Abstract
The network structure of the plant circadian clock is complex and direct regulatory interactions between individual components have proven particularly difficult to predict from genetic analyses. Here, we systematically investigate in vivo binding interactions between the morning-specific transcription factor, LATE ELONGATED HYPOCOTYL (LHY) and the promoters of other components of the network. We then demonstrate the functionality of these interactions by testing the responsiveness of the target gene to an ethanol-induced change in expression level of the LHY protein. We uncover novel, negative autoregulatory feedback loops from LHY and the closely related CIRCADIAN CLOCK ASSOCIATED-1 (CCA1) onto their own and each other’s expression. Furthermore we show that LHY acts as a repressor of all other clock components, including PSEUDO-RESPONSE REGULATORs (PRRs) 9 and 7, which were previously thought to be positive regulatory targets. These experimental results lead to a substantial revision of the morning loops of the clock.
Introduction
One fascinating aspect of Biology is the ability of most organisms to keep time and to anticipate predictable changes in environmental conditions. Daily rhythms, controlled by an endogenous circadian clock, have been identified in a wide range of organisms ranging from cyanobacteria to plants, fungi and mammals. The molecular mechanism of these clocks has been extensively studied over the past 20 years, and was shown to be largely based on networks of negative, interlocked transcriptional-translational feedback loops, where positive and negative components regulate each other’s expression to generate approximately 24 hour oscillations [1].
The plant circadian clock is composed of a set of proteins distinct from its animal and fungal counterparts. Recent work suggested that its oscillatory mechanism is also distinct in its architecture, in that its core feedback loop is composed of three inhibitory steps [2–4]. The two MYB transcription factors, LATE ELONGATED HYPOCOTYL (LHY) and CIRCADIAN CLOCK ASSOCIATED-1 (CCA1) peak in the morning, and act to repress expression of a pseudo response regulator (PRR1, also known as TIMING OF CAB-1, or TOC1) during the day, by binding to an Evening Element (EE) motif in the promoter of its gene. As LHY/CCA1 protein levels decline towards the evening, TOC1 accumulates and acts to repress transcription from their respective promoters. TOC1 transcription is then down-regulated late at night by an Evening Complex (EC) composed of three proteins, LUX and EARLY FLOWERING (ELF) 3 and 4 and this enables transcription of LHY and CCA1 to resume at the following dawn.
Additional feedback loops are mediated by three other PRR proteins, PRR 9, 7 and 5. These proteins are expressed in sequential waves throughout the day [5], and bind to the LHY and CCA1 promoters to repress their activity. Altogether, the PRR proteins and TOC1 ensure that expression of LHY and CCA1 is repressed from the late morning until the following dawn [6]. Recent work also identified a number of rhythmically expressed transcriptional activators that also contribute to the function of clock. REVEILLE (RVE) 4, 6 and 8 up-regulate the afternoon and evening specific genes PRR5, TOC1, GI, ELF4 and LUX as well as the morning-specific PRR9; The Light-regulated WD1 (LWD1) and LWD2 proteins activate the expression of PRR9, PRR5 and TOC1, and the LNK transcription factors 1 and 2, the expression of PRR5 and ELF4 [7–11].
Genetic methods can prove unreliable when investigating regulatory interactions as part of highly interconnected gene networks such as the plant circadian clock [3]. In order to further investigate the structure of this network, we tested the direct binding of LHY and CCA1 to genes encoding other oscillator components. We then confirmed the regulatory function of these physical interactions.
Results and Discussion
LHY binds to the promoter of all clock genes including itself and CCA1
We investigated the binding of LHY to individual promoters in chromatin immunoprecipitation (ChIP) experiments. This confirmed known interactions with the promoter of TOC1 [12, 13], PRR7 and 9 [14], ELF4 [15], ELF3, GI [16] and LUX [17] and verified interactions with the PRR3, PRR5 and CCA1 promoters that were predicted based on the presence of EE or EE-like motifs (Fig 1A). Similar ChIP analyses using cca1-1 CCA1pro::CCA1-HA-YFP plants [18] and an antibody to the YFP tag showed that LHY and CCA1 have similar binding preferences (Fig 1B). This suggests that LHY and CCA1 mediate identical regulatory connections as part of the oscillatory mechanism of the clock, and supports the previous suggestion that their function as part of the clock mechanism is largely redundant [19, 20].
Fig 1. Binding of LHY and CCA1 to the promoters of clock-associated genes.
