were blinded to treatment assignments until disease progression. After disease progression and unblinding, patients in arm C who continued to meet eligibility criteria could choose to receive open-label AMG 386 at 10 mg/kg qw plus sorafenib. Doses of AMG 386 could be withheld and doses of sorafenib could be withheld/modified per protocol-specified rules. Dose modifications for AMG 386 were not permitted.
Study Endpoints
The primary endpoint was progression-free survival (PFS) per investigator assessment defined as the time from randomization to disease progression per RECIST or death. Independent centralized radiologic review (RadPharm, Princeton, NJ) to confirm PFS and objective response rate (ORR) was a protocol-specified option. Secondary endpoints included: overall survival (time from randomization to death), ORR, duration of response, change in tumor burden, incidence of adverse events (AEs), anti-AMG 386 antibody formation, and pharmacokinetics (AMG 386 and sorafenib). Pharmacodynamic biomarkers were exploratory endpoints.
Assessments
Disease status and progression according to RECIST version 1.0 was assessed by computed tomography or magnetic resonance imaging of the chest, abdomen, and pelvis every 8 weeks and during long-term follow-up of patients who discontinued treatment before disease progression. Follow-up was up to 48 months after randomization.
All AEs occurring from randomization to safety follow-up were recorded, classified, and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0.
Anti-AMG 386 antibodies and AMG 386 concentrations in the serum were analyzed using previously described techniques.7 Plasma sorafenib concentrations were determined using a validated liquid chromatography tandem mass spectrometry method. Serum concentrations of pharmacodynamic biomarkers were assessed by using enzyme-linked immunosorbent assay or multiplexed sandwich immunoassays as described.8
Statistical Analysis
The intent of the primary statistical analysis was to estimate the treatment effect on PFS of AMG 386 (at 2 doses) combined with sorafenib compared with placebo plus sorafenib. A minimum of 150 patients were needed for a hypothesized AMG 386/placebo PFS hazard ratio (HR) of 0.79 and a 2-sided 80% confidence interval (CI) with a maximum half-width of 0.23 (comparing arms A and B combined versus arm C) to generate estimates of the treatment effect. The primary analysis was planned at 113 PFS events. The study had approximately 49% power for a 2-sided 20% significance level log-rank test of the treatment effect for arms A and B versus arm C (HR for PFS of 0.79).
Efficacy endpoints were analyzed for the intent-to-treat population. Analyses of AEs included all randomized patients who received at least 1 dose of AMG 386 or sorafenib. The Cox regression model stratified by MSKCC risk was used to estimate HRs and 2-sided 80% and 95% CIs (calculated post facto) for comparisons between treatment arms. Tarone’s test stratified by MSKCC risk was used to descriptively assess increasing trends in PFS among the treatment arms. Kaplan-Meier estimates for the medians for PFS and overall survival were derived according to a previously described method.9 Exact binomial 95% CIs were calculated for ORR. Wilson’s score method with continuity correction was used to calculate 95% CIs for the differences in ORR between arms.
RESULTS
Patients
Between May 2007 and November 2008, 152 patients were randomized (arms A/B/C, n = 50/51/51) and received at least 1 dose of treatment; 1 patient randomized to placebo (arm C) withdrew from the study before having received any treatment. Demographics and baseline clinical characteristics were generally consistent across treatment arms (Table 1). However, the proportion of patients with >3 sites of metastasis (arms A/B/C, 22%/24%/12%) was higher and the sum of longest diameters of target lesions was greater among patients randomized to AMG 386, compared with those receiving placebo.
Table 1.
Baseline Demographics and Clinical Characteristics
| Characteristic | Arm A AMG 386, 10 mg/kg qw + Sorafenib (n = 50) | Arm B AMG 386, 3 mg/kg qw + Sorafenib (n = 51) | Arm C Placebo + Sorafenib (n = 51) |
|---|---|---|---|
| Men, % | 82 | 69 | 75 |
| Race/ethnicity, % | |||
| White | 98 | 92 | 94 |
| Black | 0 | 4 | 4 |
| Hispanic | 2 | 4 | 2 |
| Median (range) age, y | 60 (39–80) | 58 (28–84) | 59 (38–84) |
| Median (range) time since primary diagnosis, mo | 11.6 (1–323) | 7.5 (1–157) | 10.5 (1–106) |
| Number of sites of metastases, % | |||
| 1 | 20 | 22 | 24 |
| 2 | 42 | 27 | 31 |
| 3 | 16 | 27 | 31 |
| >3 | 22 | 24 | 12 |
| Unavailable | 0 | 0 | 2 |
| Most common metastatic sites, % | |||
| Bone | 20 | 25 | 27 |
| Liver | 16 | 31 | 18 |
| Lung | 82 | 73 | 71 |
| Lymph nodes | 46 | 49 | 47 |
| Median (range) sum of longest diameters of target lesions at baseline, mm | 85 (15–382) | 108 (11–466) | 80 (11–359) |
| Eastern Cooperative Oncology Group performance status, % | |||
| 0 | 62 | 57 | 55 |
| 1 | 38 | 43 | 45 |
| Memorial Sloan-Kettering Cancer Center prognostic risk classification, % | |||
| Good | 40 | 39 | 37 |
| Intermediate | 60 | 61 | 61 |
| Poor | 0 | 0 | 2a |
Protocol violation.
