Abstract
Background
S. aureus is a pathogen in humans and animals that harbors a wide variety of virulence factors and resistance genes. This bacterium can cause a wide range of mild to life-threatening diseases. In the latter case, fast diagnostic procedures are important. In routine diagnostic laboratories, several genotypic and phenotypic methods are available to identify S. aureus strains and determine their resistances. However, there is a demand for multiplex routine diagnostic tests to directly detect staphylococcal toxins and proteins.
Methods
In this study, an antibody microarray based assay was established and validated for the rapid detection of staphylococcal markers and exotoxins. The following targets were included: staphylococcal protein A, penicillin binding protein 2a, alpha- and beta-hemolysins, Panton Valentine leukocidin, toxic shock syndrome toxin, enterotoxins A and B as well as staphylokinase. All were detected simultaneously within a single experiment, starting from a clonal culture on standard media. The detection of bound proteins was performed using a new fluorescence reading device for microarrays.
Results
110 reference strains and clinical isolates were analyzed using this assay, with a DNA microarray for genotypic characterization performed in parallel. The results showed a general high concordance of genotypic and phenotypic data. However, genotypic analysis found the hla gene present in all S. aureus isolates but its expression under given conditions depended on the clonal complex affiliation of the actual isolate.
Conclusions
The multiplex antibody assay described herein allowed a rapid and reliable detection of clinically relevant staphylococcal toxins as well as resistance- and species-specific markers.
Introduction
Routine laboratories focus on culturing and identifying bacterial species, as well as obtaining their susceptibility profiles. Some susceptibility test results, such as oxacillin/methicillin resistance in staphylococci, vancomycin resistance in enterococci or carbapenem resistance in enterobacteria, require additional assays for confirmation due to their high relevance for therapy of individual patients, and for infection control. This can be done by molecular methods or using antibody-based assays. Molecular methods require sophisticated and expensive equipment. Currently, antibody-based tests are widely used. Examples include agglutination assays or lateral flow (LF) tests, e.g., for confirmation of the presence of modified penicillin binding protein (PBP2a) conferring oxacillin/methicillin resistance in Staphylococcus aureus/MRSA [1].
Staphylococcus aureus (S. aureus) is a common opportunistic pathogen. It colonizes approximately 30% of a healthy human population [2], but can also cause nosocomial or community-acquired infections. Clinically, S. aureus is associated with skin and soft tissue infections, food intoxications, and life-threatening diseases like pneumonia, endocarditis or septicemia. Carriers of S. aureus, in particular hospitalized, dialysis and catheter patients, show an increased risk of invasive infections [3] but a lower risk of septicemia-related death [4].
A major problem with S. aureus is the high rate of resistance to methicillin and other beta-lactam antibiotics (MRSA), especially in nosocomial settings. Normally, methicillin inhibits the cell wall synthesis of the bacteria by binding to their penicillin-binding proteins (PBPs). The gene mecA encodes for a modified penicillin-binding protein (PBP2a). PBP2a performs the function of PBP by synthesizing peptidoglycan, therefore methicillin cannot bind anymore [5]. In life-threatening situations it is important to rapidly detect the presence of mecA in order to ensure efficient, i.e., non-beta-lactam-based therapy. Additionally, MRSA-positive patients should be isolated in separate rooms to avoid a transmission to other patients. In routine diagnostics, the beta-lactam resistance, caused by PBP2a, is detected by agar diffusion or micro dilution tests, and the presence of mecA/PBP2a is then confirmed by either PCR, agglutination or lateral flow assays [6].
In addition to mecA, a highly divergent homologue, mecC, was recently identified [7–8]. This gene also encodes for beta-lactam resistance. Due to its low homology to mecA, mecC caused concern in diagnostics. While selective media and susceptibility tests can indicate methicillin resistance in mecC strains, confirmatory tests frequently do not identify them [9]. Beside mecA/C, some staphylococci (e.g., S. sciuri and S. vitulinus) can harbor other mecA alleles (“mecA1”) that do not encode resistance to beta lactam compounds [10–11].
MRSA has become a global problem—first in hospitals, but for approximately the last 25 years, also in non-hospitalized individuals. The so-called community-acquired MRSA (CA-MRSA) strains are often found to be more virulent than hospital-acquired MRSA (HA-MRSA), and can even infect young and otherwise healthy people. Typical properties of these clones are the presence of the smaller sized type IV or V staphylococcal cassette chromosomes mec (SCCmec) and, in many but not all strains, of the Panton Valentine leukocidin (PVL) [12],[13–24]. PVL is a leukocidin of special medical relevance that has been previously reviewed in detail [12], [13–22],[23].
Relevant virulence factors in S. aureus range from hemolysins, e.g., alpha- and beta-hemolysins (HLA, HLB) [25–29],[30], and other enzymes that digest host tissues to yield nutrients to proteins that disrupt or manipulate the host immune system [31–33]. These proteins include Superantigens, such as toxic shock syndrome toxin (TSST) [4], [34–37], staphylococcal enterotoxins (SEs) [38–41], and leukocidins. Superantigens lead to an antigen-unspecific T-cell activation followed by an immense cytokine release [42]. Currently, the detection of staphylococcal toxins relies largely on molecular methods, i.e., PCR or array hybridization [43]. These approaches are mainly restricted to research and/or reference laboratories. In routine laboratories, options for detecting staphylococcal toxins are limited, since there are no routine diagnostic tests to confirm the presence of multiple staphylococcal toxins. Enzyme-linked Immunosorbent Assays (ELISAs) or Lateral Flow tests are mainly focusing on one target only [44–45].
A multiplex test for Staphylococcal toxins could be helpful, because infections with S. aureus producing toxins should be treated differently than infections with S. aureus lacking those toxins. The presence of PVL mandates special infection control and eradication measures (HPA guideline: https://www.gov.uk/government/collections/panton-valentine-leukocidin-pvl-guidance-data-and-analysis), or a clinical condition related to PVL or TSST1 might be treated with gamma globulin and/or compounds inhibiting toxin biosynthesis (such as rifampicin, clindamycin) in addition to the standard regimen [46]. Given the clinical relevance of antibiotic resistance in S. aureus and of its various exotoxins, an assay for the detection of the respective proteins could be of high interest.
The aim of this study was to develop a new, rapid and economic fluorescence-based assay for qualitative or semi-quantitative analysis of expressed proteins, starting with clonal cultures obtained by routine laboratory procedures. A designated new reader and software were developed for the analysis of fluorescence microarray images. An antibody microarray was designed to allow simultaneous detection of PBP2a, important secreted virulence factors (TSST, PVL, SEA, SEB, HLA, HLB, Staphylokinase (SAK)) as well as a species marker that serves as a positive control (staphylococcal protein A, SPA).
In parallel to the phenotypic detection using the protein microarrays, the presence of genes and alleles coding for virulence factors was investigated during this study using DNA microarrays. A direct comparison of genotypic and phenotypic data for the targets was therefore possible.
Materials & Methods
Strains
In this study, 110 bacterial strains/isolates were tested. These included 105 S. aureus, 2 S. epidermidis (ATCC35984 and ATCC12228), 1 S. sciuri, 1 S. capitis and 1 E. coli (BL21-DE3, National Laboratory New York, USA) as negative control. Most of the strains/isolates originated from clinical diagnostic samples, but some well characterized reference strains were also tested. All of them were genotyped by DNA microarray hybridization (StaphyType Kit, Alere Technologies GmbH, Jena, Germany). This provided information regarding the presence or absence of relevant virulence and resistance genes, including those that encode PVL, TSST, HLA, HLB, SEA, SEB, SAK, PBP2a and Protein A, as well as the affiliation to clonal complexes and strains.
Culture conditions
In previous experiments, different growth media were tested for strain culturing and detection of proteins [21],[25]. Based on these results, strains and isolates were incubated on Columbia Blood agar (Oxoid, Wesel, Germany) at 37°C for 18–24 h. One loop of bacterial material was inoculated into 130 μl phosphate buffered saline (1x PBS) or into 65 μl sodium hydroxide (NaOH; 100mM), respectively, and vortexed. To the NaOH suspension, 65 μl of buffered phosphate buffer (pH 5.5; 1M di-sodium hydrogen phosphate and sodium di-hydrogen phosphate) was added for neutralization (pH 7), followed by additional vortexing.