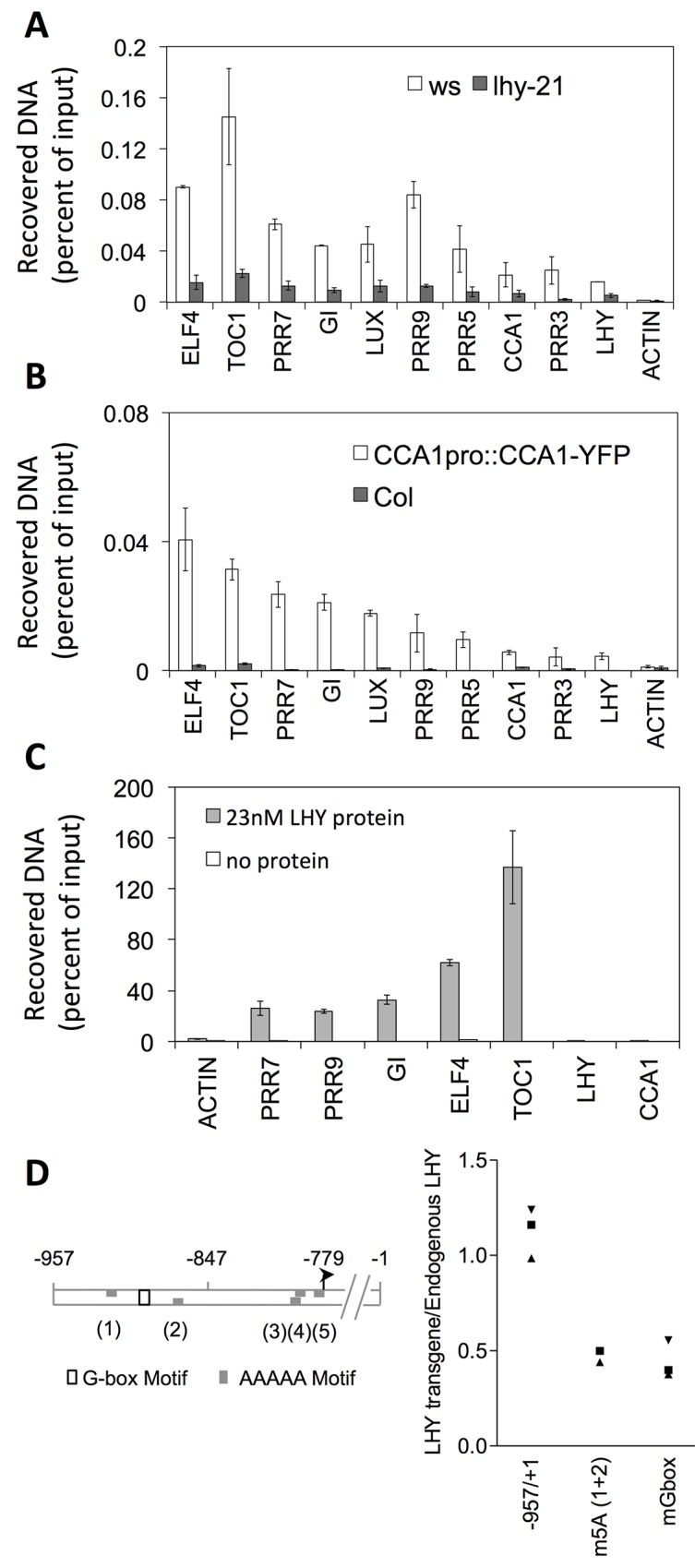
(A) In vivo binding of LHY to the promoters of clock-associated genes was tested by ChIP-qPCR analyses of wild-type samples using a polyclonal antibody to the LHY protein. Plants were grown under 12L12D light-dark cycles and tissue was sampled two hours after dawn, corresponding to the time when LHY protein levels are at their maximum [31]. The ACTIN locus was used as a negative control. In order to demonstrate the specificity of the antibody, ChIP enrichments for wild-type (Ws) plants were compared to those for the insertion mutant, lhy-21. Error bars represent standard errors from two independent biological experiments. (B) In vivo binding of CCA1 was tested by performing ChIP-qPCR analyses on cca1-1 CCA1pro::CCA1-HA-YFP or wild-type plants using an antibody to the YFP tag. Enrichment for the ACTIN2-7 locus is shown as a negative control. Error bars represent standard errors from two independent biological experiments. (C) In vitro binding of LHY to purified genomic DNA. Bacterially expressed, His-tagged LHY protein was used to pull down sheared, purified genomic DNA. The resulting enrichment for different target promoter sequences was quantified by real time PCR using the same primers as used for ChIP analyses, and expressed as a percentage of input material. This experiment detected binding to all LHY binding targets identified in ChIP experiments, except for the LHY and CCA1 promoters. Error bars represent standard deviations from two independent binding experiments. (D) Mutations of the G-box and 5A sites disrupt in vivo binding of the LHY protein to its own promoter. The diagram at the top of the panel shows the relative positions of G-box and 5A motifs in the LHY promoter. Positions are numbered relative to the ATG and the arrow represents the transcriptional start site. ChIP experiments were carried out to assay binding of the LHY protein to wild-type and mutated LHY::luc transgenes. In order to account for differences in sample preparation, enrichment for LHY::luc transgene sequence was expressed relative to endogenous LHY promoter sequence. Relative enrichment levels close to 1 were obtained using the wild-type construct (-957/+1), indicating equivalent enrichment for the endogenous and the transgenic copies of the LHY promoter. However, mutation of the G-box flanking regions (ACCACGTGTC to GTCACGTGAC) reduced binding of the LHY protein by over 50%. Mutation of both flanking 5A sites [5A (1) CCAAAAA to TGTCAAA and 5A(2) TTTTTCC to TTTGACA] had a similar effect. Each data point represents the relative enrichment for one transgenic line. Error bars from Q-PCR analyses have been omitted for clarity.