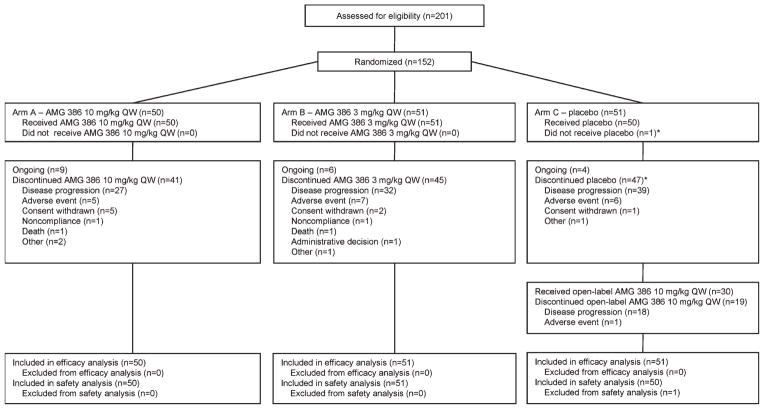
At the time of analysis, 19 patients continued to receive treatment (arms A/B/C, n = 9/6/4; Fig. 1). The most common reasons for treatment discontinuation were disease progression and AEs. Patients in arms A, B, and C received a median (range) of 35 (2–108), 31 (1–115), and 34 (2–101) infusions of AMG 386 or placebo, respectively. The overall median (range) follow-up time was 75 (1–124) weeks. Thirty patients in arm C received open-label AMG 386 (10 mg/kg qw) after disease progression, 11 of whom continued to receive AMG 386 at the time of analysis.
Figure 1.
Algorithm shows disposition of patients in the study. *One patient was reported as having ended placebo before receiving placebo (ie, the patient died before treatment started).
Efficacy
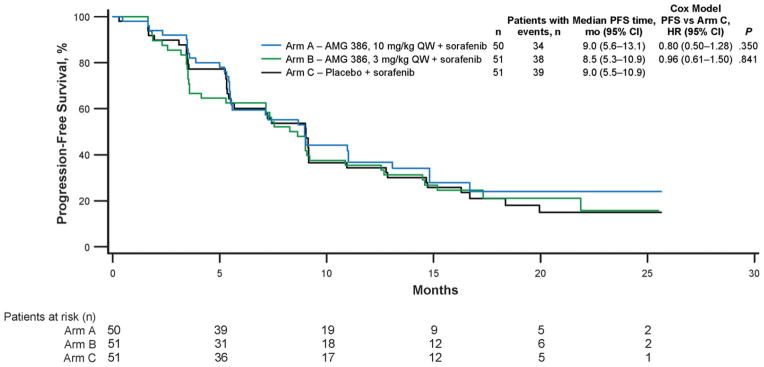
At the analysis cutoff time, 34 patients in arm A, 38 in arm B, and 39 in arm C have had disease progression. Median PFS time was similar across treatment arms (9.0, 8.5, and 9.0 months in arms A, B, and C, respectively; Fig. 2). The HR for arms A and B combined versus arm C was 0.88 (95% CI, 0.60–1.30; P = .52). There was no evidence of a dose-response relationship across the 3 treatment arms (Tarone’s test, P = .195). A protocol-specified sensitivity analysis that used an independent centralized read of all images showed a median PFS of 9.0 months (95% CI, 5.4–15.0 months) in arm A, 9.0 months (5.4–14.4 months) in arm B, and 7.2 months (5.4–12.8 months) in arm C. Eleven months after the primary analysis, 38% of patients in arm A, 45% in arm B, and 55% in arm C had died. Interim median (95% CI) overall survival was 29.2 months (22.2-NE [not estimable]) in arm B and 27.1 months (19.7-NE) in arm C but was not yet reached in arm A (95% CI, 24.3-NE).