Antibodies
Monoclonal antibodies (AB) for the targets PBP2a, SAK, HLB, HLA, SEB, SEA, TSST and lukF-PV were generated via phage display [47] as previously described [44],[21],[25]. SPA antibodies originated from three polyclonal chicken sera (courtesy of Alere Scarborough/Binax). First, all antibodies were screened to find the optimal (i.e., most specific and sensitive) combination of capture and detection antibodies for each target [44],[21],[25]. Then, all selected antibodies were tested in a mixture to find optimal conditions with regard to both, usage and functionality. The capture antibodies were spotted onto the microarrays, each 3–4 times redundantly and in two different concentrations, 0.5 mg/ml and 0.05 mg/ml, respectively. The use of different concentrations aimed at minimizing effects of steric interference. In order to reach a total protein concentration of 0.5 mg/ml (which is required for the spotting procedure), bovine serum albumin was added (0.45 mg/ml) to the antibodies at higher dilutions. For the number of spotted antibodies per target see Fig 1. For each target, a secondary detection antibody was labelled using Sulf-NHS-LC-Biotin (Pierce, Bonn, Germany) according to the manufacturer’s instructions. A mixture of all 9 biotin-labelled detection antibodies was prepared. The final concentrations of the antibodies were as follows: anti-lukF-PV, 0.2 ng/μl; anti-PBP2a, 0.02 ng/μl; anti-Protein A, 0.5 ng/μl; anti-TSST, 0.1 ng/μl; anti-SEA, 0.05 ng/μl; anti-SEB, 0.05ng/μl; anti-SAK, 0.1 ng/μl; anti-HLA, 0.2 ng/μl; and anti-HLB, 0.07 ng/μl.
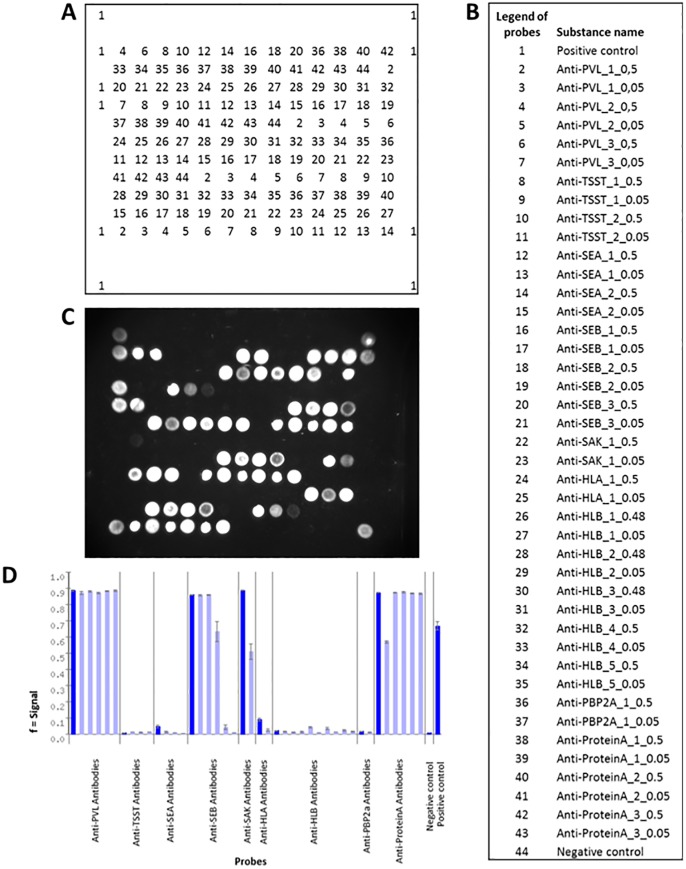
Fig 1. Layout of the protein microarray.
(A) Positions of each substance on the chip. (B) Legend to the probes. (C) Picture of a processed fluorescent microarray. (D) Bar graph with signal intensities for the expressed proteins.
Array procedures
500 μl washing buffer (Protein Binding Kit, Alere Technologies GmbH, Jena, Germany) was added to the arrays and incubated at 37°C and 400 rpm on a shaker for 5 min. 100 μl of blocking solution (Protein Binding Kit, Alere Technologies GmbH) was then added and incubated at 37°C and 300 rpm for 5 min. Meanwhile, culture suspensions in PBS and NaOH (as described above) were prepared. Both culture suspensions were diluted 1:10 in the mixture of biotinylated detection antibodies, and subsequently incubated at 37°C and 400 rpm for 10 min in a separate reaction tube. First, 50 μl of the NaOH-treated sample was added to the array and incubated at 37°C and 300 rpm for 5 min, then the sample was removed and 50 μl of the sample aliquot that was treated with PBS was added and incubated at 37°C and 300 rpm for additional 25 min. The array was then washed with 500 μl washing buffer (5 min, 37°C, 400 rpm). For the detection of specifically bound proteins, 10 μl of purpose-made fluorescent beads (Alere Technologies GmbH), labelled with Cy3 and streptavidin, were added to the array and incubated at 37°C and 300 rpm for 30 min. The arrays were then washed again with 500 μl washing buffer (5 min, 37°C, 400 rpm). The washing buffer was replaced by 100 μl fresh washing buffer and images were taken by a purpose-made reading device, the ATR-Fluo-Reader (Alere Technologies GmbH; http://alere-technologies.com/en/products/lab-solutions/reader-systems/atr-fluo-reader.html; Ehricht et al., 2014).
Analysis
The images taken by the ATR-Fluo-Reader at exposure times of 500 ms and 1,000 ms, were transferred to a computer for further analysis. Iconoclust software (Alere Technologies GmbH) was used according to manufacturer’s instructions with an assay-specific script. The signals (S) of the spots were determined by: S = M-BG whereby M is the average intensity of the spot and BG the intensity of the local background. Thus, the signals range between 0 (negative) and 1 (maximum signal). For each target, specific cut-off values were determined (Table 1). Resulting signal values were considered positive if previously determined cut-offs were reached or surpassed. A software tool was generated for the automatic analysis of the data resulting from the fluorescent microarrays. This software provides reports giving information on the presence of tested proteins based on the previously determined cut-offs. Additionally, results were manually compared to results of the DNA array based genotyping of the respective isolates.
Table 1. Specific cut-off values for each target.
| Target | Cut off |
|---|---|
| Positive control | > 0.25 |
| Negative control | < 0.1 |
| lukF-P83 | Anti-PVL_2 > 0.2 * |
| lukF-PV | at least 2 anti-PVL antibodies > 0.2 |
| Protein A | at least 1 anti-Protein A antibody > 0.1 |
| PBP2a | at least 1 anti-PBP2a antibody > 0.2 |
| TSST | at least 1 anti-TSST antibody > 0.2 |
| SEA | at least 1 anti SEA antibody > 0.2 |
| SEB | at least 1 anti-SEB antibody > 0.2 |
| HLA | at least 1 anti-HLA antibody > 0.2 |
| HLB | at least 1 anti-HLB antibody > 0.2 |
| SAK | At least 1 anti-SAK antibody > 0.2 |
*Anti-PVL_2 Antibody detects exclusive epitopes of lukF-P83. Positive signals for two of the anti-PVL antibodies, 1, 2 or 3, indicate for the target lukF-PV.
Results
Assay optimization
The protocol was optimized to facilitate rapid detection of all nine targets simultaneously. Starting from clonal cultures, the bacterial material was inoculated into different solutions to find optimal conditions for the detection of all different membrane and secreted proteins within one and the same assay. NaOH and PBS treated cells were initially tested separately and afterwards in combination. This resulted in partially different performances for the single proteins. After the NaOH treatment, the targets PBP2a, HLA, HLB, SEA, SEB, SAK, protein A and TSST were detected. However, lukF-PV as well as lukF-P83 were not reliably detectable when treating known lukF-PV or lukF-P83 positive strains/isolates with NaOH. In the PBS buffer suspension, all requested targets except PBP2a were detectable.
