Abstract
Pathologically elevated glutamate concentrations in the brain's extracellular fluid are associated with several acute and chronic brain insults. Studies have demonstrated that by decreasing the concentration of glutamate in the blood, thereby increasing the concentration gradient between the brain and the blood, the rate of brain-to-blood glutamate efflux can be increased. Blood glutamate scavengers, pyruvate and oxaloacetate have shown great promise in providing neuroprotection in many animal models of acute brain insults. However, glutamate scavengers’ potential systemic toxicity, side effects and pharmacokinetic properties may limit their use in clinical practice. In contrast, extracorporeal methods of blood glutamate reduction, in which glutamate is filtered from the blood and eliminated, may be an advantageous adjunct in treating acute brain insults. Here, we review the current evidence for the glutamate-lowering effects of hemodialysis, peritoneal dialysis and hemofiltration. The evidence reviewed here highlights the need for clinical trials.
Keywords: blood glutamate scavenging, extracorporeal methods, hemodialysis, hemofiltration, neuroprotection, peritoneal dialysis
Traumatic brain injury and stroke are leading causes of worldwide morbidity and mortality [1]. In both humans and animals, it is well known that a dramatic increase in the brain's extracellular glutamate concentration follows acute traumatic or ischemic brain insults [2–5]. This increase in extracellular glutamate sets off a cascade of intracellular events, resulting in neuronal apoptosis and tissue necrosis [6]. Current therapies are ineffective in reducing the long-term negative effects of glutamate-mediated secondary brain injury that results from these conditions.
In recent years, many studies have focused on better understanding of the brain's inherent mechanisms of removing excess glutamate from the extracellular fluid (ECF) during pathological states [7,8]. Following the clinical shortcomings of NMDA receptor antagonists [9], studies have shifted their focus to better understand methods that reduce the negative effects of excess brain glutamate without interfering with glutamate's positive effects.
Over the past decade, many studies have greatly contributed to a better understanding of the naturally occurring brain-to-blood glutamate efflux [7,8,10]. Various methods have successfully demonstrated that by scavenging blood glutamate, a favorable concentration gradient between the brain and blood can be established. This, in turn, is thought to increase the brain-to-blood glutamate efflux, which results in a reduction of pathologically elevated brain glutamate concentrations [7,8]. Extracorporeal methods, including hemodialysis (HD), peritoneal dialysis (PD) and continuous hemofiltration (HF), offer unique advantages over pharmacological methods of blood glutamate reduction.
Glutamate homeostasis & toxicity
Glutamate is both a non-essential amino acid and the primary excitatory neurotransmitter in the CNS. In addition to serving as an energy reserve, glutamate plays an important role in several physiological processes in the brain including regulation of neuronal transmission and the development of CNS plasticity [11,12]. Glutamate also serves as a precursor for GABA, the primary inhibitory transmitter in the brain.
Excess glutamate in the brain's ECF is known to be neurotoxic to surrounding tissues [10]. Abnormally elevated glutamate in the brain's ECF is the hallmark of several acute [2–4,13] and chronic neurodegenerative conditions [14–18]. As such, tight homeostatic mechanisms are required to balance maintaining the positive effects of glutamate while preventing the deleterious effects. Although glutamate exists in high concentrations in the brain (5–15 mmol/kg) [12], only a small amount exists in the ECF. Through a series of complex interactions between neurons, astrocytes and the blood, ECF glutamate in the brain is maintained at concentrations 1000-fold less than in the intracellular fluid [17].
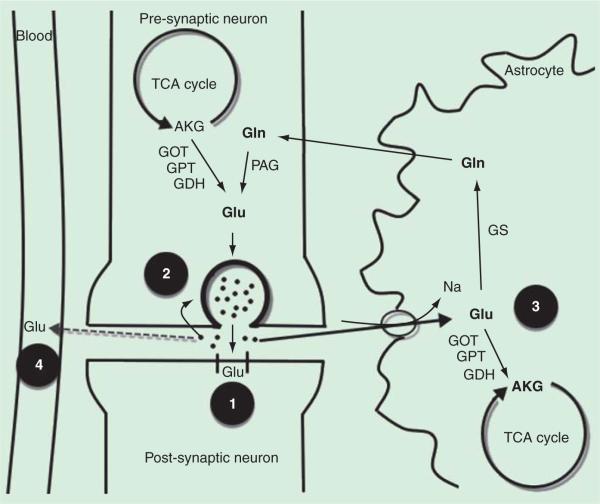
During neurotransmission, glutamate is released from vesicles into the synaptic space, where only a tiny fraction binds to postsynaptic receptors. While some glutamate is transported back to the neurons to be repackaged into vesicles, most of the glutamate is recycled for neurotransmission through the glutamate–glutamine cycle (figure 1). Glutamate is shuttled to neighboring astrocytes via Na+-dependent transporters, whereby the glutamate is converted to glutamine by the enzyme glutamate synthetase [17,19]. The glutamine can then be released back into the systemic circulation via the jugular vein or transported back to neurons [20], where it is converted by phosphate-activated glutaminase back to glutamate. This process is termed ‘glutamate-glutamine cycling’ and is thought to be critical in meeting the neurons’ metabolic demands while maintaining extracellular glutamate at concentrations below toxic levels.
