Abstract
Background
We assessed the association between the duration of diarrhoea and the risk ofpneumonia incidence among children <5 years of age.
Methods
We analysed data from a cluster randomized controlled trial in Karachi, Pakistan, which assessed the effect of promoting hand washing with soap (antibacterial and plain) on child health. Field workers visited households with children <5 years of age weekly and asked primary caregivers if their child had diarrhoea, cough or difficulty breathing in the preceding week. We used the WHO clinical case definitions for diarrhoea and pneumonia. We used adjusted time-to-event analyses with cumulative diarrhoea prevalence over the previous 2 and 4 weeks as exposure and pneumonia as outcome. We calculated the attributable risk of pneumonia due to recent diarrhoea across the intervention groups.
Results
873 households with children <5 years were visited. Children had an increased risk of pneumonia for every additional day of diarrhoea in the 2 weeks (1.06, 95% CI: 1.03–1.09) and 4 weeks (1.04, 95% CI: 1.03–1.06) prior to the week of pneumonia onset. The attributable risk of pneumonia cases due to recent exposure to diarrhoea was 6%. A lower associated pneumonia risk following diarrhoea was found in the control group: (3%) compared with soap groups (6% in antibacterial soap, 9% in plain soap).
Conclusion
Children <5 years of age are at an increased risk of pneumonia following recent diarrhoeal illness. Public health programmes that prevent diarrhoea may also reduce the burden of respiratory illnesses.
Keywords: Diarrhoea, pneumonia, childhood diseases, malnutrition
Introduction
Pneumonia and diarrhoea are the leading causes of deaths in children <5 years of age in low-income settings.1 Prevention and management of these infectious diseases are key public health priorities to reduce childhood mortality. Co-morbidity of these diseases may increase the number of child deaths due to shared risk factors such as malnutrition.2,3 Diarrhoeal illness and malnutrition have both been identified as separate risk factors for pneumonia in children <5 years of age.4 A study conducted in both Ghana and Brazil found that children <5 years of age who had recent diarrhoeal illness were at a higher risk for subsequent acute respiratory illnesses in Ghana, but not Brazil.5 If the association identified in Ghana is present in other locations of high child mortality, then diarrhoea prevention may be a useful strategy to prevent childhood respiratory diseases.
Hand washing with soap can reduce the incidence of both diarrhoea and acute respiratory illnesses in children<5.6–8 We analysed data from a randomized controlled trial in Karachi, Pakistan, that demonstrated a reduction of childhood diarrhoea and pneumonia through hand washing. We used dynamic time-to-event analyses similar to those used by Schmidt et al. to explore if children <5 were at an increased risk of pneumonia following a diarrhoeal episode.5 We also explored the effect of different levels of hand hygiene on the diarrhoea-associated risk of pneumonia.
Materials and Methods
Primary study site and data collection
We used secondary data from a randomized control trial (RCT), the Karachi Soap Health study, that has been described previously.9 Briefly, the RCT was conducted in 2003, in multi-ethnic squatter settlements in Karachi, with three arms: 300 households with hand-washing promotion with antibacterial soap, 300 households with promotion with plain soap and 306 households as the control group. Eligible households had at least two children younger than 15 years. Fieldworkers visited each household weekly over a 12-month period from April 2002 to April 2003. They asked the primary caregiver whether the child had symptoms of cough, difficulty breathing or ⩾3 loose stools within 24 hours, during the previous week and if so, for how many days. Fieldworkers counted the number of breaths per 60 s for children with a cough or difficulty breathing. They weighed the children at baseline and every 4 months using a Salter scale for children <3 years of age and a bathroom scale for older children.
Operational definitions
The study used the WHO clinical case definition which defines pneumonia as cough or difficulty breathing with a raised respiratory rate (>60 per min in children younger than 60 days, >50 per min for those aged 60–364 days, and >40 per min for those aged 1–5 years).10 We defined diarrhoea as the occurrence of ⩾3 loose stools over 24 hours. The weight-for-age Z-scores for a child was calculated consistently with the original Karachi Soap Health study analysis, according to the 2003 National Centre for Health Statistics standards.