The ChIP experiments also identified a novel interaction with an evolutionarily conserved region of the LHY promoter [21], which was surprising because no known binding motif was present within this genomic region. To test whether LHY might be recruited to its own promoter via interactions with other DNA-binding proteins, we tested the in vitro binding of purified LHY protein to purified genomic DNA. Pull-down experiments using bacterially expressed, His-tagged LHY protein as bait resulted in significant enrichment for all of the binding targets identified Fig 1A, except for the LHY and CCA1 promoters (Fig 1B). This result suggests that binding of LHY to the LHY and CCA1 promoters requires protein cofactors.
Known regulatory motifs within the LHY promoter include a G box and multiple 5A motifs [21]. Disruption of these motifs by site-directed mutagenesis of a LHY:luciferase (LHY:luc) reporter construct was previously shown to result in reduced amplitude of the luminescence rhythm in transgenic plants. To test whether either of these motifs might act to recruit LHY to its own promoter, we compared in vivo binding of the LHY protein to wild-type and mutated LHY:luc reporter constructs in ChIP experiments (Fig 1C). The enrichment level obtained for the wild-type LHY:luc transgene was similar to that for the endogenous LHY promoter, as indicated by a relative enrichment level close to 1. However mutation of the G-box motif in the LHY::luc construct reduced the enrichment level 2 to 3 fold. Mutation of the two 5A motifs flanking the G-box reduced enrichment to a similar extent. These results suggest that LHY binding to its own promoter is mediated, at least in part, through interactions with G box- and 5A-binding factors. Similar motifs present within the promoter of CCA1 may also account for LHY binding to that promoter [21].
LHY represses transcription of all of the PRR genes
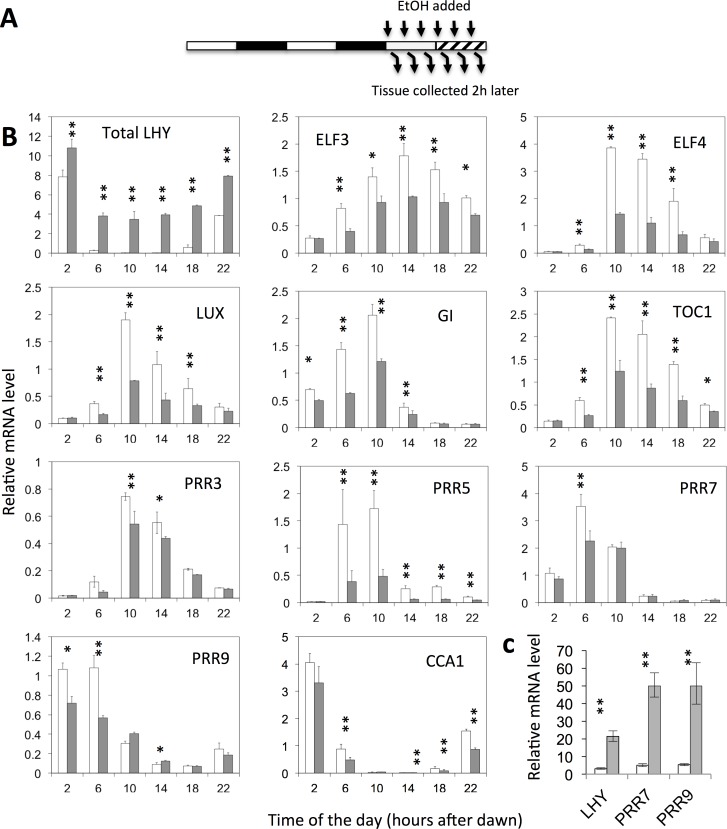
In order to test the regulatory function of promoter-binding interactions, we assayed the rapid changes in mRNA levels that followed induction of an ethanol-responsive LHY transgene (ALCpro::LHY). This experimental design enabled us to circumvent possible artifacts of constitutive overexpression or knock-down experiments, such as indirect effects of LHY that may be mediated by other components of the network. In order to uncover possible time-of-the-day dependency (i.e., gating) of the effect of LHY on its different regulatory targets, similar induction experiments were carried out at 4 hour intervals over the duration of the circadian cycle (Fig 2A).
Fig 2. LHY represses expression of other clock components.
(A) Experimental design. Wild-type and Alcpro::LHY transgenic plants were grown under 12L12D light-dark cycles as illustrated by the white and black bars in the diagram. They were transferred to constant light at the start of the experiment. Expression of the Alcpro::LHY transgene was induced using 6% ethanol (v/v). Different sets of plants were treated at 4-hour intervals over the duration of one circadian cycle, and tissue was harvested 2 hours later. (B) mRNA levels were determined using Nanostring technology and normalized relative to UBC12. Times indicate when the tissue was harvested. (C) shows levels of PRR7 and PRR9 mRNA expression 26 h after induction of the Alcpro::LHY transgene at ZT17. Open bars indicate wild-type data (+EtOH), filled bars Alcpro::LHY data (+EtOH). Transcript levels from Quantitative RT-PCR analyses were normalized relative to ACTIN. Data shown are averages and standard errors from two independent biological replicates. * indicates p <0.05 and ** p<0.01 as determined by t-tests. An additional experiment comparing comparing effects of ethanol on PRR7 and PRR9 expression in Alcpro:::LHY plants, Alcpro::GUS and wild-type plants, 2, 6 and 10 hours after dawn is provided as S1 Fig.