Figure 2.
Graph shows progression-free survival.
The confirmed ORR in arms A, B, and C was 38%, 37%, and 25%, respectively (Table 2). The confirmed ORR per independent radiologic review in arms A, B, and C was 22%, 22%, and 18%, respectively. The mean maximum reduction from baseline in the sum of longest diameters of target lesions was −34.3% in arm A, −29.2% in arm B, and −23.8% in arm C.
Table 2.
Best Tumor Response per RECIST
| Arm A AMG 386, 10 mg/kg qw + Sorafenib (n = 50) | Arm B AMG 386, 3 mg/kg qw + Sorafenib (n = 51) | Arm C Placebo + Sorafenib (n = 51) | |
|---|---|---|---|
| Objective response, % | |||
| Complete response | 0 | 2 | 2 |
| Partial response | 38 | 35 | 24 |
| Stable disease | 48 | 45 | 59 |
| Progressive disease | 8 | 10 | 10 |
| Unevaluablea | 0 | 0 | 2 |
| Not done | 6 | 8 | 4 |
| Objective response rate, % (95% CI) | 38 (25–53) | 37 (24–52) | 25 (14–40) |
| Comparison with placebo, (95% CI) | (−6.9 to 30.8) | (−7.5 to 30.0) | |
| Duration of response, mo (95% CI)b | 8.9 (7.4-NE) | 7.4 (5.9-NE) | 9.4 (5.5-NE) |
Abbreviations: CI, confidence interval; NE, not estimable; RECIST, Response Evaluation Criteria in Solid Tumors.
Includes patients with a response assessment of complete response, partial response, or stable disease before the scheduled first assessment of response without an additional response assessment.
Time from the first confirmed objective response to disease progression/death.
Open-Label AMG 386
Thirty patients in arm C received open-label AMG 386 at 10 mg/kg qw plus sorafenib after disease progression. One patient, who had a best response of stable disease during the blinded study period, achieved a partial response with a 40% reduction in tumor burden (small-volume lung metastases) after initiation of open-label treatment. Seventeen (57%) patients achieved stable disease and 11 (37%) had progressive disease after crossover; 31% of patients had some reduction in tumor burden. Among the 30 crossover patients, 6 initially had had partial responses during the blinded study period with median (range) reductions in tumor burden of 45% (39%–48%). Of those, 5 achieved stable disease after open-label treatment was initiated, with median (range) reductions in tumor burden of 0% (−12% to 6%); 1 patient did not have a subsequent disease assessment. Median PFS (time from start of open-label treatment to disease progression per RECIST or death) for crossover patients was 3.5 months (95% CI, 2.6–6.7 months).
Adverse Events
The incidence of AEs of any grade was similar across the 3 treatment arms (Table 3); however, more patients receiving placebo plus sorafenib had grade ≥3 AEs (66%, 73%, and 86% in arms A, B, and C, respectively). Adverse events that were more common (by an incidence rate ≥10%) in the combined AMG 386 arms than in the placebo arm were mucosal inflammation (23% versus 8%, respectively), nausea (32% versus 20%), insomnia (18% versus 2%), upper abdominal pain (15% versus 4%), and oropharyngeal pain (11% versus 0%). Adverse events grade ≥3 that have been previously associated with angiogenesis inhibition, including arterial and venous thromboembolic events, hemorrhagic events, and impaired wound healing occurred with a similar cumulative frequency across treatment arms (Table 3). More patients who received AMG 386 developed peripheral edema (all grade ≤2) and proteinuria (all grade ≤2, with the exception of 1 grade 3 event in arm A). Three patients had gastrointestinal perforations (Table 3). A grade 3 anal abscess in arm A was considered serious and possibly related to AMG 386 but did not result in treatment discontinuation. One patient in arm C had grade 3 anal fistula and abscess that were both considered serious.
Table 3.