We defined the index week as the week of pneumonia onset. Prior to the index week we identified both a 2-week and a 4-week period. We defined longitudinal prevalence of diarrhoea as the number of days with diarrhoea for a child during the 2- or 4-week time period before the index week. We calculated diarrhoea and pneumonia incidence by week. In the incidence calculation we used the total person-years of observation as the denominator.
Children who did not have the specific syndrome in the preceding week, but developed the syndrome (diarrhoea or pneumonia), were regarded as having a new episode. Thus children with symptoms of diarrhoea or pneumonia at the first visit were not included for incidence calculation of diarrhoea or pneumonia. We defined the proportion of time ill due to diarrhoea or pneumonia as the sum of the number of days in the weeks reported ill over the total follow-up period.
Statistical analysis
We used a dynamic time-to-event analysis using the Prentice-Williams-Peterson Gap Time (PWP-GT) model, to describe the risk of pneumonia associated with longitudinal prevalence of diarrhoea in the previous 2- and 4-week window of pneumonia onset.11 The PWP-GT model accounts for recurrent events by placing a person who has experienced N pneumonia episodes into the risk set for the N+1st episode. For example, after a child’s first pneumonia episode, he or she is placed in the risk set for a second pneumonia episode. The failure time in the new stratum is the time between the first pneumonia episode and the second. The PWP-GT model estimates a common hazard ratio for all strata, but allows a different baseline hazard in each stratum. Schmidt et al. used the Prentice-Williams-Peterson total time approach (PWP-CP) that compares children with the same number of previous acute lower respiratory infection (ALRI) episodes on the same calendar date. On the contrary, PWP-GT compares children with the same number of pneumonia episodes and the same amount of time since their previous pneumonia episode or from the beginning of observation if there were no previous diarrhoeal episodes.12–16 We used backward elimination (P < 0.2) to select explanatory variables in the model. We included household income, father’s education, whether the mother could read the newspaper, and the soap groups included in the RCT to account for potential confounding effect. We included the soap groups because provision of soap and hand-washing promotion lowered the risk of both illnesses.6 We used robust variance estimates to adjust for clustering at the child level.
We used two models for our analyses: the first model estimated the hazard ratio of pneumonia on additional days of diarrhoea exposure in the previous 2- or 4-week window; the second model treated the number of diarrhoeal days as a categorical variable to estimate the hazard ratio of pneumonia of each child with a given number of diarrhoeal days compared with those without diarrhoea in the previous 2 or 4 weeks. We checked the proportional hazard assumptions with tests based on the scaled Schoenfeld residuals.17 Since event specific hazard rate estimates for pneumonia can be unreliable for greater than 4 events, we did not include more than 4 pneumonia events for 48 children (3%).14
We calculated age specific hazard ratios for children aged <12 months and from 12 to 60 months because pneumonia is harder to diagnose in children aged <12 months who also suffer from higher mortality. We calculated the population attributable fraction (PAF), i.e. the proportion of pneumonia episodes due to diarrhoea, using the formula PAF=p* (HR-1)/HR, where p is the proportion of cases exposed and HR the adjusted hazard ratio.18,19 We also calculated confidence intervals around the PAF across intervention groups.20 We used STATA 10 (StataCorp LP, College Station, TX) to conduct the statistical analyses.
Ethical considerations
In the original study the heads of households provided informed consent and ill children were assessed by fieldworkers and referred to the appropriate level of health care. The study protocol was approved by HOPE’s Human Research Review Board and CDC’s Institutional Review Board (protocol number 3348). This secondary analysis posed no additional ethical considerations.
Results
This analysis included 873 participating households with children <5 years of age. There were 553 children in 290 households in the antibacterial soap group, 556 children in 289 households in the plain soap group and 525children in 294 households in the control group, contributing a total of 63 161 person-weeks. Of these weeks, 88%were not preceded by any diarrhoeal episode within the previous 2 weeks. The incidence rate for diarrhoeal illness was higher than the incidence rate for pneumonia (2.8 episodes/person-year vs 0.9 episodes/person-year) (Table 1).