Figure 1. Mechanisms of glutamate clearance from the extracellular space.
Pre-synaptic neuronal glutamate (Glu) is packaged into vesicles, where it is released in the synaptic space. In the synaptic space, extracellular glutamate is removed via several mechanisms. 1) A small amount of glutamate binds to receptors on the post-synaptic neuron to enable synaptic transmission. 2) While some Glu is directly uptaken by the presynaptic neuron and repackaged into vesicles, 3) most of the remaining glutamate is shuttled to neighboring astrocytes via sodium (Na) transporters. In astrocytes, the Glu can enter the tricarboxylic acid (TCA) cycle by conversion to α-ketoglutarate (AKG) via glutamate oxaloacetate transaminase (GOT), glutamate pyruvate transaminase (GPT) and glutamate dehydrogenase (GDH). Glu can alternatively be converted glutamine (Gln) by glutamate synthetase (GS), an astrocyte-specific enzyme, and the Glu is then shuttled back to the neuron. In the neuron, Gln is converted back to Glu by phosphate-activated glutaminase (PAG), completing the Glu-Gln cycle. 4) During conditions of excess extra-cellular glutamate, in which extracellular glutamate in the brain exceeds the concentration in the blood, glutamate is transported to the blood via facilitated diffusion. Various methods of reducing Glu concentrations in the blood, including blood Glu scavengers and extracorporeal methods, have been shown to facilitate the brain to blood Glu efflux. An increased rate of brain to blood Glu efflux may be effective in providing neuroprotection after an acute brain insult (see text for further discussion).
Mechanisms of glutamate neurotoxicity reduction
NMDA receptor blockade
Currently, there are no effective treatment strategies that effectively reduce the secondary brain injury that results from glutamate-mediated brain injury. The administration of NMDA-receptor antagonists failed to demonstrate a therapeutic benefit in humans and were often shown to worsen clinical outcomes [21]. The failed neuroprotective effects of NMDA-receptor antagonists have been attributed to an interference with glutamate-facilitated neuronal signaling, maintenance of cellular integrity and neuronal repair during injury [21,22]. Furthermore, even if NMDA receptor antagonists were effective in reducing glutamate's excitotoxic effects, its short therapeutic half-life would likely have greatly limited its use in clinical practice [9].
Blood glutamate scavenging
The maintenance of ECF glutamate within physiological concentrations is of vital importance to prevent glutamate's neurotoxic effects. When glutamate concentrations are pathologically elevated in the brain's ECF, there are several inherent mechanisms by which this glutamate is reduced. One such mechanism utilizes sodium-dependent transports located on the antiluminal side of brain capillaries [23]. In addition to glutamate transporters on astrocytes and neurons, the glutamate transporters on brain capillaries provide an important role by which pathologically elevated glutamate is reduced in the brain (figure 1). When concentrations of glutamate in the brain's ECF are in excess, glutamate is transported into the endothelial cell, where it accumulates. When a concentration gradient is established between the endothelial cell and the blood, glutamate is transported down its concentration gradient into the peripheral blood by facilitated diffusion [24]. Studies have demonstrated that this brain-to-blood glutamate efflux is a rapid and naturally occurring event [25,26].
By increasing the concentration gradient of glutamate between the brain ECF and blood, Gottlieb et al. demonstrated that the rate of brain-to-blood glutamate efflux can be increased [26]. This was achieved by exploiting blood enzymes glutamate-pyruvate transaminase (GPT) and glutamate-oxaloacetate transaminase (GOT), which, in the presence of their co-substrates pyruvate and oxaloacetate respectively, convert glutamate to 2-ketoglutarate. This reaction is known to occur in various tissues in the body, including the blood. By injecting pyruvate and oxaloacetate in the peripheral blood, Gottlieb et al. successfully increased the rate of elimination of glutamate from the brain ECF. For the first time, it was established that a manipulation of blood glutamate concentrations could dramatically reduce brain ECF glutamate concentrations.
The neuroprotective effects that result from the administration of pyruvate and oxaloacetate, termed ‘blood glutamate scavengers’, have been previously reviewed at length [7,8]. In short, intravenous administration of blood glutamate scavengers was shown to reduce blood glutamate concentrations, improve neurological outcomes and increase the number of surviving hippocampal neurons after traumatic brain injury in animals [22,27–29]. Similarly, in animal models of stroke, intravenous administration of blood glutamate scavengers resulted in decreased brain and blood glutamate concentrations, reduced infarct size, less cerebral edema, reduced sensorimotor deficits, improved neurological outcomes and reduced mortality compared with control animals [30–32].
In a rat model of subarachnoid hemorrhage, Boyko et al. demonstrated that peripheral blood glutamate scavenger administration resulted in a reduction in blood and brain glutamate, decreased blood–brain barrier, less brain edema and improved neurological outcomes [33]. Limited studies have further demonstrated that blood glutamate scavengers may have neuroprotective effects in animal studies of epilepsy [34], glioma [35] and organophosphate poisoning [36]. Blood glutamate scavenging has several unique attributes that make it particularly attractive as a potential therapeutic strategy. Unlike NMDA-receptor antagonists, blood glutamate scavengers do not interfere with normal neuronal transmission. Furthermore, because blood glutamate scavengers are thought to act only in the blood, glutamate is only removed from areas in the brain where it exists in excess [26]. Glutamate scavenging is also thought to be self-limiting and slows down as the brain-to-blood glutamate concentration gradient is reduced [22]. In this way, it seems unlikely that treatment with blood glutamate scavenges could result in a reduction of brain glutamate below normal physiological concentrations.