Patient Incidence of Adverse Events
| Arm A AMG 386, 10 mg/kg qw + Sorafenib (n = 50) | Arm B AMG 386, 3 mg/kg qw + Sorafenib (n = 51) | Arm C Placebo + Sorafenib (n = 50) | ||||
|---|---|---|---|---|---|---|
| Patients with any adverse event, % | 98 | 98 | 100 | |||
| Grade 3 | 56 | 57 | 74 | |||
| Grade 4 | 6 | 14 | 10 | |||
| Grade 5 | 4 | 2 | 2 | |||
| Adverse events occurring in ≥20% of patients in ≥1 treatment arm, % | All Grades | Grade ≥3 | All Grades | Grade ≥3 | All Grades | Grade ≥3 |
| Diarrhea | 70 | 8 | 67 | 10 | 56 | 8 |
| Palmar-plantar erythrodysesthesia syndrome | 52 | 12 | 47 | 16 | 54 | 28 |
| Alopecia | 50 | 0 | 45 | 0 | 50 | 2 |
| Hypertension | 42 | 18 | 49 | 20 | 46 | 14 |
| Decreased appetite | 38 | 2 | 27 | 0 | 20 | 0 |
| Nausea | 30 | 2 | 33 | 2 | 20 | 2 |
| Rash | 32 | 0 | 31 | 6 | 30 | 8 |
| Fatigue | 30 | 2 | 24 | 4 | 22 | 0 |
| Asthenia | 30 | 2 | 22 | 4 | 20 | 2 |
| Pruritus | 26 | 0 | 25 | 0 | 24 | 2 |
| Mucosal inflammation | 26 | 2 | 20 | 0 | 8 | 2 |
| Cough | 26 | 0 | 12 | 0 | 10 | 0 |
| Dry skin | 24 | 0 | 22 | 0 | 18 | 2 |
| Constipation | 24 | 0 | 12 | 0 | 22 | 2 |
| Insomnia | 24 | 2 | 12 | 0 | 2 | 0 |
| Vomiting | 20 | 2 | 22 | 2 | 18 | 2 |
| Pain in extremity | 22 | 2 | 16 | 0 | 16 | 2 |
| Stomatitis | 20 | 2 | 12 | 0 | 16 | 2 |
| Upper abdominal pain | 20 | 2 | 10 | 2 | 4 | 0 |
| Adverse events of specific interest, % | ||||||
| Gastrointestinal perforation | 4a | 2 | 0 | 0 | 2b | 2 |
| Arterial thromboembolic events | 8 | 8c | 6 | 4c | 4 | 4c |
| Venous thromboembolic events | 4 | 2 | 4 | 4 | 0 | 0 |
| Cardiac toxicity | 2 | 2d | 0 | 0 | 0 | 0 |
| Hemorrhagic events | 12 | 0 | 14 | 2 | 20 | 2 |
| Impaired wound healing | 4 | 0 | 6 | 2 | 2 | 0 |
| Proteinuria | 16 | 2 | 14 | 0 | 8 | 0 |
| Peripheral edema | 18 | 0 | 16 | 0 | 12 | 0 |
| Hypokalemia | 4 | 2 | 8 | 2 | 4 | 0 |
| Infusion reactions | 6 | 0 | 2 | 0 | 8 | 2 |
Includes 1 grade 1 anal fistula and 1 grade 3 anal abscess.
Grade 3 anal fistula and abscess.
Includes 1 grade 4 myocardial infarction.
Grade 5 cardiopulmonary failure.
There were 34 on-study deaths; most were attributed to disease progression (arms A/B/C, n = 7/ 11/3). Four patients had grade 5 AEs: 2 in arm A (cardiopulmonary failure and sudden death) and 1 each in arm B (abdominal pain considered possibly related to AMG 386) and arm C (general physical health deterioration). Serious AEs occurred in 36%, 49%, and 28% of patients in arm A, B, and C, respectively. Those that were reported in ≥3 patients who received AMG 386 at either dose included myocardial infarction (arm A/B/C, n = 3/2/2), abdominal pain (n = 0/3/0), and pyrexia (n = 1/2/0). The proportions of patients who discontinued treatment because of AEs were 12%/18%/8%.
Among patients with available postbaseline immunoassay samples, 3 of 96 receiving AMG 386 and 3 of 46 receiving placebo developed anti–AMG 386 binding antibodies. In each treatment group (AMG 386 versus placebo), 2 patients had transient anti–AMG 386 antibodies (ie, the assay was negative at the last time point tested). No AMG 386–neutralizing antibodies were detected during the study.