Table 1.
Characteristics of children <5, Soap Health Study, Karachi, Pakistan, April 2002–03
| Characteristics | |
|---|---|
| Number of children<5 years | 1634 |
| Median child age (years) | 3.1 |
| Father can read | 58% |
| Mother can read | 36% |
| Monthly household income less than US$50 | 47% |
| Diarrhoea | |
| Person-years | 1128 |
| Number of episodes | 3203 |
| Incidence rate/PYa | 2.8 |
| Longitudinal prevalence (%) | 4.4 |
| Mean duration of episodes (days) | 5.7 |
| Pneumonia | |
| Person-years | 1184 |
| Number of episodes | 1071 |
| Incidence rate/PYa | 0.91 |
| Malnutrition | |
| Weight-for-age Z-score <−2 (%) | 30 |
PY, person-year observation.
Among the study children, 23% had diarrhoea in the 2 weeks prior to their pneumonia onset (Table 2). Any diarrhoea within 2 weeks of pneumonia onset was associated with an adjusted hazards ratio of HR = 1.31 (95% CI: 1.12–1.54).
Table 2.
Population attributable fraction by intervention groups in Karachi Soap Health Study, Pakistan, 2003
| Pneumonia outcomes | Antibacterial soap | Soap group | Control group | Combined |
|---|---|---|---|---|
| Proportion of pneumonia cases with recent diarrhoea | 0.14 | 0.18 | 0.28 | 0.23 |
| HRa | 1.68 | 1.97 | 1.11 | 1.31 |
| Population attributable fractionb | 6% | 9% | 3% | 6% |
| 95% confidence interval | 5.81–5.83 | 9.14–9.17 | 2.81–2.81 | 5.59–5.60 |
HR, adjusted hazard ratio.
Population attributable fraction = (p * (HR-1)/HR).
Every additional day of diarrhoea during the 2 weeks preceding the index week increased the adjusted hazard ratio of pneumonia by a factor of 1.06 (95% CI: 1.03–1.09) (Figure 1). This association was adjusted for age, sex, weight-for-age Z-score, parents’ literacy, income and soap group. The adjusted hazard ratio of pneumonia was 1.04 (95% CI: 1.03–1.06) with every additional day of diarrhoea in the preceding 4 weeks adjusted for the same confounders. Inclusion of weight-for-age Z-scorein the model, a measure of nutritional status, did not change this association for either of the time frames. Additionally, controlling for which month each pneumonia episode occurred in did not alter the estimated hazard ratio.
Figure 1.
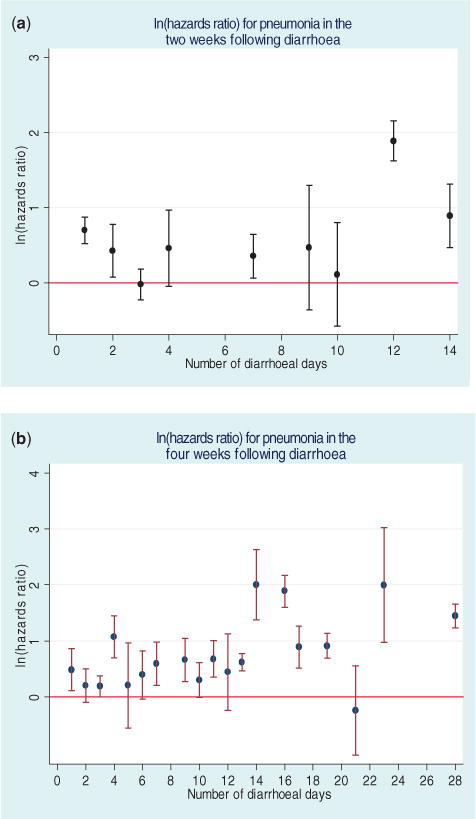
The risk of pneumonia in children aged <5 years in Pakistan, depending on number of diarrhoea days in the (a) past 2 weeks, and (b) in the 4 weeks prior to the index day, 2003
The age specific adjusted hazard ratio of pneumonia for children aged <12 months was higher at 1.09 (95%CI: 1.02–1.17) for every additional diarrhoea day in the past 2 weeks, whereas for children in the age group of 12–60 months it was 1.05 (95% CI: 1.03–1.09). This risk was not elevated in our estimates for the 4-week time frame: 1.03 (95% CI: 0.98–1.09) for <12-month-old children and 1.04 (95% CI: 1.03–1.06) for children 12–60 months of age.