Despite the promising results observed in animal studies, the use of blood glutamate scavengers in the clinical setting may have certain limitations. Several authors have pointed out that glutamate scavengers are only neuroprotective when administered within a short therapeutic window [27,28,30]. Furthermore, although studies have demonstrated a seemingly favorable side effect profile in rats, systemic effects of glutamate scavengers in humans are currently unknown. Pyruvate and oxaloacetate are known intermediates of the tricarboxylic acid cycle and therefore may have undesirable actions in tissues other than the blood. The lack of safety studies with oxaloacetate and pyruvate has greatly limited the ability to perform large-scale clinical investigations in humans [7].
Extracorpeal methods of blood glutamate reduction
In addition to blood glutamate scavengers, investigators have identified several additional factors that may reduce blood glutamate concentrations and increase the brain-to-blood glutamate efflux, including estrogen and progesterone [37,38], β-2 adrenergic receptor activation [39,40], hypothermia [13] and insulin and glucagon [41,42]. Another exciting and potentially revolutionary therapeutic modality to reduce the neurotoxic effects of glutamate after acute brain insults is with extracorporeal methods of blood glutamate reduction. These methods are thought to filter and eliminate pathologically elevated glutamate from the blood. Similar to blood glutamate scavengers, extracorporeal methods would thereby increase the concentration gradient between the brain and blood and increase the brain-to-blood glutamate efflux. The efficacy of blood glutamate reduction with HD, PD and HF has been studied in humans and animals [7,43–45].
Recently, there has been much interest in understanding the effects of extracorporeal methods of solute clearance from the blood. In addition to the well-established effects of dialysis on human metabolism of inorganic elements [46], extracorporeal methods of blood glutamate scavenging offer several potential advantages over pharmacologic agents in the treatment of acute brain insults. In contrast to pharmacological blood glutamate scavengers, which utilize reversible enzyme processes, extracorporeal methods definitely remove glutamate from the blood. Furthermore, extracorporeal methods can be utilized over several hours to days, unlike pharmacological methods that are limited by the pharmacokinetic properties. Pharmacological blood glutamate scavengers are further subject to side effects and unknown peripheral actions. Extracorporeal methods, however, are well established and are widely used in filtering various substances from the blood.
For extracorporeal methods of blood glutamate reduction to be effective in providing neuroprotection, a number of conditions must be met [47]. First, the acute brain condition must result in significantly elevated brain glutamate concentrations, and a concentration gradient between the brain and the blood must be established. Second, the extracorporeal methods must be capable of significantly reducing blood glutamate concentrations to the extent that it increases the rate of the brain-to-blood glutamate, thereby reducing pathologically elevated glutamate at the site of the brain injury. Lastly, the extent of the injury should not be exacerbated by the method of glutamate filtration and removal. There is increasing evidence that these criteria may be fulfilled, and that extracorporeal methods of blood glutamate reduction may be important adjuvant tools in the treatment of acute brain insults.
Hemodialysis
HD has been effectively used in patients with end-stage renal disease (ESRD) to restore normal intracellular and ECF metabolite concentrations [48]. When the blood is exposed to a semipermeable membrane tube containing dialysate solution, solutes are transported from the blood into the dialysate and from the dialysate into the blood. Small molecules, including glutamate, can thereby freely diffuse across the membrane according to the relative concentration gradient between the blood and dialysate solution. During conditions in which glutamate in the blood is pathologically elevated, it is thought that HD can remove glutamate from the blood by diffusion across the dialysis membrane and into the dialysate solution.
Rogachev et al. [43] recently investigated the efficacy of using HD to eliminate excess plasma glutamate in humans. The authors reported that blood glutamate concentrations were significantly reduced during HD, and that low concentrations were maintained during the first few hours of a standard HD session.
In their study, blood samples were collected from 45 patients with Stage V chronic kidney disease immediately after initiation of HD, and hourly for a total of five blood samples. Plasma concentrations of glutamate, glucose, GOT and GPT were analyzed. Blood samples from 25 healthy volunteers without chronic renal failure were used as controls for determining baseline blood concentrations of glutamate, GOT and GPT. During the first 4 h of HD (especially in the first hour), glutamate concentrations significantly decreased regardless of the size of filter pores, blood flow rate or gender. During the fourth hour, although glutamate was still significantly low compared with baseline concentrations, glutamate was significantly higher than in the third hour.
