Abstract
Purpose of review
Recent methodological advances in computational simulations are enabling increasingly realistic simulations of hemodynamics and physiology, driving increased clinical utility. We review recent developments in the use of computational simulations in pediatric and congenital heart disease, describe the clinical impact in modeling in single ventricle patients, and provide an overview of emerging areas.
Recent Findings
Multiscale modeling combining patient specific hemodynamics with reduced order (i.e. mathematically and computationally simplified) circulatory models has become the defacto standard for modeling local hemodynamics and “global” circulatory physiology. We review recent advances that have enabled faster solutions, discuss new methods, (e.g. fluid structure interaction and uncertainty quantification), which lend realism both computationally and clinically to results, highlight novel computationally-derived surgical methods for single ventricle patients, and discuss areas in which modeling has begun to exert its influence including Kawasaki disease, fetal circulation, tetralogy of Fallot, (and pulmonary tree), and circulatory support.
Summary
Computational modeling is emerging as a crucial tool for clinical decision-making and evaluation of novel surgical methods and interventions in pediatric cardiology and beyond. Continued development of modeling methods, with an eye towards clinical needs, will enable clinical adoption in a wide range of pediatric and congenital heart diseases.
Keywords: Single-ventricle, hemodynamics, multiscale modeling, Kawasaki disease, pediatric ventricular assist devices, fetal circulation, pediatric valves, Tetralogy of Fallot, pulmonary arteries, computational fluid dynamics
INTRODUCTION
Engineering-based investigations offer powerful tools for predictive and personalized medicine, with potential to augment medical imaging data, uncover links between mechanics and biological response, and provide clinical decision support. In this review, we summarize the recent advances in computational simulation, and engineering-based tools applied to pediatric and congenital heart disease.
The field of congenital heart disease was first introduced to computational modeling by Marc de Leval and colleagues in the mid-1990s.[1,2] In this early pioneering work, hemodynamics in the classic Fontan circulation were simulated using computational fluid dynamics, and a novel offset modification between the inferior and superior vena cava was introduced. Findings of reduced energy loss in this so-called “offset design,” due to minimizing flow collision in the Fontan junction led to a change in practice in the surgical community, arguably the first example of a simulation-based design leading to a change in clinical practice. While groundbreaking at the time, the methods were simplistic and significant advances in modeling methods for cardiovascular simulation have subsequently been developed. In particular, patient-specific modeling tools allowing for image-based model construction and simulation[3], increasingly advanced methods incorporating fluid structure interaction[4–6], and multiscale models of circulatory physiology and the heart[7,8] now yield increasingly realistic simulations.
With recent advances in numerical methods and computing power, computational simulations are now poised to gain greater clinical acceptance than ever before. In the adult cardiovascular field, this is best shown by the successful clinical trials and subsequent 2014 FDA approval of a simulation service by HeartFlow, Inc. using computed tomography and simulation based fractional flow reserve (FFRCT) as a non-invasive evaluation of coronary artery disease.[9–11] A similar path forward for simulation adoption in pediatrics is certainly possible, however the far more complex physiology and smaller patient populations make this more challenging.
In the pages that follow, we highlight advancements and challenges in computational and engineering methodology, patient specific modeling, and their clinical application over the recent past. We first aim to highlight the most significant methodological advances that have relevance to solving clinical problems, and provide a review of modeling methods accessible to the clinician. We then highlight application areas in single ventricle physiology, tetralogy of Fallot, pulmonary arteries, aortic and coronary disease, valves and devices, and fetal circulation. We note that while single ventricle physiology has received the bulk of the attention and effort in the modeling community over the past two decades, the field is poised for an explosion of activity in additional, previously unexplored, areas of pediatric and congenital heart disease. We therefore have made a concerted effort to provide an overview, when possible, of activities in multiple areas of pediatric and congenital heart disease. We conclude by providing our outlook on the current state of the field and future areas of need, and by highlighting emerging areas and new directions in both modeling methodology and clinical application.