The protocol was optimized as follows to detect all expressed targets simultaneously: Bacterial culture material was harvested from Columbia blood agar plates (incubated for 18- 24h, 37°C) and divided into two aliquots: one in 1x PBS and one in 100 mM NaOH, respectively. Subsequently, a buffer (see Material/Methods) was added to the NaOH suspension for neutralization. Then, both suspensions were added one after the other to the same array for specific binding of all antigens to their corresponding antibodies (see Methods).
Dilution series with known concentrations of each target (data not shown but can be provided upon request) resulted in following limits of detection: PVL: 0.5 ng/ml, TSST: 0.05 ng/ml, HLA: 0.1 ng/ml, HLB: 0.1 ng/ml, SEA: 0.01 ng/ml, SEB: 0.01 ng/ml, SAK: 0.05 ng/ml and PBP2a: 0.1 ng/ml. No data were obtained for SPA because SPA detection antibodies were polyclonal. In addition, different staphylococcal strains might present with different detection limits because of the presence of multiple binding sites per SPA molecule that might vary in both number and affinity. The use of culture material directly from agar plates ensured that the antigen concentrations was much higher than the detection limit.
Screening of staphylococcal strains using the protein array and the DNA genotyping array
The results of the antibody arrays and a comparison to the genotypic characterization are summarized in Table 2. 110 isolates and strains were tested using microarrays, the antibody array for phenotyping and the DNA array for genotyping, in parallel. The results of both arrays showed a general concordance of phenotype and genotype for all 110 tested isolates/strains (Table 2). The tested isolates represented diverse clonal complexes (CC1, CC5, CC8, CC9, CC25, CC30, CC 121, CC130, CC133, CC152, CC361, CC479, CC705) from different origins and included MSSA and MRSA.
Table 2. Comparison of genotyping data and fluorescent protein microarray results for various staphylococcal isolates.
| № | ID | typing | Protocol | SPA | mecA/ PBP2a | PVL | TST | SEA | SEB | SAK | HLA | HLB | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D | Pr | D | Pr | D | Pr | D | Pr | D | Pr | D | Pr | D | Pr | D | Pr | D | Pr | ||||
| 1 | 160886 | CC1-MSSA | NaOH/PBS | P | P | N | N | P | P | P | P | P 1 | P | N | N | P | P | P | P | T | N |
| 2 | 199573 | CC1-MSSA | NaOH/PBS | P | P | N | N | N | N | N | N | P | P | N | N | P | P | P | P | T | N |
| 3 | 215392 | CC1-MSSA | NaOH/PBS | P | P | N | N | N | N | N | N | P 1 | N | N | N | P | P | P | N | T | N |
| 4 | 173633 | CC1-MSSA | NaOH | P | P | N | N | N | N | N | N | P 1 | P | N | N | P | P | P | P | T | N |
| 5 | Sanger476 | CC1-MSSA-SCCfus | NaOH | P | P | N | N | N | N | N | N | P 1 | P | N | N | P | P | P | N | P | N |
| 6 | 231545 | CC1-MSSA-SCCfus | NaOH/PBS | P | P | N | N | N | N | N | N | P | P | P | P | P | P | P | P | T | N |
| 7 | 231549 | CC1-MSSA-SCCfus | NaOH/PBS | P | P | N | N | N | N | N | N | P | P | P | P | P | P | P | P | T | N |
| 8 | 231554 | CC1-MSSA-SCCfus | NaOH/PBS | P | P | N | N | N | N | N | N | P | P | P | P | P | P | P | P | T | N |
| 9 | MW2 | CC1-MRSA-IV [PVL+], USA400 | NaOH/PBS | P | P | P | P | P | P | N | N | P | P | N | N | P | P | P | N | T | N |
| 10 | 124931 | CC1-MRSA-IV+SCCfus [PVL+] | NaOH/PBS | P | P | P | P | P | P | N | N | P 1 | P | N | N | P | P | P | P | T | N |
| 11 | N315 | CC5-MRSA-II, New York-Japan Clone | NaOH | P | P | P | P | N | N | P | P | P 2 | N | N | N | P | P | P | P | T | N |
| 12 | ATCC700699 | CC5-MRSA-II, New York-Japan Clone | NaOH/PBS | P | P | P | P | N | N | P | P | P 1 | P | N | N | P | P | P | N | T | N |
| 13 | 223878 | CC5-MSSA with a truncated SCC element | NaOH | P | P | N | N | N | N | N | N | P 1 | P | N | N | P | P | P | P | T | N |
| 14 | 241318 | CC5-MRSA-IV, Paediatric clone [PVL+] | NaOH/PBS | P | P | P | P | P | P | N | N | P 2 | N | N | N | P | P | P | A | T | N |
| 15 | 254809 | CC5-MRSA-V+SCCfus | NaOH/PBS | P | P | P | P | N | N | N | N | N | N | P | P | P | P | P | A | A | N |
| 16 | NCTC8325 | CC8-MSSA | NaOH | P | P | N | N | N | N | N | N | N | N | N | N | P | P | P | P | T | N |
| 17 | 199572 | CC8-MSSA | NaOH/PBS | P | P | N | N | N | N | N | N | P | P | N | N | P | P | P | N | T | N |
| 18 | FPR3757 | CC8-MRSA-IV (PVL+/ACME+), USA300 | NaOH/PBS | P | P | P | P | P | P | N | N | N | N | N | N | P | P | P | P | T | N |
| 19 | 200914 | CC8-MRSA-IV (PVL+/ACME+), USA300 | NaOH/PBS | P | P | P | P | P | P | N | N | N | N | N | N | P | P | P | P | T | N |
| 20 | 200915 | CC8-MRSA-IV (PVL+/ACME+), USA300 | NaOH/PBS | P | P | P | P | P | P | N | N | N | N | N | N | P | P | P | P | T | N |
| 21 | 200917 | CC8-MRSA-IV (PVL+/ACME+), USA300 | NaOH/PBS | P | P | P | P | P | P | N | N | N | N | N | N | P | P | P | P | T | N |
| 22 | 205975 | CC8-MRSA-IV [PVL+/ACME+], USA300 | NaOH/PBS | P | P | P | P | P | P | N | N | N | N | N | N | P | P | P | P | T | N |
| 23 | 227497 | CC8-MRSA-IV (PVL+/ACME+), USA300 | NaOH | P | P | P | P | P | P | N | N | N | N | N | N | P | P | P | P | T | N |
| 24 | 176042 | CC8-MRSA-IV [PVL+/ACME+], USA300 | NaOH/PBS | P | P | P | P | P | P | N | N | N | N | N | N | P | P | P | P | T | N |
| 25 | 200916 | CC8-MRSA-IV (PVL+/ACME+), USA300 | NaOH/PBS | P | P | P | P | P | P | N | N | N | N | N | N | P | P | P | N | T | N |
| 26 | 200918 | CC8-MRSA-IV (PVL+/ACME+), USA300 | NaOH/PBS | P | P | P | P | P | P | N | N | N | N | N | N | P | P | P | N | T | N |
| 27 | 200919 | CC8-MRSA-IV (PVL+/ACME+), USA300 | NaOH/PBS | P | P | P | P | P | P | N | N | N | N | N | N | P | P | P | N | T | N |
| 29 | 124407 | CC8-MRSA-IV (PVL+/ACME+), USA300 | NaOH | P | P | P | P | P | P | N | N | N | N | N | N | P | P | P | P | T | N |
| 30 | COL | CC8/ST250-MRSA-I, Early/Ancestral MRSA | NaOH/PBS | P | P | P | P | N | N | N | N | N | N | P | P | N | N | P | A | P | N |
| 31 | 150242 | CC9-MRSA-IX | NaOH | P | P | P | P | N | N | N | N | N | N | N | N | N | N | P | N | P | N |
| 32 | 199027 | CC25-MSSA | NaOH/PBS | P | P | N | N | N | N | N | N | N | N | N | N | P | P | P | N | T | N |
| 33 | 199571 | CC30-MSSA | NaOH | P | P | N | N | N | N | P | P | P | P | N | N | P | P | P | N | T | N |