Pharmacodynamic Biomarkers
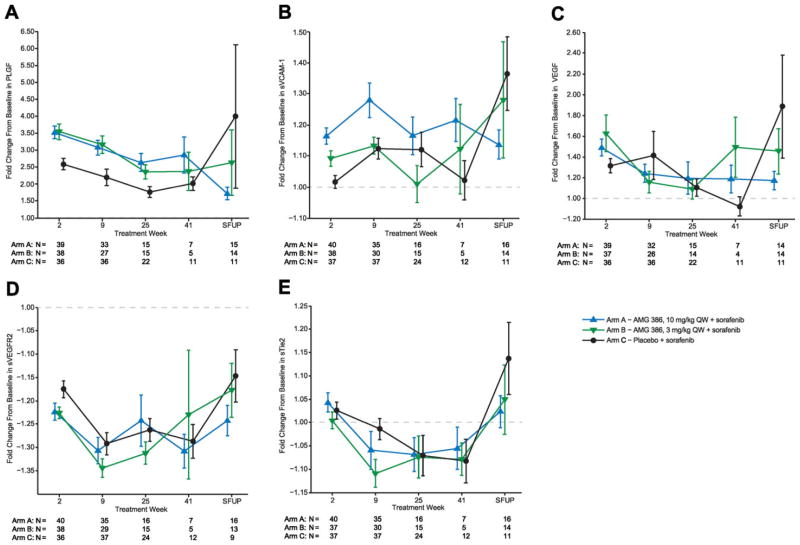
Eight serum biomarkers were tested; pharmacodynamic changes are shown for 5 biomarkers (Fig. 3). Placental growth factor (PLGF) was notably increased above baseline in all 3 treatment arms, with the largest increase seen in arms A and B, suggesting an additive effect of AMG 386. Soluble vascular cell adhesion molecule 1 (sVCAM-1) showed a similar but less pronounced response pattern, specifically at early time points. Pharmacodynamic changes in soluble VEGF receptor 2, VEGF, and soluble Tie2 were small in magnitude regardless of treatment. Levels of serum soluble VEGF receptor 1, soluble Kit, and soluble intercellular adhesion molecule 1 (sICAM-1) did not change with treatment (data not shown).
Figure 3.
Mean (SE) fold-change from baseline in (A) PLGF, (B) sVCAM-1, (C) vascular endothelial growth factor (VEGF), (D) soluble VEGF receptor 2, and (E) sTie2, among patients receiving AMG 386 at 10 mg/kg qw plus sorafenib, AMG 386 at 3 mg/kg qw plus sorafenib, or placebo plus sorafenib. SFUP indicates safety follow-up.
Pharmacokinetics
AMG 386 had dose-proportional pharmacokinetic properties, and Cmin and Cmax were comparable to the phase 1 monotherapy study,7 except for Cmin values at 10 mg/kg. In the 3 mg/kg arm, median AMG 386 Cmax and Cmin values were 81.0 and 6.85 μg/mL, respectively (week 5), and 88.3 and 9.52 μg/mL, respectively (week 9). In the 10 mg/kg arm, median AMG 386 Cmax and Cmin values were 284 and 24.3 μg/mL, respectively (week 5), and 295 and 30.7 μg/mL, respectively (week 9). The median values of sorafenib Cmin at week 5 were 6.91, 5.97, and 8.69 μg/mL in arms A, B, and C, respectively.
DISCUSSION
In this estimation study of previously untreated patients, sorafenib plus AMG 386 was tolerable without evidence of pharmacokinetic interactions, and there was no enhanced treatment effect as measured by PFS. However, there appeared to be an effect on ORR and tumor burden with AMG 386 treatment, particularly with 10 mg/kg qw dosing. Among the 30 patients in the placebo arm who elected to receive AMG 386 at 10 mg/kg qw after disease progression, 1 patient (3%) demonstrated an objective response and 31% had a reduction in tumor burden. These results suggest that mRCC treatment strategies incorporating dual inhibition of angiopoietin/Tie2 and VEGF signaling pathways may be feasible.
Both median PFS (9.0 months) and ORR (25%) in the placebo plus sorafenib arm were higher than reported in a randomized phase 2 study of first-line treatment of RCC with sorafenib or interferon alpha,10 as well as in a phase 211 and a phase 3 study12 of previously treated patients receiving sorafenib (median PFS times, 5–6 months; ORR, 5%–10%). However, one other study group reported phase 2 results similar to ours (median PFS, 7.39 months; ORR, 30%).13 In subsequent independent radiologic review of our own study data, median PFS and ORR in the placebo plus sorafenib arm remained higher (7.2 months and 18%, respectively) than most of the reported historical data. It is notable that some baseline disease characteristics were imbalanced across arms, a chance consequence of small study size. Specifically, the proportion of patients with >3 metastatic sites was greater among those randomized to AMG 386, as was baseline tumor burden. Whether this may have affected the PFS outcome is unknown. Alternatively, outcomes in arm C may simply be a result of the small study size. The efficacy of sorafenib as monotherapy in the first-line setting is currently being assessed in 2 phase 3 studies (ClinicalTrials.gov NCT00678392, NCT01030783).