Since the authors used a constant flow of dialysate without recirculation, and each time point that the fluid entered the dialyzer was devoid of glutamate, the dialysate could not have been oversaturated. The authors postulated that a rebound increase in glutamate mobilized from body tissues might account for this phenomenon. Another plausible explanation is that the removal of urea from the plasma by HD exceeds its removal from the tissues. This delay in the removal of urea from the tissue in turn would create an osmotic gradient between the cells and the plasma, resulting in the development of an osmotic disequilibrium. Glutamate is a known organic osmolyte that plays an important role in osmotic adaptation [49,50]. It is reasonable therefore to suggest that neurons secrete glutamate to the CSF and subsequently to the plasma in response to the hypo-osmotic stress during HD. The late increase in glutamate levels may be a defense mechanism to counteract this disequilibrium syndrome. As such, it would be expected that in chronic HD patients, the increase in blood glutamate levels would be more dramatic than that observed in new HD patients. If this hypothesis were true, then the potential therapeutic use of HD in decreasing brain glutamate levels after brain injury would be even more appealing. To evaluate this hypothesis, future studies should evaluate the glutamate clearance pattern in nonuremic patients during a conventional dialysis session, and during continuous therapy such as HF or hemodiafiltration.
Interestingly, patients with ESRD on HD were shown to have higher concentrations of blood glutamate compared with healthy controls [43,51]. There are several factors that may account for this increased glutamate in patients with chronic renal failure on HD, including altered lipid metabolism, decreased muscle mass and insulin resistance [51]. Chronically elevated levels of glutamate might have an important role in the development of uremic dementia in this population.
Peritoneal dialysis
PD is a commonly used form of home dialysis in patients with ESRD, with the goal to establish normal intracellular and extracellular metabolite concentration that would normally be done by a functioning kidney [47]. Similar to HD, PD makes use of a semipermeable dialysis catheter to exchange solutes between a patient and a dialysate solution. Prior to beginning PD, a catheter must first be inserted into the peritoneal cavity. Typically after 10–14 days, during which time the catheter site heals, the catheter can be used. There are some important advantages of PD over HD when considering its possible utilization of blood glutamate reduction to provide neuroprotection.
Although HD has been shown to effectively reduce blood glutamate levels, which may improve neurological outcomes after acute brain insults, it requires that patients be heparinized. Therefore, the use of HD may be limited in the setting of acute brain injury and would likely be contraindicated in hemodynamically unstable patients. In this context, PD may be an attractive alternative method of reducing blood glutamate. PD does not require heparin, nor does it negatively impact the hemodynamic stability of critically ill patients. Furthermore, the insertion of a drain in the peritoneal cavity is minimally invasive and can easily be performed at the bedside in a time-frame of approximately 20 min.
A recent study demonstrated that the use of PD resulted in decreases in blood glutamate [44,52], with a corresponding increase of glutamate in the dialysis solution [44]. Rogachev et al. demonstrated that the blood glutamate concentration was significantly reduced by 60 min after the infusion of dialysis solution, but slowly returned toward baseline values as time progressed.
Concentrations of glutamate in the dialysis solution, on the contrary, increased significantly by 60 min and gradually reached maximum levels at 180 min. By 240 min, presumably when the dialysis solution became saturated with glutamate, the concentrations of glutamate in the dialysis solution began to decrease. This resulted in the accumulation of ultrafiltrate in the peritoneal cavity, which diluted the concentrations of absorbed glutamate. In accordance with the observation that maximal reduction of glutamate in the blood was seen at 60 min after the initiation of PD, and assuming that this observation is likely the result of a saturation of dialysis solution, the authors recommended that the dialysis solution in the peritoneal cavity be replaced often (every 60 min) to avoid saturation and a reduced blood glutamate-lowering effect. For this purpose, a standard Cycler PD with short dwelling time may be particularly useful.
Godino et al. observed similar findings in humans during PD [52]. The authors investigated the effects of PD in a rat model of stroke. The reduction of blood glutamate levels observed with PD was associated with a decrease in infarct area [52].
These studies provide promising evidence to suggest that HD and PD may be effective modalities in reducing blood glutamate concentrations, thereby increasing the brain-to-blood glutamate efflux and reducing brain glutamate concentrations. These extracorporeal methods are especially promising because their blood glutamate-reducing effects were long lasting relative to the transient effects observed after the administration of blood glutamate scavengers. Furthermore, extracorporeal methods of blood glutamate reduction are advantageous in that they avoid the possible toxic systemic effects that may accompany the peripheral administration of blood glutamate scavengers.
Hemofiltration
As discussed above, HD may have several limitations in critically ill patients with an acute brain insult. First, many of the patients admitted with acute brain injury suffer some degree of hemodynamic instability, typically due to hypovolemia or shock. Furthermore, anticoagulation is required for HD therapy to prevent clot formation in the set's tubing. Anticoagulation may be detrimental to patients suffering from multiple trauma or isolated head injury due of risk of hemorrhage.
For these patients, continuous HF may offer a preferable therapeutic approach. In contrast to dialysis, which utilizes diffusion to transport solutes, HF uses principles of convection to create a positive hydrostatic pressure to drive solutes from the blood to the filtrate via a filter membrane [53]. There is evidence that HD may be superior to HD in clearing medium-sized and larger molecules and may be more effective in clearing large inflammatory cytokines in critically ill patients [54]. Considering that the diameter of pores in the membranes of filter used for HF is larger than those used for HD, we postulated that HF may be a surrogate for HD to decrease blood glutamate concentrations. HF results in a lower incidence of hemodynamic instability compared with HD and may be utilized with only minimal anticoagulation. Moreover, in contrast to HD, which typically lasts for only 4 h, HF may be utilized continuously for long periods of time (up to several days). HF may therefore provide a longer lasting glutamate-reducing effect, thereby promoting optimal neuroprotection.