Children with a history of diarrhoea preceding their episode of pneumonia had a similar duration of pneumonia (5.8 days) compared with children whose pneumonia episodes were not preceded by diarrhoea (5.7 days).
The combined estimated population attributable fraction was 0.23*0.31/1.31=0.056. The population attributable fractions were higher in soap groups compared with the control group (Table 2).
Discussion
These results support the hypothesis that diarrhoea increases the risk of pneumonia in children <5 years of age during the 2–4 weeks following an episode of diarrhoea. The increased hazard ratios for pneumonia following episodes of diarrhoea for children aged <5 years in Pakistan (HR: 1.06; 95% CI: 1.03–1.09) were comparable with those in Ghana (HR: 1.08; 95% CI: 1.00-]−1.15).5 Both Pakistan and Ghana had weekly household visits and included children aged <6 months. Brazil’s household visits were more frequent, three times per week, and excluded children aged <6 months (Table 3).
Table 3.
Methodological and epidemiological characteristics of study population in Pakistan (2003), Ghana (1990) and Brazil (1990)
| Characteristics/Outcomes | Pakistan | Ghana | Brazil |
|---|---|---|---|
| Study setting | Urban | Rural | Rural |
| Children <5 years study population | Included children <6 months | Included children <6 months | Excluded children <6 months |
| Diarrhoea definition | WHO | Mother’s perception | WHO |
| ALRI definition | WHO pneumonia definition | Rapid breathing plus danger signs | Rapid breathing plus danger signs |
| Study design | Time to event | Time to event | Time to event |
| Number of children | 1634 | 1877 | 1209 |
| Person-years | 1128 | 1455 | 1104 |
| Diarrhoea | |||
| Incidence rate/PYa | 2.8 | 9.0 | 7.0 |
| Longitudinal prevalence (%) | 4.4 | 17 | 5 |
| Mean duration of episodes (days) | 5.7 | 6.1 | 2.7 |
| Pneumonia | |||
| Number of episodes | 1071 | 162 | 128 |
| Incidence rate/PYa | 0.91 | 0.11 | 0.12 |
| Malnutrition | |||
| Weight-for-age Z-score <−2 (%) | 30 | 30 | 13 |
| Adjusted 2-week hazard ratio of pneumonia | 1.06 (95% CI: 1.03–1.09) | 1.08 (95%CI 1–1.15) | |
| Population attributable risk of pneumonia/ALRI from diarrhoea | 5.4 | 26 |
PY, person-years of observation
There was a 4-fold difference in the population attributable fraction between Ghana (26%) and Pakistan (6%) (Table 2). This difference likely reflects the difference in both the incidence and longitudinal prevalence of diarrhoea in the two countries. The incidence of pneumonia was eight times higher in Pakistan and the longitudinal prevalence of diarrhoea was three times higher in Ghana (Table 3). The much higher rate of diarrhoea in Ghana could have caused more pneumonia and so produced the observed higher population attributable fraction. The hazard ratio in Pakistan and Ghana were similar, so if we had observed a longitudinal prevalence of diarrhoea in Pakistan similar to what was seen in Ghana we would expect to see a comparable attributable fraction. In addition, the definition of diarrhea in the Ghanaian study was according to the mother’s definition, which is less restrictive than the WHO definition (Table 3). This may also account for the higher incidence and longitudinal prevalence in Ghana and the lower population attributable risk in Pakistan.