| 34 | 254033 | CC30-MSSA | NaOH/PBS | P | P | N | N | N | N | P | P | P | P | N | N | P | P | P | N | T | N |
| 35 | 199575 | CC30-MSSA | NaOH | P | P | N | N | N | N | P | P | P | P | N | N | P | P | P | N | T | N |
| 36 | 199576 | CC30-MSSA | NaOH | P | P | N | N | N | N | P | P | P 2 | N | N | N | P | P | P | N | T | N |
| 37 | 252785 | CC30-MSSA | NaOH/PBS | P | P | N | N | N | N | P | P | P | P | N | N | P | P | P | N | T | N |
| 38 | 254801 | CC30-MRSA | NaOH/PBS | P | P | P | P | P | P | N | N | P | P | N | N | P | P | P | N | P | N |
| 39 | 198848 | CC30-MRSA-IV [PVL+] | NaOH/PBS | P | P | P | P | P | P | N | N | N | N | N | N | P | P | P | N | P | N |
| 40 | 234946 | CC30-MSSA, [lukF-P83/lukM+] | NaOH/PBS | P | P | N | N | P b | N | N | N | N | N | N | N | N | N | P | N | P | N |
| 41 | 200922 | CC30-MRSA-IV-SCCfus [PVL+] | NaOH/PBS | P | P | P | P | P | P | P | P | N | N | N | N | P | P | P | N | T | N |
| 42 | 200923 | CC30-MRSA-IV-SCCfus [PVL+] | NaOH/PBS | P | P | P | P | P | P | P | P | N | N | N | N | N | N | P | N | P | N |
| 43 | 200924 | CC30-MRSA-IV-SCCfus [PVL+] | NaOH/PBS | P | P | P | P | P | P | P | P | N | N | N | N | P | P | P | N | P | N |
| 44 | 215384 | CC30-MRSA-IV | NaOH | P | P | P | P | N | N | P | P | N | N | N | N | P | N | P | N | T | N |
| 45 | 200920 | CC30-MRSA-IV [PVL+], Southwest Pacific Clone | NaOH/PBS | P | P | P | P | P | P | N | N | N | N | N | N | P | P | P | N | T | N |
| 46 | 200921 | CC30-MRSA-IV [PVL+], Southwest Pacific Clone | NaOH/PBS | P | P | P | P | P | P | N | N | P | P | N | N | P | P | P | N | T | N |
| 47 | 200925 | CC30-MRSA-IV [PVL+], Southwest Pacific Clone | NaOH/PBS | P | P | P | P | P | P | N | N | P | P | N | N | P | P | P | N | P | N |
| 48 | Sanger252 | ST36/39-MRSA-II, UK-EMRSA-16 | NaOH/PBS | P | P | P | P | N | N | N | N | P 1 | P | N | N | P | P | P | N | T | N |
| 49 | 236783 | CC88-MRSA-IV [PVL+], [lukF-PV+, lukS-PV-] | NaOH/PBS | P | P | P | P | P 4 | N | N | N | P 2 | N | N | N | P | P | P | N | T | N |
| 50 | 238233 | CC121-MSSA [PVL+] | NaOH/PBS | P | P | N | N | P | P | N | N | N | N | N | N | P | P | P | N | T | N |
| 51 | 238234 | CC121-MSSA [PVL+] | NaOH/PBS | P | P | N | N | P | P | N | N | N | N | N | N | P | P | P | N | T | N |
| 52 | 238235 | CC121-MSSA [PVL+] | NaOH/PBS | P | P | N | N | P | P | N | N | N | N | N | N | P | P | P | N | T | N |
| 53 | 238236 | CC121-MSSA [PVL+] | NaOH/PBS | P | P | N | N | P | P | N | N | N | N | P | P | P | P | P | N | T | N |
| 54 | 238237 | CC121-MSSA [PVL+] | NaOH/PBS | P | P | N | N | P | P | N | N | N | N | P | A | P | P | P | N | T | N |
| 55 | 238238 | CC121-MSSA [PVL+] | NaOH/PBS | P | P | N | N | P | P | N | N | N | N | P | P | P | P | P | N | T | N |
| 56 | 238239 | CC121-MSSA [PVL+] | NaOH/PBS | P | P | N | N | P | P | N | N | N | N | P | P | P | P | P | N | T | N |
| 57 | 238240 | CC121-MSSA [PVL+] | NaOH/PBS | P | P | N | N | P | P | N | N | N | N | P | P | P | P | P | N | T | N |
| 58 | 238241 | CC121-MSSA [PVL+] | NaOH/PBS | P | P | N | N | P | P | N | N | N | N | P | P | P | P | P | N | T | N |
| 59 | 238242 | CC121-MSSA [PVL+] | NaOH/PBS | P | P | N | N | P | P | N | N | N | N | P | A | P | P | P | N | T | N |
| 60 | 238243 | CC121-MSSA [PVL+] | NaOH/PBS | P | P | N | N | P | P | N | N | N | N | P | A | P | P | P | N | T | N |
| 61 | 238244 | CC121-MSSA [PVL+] | NaOH/PBS | P | P | N | N | P | P | N | N | N | N | P | P | P | P | P | N | T | N |
| 62 | 238245 | CC121-MSSA [PVL+] | NaOH/PBS | P | P | N | N | P | P | N | N | N | N | P | P | P | P | P | N | T | N |
| 63 | 238246 | CC121-MSSA [PVL+] | NaOH/PBS | P | P | N | N | P | P | N | N | P 2 | N | P | P | P | P | P | N | T | N |
| 64 | 238247 | CC121-MSSA [PVL+] | NaOH/PBS | P | P | N | N | P | P | N | N | N | N | P | P | P | P | P | N | T | N |
| 65 | 238248 | CC121-MSSA [PVL+] | NaOH/PBS | P | P | N | N | P | P | N | N | N | N | P | P | P | P | P | N | T | N |
| 66 | 238249 | CC121-MSSA [PVL+] | NaOH/PBS | P | P | N | N | P | P | N | N | N | N | N | N | P | P | P | N | T | N |
| 67 | 238251 | CC121-MSSA [PVL+] | NaOH/PBS | P | P | N | N | P | P | N | N | N | N | P | A | P | P | P | N | T | N |
| 68 | 238252 | CC121-MSSA [PVL+] | NaOH/PBS | P | P | N | N | P | P | N | N | N | N | P | A | P | P | P | N | T | N |
| 69 | 238253 | CC121-MSSA [PVL+] | NaOH/PBS | P | P | N | N | P | P | N | N | N | N | P | A | P | P | P | N | T | N |
| 70 | 253431 | CC121-MSSA [PVL+] | NaOH/PBS | P | P | N | N | P | P | N | N | N | N | P | P | P | P | P | N | T | N |
| 71 | 238254 | CC121-MSSA | NaOH/PBS | P | P | N | N | N | N | N | N | N | N | N | N | P | P | P | N | T | N |
| 72 | 238255 | CC121-MSSA | NaOH/PBS | P | P | N | N | N | N | N | N | N | N | N | N | P | P | P | N | T | N |
| 73 | 238256 | CC121-MSSA | NaOH/PBS | P | P | N | N | N | N | N | N | P 2 | N | N | N | P | P | P | N | T | N |
| 74 | 224815 | CC130-MRSA-XI [mecC +] | NaOH | P | P | N | N | N | N | N | N | N | N | N | N | N | N | P | N | P | P |
| 75 | 225779 | CC130-MRSA-XI, [mecC +] | NaOH | P | P | N | N | N | N | N | N | N | N | N | N | N | N | P | N | P | P |
| 76 | 124627 | CC133-MSSA [lukF-P83/lukM+] | NaOH/PBS | P | P | N | N | P b | P b | P | P | P 3 | P | N | N | N | N | P | N | P | P |
| 77 | 215375 | CC133-MSSA, [lukF-P83/lukM+] | NaOH/PBS | P | P | N | N | P b | P b | P | P | N | N | N | N | N | N | P | N | P | P |
| 78 | 252784 | CC133-MSSA [lukF-P83/lukM+] | NaOH/PBS | P | P | N | N | P b | P b | P | P | N | N | P X | N | N | N | P | N | P | P |
| 79 | 215373 | CC133-MSSA, [lukF-P83/lukM+] | NaOH/PBS | P | P | N | N | P b | N | N | N | P 3 | P | N | N | P | P | P | N | P | N |
| 80 | 238250 | CC152-MSSA [PVL+] | NaOH/PBS | P | P | N | N | P | P | N | N | N | N | N | N | P | P | P | N | P | P |
| 81 | 124474 | CC361-MRSA-IV, WA MRSA-29 | NaOH/PBS | P | P | P | P | N | N | P | P | N | N | P | P | P | P | P | N | T | N |
| 82 | 225776 | CC398-MRSA-IV | NaOH | P | P | P | P | N | N | N | N | N | N | N | N | N | N | P | N | P | P |
| 83 | 199873 | CC398-MRSA-V | NaOH | P | P | P | P | N | N | N | N | N | N | N | N | N | N | P | N | P | N |
| 84 | 199874 | CC398-MRSA-V | NaOH | P | P | P | P | N | N | N | N | N | N | N | N | N | N | P | N | P | N |