HF is widely used in critically ill patients for both renal and non-renal indications. Traditionally, HF has been used in patients with acute renal failure associated with several conditions, including heart failure, volume overload, chronic liver failure and brain swelling [55]. Non-renal indications include systemic inflammatory response, hyperkalemia, sepsis and septic shock, multi-organ failure and adult respiratory distress syndrome [55].
Early initiation of HF for the removal of excess glutamate from the plasma may be a useful adjuvant therapy for acute neurodegenerative conditions. Currently, there are no published reports that examine the effects of HF on blood glutamate levels and neurological outcome after acute brain insults. However, two such studies are currently underway. The first study is investigating the effects of continuous HF in ICU patients irrespective of their basic pathology on blood glutamate concentrations. The second study is a Phase II, prospective, interventional, double-blinded and randomized clinical trial evaluating the effect of HF in the treatment of acute ischemic stroke. The primary endpoints of the study are the safety of HF in these patients, and its efficacy in improving neurological outcomes after ischemic stroke. In the study, the HF protocol will be initiated within the first 6 h after the onset of stroke in 300 subjects randomized in a 1:1 ratio to treatment (HF) and control (non-HF) groups. Patients in the treatment group will receive HF for the duration of 24 h. Thus far, 30 patients have been enrolled in the study.
Conclusions
Extracorporeal methods of blood glutamate scavenging are thought to increase the brain-to-blood glutamate efflux, which may provide neuroprotection after an acute brain insult. These modalities may reduce brain glutamate in a manner similar to that observed with the treatment of pharmacological blood glutamate scavengers, but may be advantageous in that they avoid possible systemic effects and toxicity. Clinical trials are greatly needed to evaluate the clinical effects of extracorporeal methods of blood glutamate scavenging in humans. Furthermore, knowledge of the relationship between glutamate, glutamate scavengers, GOT and GPT provide important insights that may be useful in guiding the clinical diagnosis, prognosis and treatment protocols of acute brain insults in humans.
Expert commentary
Currently, there are few clinical studies that have sought to determine the blood glutamate-lowering effects of extracorporeal methods as well as determine its efficacy in improving neurological outcomes after an acute brain insult. There are two clinical studies that are currently being conducted to better study the blood glutamate-lowering effects of HF. The first study is investigating the effects of continuous HF in critically ill patients. The second study is a Phase II, prospective, double-blinded randomized control trial evaluating the effect of HF in the treatment of acute ischemic stroke. These and other randomized control trials will be vital in determining optimal methods of providing neuroprotection while limiting the treatments’ potential systemic side effects.
Five-year view
Over the next 5 years, extracorporeal methods of blood glutamate reduction may emerge as a novel and effective therapeutic treatment of acute neurological conditions. However, to date, there have been few studies in humans and there is much that is unknown. Randomized clinical control trials in humans are needed to further determine the efficacy of brain and blood glutamate reduction, degree of neurological improvement, the therapeutic window of treatment and potential side effects. As such, clinical studies over the next several years will be vital in determining whether this exciting therapeutic approach will be useful in the clinical setting.
Key issues.
In recent years, there has been much focus on better understanding the brain's inherent mechanisms of removing excess glutamate from the extracellular fluid during pathological states.
By establishing a favorable brain-to-blood glutamate efflux, the rate of reducing pathologically elevated glutamate in the brain's extracellular fluid can be dramatically increased.
Various pharmacological blood glutamate scavengers have been demonstrated to successfully reduce brain glutamate and provide neuroprotection in animal models of brain injury including stroke, traumatic brain injury and subarachnoid hemorrhage.
Extracorporeal methods, including hemodialysis (HD), peritoneal dialysis and continuous hemofiltration (HF), offer unique advantages over pharmacological methods of blood glutamate reduction including irreversible glutamate removal, as well as fewer peripheral effects and side effects.
Humans with end-stage renal failure have been shown to have higher concentrations of blood glutamate compared with healthy controls, and HD has been effective in eliminating this excess plasma glutamate.
Peritoneal dialysis, which neither impacts the hemodynamic stability of critically ill patients nor requires heparin, has been effective in reducing blood glutamate in humans and animal studies.
HF may be superior to dialysis in clearing medium and larger sized molecules, and may be a surrogate for HD to decrease blood glutamate concentrations.