The densely populated urban settlements in Pakistan may better support transmission of respiratory pathogens than the rural Ghanaian context. Additionally, the lower incidence of pneumonia in Ghana may be because Schmidt et al. used a more specific case definition that included danger signs, such as chest in-drawing and stridor, indicative of severe acute lower respiratory illness. We used the more sensitive, less specific WHO case definition for pneumonia, which may classify some children as having pneumonia who would not meet the criteria used by Schmidt et al.21 (Table 3).
In addition, there may have been nutritional differences since the Ghana site was chosen for a high prevalence of vitamin A deficiency, which has been associated with an increased risk for both diarrhoeal and respiratory illness.22 Malnutrition prevalence was higher in Ghana and Pakistan than in Brazil (Table 3). Malnourished children also have a greater incidence and increased duration and severity of diarrhoeal diseases.23–26 Other biological factors contributing to this increased risk include compromised immune responses or micronutrient deficiencies such as zinc and vitamin A in children with marginal diets.27–29 The timing of our anthropometric measurements did not allow us to assess if the observed diarrhoeal episodes were associated with acute deteriorations in anthropometric indices, but we would expect children to have lower nutritional stores of many nutrients following episodes of diarrhoea. Dehydration caused by diarrhoea may also increase the risk of respiratory illnesses.30 The increase for risk of pneumonia following episodes of diarrhoea in Pakistan is consistent with results from a prospective case control study in 2001–2002 of children aged <5 years in Israel, that found that ⩾1 diarrhoea episodes between 8 and 31 days before the enrolment was associated with community acquired alveolar pneumonia. Their results also emphasized that poor nutritional status, detected through anthropometric measurements, increased the risk of pneumonia in children.
Our calculation of the population attributable fraction suggests that 6% of the pneumonia cases in children <5 years old in this population could have been avoided if the diarrhoeal illness of children had been prevented. Our findings are consistent with several observational studies that have noted reductions in child mortality from pneumonia after safe water interventions.31 The Mills-Reincke phenomenon suggests that for every diarrhoeal death from waterborne diseases prevented, additional pneumonia-related infant deaths were averted.31 Increased rates of hand washing in the soap groups could have minimized transmission of pathogens through respiratory droplets and feco-oral routes, which led to reductions in the proportion of both diarrhoea and pneumonia.
The population attributable fraction was higher in the soap groups compared with the control group, driven by the increased hazard ratio of pneumonia following diarrhoeal episodes in soap groups. Hand washing with soap has two mechanisms for preventing pneumonia. First, washing hands with soap removes respiratory pathogens and so directly interrupts transmission.32,33 Second, hand washing with soap reduces incidence of diarrhoea and this diarrhoea prevention reduces the subsequent development of pneumonia. One interpretation of our observations is that hand washing with soap was primarily acting through the pathway of directly interrupting transmission. Once those episodes were removed, the remaining pneumonia episodes were more likely to be mediated through increased susceptibility from diarrhoea. It suggests that the two pathways are largely independent.
This study has several limitations. Schmidt et al. employed definitions for pneumonia that would identify respiratory illness that was more severe than the definition used in the Karachi Soap Health Study. We did not find evidence that this difference affected our population attributable fraction since the mean number of days of illness with pneumonia, a proxy for severity of respiratory illness, was similar in groups with and without recent diarrhoea. However, the WHO definition is prone to misclassification and may have lowered the precision of our estimates.21 We cannot directly compare out findings with Coles et al., because they used a more specific diagnostic tool to enrol community acquired alveolar pneumonia in a hospital setting into their study, which possibly detected more severe pneumonia cases. There is evidence that diarrhoea is underreported beyond 48 hours, which could have reduced our diarrhoea incidence estimations.34,35 However, both Ghana and Pakistan also reported diarrhoea on a weekly basis.