Toxicities in our study were consistent with those anticipated for AMG 386 and sorafenib as monotherapy. The incidence and severity of specific AEs that have previously been reported with AMG 386 treatment, such as peripheral edema and proteinuria, were consistent with that described in earlier studies.7,14,15 Dose-related trends in toxicity were not apparent. Adverse events known to occur among mRCC patients receiving sorafenib10–12 (including hypertension, rash, and palmar-plantar erythrodysesthesia syndrome) were balanced across the treatment arms. Gastrointestinal perforations have been previously reported with sorafenib16,17 and other VEGF pathway inhibitors in mRCC.18–20 In this study, 1 placebo-treated and 2 AMG 386–treated patients (both received 10 mg/kg qw) had gastrointestinal perforations (ie, anal fistulae or abscesses). Four patients had fatal AEs; 3 received AMG 386 treatment, but only 1 AE (abdominal pain, arm B) was considered possibly related to AMG 386. Recent phase 1 studies have reported exacerbated toxicity in patients with mRCC receiving combinations of 2 VEGF pathway inhibitors, such as bevacizumab plus sunitinib21 or bevacizumab plus sorafenib,22 suggesting that extensive blockade of VEGF signaling may not be tolerable in this setting. Our phase 2 study shows that concomitant administration of the angiopoietin-1/2 inhibitor AMG 386 and sorafenib appears feasible. This is consistent with early data from a phase 1b study that tested AMG 386 plus sorafenib or sunitinib in mRCC.6
Two of the circulating biomarkers we analyzed underwent notable pharmacodynamic changes. Increases from baseline in PLGF and sVCAM-1 were greater in the AMG 386 plus sorafenib arms compared with placebo (plus sorafenib), suggesting an additive effect of AMG 386 on these markers. Both PLGF and sVCAM-1 have important roles in tumor angiogenesis and have been proposed as potential prognostic markers for outcome.23,24 VCAM-1 is highly expressed in endothelial cells, is up-regulated in immune-resistant RCC cell lines, and is also thought to be involved in immune escape.25 Levels of sVCAM-1 have been shown to increase in RCC patients receiving sunitinib26 and in breast cancer patients receiving bevacizumab.27,28 Further investigation will be required to establish clinical utility of these pharmacodynamic markers.
AMG 386 pharmacokinetics showed that median Cmin values in the 10 mg/kg arm at week 5 and subsequent time points were approximately 2-fold higher than those reported in the phase 1 monotherapy study,7 which was confirmed using population pharmacokinetics modeling (data not shown). These results suggest slightly higher AMG 386 exposure in patients with RCC, consistent with the hypothesis that AMG 386 may be, at least in part, cleared through the kidney, because creatinine clearance is significantly associated with AMG 386 clearance.29
The study was limited by its small size, chosen to provide an estimate of efficacy as measured by PFS; it was not designed to formally compare outcomes across study arms. AMG 386 was only tested at a dose of up to 10 mg/ kg qw; however, the possibility that higher doses might improve outcomes, as suggested by an exposure-response analysis of the AMG 386 phase 2 ovarian cancer study,15,29 cannot be excluded. AMG 386 at doses up to 15 mg/kg qw in combination with sunitinib (a standard-of-care therapy in mRCC) is currently being investigated in a phase 2 open-label study (ClinicalTrials.gov, NCT00853372).
In summary, AMG 386 plus sorafenib was tolerable. The effect of treatment on PFS was estimated to be similar in the AMG 386 and placebo arms. Results for ORR and tumor burden reduction with AMG 386 treatment were encouraging and suggestive of antitumor activity. Outcomes among patients randomized to placebo plus sorafenib who received AMG 386 plus sorafenib after disease progression may provide insight into resistance to VEGF receptor inhibitor therapy in mRCC.
Acknowledgments
We thank Don Zhong for antibody analysis; Teresa Wong and Cindy Kitahara for bioanalytical analyses; Rebeca Melara for pharmacokinetic analyses (all Amgen); and Ali Hassan, whose work was funded by Amgen, and Beate Quednau (Amgen) for assistance preparing the manuscript.
FUNDING SOURCES
This study was funded by Amgen Incorporated.
Footnotes
This study is registered at ClinicalTrials.gov, identifier NCT00467025.