ADVANCES IN MODELING METHODS
Background
The application of computational fluid dynamics to cardiovascular blood flow simulation spans a wide range of adult diseases including coronary artery disease[12–15], abdominal and cerebral aneurysms[16–18], and peripheral vascular disease[19]. Image-based modeling allows for construction of patient specific anatomy directly from image data, and subsequent simulation of blood flow in these complex models using computational fluid dynamics (CFD)[20]. Once the geometric anatomic model has been constructed, so-called “boundary conditions” must be prescribed at all inlets and outlets of the model. Boundary conditions are prescriptions of flow, pressure, or relations between them, and provide mathematical representations of the physiology outside of the computational domain (e.g. pulmonary vascular resistance). Boundary conditions must also be prescribed on the vessel walls, assigning material properties to simulate wall deformation though obtaining patient specific material properties remains a challenge.
Limitations of cardiovascular CFD models can include the lack of patient specific material properties, uncertainties and variability in boundary and physiologic conditions, and Newtonian fluid models. High computational expense and the time required for model construction often pose a challenge for application to clinical studies with large patient cohorts. In addition, there is often limited knowledge on the biological and physiological response to changing hemodynamic conditions, and hence these effects are often neglected. For example, most current CFD models do not properly account for vessel wall growth and remodeling, venovenous or aortopulmonary collaterals, thrombus formation in response to injury or flow stasis, or auto-regulatory and adaptive mechanisms.
There is also a pressing need for increased validation of CFD and multiscale models, particularly against clinical and in vivo data. Validation of CFD has generally relied on in vitro experiments, with more complex models, such as single ventricle physiology, implemented in mock circulatory loops[21–23]. Flow visualization and measurement are typically performed using phase-contrast MRI or particle image velocimetry (PIV). Validation against both in vitro and in vivo data have shown excellent agreement, though these efforts should be expanded in future work.
Multiscale modeling
While early studies of the Fontan circulation by de Leval, Dubini, and colleagues focused primarily on energy loss, recent work has applied multiscale modeling to capture both the local hemodynamics and the global physiologic response. This approach enables prediction of clinically relevant quantities such as pressure volume loops, oxygen saturation, pulmonary to systemic flow ratios, and changing pressure levels. Multiscale modeling for single ventricle clinical decision support was the focus of a recent 5-year Leducq-Foundation-funded network. The cumulative efforts of this network demonstrated the application of multiscale modeling in larger cohorts of patients, and for comparatively evaluating the performance of surgical and interventional methods for single ventricle patients. Recent advances have enabled incorporation of lumped parameter network (LPN) models as boundary conditions for CFD, allowing for computation of local hemodynamics, as well as global physiologic variables.[24] [25] [26] For further details, we refer the reader to a recent review of advances in multiscale modeling.[27]
Accounting for Uncertainties
The cardiovascular modeling process requires assimilation of patient specific clinical and imaging data. Image data is segmented to construct 3D models, clinical data, including blood pressures, heart rates, and flow and pressure waveforms, are required for boundary condition selection, and material properties must be assigned to vessel walls for deformable wall simulations. The modeling process has traditionally neglected the myriad uncertainties arising in this process, and foregone the variability of human physiology. However, recent studies have begun to account for uncertainties with greater rigor, both for model tuning (i.e. parameter estimation) and in assessing errors in simulation predictions.[28–30] [31,32] Parameter estimation has also been successfully applied in the setting of fluid structure interaction for selection of patient specific material properties to match time-resolved clinical imaging data.[33] These approaches have the potential to provide clinicians with accuracy on the level of trust that can be placed in simulation predictions, thereby fostering increased clinical acceptance and establishing standards for model fidelity.