Our methodology differed from that used by Schmidt et al. Before adopting the Prentice-Williams-Peterson gap time as our final model of interest, we compared three statistical models: Prentice-WilliamsPeterson total time model (PWP-CP) used by Schmidt et al; Prentice-Williams-Peterson gap time model (PWP-GT); and generalized estimating equations (GEE) approach. All the models provided similar estimates of the hazards/odds ratios (results not shown). Schmidt et al. used calendar time as the time scale in order to control for seasonality in the incidence of diarrhoea and ALRI. To account for seasonality, we included the month of each pneumonia episode in the regression model. However, controlling for the month of each pneumonia episode did not alter the estimated hazard ratios. Moreover, immune responses to previous pneumonia episodes, which may influence the risk of consequent pneumonia in a child, may be better accounted for in the PWP-GT than the PWP-CP model.
These data suggest that in a setting where there is a high incidence of malnutrition, episodes of diarrhea increase the risk of pneumonia in the subsequent 2–4 weeks in children aged <5 years. Efforts to achieve millennium development goal 4, to reduce child mortality by two-thirds by 2015, focus on pneumonia, the dominant cause of child mortality. Strategies include vaccination, nutrition, reducing environmental pollution and case detection and management.36 In light of our findings, preventive interventions targeting diarrhoea are of even higher importance than that captured by their effectiveness in preventing diarrhoea, because they could have a multiplier effect on disease reduction in children aged <5 years. Zinc treatment for acute diarrhea, which reduces the duration and severity of acute diarrhoea and reduces the risk of subsequent infections including pneumonia, should be encouraged.37,38 Health communication messages for caregivers could alert them to signs of pneumonia, especially for those children aged <5 years who have recently experienced an episode of diarrhoea, and encourage prompt care seeking.
KEY MESSAGES.
Communities that have a high risk of childhood diarrhoea also have a high risk of pneumonia.
Children <5 years of age are at an increased risk of pneumonia following recent diarrhoeal illness.
Public health programmes that prevent diarrhoea may also reduce the burden of respiratory illnesses.
Acknowledgments
We thank the HOPE staff workers for the data collection and fieldwork and the community members for their participation. We acknowledge the contribution of Dorothy Southern’s review of this manuscript and M. Yushuf Sharker’s support with the statistical analyses.
Funding: Procter and Gamble and the Centers for Disease Control and Prevention funded this study.
Footnotes
Conflict of interest: None declared.
References
- 1.Bryce J, Boschi-Pinto C, Shibuya K, Black RE. WHO estimates of the causes of death in children. Lancet. 2005;365:1147–52. doi: 10.1016/S0140-6736(05)71877-8. [DOI] [PubMed] [Google Scholar]
- 2.Fenn B, Morris SS, Black RE. Comorbidity in childhood in northern Ghana: magnitude, associated factors, and impact on mortality. Int J Epidemiol. 2005;34:368. doi: 10.1093/ije/dyh335. [DOI] [PubMed] [Google Scholar]
- 3.Mulholland K. Commentary: comorbidity as a factor in child health and child survival in developing countries. Int J Epidemiol. 2005;34:375. doi: 10.1093/ije/dyi028. [DOI] [PubMed] [Google Scholar]