Induction of the ALCpro::LHY transgene led to the repression of other components of the network, including ELF3, ELF4, LUX, GI, TOC1, PRR3, PRR5, PRR7, PRR9 and CCA1 (Fig 2B and S1 Fig). Down-regulated transcript levels were observed within 2 hours of ethanol addition, and most pronounced effects were observed at times corresponding to peak transcript levels.
Previous work showed that expression of the PRR7 and PRR9 transcripts was elevated during the night in LHY- and CCA1-overexpressing (LHY-ox and CCA1-ox) plants, and reduced during the day in cca1-1 lhy-R double mutants [22]. In agreement with these observations, we found that induction of Alcpro::LHY expression led to elevated PRR7 and PRR9 transcript levels, 26 hours after ethanol addition (Fig 2C). Based on these results, we suggest that LHY acts as a direct repressor of PRR7 and PRR9 transcription, and that the elevated expression of these transcripts in LHY-ox plants reflects indirect effects, mediated by feedback.
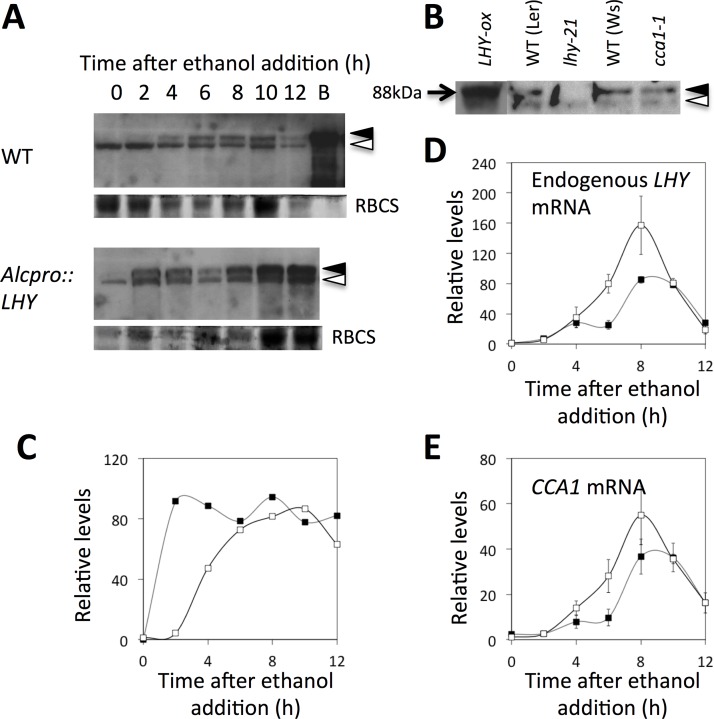
LHY and CCA1 repress their own and each other’s expression
CCA1 expression was down-regulated rapidly following Alcpro::LHY induction (Fig 2B). This showed that LHY acts as a direct repressor of CCA1 transcription. Downregulation of the LHY transcript was also observed following induction of the Alcpro::LHY transgene in the middle of the subjective night (at ZT17, i.e. 17 hours after dawn) (Fig 3C). Reduced LHY transcript levels were observed 4–8 h after ethanol addition (Fig 3C), mirroring effects on CCA1 transcript levels (Fig 3D). Experiments testing the effects of induction of an ethanol-inducible Alcpro::CCA1 transgene produced similar results (S2 Fig). Altogether, these results suggest that, although they don’t bind DNA at their own promoters, both LHY and CCA1 repress of their own and of each other’s transcription by forming physical interactions with other transcription factors.
Fig 3. Induction of the ALCpro::LHY transgene abrogates the peak of LHY and CCA1 expression at dawn.
Ethanol (1% v/v) was added to plants 17 hours after dawn, i.e. just before the normal rise in LHY transcription. (A, B) Immunoblot showing changes in LHY protein levels after ethanol addition, and control experiment showing the specificity of the LHY antibody. The LHY protein is indicated by filled triangles, and a constitutive, cross-reactive band is indicated by open triangles. B indicates bacterially expressed LHY protein. As a loading control, the lower part of the gel was stained with Coomassie blue to reveal the RBCS protein. (C) Quantification of LHY protein levels from (A). LHY protein levels were normalized to the cross-reactive band and expressed relative to wild-type levels at time zero. (D, E) Changes in endogenous LHY and CCA1 mRNA levels as determined by quantitative RT-PCR. Transcript levels were normalized to the ACTIN transcript and to levels in control plants at time zero. Open symbols indicate wild-type and filled symbols, Alcpro::LHY data. Data shown are averages and standard errors from triplicate quantitative RT-PCR analyses. A replicate experiment in shown in S2A–S2C Fig.