While current models predict quantities of potential clinical interest, including wall shear stresses, oxygen levels, pressure levels, and PV loops, these quantities have traditionally been reported with no accompanying statistics to indicate confidence level. The failure to provide statistics leaves clinicians with little basis on which to evaluate them, and is a barrier to clinical adoption. If simulations are to inform decisions on interventions such as stent placement, it is important to provide measures of confidence that can be used to weigh model predictions against other clinical factors. Recent studies have proposed computational frameworks for performing “uncertainty quantification” (UQ). Using these methods, one performs a carefully selected set of simulations with varied parameter values to compile converged statistics on model predictions. For example, this approach could provide a clinician with a 95% confidence interval on a prediction of post-operative pressure levels in a virtual surgery application. Recent work has extended these approaches to propagate uncertainty directly from the clinical data to the simulation outputs.[34]
Emerging areas
While patient specific modeling and computational fluid dynamics simulations are now relatively mature technology, important biological and physiological processes that interplay with hemodynamic forces are often neglected. These include growth and remodeling (G&R), thrombosis, and endothelial cell response. A few recent studies have begun to address these areas. Vascular growth and remodeling describes changes in thickness, radius and wall composition of a vessel wall in response to changing hemodynamic forces. Recent work has expanded models of arterial growth and remodeling to veins[35], coupled CFD and G&R models in the context of adult cardiology,[36] and applied rigorous approaches for parameter selection.[37] Applications of G&R to pediatrics will require modifications to existing models to account for differing biologic and material responses in children. However, there is immense potential for these models, in conjunction with CFD, to enable predictions of pulmonary artery and venous remodeling, as well as formation of venovenous collaterals. In addition, recent work demonstrated the first use of tissue-engineered grafts in Fontan patients, and simulations with G&R will play an important role in optimizing graft properties.[38,39]
Modeling thrombus formation is a complex problem, with the need to account for numerous chemical reactions involved in the coagulation cascade. These processes are highly multiscale, with chemical reactions often occurring on much faster time scales than clot formation, imposing special simulation challenges. However, several groups have shown success in this arena, including recent studies of Alber, Fogelson, and colleagues.[40,41] While most have focused on the extrinsic, or injury-mediated, pathway for clot formation, there is also a need for model development to handle the intrinsic, or flow-mediated, pathway. This would be particularly relevant in pediatrics for the study of thrombus development in grafts, ventricular assist devices (VADs) and areas of flow stasis such as coronary artery aneurysms. Future work should expand current models to handle complex patient specific geometries and a greater range of clinical scenarios.
The above modeling developments have enabled increased application to a range of diseases with greater model fidelity. The next sections outline application of these new methods to specific disease applications in pediatric and congenital heart disease.
SINGLE VENTRICLES
Single ventricle physiology has been by far the most active application for simulations in congenital heart disease, with several key recent developments. Below, we highlight recent contributions in all three stages of single ventricle repair. Several recent reviews provide an overview of modeling techniques in single ventricle physiology[42–44] as well as recent clinical guidelines.[45,46]
Neonatal stage-one physiology continues to pose clinical challenges, with the highest mortality of the three stages and the need to balance delicate shunt physiology. The recent study of Hsia and colleagues used multiscale modeling to compare the Sano, Blalock Taussig (BT) shunt, and Hybrid Norwood procedures, demonstrating reduced oxygen deliver and higher cardiac workload with the Hybrid procedure compared to the other approaches.[47] Computational modeling has also been used to propose and evaluate novel surgical concepts, demonstrating the utility of simulations to test high-risk concepts that would otherwise be technically or ethically infeasible. The recent introduction of the Assisted Bidirectional Glenn (ABG) procedure of Moghadam et al[48] proposed a combination of bidirectional Glenn with a systemic-to-pulmonary shunt to create an ejector pump to increase pulmonary blood flow. Simulations and in vitro experiments demonstrated decreased cardiac workload, increased pulmonary blood flow, and increased oxygen delivery.[49] If used as a stage one surgery, this could offer the possibility of condensing the first two stages of single ventricle repair into one, although substantially more evidence and animal studies are needed prior to considerations of clinical translation.
Multiscale modeling has also been applied to study the stage-two Glenn and Hemi-Fontan procedures. Recent work of Schiavazzi et al. examined the impact of varying levels of pulmonary stenosis in the bidirectional Glenn procedure.[50] Results indicated that degree of stenosis higher than 65% area reduction was not sufficient to change most parameters of clinical interest, including oxygen levels and cardiac workload. This may suggest a less aggressive approach to pulmonary arterio-plasty, however we also note these models do not yet account for development of venovenous collaterals or pulmonary artery adaptations that may impact hemodynamics.[51] Another recent study used multiscale modeling to compare the Glenn and Hemi-Fontan procedures (Figure 1), with similar findings: energy loss differences between these two surgical approaches did not result in significant differences in global hemodynamics or cardiac workload, suggesting that energy loss may not be a primary determinant of surgical method in stage-two patients.[52]
FIGURE 1.