| 85 | 225778 | CC398-MRSA-V | NaOH | P | P | P | P | N | N | N | N | N | N | N | N | N | N | P | N | P | P |
| 86 | 210067 | CC479-MSSA [lukF-P83/lukM+] | NaOH/PBS | P | A | N | N | P b | P b | N | N | N | N | N | N | N | N | P | N | P | P |
| 87 | 178400 | CC479-MSSA [lukF-P83/lukM+] | NaOH/PBS | P | N | N | N | P b | P b | N | N | N | N | N | N | N | N | P | N | P | P |
| 88 | 138830 | CC479-MSSA [lukF-P83/lukM+] | NaOH/PBS | P | P | N | N | P b | A b | N | N | N | N | N | N | N | N | P | N | P | P |
| 89 | 138831 | CC479-MSSA [lukF-P83/lukM+] | NaOH/PBS | P | P | N | N | P b | A b | N | N | N | N | N | N | N | N | P | N | P | P |
| 90 | RF122 | CC705-MSSA [lukF-P83/lukM+] | NaOH/PBS | P | P | N | N | P b | A | P | P | N | N | N | N | N | N | P | N | P | P |
| 91 | 196911 | CC705-MSSA [lukF-P83/lukM+] | NaOH/PBS | P | P | N | N | P b | P b | P | P | N | N | N | N | N | N | P | N | P | P |
| 92 | 196916 | CC705-MSSA [lukF-P83/lukM+] | NaOH/PBS | P | P | N | N | P b | P b | N | N | N | N | N | N | N | N | P | N | P | P |
| 93 | 119087 | CC705-MSSA [lukF-P83/lukM+] | NaOH | P | P | N | N | P b | P b | N | N | N | N | N | N | N | N | P | P | P | P |
| 94 | 138826 | CC705-MSSA [lukF-P83/lukM+] | NaOH/PBS | P | A | N | N | P b | P b | P | P | N | N | N | N | N | N | P | N | P | P |
| 95 | 101818 | ST8-MRSA-IIB, Irish AR13/14 | NaOH/PBS | P | P | P | P | N | N | N | N | P 1 | P | N | N | P | P | P | N | T | N |
| 96 | 124414 | ST59-MRSA-IV+V, WA MRSA-15 | NaOH/PBS | P | P | P | P | N | N | N | N | P 1 | P | P | P | P | P | P | N | P | P |
| 97 | 215382 | ST80-MRSA-IV [PVL+], “European Clone” | NaOH | P | P | P | P | P | P | N | N | N | N | N | N | P | P | P | N | T | N |
| 98 | 224519 | ST239-MRSA-III agr/hld deletion variant | NaOH/PBS | P | P | P | P | N | N | N | N | P | P | N | N | P | P | P | N | T | N |
| 99 | 200926 | ST772-MRSA-V [PVL+], Bengal Bay Clone/WA MRSA-60 | NaOH/PBS | P | P | P | P | P | P | N | N | P | P | N | N | N | N | P | N | T | N |
| 100 | 238824 | ST772-MRSA-V [PVL+], Bengal Bay Clone/WA MRSA-60 | NaOH/PBS | P | P | P | P | P | P | N | N | P | P | N | A | N | N | P | N | T | N |
| 101 | 200930 | ST772-MRSA-V [PVL+], Bengal Bay Clone/WA MRSA-60 | NaOH/PBS | P | P | P | P | P | P | N | N | P | P | N | N | N | N | P | N | A | N |
| 102 | 145330 | ST772-MRSA-V [PVL+], Bengal Bay Clone/WA MRSA-60 | NaOH/PBS | P | P | P | P | P | P | N | N | P 1 | P | N | N | N | N | P | N | T | N |
| 103 | 200927 | ST772-MRSA-V [PVL+], Bengal Bay Clone/WA MRSA-60 | NaOH/PBS | P | P | P | P | P | P | N | N | P | P | N | N | N | N | P | N | T | N |
| 104 | 200928 | ST772-MRSA-V [PVL+], Bengal Bay Clone/WA MRSA-60 | NaOH/PBS | P | P | P | P | P | P | N | N | P | P | N | N | N | N | P | N | A | N |
| 105 | 200929 | ST772-MRSA-V [PVL+], Bengal Bay Clone/WA MRSA-60 | NaOH/PBS | P | P | P | P | P | P | N | N | P | P | N | N | N | N | P | N | A | N |
| 106 | 200931 | ST772-MRSA-V | NaOH/PBS | P | P | P | P | N | N | N | N | N | N | N | N | N | N | P | N | A | N |
| 107 | ATCC35984 | S. epidermidis, [mecA+] | NaOH | N | N | P | P | N | N | N | N | N | N | N | N | N | N | N | N | N | N |
| 108 | ATCC12228 | S. epidermidis | NaOH | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N |
| 109 | 225781 | S. sciuri | NaOH | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N |
| 110 | 223885 | S. capitis | NaOH | N | N | N | N | N | N | N | N | N | N | N | N | P X | N | N | N | N | N |
| 111 | BL21-DE3 | E. coli | NaOH | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N |
1 = sea-FRI100;
2 = sea-N315;
3 = sea320;
4 = lukF-PV+/lukS-PV-;
A = ambiguous;
B = lukF-P83/lukM;
D = DNA;
N = negative;
P = positive;
Pr = Protein;
T = truncated;
X = one DNA probe out of 2 only
In addition to the S. aureus isolates, an E. coli strain (BL21-DE3) and one S. capitis isolate were tested on both arrays as negative controls. For these strains, none of the target genes and none of the corresponding proteins were detectable. Two S. epidermidis strains (ATCC35984 and ATCC12228) were also tested. The strain ATCC35984 harbored the mecA gene. This gene, as well as the corresponding protein PBP2a, was correctly identified. Strain ATCC12228 was negative for all tested targets.
Identification and detection of variants of targets
For three targets, distinct variants are known. These included mecA/PBP2a, PVL and SEA.
In addition to mecA, there is “mecA1” in various animal staphylococci that does not confer resistance to methicillin, as well as mecC. In a “mecA1”-positive/mecA-negative S. sciuri isolate, no PBP2a was detected. Two isolates of CC130-MRSA-XI that carried the mecC gene as part of a SCCmec XI element did not yield signals with PBP2a antibodies. That indicates a specific recognition of mecA encoded PBP2a by the antibody and no cross-reaction with the gene products of “mecA1” or mecC.
In lukF-P83/lukM-positive S. aureus isolates, lukF-P83 was detected by one of the three PVL antibodies, thus facilitating both its detection, as well as its differentiation from PVL.
For SEA, different alleles can be recognized that will be designated here sea-FRI100 (GenBank accession number L22565.1 [482…526]), sea-320 (GenBank accession number CP001996.1 [1144119…1144898]) and sea-N315 (GenBank accession number BA000018.3 [2011380…2012153]). Several S. aureus isolates with all of these sea alleles were tested. Anti-SEA-antibodies detected SEA corresponding to alleles sea-FRI100 and sea-320, whereas SEA of allele sea-N315 was not identified.
Discussion
The multiplex protein microarray as described herein is a test for the fast and direct detection of relevant staphylococcal proteins from clonal culture material. These include PBP2a, allowing the use of the assay as a confirmatory test for the identification of MRSA after obtaining a doubtful susceptibility test result. In addition, the S. aureus species marker SPA [48], [49] and several clinically relevant toxins are included, i.e., PVL, TSST, HLA, HLB and enterotoxins A and B. No simple non-molecular assays such as ELISAs, regardless whether using single- or multiplex assays, are currently available in routine settings.