Conclusion
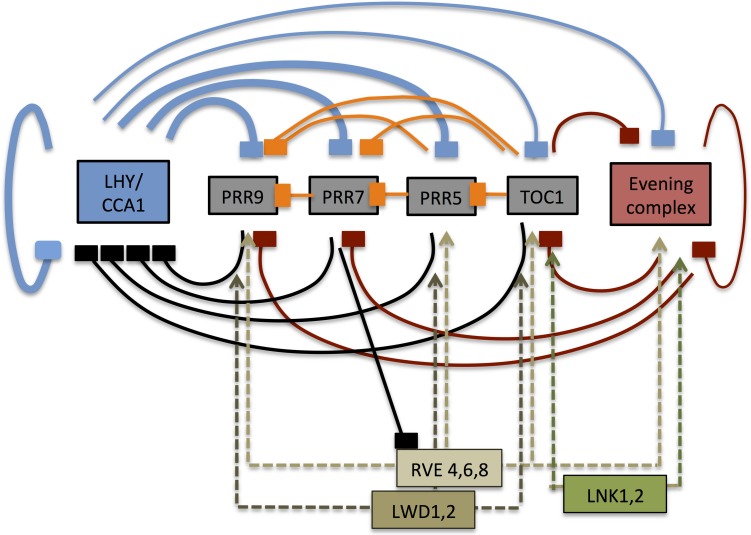
Our experimental results lead to substantial alterations of the morning loops of the plant circadian clock, as summarized in Fig 4.
Fig 4. The transcriptional network of the plant circadian clock.
Revised regulatory connections arising from this work are indicated by heavier lines. We add a novel, direct autoregulatory loop from LHY/CCA1 onto their own expression and we introduce a change in the sign of the regulation of PRR5, 7 and 9 transcription by LHY and CCA1. Furthermore, we show that the PRR5 gene is also down-regulated by LHY/CCA1. Each box indicates a gene. Pointed arrows indicate positive regulation and blunt arrows, transcriptional repression. Blue arrows represent regulation by LHY/CCA1, red arrows regulation by the evening complex (EC) and black arrows regulation by the PRRs and TOC1. Orange arrows indicate regulatory interactions between the PRR genes, and dashed arrows indicative positive regulation by the RVE, LNK or LWD proteins.
LHY and CCA1 were until now thought to promote transcription of PRR7 and PRR9. This was based on the observation, that expression of the PRR7 and PRR9 transcripts was elevated during the night in LHY- and CCA1-overexpressing (LHY-ox and CCA1-ox) plants, and reduced during the day in cca1-1 lhy-R double mutants [22]. However, our results show that induction of LHY expression causes immediate down-regulation of all of the PRR genes, including PRR9, 7, 5 and PRR1/TOC1. This demonstrates that LHY functions as a transcriptional repressor of these genes. We propose that the elevated levels of PRR7 and PRR9 transcripts in LHY- and CCA1-overexpressing plants reflect long-term, indirect effects due to altered expression of other components of the network.
LHY and CCA1 were previously shown to act as negative regulators of their own expression [23, 24], but the generally accepted model was that this regulation was indirect. LHY and CCA1 are known to function as part of a negative feedback loop, in which their expression is repressed by the PRR proteins and TOC1 during the day, and this repression is lifted when the EC represses TOC1 transcription late at night [2]. The new finding, that LHY acts as a repressor of all of the PRR genes raises an issue with this model, as it implies that LHY switches off expression of all of its inhibitors. This implies that once LHY and CCA1 expression is switched on in the morning, their expression will remain high and the repression of the PRR genes will be maintained. Oscillatory behavior will be prevented unless some other mechanism is present, either to shut down expression of LHY and CCA1 or to override their effect on PRR 9 and PRR7 transcription. Our finding, that LHY and CCA1 act to directly downregulate their own transcription provides one such mechanism.
This direct autoregulation of LHY and CCA1 and the negative regulation of PRR7 and PRR9 expression by LHY were both incorporated into a recent mathematical model for the clock, albeit without experimental justification [25]. This model demonstrates that both of these features are compatible with the temporal patterns of oscillations of the clock genes. However, analyses of the model have so far focused on the role of positive regulators of the PRR genes, also newly incorporated into the model. Further modeling will be required in order to fully understand the functional implications of the revised network architecture. In the revised structure each of the PRR genes (including TOC1) is regulated in a highly similar fashion expression by LHY/CCA1 and by the evening complex. This now blurs the functional distinction between the so-called morning loops of the clock, mediated by PRR9 and PRR7, and the evening loops mediated by TOC1 [26]. The similar structure of the PRR and TOC1 feedback loops would suggest redundant functions as part of the oscillatory mechanism of the clock, and yet we know that mutations in these different genes result in distinct period phenotypes [27, 28]. The key to their different functions lies in the differential timing of their expression, with the night-specific TOC1 and PRR5 controlling the onset of LHY expression and the morning-specific PRR9 controlling its offset [6]. We still need to develop a better understanding of the mechanism by which the sequential waves of PRR gene expression are generated.