- 4.Coles CL, Fraser D, Givon-Lavi N, et al. Nutritional status and diarrheal illness as independent risk factors for alveolar pneumonia. Am J Epidemiol. 2005;162:999–1007. doi: 10.1093/aje/kwi312. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt W-P, Cairncross S, Barreto ML, Clasen T, Genser B. Recent diarrhoeal illness and risk of lower respiratory infections in children under the age of 5 years. Int J Epidemiol. 2009;38:766–72. doi: 10.1093/ije/dyp159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luby S, Mubina A, Feikin DR, et al. Effect of handwashing on child health: a randomised controlled trial. Lancet. 2005;366:225–33. doi: 10.1016/S0140-6736(05)66912-7. [DOI] [PubMed] [Google Scholar]
- 7.Ejemot RI, Ehiri JE, Meremikwu MM, Critchley JA. Cochrane review: Hand washing for preventing diarrhoea. Evid Based Child Health. 2009;4:893–939. doi: 10.1002/14651858.CD004265.pub2. [DOI] [PubMed] [Google Scholar]
- 8.Rabie T, Curtis V. Handwashing and risk of respiratory infections: a quantitative systematic review. Trop Med Int Health. 2006;11:258–67. doi: 10.1111/j.1365-3156.2006.01568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luby S, Agboatwalla M, Painter J, Altaf A, Billhimer W, Hoekstra R. Effect of intensive handwashing promotion on childhood diarrhea in high-risk communities in Pakistan: a randomized controlled trial. JAMA. 2004;291:2547. doi: 10.1001/jama.291.21.2547. [DOI] [PubMed] [Google Scholar]
- 10.Gove S. Integrated management of childhood illness by outpatient health workers: technical basis and overview. The WHO Working Group on Guidelines for Integrated Management of the Sick Child. Bull World Health Organ. 1997;75(Suppl 1):7–24. [PMC free article] [PubMed] [Google Scholar]
- 11.Prentice R, Williams B, Peterson A. On the regression analysis of multivariate failure time data. Biometrika. 1981;68:373. [Google Scholar]
- 12.Kelly P, Lim L. Survival analysis for recurrent event data: an application to childhood infectious diseases. Stat Med. 2000;19:13–33. doi: 10.1002/(sici)1097-0258(20000115)19:1<13::aid-sim279>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 13.Lim HJ, Liu J, Melzer-Lange M. Comparison of methods for analyzing recurrent events data: Application to the emergency department visits of pediatric firearm victims. Accid Anal Prev. 2007;39:290–99. doi: 10.1016/j.aap.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 14.Lin D. Cox regression analysis of multivariate failure time data: the marginal approach. Stat Med. 1994;13:2233–47. doi: 10.1002/sim.4780132105. [DOI] [PubMed] [Google Scholar]
- 15.Cox D. Regression models and life-tables. J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 16.Lin DY, Wei LJ. The Robust Inference for the Cox Proportional Hazards Model. J Am Stat Assoc. 1989;84:1074–78. [Google Scholar]
- 17.Grambsch P, Therneau T. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515. [Google Scholar]
- 18.Benichou J. Biostatistics and epidemiology: measuring the risk attributable to an environmental or genetic factor. C R Biologies. 2007;330:281–98. doi: 10.1016/j.crvi.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 19.Samuelsen SO, Eide GE. Attributable fractions with survival data. Stat Med. 2008;27:144767. doi: 10.1002/sim.3022. [DOI] [PubMed] [Google Scholar]
- 20.Newcombe RG. Re: Confidence limits made easy: interval estimation using a substitution method. Am J Epidemiol. 1999;149:884. doi: 10.1093/oxfordjournals.aje.a009906. [DOI] [PubMed] [Google Scholar]
- 21.Perkins BA, Zucker JR, Otieno J, et al. Evaluation of an algorithm for intergrated management of chidhood illnes in an area of Kenya with high malaria transmission. Bull World Health Organ. 1997;75(Suppl 1):33–42. [PMC free article] [PubMed] [Google Scholar]
- 22.Sommer A, Katz J, Tarwotjo I. Increased risk of respiratory disease and diarrhea in children with preexisting mild vitamin A deficiency. Am J Clin Nutr. 1984;40:1090. doi: 10.1093/ajcn/40.5.1090. [DOI] [PubMed] [Google Scholar]