Currently, there is a set of genotypic methods in bacterial routine diagnostics for strain identification and determination of relevant virulence and resistance markers. Common techniques are PCR or DNA microarray hybridization. Most of these assays give information about the presence of species-specific virulence or resistance genes, but not about their expression. For protein expression and functionality, molecular phenotypic techniques are well established. ELISAs, lateral flow tests for protein detection in general, or automated micro dilution techniques, with regard to resistance markers, are current methods. A main disadvantage of ELISAs or LFs is that only one, or a low number of targets can be tested in one experiment. The multiplex protein array developed herein allows a rapid, parallel and economic performance to detect several markers within a single experiment. A pure culture harvested from a blood agar plate can be used to perform the assay. These are optimal preconditions for integrating the assay into routine laboratories.
The array contains antibodies against epitopes of protein A. This cell wall located protein is used in this assay as species marker for S. aureus. All S. aureus isolates tested in this study gave positive signals for SPA. By contrast, the tested coagulase-negative staphylococci (CoNS), S. epidermidis, S. sciuri and S. capitis, as well as another control strain, E. coli, tested negative for this S. aureus species-specific marker. Therefore, these anti-protein A-antibodies act as positive controls for S. aureus isolates in the experiments.
The qualitative detection of proteins using the antibody microarray was verified by checking the presence of corresponding genes using the DNA microarray. Generally, the results described herein showed very good concordance of genotypic and phenotypic results.
The protein microarray allows the detection of PBP2a, which is related to methicillin resistance in tested isolates. Thus, the protein microarray can be used as a confirmatory assay after methicillin resistance detection in microdilution or agar diffusion tests. Fast discrimination between MSSA and MRSA plays an important role in hospitals. New patients can be screened and, if necessary (i.e., in case of a MRSA-positive result), be isolated from other patients to avoid transmissions or even outbreaks. In addition, appropriate treatment can be initiated. Recently, a new mecA homologue, mecC, was discovered. It is located on a novel SCCmec element type XI and also confers resistance to methicillin [7],[8]. This mecC encoded protein could not be detected using the assay described herein. The antibodies currently used are specific for mecA-encoded PBP2a and do not cross-react with the gene product of mecC. We did not aim to cover mecC at this stage. The reason being a very low prevalence that can be estimated to be in an order of magnitude of about 0.1% to 1% of MRSA isolates only [9],[50]. However, in case of an increasing prevalence, it would be feasible to add an antibody that recognizes the gene product of mecC. S. sciuri harbors “mecA1”, i.e., one of several deviant mecA alleles that do not confer beta lactam resistance [10–11]. One S. sciuri isolate was tested which did not yield a signal for PBP2a. This indicates that possible S. sciuri contaminants would not interfere with the mecA/PBP2a detection.
PVL is a marker for virulent S. aureus strains including CA-MRSA. A guideline was recently issued in the UK for the management of patients infected with PVL-positive S. aureus (https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/322363/PVL_LRTI_risk_assessment_protocol.pdf). For the implementation of this guideline, a diagnostic test for the detection of PVL is necessary. Currently, testing for the presence of PVL is performed in molecular/reference laboratories. The assay described herein provides the opportunity to rapidly detect PVL in routine settings without the use of PCR techniques, and therefore to adequately and timely manage patients with PVL-positive S. aureus. An increasing prevalence of strains that harbor both PVL and PBP2a, was observed [51], and this assay is the first available, non-molecular test able to detect both relevant proteins in parallel.
In veterinary medicine, leukocidins are also relevant virulence factors. The leukocidin lukF-P83/lukM is associated with veterinary disease such as bovine mastitis. It appears to be a marker for epidemic strains within a herd [23]. The protein microarray might be a useful phenotypic test in veterinary medicine to differentiate between lukF-P83/lukM-positive and—negative strains. Its detection could help to distinguish an isolated, sporadic case, following a transmission from the farmer, or secondary to an injury from an infection caused by a virulent strain with potential for epidemic spread within a herd [23]. Isolates of CC479 and CC705, harboring the genes lukF-P83/lukM, also showed positive signals for lukF-P83 expression.
Rapid detection of staphylococcal enterotoxins A and B is advisable in the case of foodborne intoxication. Their detection is helpful for epidemiological monitoring of such intoxications and might help to elucidate outbreaks. Currently, cultured isolates can be screened for the production of enterotoxins A and B. Antibodies to additional enterotoxins could be generated and added at a later stage, and protocols for the direct detection of enterotoxins in suspected food should be developed and evaluated.
Rapid detection of TSST in clinical isolates might also be helpful. For instance, cases of toxic shock syndrome can superficially resemble Lyell syndrome. Toxic shock syndrome requires aggressive antibiotic chemotherapy, whereas in Lyell syndrome discontinuation of any unnecessary medication is warranted. A rapid assay for detection of TSST production from S. aureus primary cultures could facilitate a rapid decision, thereby aiding management of patients with these severe conditions.
The hla gene was present in all isolates tested and therefore can be regarded as a potential species-specific marker for S. aureus. However, for alpha toxin, major discrepancies between the assays for the gene and the corresponding protein were detected. Results from a previous study were confirmed, that showed that the levels of HLA expression depended on the CC affiliation. Isolates belonging to clonal complexes such as CC1, CC5 and CC8 showed variable to high levels of alpha toxins. Isolates that belonged, e.g., to CC22, CC30, CC45, CC30, CC479 and CC705 did not yield detectable levels of alpha toxin under the given culture conditions despite their proven carriage of the hla gene. A reason for this discrepancy is not yet known. It might be related to in vitro conditions and/or different gene regulation mechanisms [25].
The sak-carrying bacteriophages are usually present in S. aureus from humans where they are inserted into the hlb coding sequence. SAK will be expressed in humans, whereas HLB is mainly expressed in animals. The results of this study confirm these previous findings. The livestock-associated strains of CC398, CC479 or CC705 express HLB but do not carry SAK. Human S. aureus isolates showed converse expression of both these proteins. The expression of either SAK or HLB can be distinguished by the multiplex array, and is more of a scientific than a diagnostic interest.
The current assay provides for a wide range of future developments and applications. The fluorescent array image results showed characteristic expression patterns for several strains. An expanded assay could use these typical “fingerprints” for presumptive strain identification. Several strains are characterized in detail by carrying different resistance and virulence genes. For example, S. aureus strain USA300 (ST8-MRSA-IV/PVL+/ACME+) carries the genes mecA, spa and sak, and expresses the corresponding proteins, as well as the exotoxins PVL and alpha toxin. In comparison, the Bengal Bay Clone/WA MRSA-60 (ST772-MRSA-V/PVL+) yields under the given culture conditions PBP2a, SPA, PVL and enterotoxin A, but no alpha toxin and no staphylokinase. Using these different expression patterns, presumptive strain identification can be done directly based on the fluorescent array images. However, use for fingerprinting purposes has limitations that require further study. Firstly, the number of target proteins should be expanded in order to identify more strains. More enterotoxins as well as exfoliative toxins might also be of clinical interest. Secondly, some target proteins are located on mobile genetic elements. Thus, their carriage in different isolates of one strain may vary. Thirdly, their expression might theoretically vary depending on clinical background, including previous medication or culture conditions. Future studies should therefore aim at expansion of the target panel and standardization issues. Additionally, the technology of a multiplex protein microarray can be useful for other microorganisms.
The assay was developed for qualitative analyses of protein expression. However a quantitative analysis of the fluorescent arrays might also be possible. Fluorescence allows an easier determination of protein quantities compared to precipitation staining which was used in previously studies.
As described, there are quantitative differences between several S. aureus strains with regard to the HLA and PVL expression [25],[21]. Among other factors, the size of the inoculum requires standardization to facilitate a comparison of results from different experiments. The use of a liquid medium is therefore advisable. Further experiments are necessary to establish a suitable medium and a corresponding protocol. For a standardized method, the quantities of individual target proteins need to be normalized with a conserved and constant species marker as an internal standard, with reference to the cell count. In future, quantitative measurements could be used to study strain-specific virulence traits and a possible influence of different antibiotics on the virulence factor expression.