Currently, two clinical studies are underway that better study the blood glutamate-lowering effects of HF: the first study is investigating the effects of continuous HF in critically ill patients, and the second study is a Phase II, prospective, double-blinded randomized control trial evaluating the effect of HF in the treatment of acute ischemic stroke.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Waxweiler RJ, Thurman D, Sniezek J, et al. Monitoring the impact of traumatic brain injury: a review and update. J Neurotrauma. 1995;12(4):509–16. doi: 10.1089/neu.1995.12.509. [DOI] [PubMed] [Google Scholar]
- 2.Baker AJ, Moulton RJ, MacMillan VH, Shedden PM. Excitatory amino acids in cerebrospinal fluid following traumatic brain injury in humans. J Neurosurg. 1993;79(3):369–72. doi: 10.3171/jns.1993.79.3.0369. [DOI] [PubMed] [Google Scholar]
- 3.Benveniste H, Drejer J, Schousboe A, Diemer NH. Elevation of the extracellular concentrations of glutamate and aspartate in rat hippocampus during transient cerebral ischemia monitored by intracerebral microdialysis. J Neurochem. 1984;43(5):1369–74. doi: 10.1111/j.1471-4159.1984.tb05396.x. [DOI] [PubMed] [Google Scholar]
- 4.Davalos A, Castillo J, Serena J, Noya M. Duration of glutamate release after acute ischemic stroke. Stroke. 1997;28(4):708–10. doi: 10.1161/01.str.28.4.708. [DOI] [PubMed] [Google Scholar]
- 5.Palmer AM, Marion DW, Botscheller ML, et al. Traumatic brain injury-induced excitotoxicity assessed in a controlled cortical impact model. J Neurochem. 1993;61(6):2015–24. doi: 10.1111/j.1471-4159.1993.tb07437.x. [DOI] [PubMed] [Google Scholar]
- 6.Faden AI, Demediuk P, Panter SS, Vink R. The role of excitatory amino acids and NMDA receptors in traumatic brain injury. Science. 1989;244(4906):798–800. doi: 10.1126/science.2567056. [DOI] [PubMed] [Google Scholar]
- 7.Boyko M, Gruenbaum SE, Gruenbaum BF, et al. Brain to blood glutamate scavenging as a novel therapeutic modality: a review. J Neural Transm. 2014;121(8):971–9. doi: 10.1007/s00702-014-1181-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leibowitz A, Boyko M, Shapira Y, Zlotnik A. Blood glutamate scavenging: insight into neuroprotection. Int J Mol Sci. 2012;13(8):10041–66. doi: 10.3390/ijms130810041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biegon A, Fry PA, Paden CM, et al. Dynamic changes in N-methyl-D-aspartate receptors after closed head injury in mice: implications for treatment of neurological and cognitive deficits. Proc Natl Acad Sci USA. 2004;101(14):5117–22. doi: 10.1073/pnas.0305741101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teichberg VI. GOT to rid the body of excess glutamate. J Cereb Blood Flow Metab. 2011;31(6):1376–7. doi: 10.1038/jcbfm.2011.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakanishi S. Molecular diversity of glutamate receptors and implications for brain function. Science. 1992;258(5082):597–603. doi: 10.1126/science.1329206. [DOI] [PubMed] [Google Scholar]
- 12.Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65(1):1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 13.Berger C, Schabitz WR, Wolf M, et al. Hypothermia and brain-derived neurotrophic factor reduce glutamate synergistically in acute stroke. Exp Neurol. 2004;185(2):305–12. doi: 10.1016/j.expneurol.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 14.Chakrabarty K, Bhattacharyya S, Christopher R, Khanna S. Glutamatergic dysfunction in OCD. Neuropsychopharmacology. 2005;30(9):1735–40. doi: 10.1038/sj.npp.1300733. [DOI] [PubMed] [Google Scholar]
- 15.Cortese BM, Phan KL. The role of glutamate in anxiety and related disorders. CNS Spectr. 2005;10(10):820–30. doi: 10.1017/s1092852900010427. [DOI] [PubMed] [Google Scholar]
- 16.de Groot J, Sontheimer H. Glutamate and the biology of gliomas. Glia. 2011;59(8):1181–9. doi: 10.1002/glia.21113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eid T, Williamson A, Lee TS, et al. Glutamate and astrocytes-key players in human mesial temporal lobe epilepsy? Epilepsia. 2008;49(Suppl 2):42–52. doi: 10.1111/j.1528-1167.2008.01492.x. [DOI] [PubMed] [Google Scholar]
- 18.Ferrarese C, Aliprandi A, Tremolizzo L, et al. Increased glutamate in CSF and plasma of patients with HIV dementia. Neurology. 2001;57(4):671–5. doi: 10.1212/wnl.57.4.671. [DOI] [PubMed] [Google Scholar]
- 19.Stobart JL, Anderson CM. Multifunctional role of astrocytes as gatekeepers of neuronal energy supply. Front Cell Neurosci. 2013;7:38. doi: 10.3389/fncel.2013.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grill V, Bjorkman O, Gutniak M, Lindqvist M. Brain uptake and release of amino acids in nondiabetic and insulin-dependent diabetic subjects: important role of glutamine release for nitrogen balance. Metabolism. 1992;41(1):28–32. doi: 10.1016/0026-0495(92)90186-e. [DOI] [PubMed] [Google Scholar]