Material and Methods
Plant material and growth conditions
Wild-type transgenic lines carrying the LHY::LUC, ALCpro::LHY and ALCpro::CCA1 constructs and the cca1-1 mutant line carrying the CCA1pro::CCA1-HA-YFP transgene have been described previously [18, 21, 29]. Seeds were sown on MS-agar plates, stratified in the dark for 3 days at 4°C and grown under 12-h photoperiods at 22°C unless otherwise stated.
Chromatin immunoprecipitation (ChIP)
Chromatin was isolated from 2-week-old seedlings harvested 2 h after dawn. Immunoprecipitation was carried out according to [30] using a polyclonal antibody to the LHY protein previously described by [31]. DNA was eluted from protein A beads in the presence of 10% Chelex according to [32] and analysed by qPCR. Results were expressed relative to the original input chromatin sample. All primers used are listed in S1 Table.
Gene expression analyses
Total RNA was extracted from seedlings using the Plant RNeasy kit (Qiagen) and contaminating genomic DNA removed by treatment with DNaseI (SIGMA). First-strand cDNA synthesis was carried out using Revert-aid H-Minus M-MuMLV Reverse transcriptase (Fermentas) and primed using random DNA hexamers. Expression levels were determined by qPCR as above and calculated relative to ACT2 (At3g18780). Alternatively, mRNA expression levels were quantified using Nanostring® technology [33] and expressed relative to UBC12 (AT3g08700).
Ethanol induction of ALCpro::LHY and ALCpro::CCA1 lines
5 ml of ethanol (1 to 6% v/v) was added directly to the roots of the plants. In order to maintain ethanol vapours, a 3 cm2 piece of filter paper soaked in ethanol was placed on the underside of the plate lid at hourly intervals. Expression of the LHY protein following induction was quantified by immunoblot analyses and was calculated relative to a constitutive cross-reacting band.
LHY protein expression in E. coli
The LHY protein was expressed in E. coli BL21(DE3)pREP4-RIL cells (Stratagene) as a C-terminal hexa-histidine fusion. It was purified by chromatography on HisTrap FF column (GE Healthcare) then on a HiTrap Q HP column (GE Healthcare).
Genomic DNA pull-down
Arabidopsis genomic DNA was isolated using the PHYTOPURE™ extraction kit (GE healthcare), sonicated to generate fragments of 100 to 600 bp2, then incubated with purified, recombinant LHY:His protein for 2 hours at 20°C. DNA-protein complexes were pulled-down using Ni-NTA magnetic beads (Dynabeads). DNA was purified using the MinElute PCR purification kit (Qiagen) prior to q-PCR analyses. The same primer sets were used as in ChIP analyses (S1 Table).
Supporting Information
Ethanol (6% v/v) was added to different groups of ALCPro::LHY plants 2, 6 or 10 hours after dawn, and changes in transcript levels were determined after 2 hours as described for Fig 2. Error bars indicate standard errors from three technical replicates.* indicates p <0.05 and ** p<0.01 as determined by t-tests.
(TIFF)
Expression of the Alcpro::LHY (A-C) or Alcpro::CCA1 transgenes (D-F) was induced by ethanol (1% v/v) at ZT 17 as described in Fig 2. (A,D) show the resulting increases in total LHY protein and CCA1 mRNA expression, respectively. (B,E) show effects of endogenous LHY mRNA levels and (C,F) on endogenous CCA1 mRNA levels. LHY protein levels were quantified as in Fig 2. LHY and CCA1 mRNA levels were assayed by quantitative PCR, normalized to ACTIN mRNA and expressed relative to wild-type levels at time zero. Specific amplification of the endogenous LHY and CCA1 mRNAs was achieved using primers to the 5’untranslated region (5’UTR) of the genes. Error bars represent standard errors of the mean from three technical replicates. (G) Immunoblot showing changes in LHY protein levels after ethanol addition. The LHY protein is indicated by filled triangles, and a constitutive, cross-reactive band is indicated by open triangles. B indicates bacterially expressed LHY protein. As a loading control, the lower part of the gel was stained with Coomassie blue to reveal the RBCS protein.