The newly developed assay as described herein allows the multiplex detection of staphylococcal proteins, and contains the option to test the influence of external factors on protein expression. This might be a useful test in routine diagnostics as well as for research purposes.
Supporting Information
(XLSX)
Acknowledgments
We thank A. Reißig and I. Engelmann for excellent technical assistance as well as A. Ruppelt for maintaining the strain collection. We are thankful to K. P. Möbius and U. Thiemer for development and construction of the fluorescence reader as well as to V. Baier for the analyzing software.
We acknowledge S. Boswihi (Department of Microbiology, Faculty of Medicine, Kuwait University, Kuwait), A. Shore and J. Geoghegan (Dublin Trinity College, Dublin, Ireland), I. Loncaric (Veterinärmedizinische Universität, Wien, Austria), G. W. Coombs (Australian Collaborating Centre for Enterococcus and Staphylococcus Species (ACCESS), Perth, Australia) and H. Hotzel (Friedrich-Loeffler Institut, Jena, Germany) for providing additional isolates.
We thank K. Clack for proof-reading the manuscript.
Data Availability
The raw data for measured signal intensities are available as an additional supplemental file. The image files, i.e., photographs of the processed arrays, will be made available upon request to the corresponding author because these files might contain identifying information such as patient´s initials or laboratory numbers.
Funding Statement
The authors received no specific funding for this work. Alere Technologies GmbH, Alere San Diego, Inc. and Infectognostics Forschungscampus provided support in the form of salaries for authors BS, SM, EM, RE and JB, but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of these authors are articulated in the ‘author contributions’ section.
References
- 1. Brown DFJ. Guidelines for the laboratory diagnosis and susceptibility testing of methicillin-resistant Staphylococcus aureus (MRSA). Journal of Antimicrobial Chemotherapy. 2005;56(6):1000–18. [DOI] [PubMed] [Google Scholar]
- 2. van Belkum A, Melles DC, Nouwen J, van Leeuwen WB, van Wamel W, Vos MC, et al. Co-evolutionary aspects of human colonisation and infection by Staphylococcus aureus. Infection, Genetics and Evolution. 2009/January;9(1):32–47. 10.1016/j.meegid.2008.09.012 [DOI] [PubMed] [Google Scholar]
- 3. Von Eiff C, Becker K, Machka K, Stammer H, Peters G. Nasal Carriage as a Source of Staphylococcus aureus Bacteremia. The New England Journal of Medicine. 2001;344(1):11–6. [DOI] [PubMed] [Google Scholar]
- 4. Verkaik NJ, de Vogel CP, Boelens HA, Grumann D, Hoogenboezem T, Vink C, et al. Anti-Staphylococcal Humoral Immune Response in Persistent Nasal Carriers and Noncarriers of Staphylococcus aureus. The Journal of Infectious Diseases. 2009;199:625–32. 10.1086/596743 [DOI] [PubMed] [Google Scholar]
- 5. Fuda C, Suvorov M, Vakulenko SB, Mobashery S. The basis for resistance to beta-lactam antibiotics by penicillin-binding protein 2a of methicillin-resistant Staphylococcus aureus. J Biol Chem. 2004;279(39):40802–6. [DOI] [PubMed] [Google Scholar]
- 6. Geiss HK, Mack D, Seifert H. Konsensuspapier zur Identifizierung von speziellen Resistenzmechanismen und zur Interpretation von Ergebnissen der Antibiotikaempfindlichkeitstestung bei grampositiven und gramnegativen Erregern. Der Mikrobiologe. 2003;13:222–39. [Google Scholar]
- 7. Shore AC, Deasy EC, Slickers P, Brennan G, O'Connell B, Monecke S, et al. Detection of staphylococcal cassette chromosome mec type XI carrying highly divergent mecA, mecI, mecR1, blaZ, and ccr genes in human clinical isolates of clonal complex 130 methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2011. August;55(8):3765–73. 10.1128/AAC.00187-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Garcia-Alvarez L, Holden MT, Lindsay H, Webb CR, Brown DF, Curran MD, et al. Meticillin-resistant Staphylococcus aureus with a novel mecA homologue in human and bovine populations in the UK and Denmark: a descriptive study. Lancet Infect Dis. 2011. August;11(8):595–603. 10.1016/S1473-3099(11)70126-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Petersen A, Stegger M, Heltberg O, Christensen J, Zeuthen A, Knudsen LK, et al. Epidemiology of methicillin-resistant Staphylococcus aureus carrying the novel mecC gene in Denmark corroborates a zoonotic reservoir with transmission to humans. Clin Microbiol Infect. 2013;19(1):E16–22. 10.1111/1469-0691.12036 [DOI] [PubMed] [Google Scholar]
- 10. Couto I, de Lencastre H, Severina E, Kloos W, Webster JA, Hubner RJ, et al. Ubiquitous presence of a mecA homologue in natural isolates of Staphylococcus sciuri. Microb Drug Resist. 1996. Winter;2(4):377–91. [DOI] [PubMed] [Google Scholar]
- 11. Monecke S, Muller E, Schwarz S, Hotzel H, Ehricht R. Rapid microarray based identification of different mecA alleles in Staphylococci. Antimicrob Agents Chemother. 2012. August 13;56(11):5547–54. 10.1128/AAC.00574-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vandenesch F, Naimi T, Enright MC, Lina G, Nimmo GR, Heffernan H, et al. Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: worldwide emergence. Emerg Infect Dis. 2003. August;9(8):978–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Boyle-Vavra S, Daum RS. Community-acquired methicillin-resistant Staphylococcus aureus: the role of Panton-Valentine leukocidin. Lab Invest. 2007. January;87(1):3–9. [DOI] [PubMed] [Google Scholar]
- 14. Kaneko J, Kamio Y. Bacterial two-component and hetero-heptameric pore-forming cytolytic toxins: structures, pore-forming mechanism, and organization of the genes. Biosci Biotechnol Biochem. 2004. May;68(5):981–1003. [DOI] [PubMed] [Google Scholar]
- 15. Diep BA, Otto M. The role of virulence determinants in community-associated MRSA pathogenesis. Trends in Microbiology. 2008/August;16(8):361–9. 10.1016/j.tim.2008.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kazakova SV, Hageman JC, Matava M, Srinivasan A, Phelan L, Garfinkel B, et al. A clone of methicillin-resistant Staphylococcus aureus among professional football players. N Engl J Med. 2005. February 3;352(5):468–75. [DOI] [PubMed] [Google Scholar]
- 17. Aiello AE, Lowy FD, Wright LN, Larson EL. Meticillin-resistant Staphylococcus aureus among US prisoners and military personnel: review and recommendations for future studies. Lancet Infect Dis. 2006;6:335–41. [DOI] [PubMed] [Google Scholar]
- 18. Roberts MC, Soge OO, No D, Beck NK, Meschke JS. Isolation and characterization of methicillin-resistant Staphylococcus aureus from fire stations in two northwest fire districts. Am J Infect Control. 2011;39(5):382–9. 10.1016/j.ajic.2010.09.008 [DOI] [PubMed] [Google Scholar]
- 19. Schaumburg F, Ngoa UA, Kosters K, Kock R, Adegnika AA, Kremsner PG, et al. Virulence factors and genotypes of Staphylococcus aureus from infection and carriage in Gabon. Clin Microbiol Infect. 2011. April 4. [DOI] [PubMed] [Google Scholar]
- 20. Ellington MJ, Hope R, Ganner M, East C, Brick G, Kearns AM. Is Panton-Valentine leucocidin associated with the pathogenesis of Staphylococcus aureus bacteraemia in the UK? J Antimicrob Chemother. 2007. August;60(2):402–5. [DOI] [PubMed] [Google Scholar]
- 21. Stieber B, Monecke S, Muller E, Baier V, Coombs G, Ehricht R. Development and usage of protein microarrays for the quantitative measurement of Panton-Valentine leukocidin. Mol Cell Probes. 2014. December 3. [DOI] [PubMed] [Google Scholar]