- 21.Ikonomidou C, Turski L. Why did NMDA receptor antagonists fail clinical trials for stroke and traumatic brain injury? Lancet Neurol. 2002;1(6):383–6. doi: 10.1016/s1474-4422(02)00164-3. [DOI] [PubMed] [Google Scholar]
- 22.Zlotnik A, Gruenbaum SE, Artru AA, et al. The neuroprotective effects of oxaloacetate in closed head injury in rats is mediated by its blood glutamate scavenging activity: evidence from the use of maleate. J Neurosurg Anesthesiol. 2009;21(3):235–41. doi: 10.1097/ANA.0b013e3181a2bf0b. [DOI] [PubMed] [Google Scholar]
- 23.O'Kane RL, Martinez-Lopez I, DeJoseph MR, et al. Na(+)-dependent glutamate transporters (EAAT1, EAAT2, and EAAT3) of the blood-brain barrier. A mechanism for glutamate removal. J Biol Chem. 1999;274(45):31891–5. doi: 10.1074/jbc.274.45.31891. [DOI] [PubMed] [Google Scholar]
- 24.Marliss EB, Aoki TT, Pozefsky T, et al. Muscle and splanchnic glutamine and glutamate metabolism in postabsorptive and starved man. J Clin Invest. 1971;50(4):814–17. doi: 10.1172/JCI106552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hosoya K, Sugawara M, Asaba H, Terasaki T. Blood-brain barrier produces significant efflux of L-aspartic acid but not D-aspartic acid: in vivo evidence using the brain efflux index method. J Neurochem. 1999;73(3):1206–11. doi: 10.1046/j.1471-4159.1999.0731206.x. [DOI] [PubMed] [Google Scholar]
- 26.Gottlieb M, Wang Y, Teichberg VI. Blood-mediated scavenging of cerebrospinal fluid glutamate. J Neurochem. 2003;87(1):119–26. doi: 10.1046/j.1471-4159.2003.01972.x. [DOI] [PubMed] [Google Scholar]
- 27.Zlotnik A, Gurevich B, Tkachov S, et al. Brain neuroprotection by scavenging blood glutamate. Exp Neurol. 2007;203(1):213–20. doi: 10.1016/j.expneurol.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 28.Zlotnik A, Gurevich B, Cherniavsky E, et al. The contribution of the blood glutamate scavenging activity of pyruvate to its neuroprotective properties in a rat model of closed head injury. Neurochem Res. 2008;33(6):1044–50. doi: 10.1007/s11064-007-9548-x. [DOI] [PubMed] [Google Scholar]
- 29.Zlotnik A, Sinelnikov I, Gruenbaum BF, et al. Effect of glutamate and blood glutamate scavengers oxaloacetate and pyruvate on neurological outcome and pathohistology of the hippocampus after traumatic brain injury in rats. Anesthesiology. 2012;116(1):73–83. doi: 10.1097/ALN.0b013e31823d7731. [DOI] [PubMed] [Google Scholar]
- 30.Boyko M, Zlotnik A, Gruenbaum BF, et al. Pyruvate's blood glutamate scavenging activity contributes to the spectrum of its neuroprotective mechanisms in a rat model of stroke. Eur J Neurosci. 2011;34(9):1432–41. doi: 10.1111/j.1460-9568.2011.07864.x. [DOI] [PubMed] [Google Scholar]
- 31.Campos F, Sobrino T, Ramos-Cabrer P, et al. Neuroprotection by glutamate oxaloacetate transaminase in ischemic stroke: an experimental study. J Cereb Blood Flow Metab. 2011;31(6):1378–86. doi: 10.1038/jcbfm.2011.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagy D, Marosi M, Kis Z, et al. Oxaloacetate decreases the infarct size and attenuates the reduction in evoked responses after photothrombotic focal ischemia in the rat cortex. Cell Mol Neurobiol. 2009;29(6-7):827–35. doi: 10.1007/s10571-009-9364-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boyko M, Melamed I, Gruenbaum BF, et al. The effect of blood glutamate scavengers oxaloacetate and pyruvate on neurological outcome in a rat model of subarachnoid hemorrhage. Neurotherapeutics. 2012;9(3):649–57. doi: 10.1007/s13311-012-0129-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carvalho AS, Torres LB, Persike DS, et al. Neuroprotective effect of pyruvate and oxaloacetate during pilocarpine induced status epilepticus in rats. Neurochem Int. 2011;58(3):385–90. doi: 10.1016/j.neuint.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 35.Ruban A, Berkutzki T, Cooper I, et al. Blood glutamate scavengers prolong the survival of rats and mice with brain-implanted gliomas. Invest New Drugs. 2012;30(6):2226–35. doi: 10.1007/s10637-012-9794-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruban A, Mohar B, Jona G, Teichberg VI. Blood glutamate scavenging as a novel neuroprotective treatment for paraoxon intoxication. J Cereb Blood Flow Metab. 2014;34(2):221–7. doi: 10.1038/jcbfm.2013.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zlotnik A, Gruenbaum BF, Mohar B, et al. The effects of estrogen and progesterone on blood glutamate levels: evidence from changes of blood glutamate levels during the menstrual cycle in women. Biol Reprod. 2011;84(3):581–6. doi: 10.1095/biolreprod.110.088120. [DOI] [PubMed] [Google Scholar]