(TIFF)
(DOCX)
Acknowledgments
ALCpro::LHY and ALCpro::CCA1 transgenic lines were generous gifts from Elaine Tobin, the CCA1pro::CCA1-HA-YFP line from Rachel Green and the ALCpro::GUS line from Vicky Buchanan-Wollaston. Nanostring analyses were carried out by the University Health Network Microarray Centre in Toronto.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The research was funded by a grant from the Biotechnology and Biological Sciences Research Council (BBSRC) to IAC and PS (Ref: BB/F022832/1). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Harmer SL, Panda S, Kay SA. Molecular bases of circadian rhythms. Annual Review of Cell and Developmental Biology. 2001;17(1):215–53. [DOI] [PubMed] [Google Scholar]
- 2. Pokhilko A, Fernandez AP, Edwards KD, Southern MM, Halliday KJ, Millar AJ. The clock gene circuit in Arabidopsis includes a repressilator with additional feedback loops. Mol Syst Biol. 2012;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huang W, Pérez-García P, Pokhilko A, Millar AJ, Antoshechkin I, Riechmann JL, et al. Mapping the Core of the Arabidopsis Circadian Clock Defines the Network Structure of the Oscillator. Science. 2012;336(6077):75–9. 10.1126/science.1219075 [DOI] [PubMed] [Google Scholar]
- 4. Carré I, Veflingstad SR. Emerging design principles in the Arabidopsis circadian clock. Seminars in Cell & Developmental Biology. 2013;24(5):393–8. 10.1016/j.semcdb.2013.03.011 [DOI] [PubMed] [Google Scholar]
- 5. Matsushika A, Makino S, Kojima M, Mizuno T. Circadian waves of expression of the APRR1/TOC1 family of pseudo-response regulators in Arabidopsis thaliana: insight into the plant circadian clock. Plant and Cell Physiology. 2000;41(9):1002–12. [DOI] [PubMed] [Google Scholar]
- 6. Nakamichi N, Kiba T, Henriques R, Mizuno T, Chua NH, Sakakibara H. PSEUDO-RESPONSE REGULATORS 9,7 and 5 are transcriptional repressors in the Arabidopsis circadian clock. Plant Cell. 2010;22:594–605. 10.1105/tpc.109.072892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Farinas B, Mas P. Functional implication of the MYB transcription factor RVE8/LCL5 in the circadian control of histone acetylation. Plant Journal. 2011;66(2):318–29. 10.1111/j.1365-313X.2011.04484.x [DOI] [PubMed] [Google Scholar]
- 8. Rawat R, Takahashi N, Hsu PY, Jones MA, Schwartz J, Salemi MR, et al. REVEILLE8 and PSEUDO-REPONSE REGULATOR5 Form a Negative Feedback Loop within the Arabidopsis Circadian Clock. PLoS Genet. 2011;7(3):e1001350 10.1371/journal.pgen.1001350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hsu PY, Devisetty UK, Harmer SL, Chory J. Accurate timekeeping is controlled by a cycling activator in Arabidopsis . eLife. 2013;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang Y, Wu J-F, Nakamichi N, Sakakibara H, Nam H-G, Wu S-H. LIGHT-REGULATED WD1 and PSEUDO-RESPONSE REGULATOR9 Form a Positive Feedback Regulatory Loop in the Arabidopsis Circadian Clock. Plant Cell. 2011;23(2):486–98. 10.1105/tpc.110.081661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rugnone ML, Faigón Soverna A, Sanchez SE, Schlaen RG, Hernando CE, Seymour DK, et al. LNK genes integrate light and clock signaling networks at the core of the Arabidopsis oscillator. Proceedings of the National Academy of Sciences. 2013;110(29):12120–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Perales M, Mas P. A Functional Link between Rhythmic Changes in Chromatin Structure and the Arabidopsis Biological Clock. Plant Cell. 2007;19(7):2111–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ni Z, Kim E-D, Ha M, Lackey E, Liu J, Zhang Y, et al. Altered circadian rhythms regulate growth vigour in hybrids and allopolyploids. Nature. 2009;457(7227):327–31. 10.1038/nature07523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Portolés S, Más P. The Functional Interplay between Protein Kinase CK2 and CCA1 Transcriptional Activity Is Essential for Clock Temperature Compensation in Arabidopsis . PLoS Genet. 2010;6(11):e1001201 10.1371/journal.pgen.1001201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li G, Siddiqui H, Teng Y, Lin R, Wan X-y, Li J, et al. Coordinated transcriptional regulation underlying the circadian clock in Arabidopsis . Nat Cell Biol. 2011;13(5):616–22. 10.1038/ncb2219 [DOI] [PubMed] [Google Scholar]
- 16. Lu SX, Webb CJ, Knowles SM, Kim SHJ, Wang Z, Tobin EM. CCA1 and ELF3 Interact in the Control of Hypocotyl Length and Flowering Time in Arabidopsis . Plant Physiology. 2012;158(2):1079–88. 10.1104/pp.111.189670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hazen SP, Schultz TF, Pruneda-Paz JL, Borevitz JO, Ecker JR, Kay SA. LUX ARRHYTHMO encodes a Myb domain protein essential for circadian rhythms. Proc Natl Acad Sci U S A. 2005;102(29):10387–92. Epub 2005/07/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yakir E, Hilman D, Kron I, Hassidim M, Melamed-Book N, Green RM. Posttranslational Regulation of CIRCADIAN CLOCK ASSOCIATED1 in the Circadian Oscillator of Arabidopsis . Plant Physiol. 2009;150:844–57. 10.1104/pp.109.137414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mizoguchi T, Wheatley K, Hanzawa Y, Wright L, Mizoguchi M, Song HR, et al. LHY and CCA1 are partially redundant genes required to maintain circadian rhythms in Arabidopsis . Developmental Cell. 2002;2(5):629–41. [DOI] [PubMed] [Google Scholar]