- 22. Rasigade JP, Trouillet-Assant S, Breurec S, Antri K, Lina G, Bes M, et al. The levels of antibodies to Panton–Valentine leukocidin (PVL) vary with PVL prevalence along a north-to-south gradient. European Journal of Clinical Microbiology & Infectious Diseases. 2015;34(5):927–33. [DOI] [PubMed] [Google Scholar]
- 23. Schlotter K, Ehricht R, Hotzel H, Monecke S, Pfeffer M, Donat K. Leukocidin genes lukf-P83 and lukM are associated with Staphylococcus aureus clonal complexes 151, 479 and 133 isolated from bovine udder infections in Thuringia, Germany. Vet Res. 2012. May 15;43(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Graveland H, Duim B, van Duijkeren E, Heederik D, Wagenaar JA. Livestock-associated methicillin-resistant Staphylococcus aureus in animals and humans. International Journal of Medical Microbiology. 2011;301(8):630–4. 10.1016/j.ijmm.2011.09.004 [DOI] [PubMed] [Google Scholar]
- 25. Monecke S, Müller E, Büchler J, Stieber B, Ehricht R. Staphylococcus aureus In Vitro Secretion of Alpha Toxin (hla) Correlates with the Affiliation to Clonal Complexes. PLoS ONE. 2014;9(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Valeva A, Palmer M, Bhakdi S. Staphylococcal R-Toxin: Formation of the Heptameric Pore Is Partially Cooperative and Proceeds through Multiple Intermediate Stages. Biochemistry. 1997;36:13298–304. [DOI] [PubMed] [Google Scholar]
- 27. Wardenburg JB, Bae T, Otto M, Deleo FR, Schneewind O. Poring over pores: alpha-hemolysin and Panton-Valentine leukocidin in Staphylococcus aureus pneumonia. Nat Med 2007;13(12):1405–6. [DOI] [PubMed] [Google Scholar]
- 28. Vandenesch F, Lina G, Henry T. Staphylococcus aureus hemolysins,bi-component leukocidins, and cytolytic peptides:a redundant arsenal of membrane-damaging virulence factors? Frontiers in Cellular and Infection Microbiology. 2012;2(12):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sharma-Kuinkel BK, Wu Y, Tabor DE, Mok H, Sellman BR, Jenkins A, et al. Characterization of Alpha-ToxinhlaGene Variants, Alpha-Toxin Expression Levels, and Levels of Antibody to Alpha-Toxin in Hemodialysis and Postsurgical Patients with Staphylococcus aureus Bacteremia. Journal of Clinical Microbiology. 2015;53(1):227–36. 10.1128/JCM.02023-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Katayama Y, Baba T, Sekine M, Fukuda M, Hiramatsu K. Beta-hemolysin promotes skin colonization by Staphylococcus aureus. J Bacteriol. 2013;195(6):1194–203. 10.1128/JB.01786-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Coleman D, Knights J, Russell R, Shanley D, Birkbeck TH, Dougan G, et al. Insertional inactivation of the Staphylococcus aureus beta-toxin by bacteriophage phi 13 occurs by site- and orientation-specific integration of the phi 13 genome. Mol Microbiol. 1991. April;5(4):933–9. [DOI] [PubMed] [Google Scholar]
- 32. Bokarewa MI, Jin T, Tarkowski A. Staphylococcus aureus: Staphylokinase. Int J Biochem Cell Biol. 2006;38(4):504–9. [DOI] [PubMed] [Google Scholar]
- 33. Luedicke C, Slickers P, Ehricht R, Monecke S. Molecular fingerprinting of Staphylococcus aureus from bone and joint infections. Eur J Clin Microbiol Infect Dis. 2010. April;29(4):457–63. 10.1007/s10096-010-0884-4 [DOI] [PubMed] [Google Scholar]
- 34. Schlievert PM, Kim MH. Reporting of toxic shock syndrome Staphylococcus aureus in 1982 to 1990. J Infect Dis. 1991;164(6):1245–6. [DOI] [PubMed] [Google Scholar]
- 35. Dinges MM, Orwin PM, Schlievert PM. Exotoxins of Staphylococcus aureus. Clin Microbiol Rev. 2000. January;13(1):16–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Monecke S, Luedicke C, Slickers P, Ehricht R. Molecular epidemiology of Staphylococcus aureus in asymptomatic carriers. European Journal of Clinical Microbiology & Infectious Diseases. 2009;28(9):1159–65. [DOI] [PubMed] [Google Scholar]
- 37. Kloppot P, Selle M, Kohler C, Stentzel S, Fuchs S, Liebscher V, et al. Microarray-based identification of human antibodies against Staphylococcus aureus antigens. Proteomics Clin Appl. 2015. [DOI] [PubMed] [Google Scholar]
- 38. Betley MJ, Mekalanos JJ. Staphylococcal enterotoxin A is encoded by phage. Science. 1985;229(4709):185–7. [DOI] [PubMed] [Google Scholar]
- 39. Shafer WM, Iandolo JJ. Chromosomal locus for staphylococcal enterotoxin B. 1978. 20(1):273–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pinchuk IV, Beswick EJ, Reyes VE. Staphylococcal Enterotoxins. Toxins. 2010;2: 2177–97. 10.3390/toxins2082177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wehner J, Neuber K. Staphylococcus aureus enterotoxins induce histamine and leukotriene release in patients with atopic eczema. British Journal of Dermatology. 2001;145:302–5. [DOI] [PubMed] [Google Scholar]
- 42. Foster TJ. Immune evasion by staphylococci. Nat Rev Microbiol. 2005;3(12):948–58. [DOI] [PubMed] [Google Scholar]
- 43. Cunha da MdLRS, Calsolari RAO, Pessoa Araujo J Jr. Detection of Enterotoxin and Toxic Shock Syndrome Toxin 1 Genes in Staphylococcus, with Emphasis on Coagulase-Negative Staphylococci. Microbiology and Immunology. 2007;51(4):381–90. [DOI] [PubMed] [Google Scholar]
- 44. Monecke S, Muller E, Buechler J, Rejman J, Stieber B, Akpaka PE, et al. Rapid detection of Panton-Valentine leukocidin in Staphylococcus aureus cultures by use of a lateral flow assay based on monoclonal antibodies. J Clin Microbiol. 2013. February;51(2):487–95. 10.1128/JCM.02285-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gilligan K, Shipley M, Stiles B, Hadfield TL, Sofi Ibrahim M. Identification of Staphylococcus aureus enterotoxins A and B genes by PCR-ELISA. Molecular and Cellular Probes. 2000;14(2):71–8. [DOI] [PubMed] [Google Scholar]
- 46. Stevens DL, Bisno AL, Chambers HF, Dellinger EP, Goldstein EJ, Gorbach SL, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59(2):147–59. 10.1093/cid/ciu296 [DOI] [PubMed] [Google Scholar]
- 47. Frénay HM, Bunschoten AE, Schouls LM, van Leeuwen WJ, Vandenbroucke-Grauls CM, Verhoef J, et al. Molecular typing of methicillin-resistant Staphylococcus aureus on the basis of protein A gene polymorphism. 15 1996;1(60–4). [DOI] [PubMed] [Google Scholar]
- 48. Harmsen D, Claus H, Witte W, Rothganger J, Turnwald D. Typing of methicillin-resistant Staphylococcus aureus in a university hospital setting by using novel software for spa repeat determination and database management. J Clin Microbiol. 2003;41:5442–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dübel S, Breitling F, Fuchs P, Braunagel M, Klewinghaus I, Little M. A family of vectors for surface display and production of antibodies. Gene. 1993;128:97–101. [DOI] [PubMed] [Google Scholar]
- 50. RKI. Eigenschaften, Häufigkeit und Verbreitung von MRSA in Deutschland—Update 2011/2012. Epidemiologisches Bulletin. 2013;21:187–96. [Google Scholar]
- 51. Tristan A, Bes M, Meugnier H, Lina G, Bozdogan B, Courvalin P, et al. Global distribution of Panton-Valentine leukocidin—positive methicillin-resistant Staphylococcus aureus, 2006. Emerg Infect Dis. 2007. April;13(4):594–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
The raw data for measured signal intensities are available as an additional supplemental file. The image files, i.e., photographs of the processed arrays, will be made available upon request to the corresponding author because these files might contain identifying information such as patient´s initials or laboratory numbers.