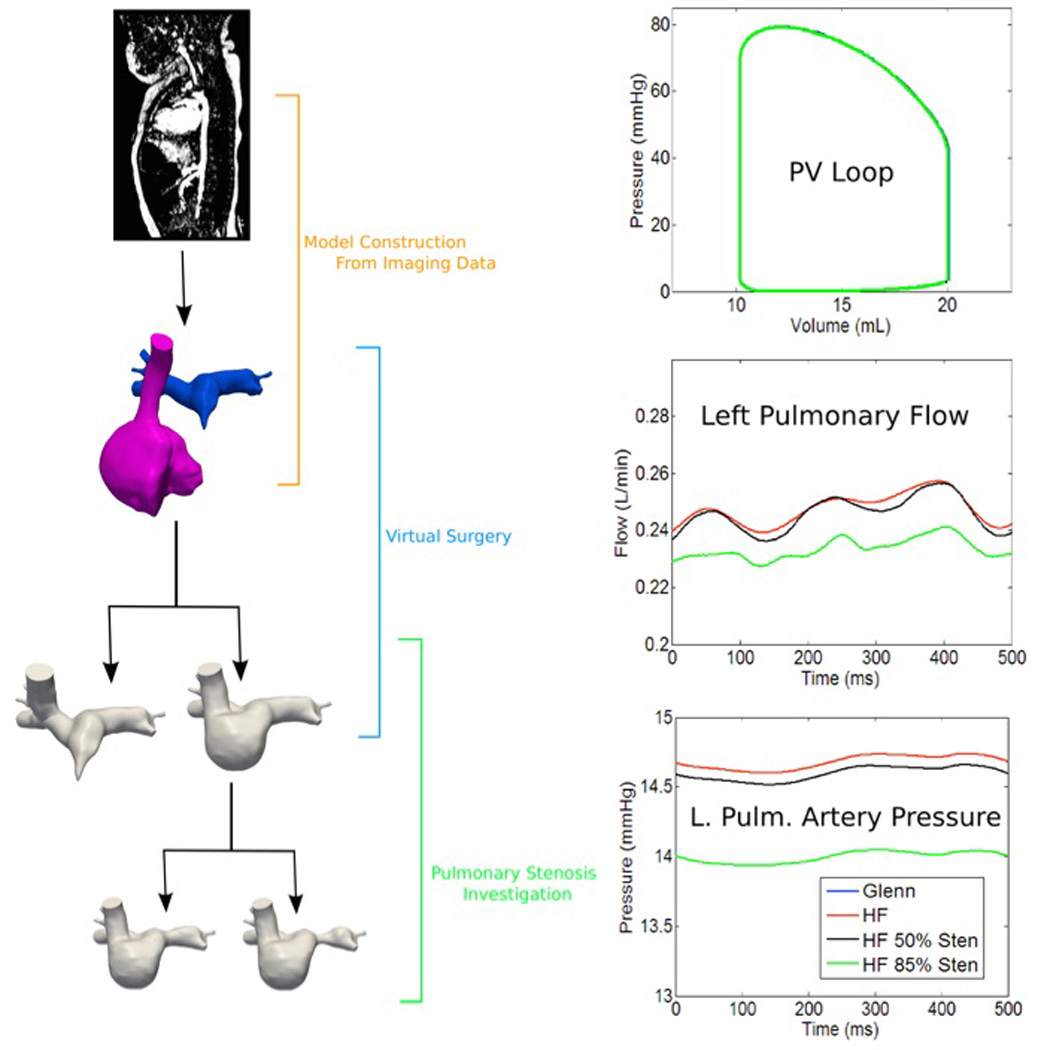
Multiscale modeling and virtual surgery is used to compare Glenn and Hemi-Fontan surgical approaches for stage two single ventricle palliation and correction of pulmonary stenosis. Results allow for comparison of cardiac workload (i.e. pressure-volume loops), pulmonary flow, and pressure.