- 20. Alabadi D, Yanovsky MJ, Mas P, Harmer SL, Kay SA. Critical role for CCA1 and LHY in maintaining circadian rhythmicity in Arabidopsis. Current Biology. 2002;12(9):757–61. [DOI] [PubMed] [Google Scholar]
- 21. Spensley M, Kim J-Y, Picot E, Reid J, Ott S, Helliwell C, et al. Evolutionarily Conserved Regulatory Motifs in the Promoter of the Arabidopsis Clock Gene LATE ELONGATED HYPOCOTYL . Plant Cell. 2009;21:2606–23. 10.1105/tpc.109.069898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Farré EM, Harmer SL, Harmon FG, Yanovsky MJ, Kay SA. Overlapping and distinct roles of PRR7 and PRR9 in the Arabidopsis clock. Current Biology. 2005;15:47–54. [DOI] [PubMed] [Google Scholar]
- 23. Wang Z-Y, Tobin EM. Constitutive expression of the CIRCADIAN CLOCK ASSOCIATED (CCA1) gene disrupts circadian rhythms and suppresses its own expression. Cell. 1998;93:1207–17. [DOI] [PubMed] [Google Scholar]
- 24. Schaffer R, Ramsay N, Samach A, Corden S, Putterill J, Carré IA, et al. The late elongated hypocotyl mutation of Arabidopsis disrupts circadian rhythms and the photoperiodic control of flowering. Cell. 1998;93:1219–29. [DOI] [PubMed] [Google Scholar]
- 25. Fogelmark K, Troein C. Rethinking Transcriptional Activation in the Arabidopsis Circadian Clock. PLoS Comput Biol. 2014;10(7):e1003705 10.1371/journal.pcbi.1003705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Locke J, Kozma-Bognár L, Gould P, B F, Kevei E, Nagy F, et al. Experimental validation of a predicted feedback loop in the multi-oscillator clock of Arabidopsis thaliana . Molecular Systems Biology. 2006;2:59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nakamichi N, Kita M, Ito S, Yamashino T, Mizuno T. PSEUDO-RESPONSE REGULATORS, PRR9, PRR7 and PRR5, Together Play Essential Roles Close to the Circadian Clock of Arabidopsis thaliana . Plant and Cell Physiology. 2005;46(5):686–98. [DOI] [PubMed] [Google Scholar]
- 28. Salome PA, McClung CR. PSEUDO-RESPONSE REGULATOR 7 and 9 are partially redundant genes esential for the temperature responsiveness of the Arabidopsis circadian clock. Plant Cell. 2005;17(791–803). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Knowles SM, Lu SX, Tobin EM. Testing Time: Can Ethanol-Induced Pulses of Proposed Oscillator Components Phase Shift Rhythms in Arabidopsis? J Biol Rhythms. 2008;23:463–71. 10.1177/0748730408326749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gendrel A-V, Lippman Z, Yordan C, Colot V, Martienssem RA. Dependence on heterochromatic Histone H3 methylation patterns on the Arabidopsis gene DDM1 . Science. 2002;297:1871–3. [DOI] [PubMed] [Google Scholar]
- 31. Kim JY, Song HR, Taylor BL, Carre IA. Light-regulated translation mediates gated induction of the Arabidopsis clock protein LHY. The Embo Journal. 2003;22(4):935–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nelson JD, Denisenko O, Bomsztyk K. Protocol for the fast chromatin immunoprecipitation (ChIP) method. Nat Protocols. 2006;1(1):179–85. [DOI] [PubMed] [Google Scholar]
- 33. Geiss GK, Bumgarner RE, Birditt B, Dahl T, Dowidar N, Dunaway DL, et al. Direct multiplexed measurement of gene expression with color-coded probe pairs. Nat Biotech. 2008;26(3):317–25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Ethanol (6% v/v) was added to different groups of ALCPro::LHY plants 2, 6 or 10 hours after dawn, and changes in transcript levels were determined after 2 hours as described for Fig 2. Error bars indicate standard errors from three technical replicates.* indicates p <0.05 and ** p<0.01 as determined by t-tests.
(TIFF)
Expression of the Alcpro::LHY (A-C) or Alcpro::CCA1 transgenes (D-F) was induced by ethanol (1% v/v) at ZT 17 as described in Fig 2. (A,D) show the resulting increases in total LHY protein and CCA1 mRNA expression, respectively. (B,E) show effects of endogenous LHY mRNA levels and (C,F) on endogenous CCA1 mRNA levels. LHY protein levels were quantified as in Fig 2. LHY and CCA1 mRNA levels were assayed by quantitative PCR, normalized to ACTIN mRNA and expressed relative to wild-type levels at time zero. Specific amplification of the endogenous LHY and CCA1 mRNAs was achieved using primers to the 5’untranslated region (5’UTR) of the genes. Error bars represent standard errors of the mean from three technical replicates. (G) Immunoblot showing changes in LHY protein levels after ethanol addition. The LHY protein is indicated by filled triangles, and a constitutive, cross-reactive band is indicated by open triangles. B indicates bacterially expressed LHY protein. As a loading control, the lower part of the gel was stained with Coomassie blue to reveal the RBCS protein.
(TIFF)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.