CONFLICT OF INTEREST DISCLOSURE
Dr. Rini has been a consultant for and has received research funding from Amgen. Dr. Tannir has been a consultant and has received honoraria from Pfizer, Abbott, and Novartis, and has received research funding from Abbott, Amgen, and Pfizer. Dr. Fishman has been a consultant for Aveo, Eisai, GlaxoSmithKline, Genentech, Seattle Genetics, Pfizer, Novartis, Prometheus, and Altor, has received honoraria from Aveo, Bayer, Pfizer, Novartis, Genentech, Eisai, and GlaxoSmithKline, and research funding from Amgen, Altor, Aveo, FDA, NCI, Southwest Oncology Group, Bayer, Pfizer, Genentech, and Eli Lilly. Dr. Escudier has been a consultant for Bayer, Pfizer, and Roche, and has received honoraria from Bayer, Roche, Pfizer, Genentech, Novartis, and Aveo. Ms. Kracht and Drs. Sun, Bass, and Puhlmann are Amgen employees/shareholders. Dr. Ravaud has been a consultant for Pfizer, Novartis, Bayer, GlaxoSmithKline, Dendreon, and Aveo, has received travel expenses from Novartis and Pfizer, and research funding from Pfizer and Roche. All other authors made no disclosures.
References
- 1.Rini BI, Campbell SC, Escudier B. Renal cell carcinoma. Lancet. 2009;373:1119–1132. doi: 10.1016/S0140-6736(09)60229-4. [DOI] [PubMed] [Google Scholar]
- 2.Rini BI. New strategies in kidney cancer: therapeutic advances through understanding the molecular basis of response and resistance. Clin Cancer Res. 2010;16:1348–1354. doi: 10.1158/1078-0432.CCR-09-2273. [DOI] [PubMed] [Google Scholar]
- 3.Currie MJ, Gunningham SP, Turner K, et al. Expression of the angiopoietins and their receptor Tie2 in human renal clear cell carcinomas; regulation by the von Hippel-Lindau gene and hypoxia. J Pathol. 2002;198:502–510. doi: 10.1002/path.1228. [DOI] [PubMed] [Google Scholar]
- 4.Bullock AJ, Zhang A, O’Neill A, et al. Plasma angiopoietin-2 (ANG2) as an angiogenic biomarker in renal cell carcinoma (RCC) [Abstract] J Clin Oncol. 2010;28:4630. [Google Scholar]
- 5.Coxon A, Bready J, Min H, et al. Context-dependent role of angiopoietin-1 inhibition in the suppression of angiogenesis and tumor growth: implications for AMG 386, an angiopoietin-1/2-neutralizing peptibody. Mol Cancer Ther. 2010;9:2641–2651. doi: 10.1158/1535-7163.MCT-10-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Appleman LJ, Gordon MS, Samlowski W, et al. Open-label phase 1b study of AMG 386, a selective angiopoietin-1/2 neutralizing peptibody, in combination with sorafenib or sunitinib in advanced renal cell carcinoma (RCC): interim results [Abstract] Ann Oncol. 2010;21:viii65, Abstract 505P. [Google Scholar]
- 7.Herbst RS, Hong D, Chap L, et al. Safety, pharmacokinetics, and antitumor activity of AMG 386, a selective angiopoietin inhibitor, in adult patients with advanced solid tumors. J Clin Oncol. 2009;27:3557–3565. doi: 10.1200/JCO.2008.19.6683. [DOI] [PubMed] [Google Scholar]
- 8.Bass MB, Sherman SI, Schlumberger MJ, et al. Biomarkers as predictors of response to treatment with motesanib in patients with progressive advanced thyroid cancer. J Clin Endocrinol Metab. 2010;95:5018–5027. doi: 10.1210/jc.2010-0947. [DOI] [PubMed] [Google Scholar]
- 9.Brookmeyer R, Crowley J. A confidence interval for the median survival time. Biometrics. 1982;38:29–41. [Google Scholar]
- 10.Escudier B, Szczylik C, Hutson TE, et al. Randomized phase II trial of first-line treatment with sorafenib versus interferon alfa-2a in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:1280–1289. doi: 10.1200/JCO.2008.19.3342. [DOI] [PubMed] [Google Scholar]
- 11.Ratain MJ, Eisen T, Stadler WM, et al. Phase II placebo-controlled randomized discontinuation trial of sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24:2505–2512. doi: 10.1200/JCO.2005.03.6723. [DOI] [PubMed] [Google Scholar]