- 38.Zlotnik A, Leibowitz A, Gurevich B, et al. Effect of estrogens on blood glutamate levels in relation to neurological outcome after TBI in male rats. Intensive Care Med. 2012;38(1):137–44. doi: 10.1007/s00134-011-2401-3. [DOI] [PubMed] [Google Scholar]
- 39.Zlotnik A, Klin Y, Gruenbaum BF, et al. The activation of beta2-adrenergic receptors in naive rats causes a reduction of blood glutamate levels: relevance to stress and neuroprotection. Neurochem Res. 2011;36(5):732–8. doi: 10.1007/s11064-010-0388-8. [DOI] [PubMed] [Google Scholar]
- 40.Zlotnik A, Klin Y, Gruenbaum BF, et al. β2 adrenergic-mediated reduction of blood glutamate levels and improved neurological outcome after traumatic brain injury in rats. J Neurosurg Anesthesiol. 2012;24(1):30–8. doi: 10.1097/ANA.0b013e318232deaa. [DOI] [PubMed] [Google Scholar]
- 41.Fanne RA, Nassar T, Heyman SN, et al. Insulin and glucagon share the same mechanism of neuroprotection in diabetic rats: role of glutamate. Am J Physiol Regul Integr Comp Physiol. 2011;301(3):R668–73. doi: 10.1152/ajpregu.00058.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zlotnik A, Gruenbaum BF, Klin Y, et al. The effects of insulin, glucagon, glutamate, and glucose infusion on blood glutamate and plasma glucose levels in naive rats. J Neurosurg Anesthesiol. 2011;23(4):323–8. doi: 10.1097/ANA.0b013e3182299b15. [DOI] [PubMed] [Google Scholar]
- 43.Rogachev B, Ohayon S, Saad A, et al. The effects of hemodialysis on blood glutamate levels in chronic renal failure: implementation for neuroprotection. J Crit Care. 2012;27(6):743.e1–7. doi: 10.1016/j.jcrc.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 44.Rogachev B, Tsesis S, Gruenbaum BF, et al. The effects of peritoneal dialysis on blood glutamate levels: implementation for neuroprotection. J Neurosurg Anesthesiol. 2013;25(3):262–6. doi: 10.1097/ANA.0b013e318283f86a. [DOI] [PubMed] [Google Scholar]
- 45.Hu SL, Wang D, Jiang H, et al. Therapeutic effectiveness of sustained low-efficiency hemodialysis plus hemoperfusion and continuous hemofiltration plus hemoperfusion for acute severe organophosphate poisoning. Artif Organs. 2014;38(2):121–4. doi: 10.1111/aor.12134. [DOI] [PubMed] [Google Scholar]
- 46.Avino P, Capannesi G, Rosada A, et al. Multivariate analysis applied to some elements in human fluids and whole bloods of hemodialysis patients determined by INAA. J Radioanal Nucl Chem. 2013;298(3):1957–68. [Google Scholar]
- 47.Davies S, Lally F, Satchithananda D, et al. Extending the role of peritoneal dialysis: can we win hearts and minds? Nephrol Dial Transplant. 2014;29(9):1648–54. doi: 10.1093/ndt/gfu001. [DOI] [PubMed] [Google Scholar]
- 48.Himmelfarb J, Ikizler TA. Hemodialysis. N Engl J Med. 2010;363(19):1833–45. doi: 10.1056/NEJMra0902710. [DOI] [PubMed] [Google Scholar]
- 49.Soupart A, Silver S, Schrooeder B, et al. Rapid (24-hour) reaccumulation of brain organic osmolytes (particularly myo-inositol) in azotemic rats after correction of chronic hyponatremia. J Am Soc Nephrol. 2002;13(6):1433–41. doi: 10.1097/01.asn.0000017903.77985.cd. [DOI] [PubMed] [Google Scholar]
- 50.McLaggan D, Naprstek J, Buurman ET, Epstein W. Interdependence of K+ and glutamate accumulation during osmotic adaptation of Escherichia coli. J Biol Chem. 1994;269(3):1911–17. [PubMed] [Google Scholar]
- 51.Gil HW, Yang JO, Lee EY, et al. The effect of dialysis membrane flux on amino acid loss in hemodialysis patients. J Korean Med Sci. 2007;22(4):598–603. doi: 10.3346/jkms.2007.22.4.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Godino Mdel C, Romera VG, Sanchez-Tomero JA, et al. Amelioration of ischemic brain damage by peritoneal dialysis. J Clin Invest. 2013;123(10):4359–63. doi: 10.1172/JCI67284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Friedrich JO, Wald R, Bagshaw SM, et al. Hemofiltration compared to hemodialysis for acute kidney injury: systematic review and meta-analysis. Crit Care. 2012;16(4):R146. doi: 10.1186/cc11458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ronco C, Tetta C, Mariano F, et al. Interpreting the mechanisms of continuous renal replacement therapy in sepsis: the peak concentration hypothesis. Artif Organs. 2003;27(9):792–801. doi: 10.1046/j.1525-1594.2003.07289.x. [DOI] [PubMed] [Google Scholar]
- 55.Patel P, Nandwani V, McCarthy PJ, et al. Continuous renal replacement therapies: a brief primer for the neurointensivist. Neurocrit Care. 2010;13(2):286–94. doi: 10.1007/s12028-010-9386-6. [DOI] [PubMed] [Google Scholar]