The Fontan, or total cavopulmonary connection (TCPC), surgery is the subject of the bulk of the literature on single ventricle computational simulations. Recent contributions highlight the first examples of direct clinical translation of a simulation-derived surgical concept, the Fontan Y-graft procedure (Figure 2). Following introduction of the Y-graft Fontan in simulation,[53,54] the first Y-graft surgeries were performed at Stanford University[55] and then at Emory[56], with short-to-medium term outcomes reported. This experience also allowed for validation of hepatic flow distribution against lung perfusion scans[57] and simulations using in vivo data.[58] A comparison of the Y-graft Fontan to traditional offset and t-junction surgical approaches also demonstrated that while differences in hepatic flow were observed, modest energy losses did not correspond with significant changes in cardiac performance when comparing competing designs.[59] However, others have identified correlations between energy loss, cardiac workload, and exercise performance,[60,61] though these findings remain under discussion.[62]
FIGURE 2.
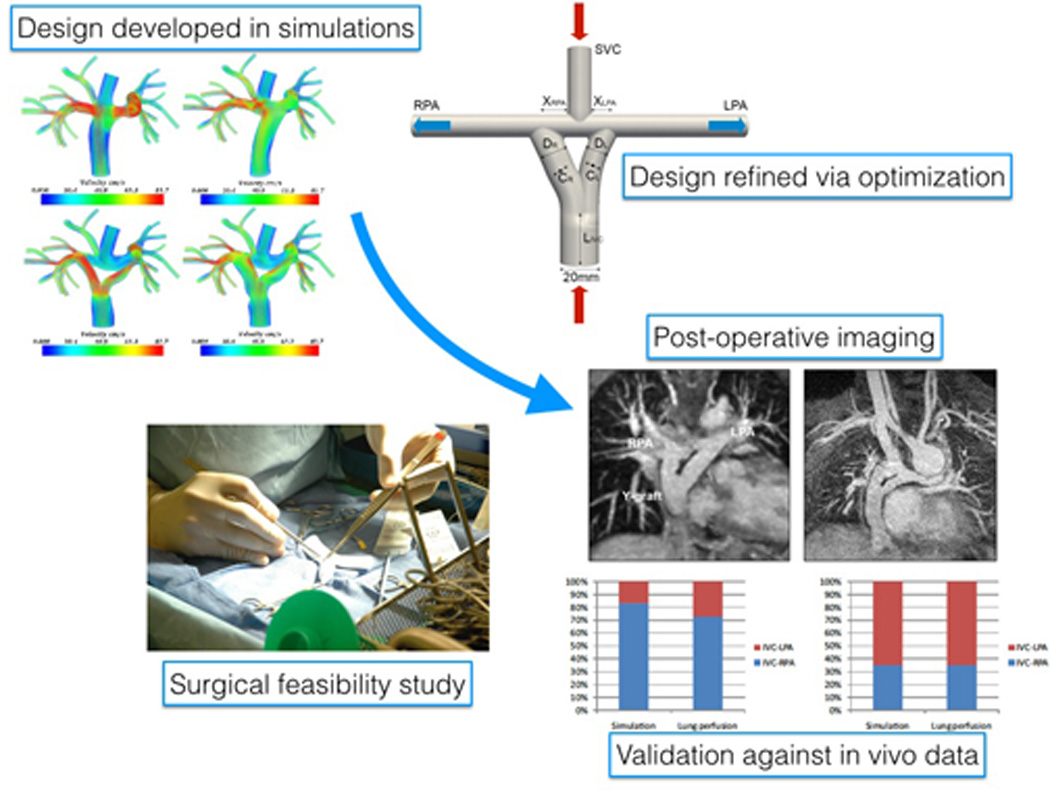
Development of the Fontan Y-graft from initial testing in simulation to design optimization, surgical implementation, post-operative imaging and validation.