- 12.Escudier B, Eisen T, Stadler WM, et al. TARGET Study Group. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 13.Jonasch E, Corn P, Pagliaro LC, et al. Upfront, randomized, phase 2 trial of sorafenib versus sorafenib and low-dose interferon alfa in patients with advanced renal cell carcinoma: clinical and biomarker analysis. Cancer. 2010;116:57–65. doi: 10.1002/cncr.24685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mita AC, Takimoto CH, Mita M, et al. Phase 1 study of AMG 386, a selective angiopoietin 1/2-neutralizing peptibody, in combination with chemotherapy in adults with advanced solid tumors. Clin Cancer Res. 2010;16:3044–3056. doi: 10.1158/1078-0432.CCR-09-3368. [DOI] [PubMed] [Google Scholar]
- 15.Karlan BY, Oza AM, Richardson GE, et al. A randomized, double-blind, placebo-controlled phase II study of AMG 386 combined with weekly paclitaxel in patients with recurrent ovarian cancer. J Clin Oncol. 2012;30:362–371. doi: 10.1200/JCO.2010.34.3178. [DOI] [PubMed] [Google Scholar]
- 16.Beck J, Procopio G, Bajetta E, et al. Final results of the European Advanced Renal Cell Carcinoma Sorafenib (EU-ARCCS) expanded-access study: a large open-label study in diverse community settings. Ann Oncol. 2011;22:1812–1823. doi: 10.1093/annonc/mdq651. [DOI] [PubMed] [Google Scholar]
- 17.Full prescribing information. Wayne, NJ: Bayer Healthcare Pharmaceuticals, Inc; 2011. Nexavar (sorafenib) [Google Scholar]
- 18.Hapani S, Chu D, Wu S. Risk of gastrointestinal perforation in patients with cancer treated with bevacizumab: a meta-analysis. Lancet Oncol. 2009;10:559–568. doi: 10.1016/S1470-2045(09)70112-3. [DOI] [PubMed] [Google Scholar]
- 19.Full prescribing information. Research Triangle Park, NC: GlaxoSmithKline; 2011. Votrient (pazopanib) [Google Scholar]
- 20.Hutson TE, Davis ID, Machiels JP, et al. Efficacy and safety of pazopanib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2010;28:475–480. doi: 10.1200/JCO.2008.21.6994. [DOI] [PubMed] [Google Scholar]
- 21.Feldman DR, Baum MS, Ginsberg MS, et al. Phase I trial of bevacizumab plus escalated doses of sunitinib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:1432–1439. doi: 10.1200/JCO.2008.19.0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sosman JA, Flaherty KT, Atkins MB, et al. Updated results of phase I trial of sorafenib (S) and bevacizumab (B) in patients with metastatic renal cell cancer (mRCC) [Abstract] J Clin Oncol. 2008;26:5011. [Google Scholar]
- 23.Okugawa Y, Miki C, Toiyama Y, et al. Serum soluble VCAM-1 as a valuable prognostic marker in colorectal carcinoma [Abstract]. Presented at: American Society of Clinical Oncology 2009 Gastrointestinal Cancers Symposium; January 15–17, 2009; San Francisco, CA. p. Abstract 319. [Google Scholar]
- 24.Jain RK, Duda DG, Willett CG, et al. Biomarkers of response and resistance to antiangiogenic therapy. Nat Rev Clin Oncol. 2009;6:327–338. doi: 10.1038/nrclinonc.2009.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu TC. The role of vascular cell adhesion molecule-1 in tumor immune evasion. Cancer Res. 2007;67:6003–6006. doi: 10.1158/0008-5472.CAN-07-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van der Veldt AA, Vroling L, de Haas RR, et al. Sunitinib-induced changes in circulating endothelial cell-related proteins in patients with metastatic renal cell cancer. Int J Cancer. 2011 doi: 10.1002/ijc.26456. [DOI] [PubMed] [Google Scholar]
- 27.Baar J, Silverman P, Lyons J, et al. A vasculature-targeting regimen of preoperative docetaxel with or without bevacizumab for locally advanced breast cancer: impact on angiogenic biomarkers. Clin Cancer Res. 2009;15:3583–3590. doi: 10.1158/1078-0432.CCR-08-2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Denduluri N, Yang SX, Berman AW, et al. Circulating biomarkers of bevacizumab activity in patients with breast cancer. Cancer Biol Ther. 2008;7:15–20. doi: 10.4161/cbt.7.1.5337. [DOI] [PubMed] [Google Scholar]
- 29.Lu J-F, Rasmussen E, Karlan BY, et al. Exposure-response relationship of AMG 386 in combination with weekly paclitaxel in recurrent ovarian cancer and its implication for dose selection. Cancer Chemother Pharmacol. 2012;69:1135–1144. doi: 10.1007/s00280-011-1787-5. [DOI] [PMC free article] [PubMed] [Google Scholar]