- 23.Bairagi R, Chowdhury MK, Kim YJ, Curlin GT, Gray RH. The association between malnutrition and diarrhoea in rural Bangladesh. Int J Epidemiol. 1987;16:477. doi: 10.1093/ije/16.3.477. [DOI] [PubMed] [Google Scholar]
- 24.Chandra R. Interactions of nutrition, infection and immune response. Immunocompetence in nutritional deficiency, methodological considerations and intervention strategies. Acta Pædiatr. 1979;68:13744. doi: 10.1111/j.1651-2227.1979.tb04975.x. [DOI] [PubMed] [Google Scholar]
- 25.Guerrant RL, Oriá RB, Moore SR, Oriá MOB, Lima AAM. Malnutrition as an enteric infectious disease with long-term effects on child development. Nutr Rev. 2008;66:487. doi: 10.1111/j.1753-4887.2008.00082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palmer D, Koster F, Alam A, Islam M. Nutritional status: a determinant of severity of diarrhea in patients with cholera. J Infectious Dis. 1976;134:8. doi: 10.1093/infdis/134.1.8. [DOI] [PubMed] [Google Scholar]
- 27.Walker CF, Black RE. Zinc and the risk for infectious disease. Annu Rev Nutr. 2004;24:255–75. doi: 10.1146/annurev.nutr.23.011702.073054. [DOI] [PubMed] [Google Scholar]
- 28.Sheikh A, Shamsuzzaman S, Ahmad SM, et al. Zinc influences innate immune responses in children with enterotoxigenic Escherichia coli-induced diarrhea. J Nutr. 2010;140:1049–56. doi: 10.3945/jn.109.111492. [DOI] [PubMed] [Google Scholar]
- 29.Glasziou PP, Mackerras DE. Vitamin A supplementation in infectious diseases: a meta-analysis. BMJ. 1993;306:366–70. doi: 10.1136/bmj.306.6874.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalhoff H. Mild dehydration: a risk factor of bronchopulmonary disorders? Eur J Clin Nutr. 2003;57:S81–S87. doi: 10.1038/sj.ejcn.1601906. [DOI] [PubMed] [Google Scholar]
- 31.Sedgwick WT, Macnutt JS. On the Mills-Reincke phenomenon and Hazen’s theorem concerning the decrease in mortality from diseases other than typhoid fever following the purification of public water-supplies. J Infect Dis. 1910;7:489–564. [Google Scholar]
- 32.Lowbury EJ, Lilly HA, Bull JP. Disinfection of hands: removal of transient organisms. BMJ. 1964;2:230–33. doi: 10.1136/bmj.2.5403.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gwaltney JM, Moskalski PB, Hendley JO. Hand-to-hand transmission of rhinovirus colds. Ann Intern Med. 1978;88:463–67. doi: 10.7326/0003-4819-88-4-463. [DOI] [PubMed] [Google Scholar]
- 34.Alam N, Henry FJ, Rahaman MM. Reporting errors in one-week diarrhoea recall surveys: experience from a prospective study in rural Bangladesh. Int J Epidemiol. 1989;18:697. doi: 10.1093/ije/18.3.697. [DOI] [PubMed] [Google Scholar]
- 35.Zafar S, Luby S, Mendoza C. Recall errors in a weekly survey of diarrhoea in Guatemala: determining the optimal length of recall. Epidemiol Infect. 2010;138:264–69. doi: 10.1017/S0950268809990422. [DOI] [PubMed] [Google Scholar]
- 36.Mulholland K. Childhood pneumonia mortality—a permanent global emergency. Lancet. 2007;370:285–89. doi: 10.1016/S0140-6736(07)61130-1. [DOI] [PubMed] [Google Scholar]
- 37.Bhutta ZA, Bird SM, Black RE, et al. Therapeutic effects of oral zinc in acute and persistent diarrhea in children in developing countries: pooled analysis of randomized controlled trials. Ame J Clin Nutr. 2000;72:1516–22. doi: 10.1093/ajcn/72.6.1516. [DOI] [PubMed] [Google Scholar]
- 38.Bhutta ZA, Black RE, Brown KH, et al. Prevention of diarrhea and pneumonia by zinc supplementation in children in developing countries: Pooled analysis of randomized controlled trials. J Pediatr. 1999;135:689–97. doi: 10.1016/s0022-3476(99)70086-7. [DOI] [PubMed] [Google Scholar]