Additional studies have assessed the performance of mechanical circulatory support and valve devices in the Fontan circulation. A viscous impeller pump was introduced by Rodefeld et al for mechanical circulatory support in Fontan patients. Subsequent studies successfully compared CFD, PIV, and mock circuit results to assess blood damage, pressure rise, and cardiac function[63–65], and have compared left vs. right heart support in simulations and mock circulatory loops[66]. Mock circulatory experiments also examined the effect of adding a valve in the Fontan conduit in the presence of respiration, showing little change in cycle-averaged pressure, though the valve did function with higher pulmonary vascular resistance.[67]
TETRALOGY OF FALLOT
Despite the larger patient population compared to single-ventricle physiology, there have been few studies in the computational literature focused on tetralogy of Fallot (ToF). Recent studies applying engineering to ToF have correlated energy loss with cardiac performance, suggesting new clinical indices that may be used to better predict the timing of surgical intervention.[68–70] Further investigation is needed in larger patient cohorts. Shape analysis has also been applied in the context of ToF to determine factors related to timing of pulmonary valve replacement, showing significant correlations between regurgitation severity and RV dilation.[71] Virtual surgery to model pericardial patch repair was also investigated, together with CFD simulations, with promising results.[72] The use of an elastic band for the right ventricle was computationally investigated varying band elasticity, length, active contraction, and a model of tissue regeneration.[73] Results showed the potential for increased ejection fraction using this approach, and called for further experimental and clinical investigation.
PULMONARY ARTERIES AND HYPERTENSION
CFD simulations were recently applied to simulate flow in up to 11 branches of the pulmonary vasculature, revealing the need for patient specific cardiac output to make accurate predictions, as well as correlations between wall shear stress, pulmonary vascular resistance, and arterial compliance.[74] Simulations also revealed significantly lower shear stress in patient specific models of pulmonary hypertension, and correlated findings with gene regulation.[75]
AORTIC AND CORONARY DISEASE
Kawasaki Disease
Recent advances in modeling coronary physiology have enabled simulations of hemodynamics in coronary artery aneurysms caused by Kawasaki disease (KD). Current clinical guidelines suggest anticoagulation therapy for KD patients with coronary aneurysm diameter greater than 8mm. Specialized boundary conditions to handle coronary physiology were recently introduced, and applied in patient-specific models to study thrombotic risk in KD. Simulations showed large differences in hemodynamic conditions, including wall shear stress and particle residence times, in aneurysmal compared to non-aneurysmal coronary arteries.[76] When simulation results were correlated with clinical data in a small cohort of KD patients, results indicated that hemodynamic parameters may be better predictors of thrombotic risk compared to aneurysm diameter, and that fusiform aneurysms may pose higher risk due to increased residence times.[77] Fluid mechanics also played an interesting role in a recent study revealing striking correlations between tropospheric wind patterns and occurrence of KD, suggesting an airborne agent as a possible etiology of KD.[78]
Coarctation of the Aorta
Coarctation of the aorta has also been largely overlooked in the modeling community, despite high potential for clinical impact. Recent studies have correlated altered hemodynamics quantified by CFD with endothelial function and protein expression in a rabbit model[79] and used CFD to evaluate changes in cardiac workload resulting from aortic arch obstruction.[80] Others have focused on the use of simulation to avoid unnecessary catheterization in surgical evaluation, achieving significant correlation between pressure drops measured by CFD and catheterization both pre- and post-treatment.[81] Szopos et al. also showed statistically significant higher wall shear stress in patients with “Gothic” compared to “Romanesque” arch repair using CFD with fluid structure interaction.[82]
VALVES AND DEVICES
Valvar abnormalities are now receiving increased attention in the modeling literature. Szeto et al demonstrated increased strain in bicuspid as compared to tricuspid valves,[83] and patient specific modeling has shown potential to improve success rates when used to aid in the placement and sizing of melody pulmonary replacement valves.[84] For a summary of recent progress in engineering approaches to valve disease, we refer the reader to a recent three-part review.[85–87]
Long et al demonstrated fluid structure interaction simulations of a pediatric ventricular assist device (VADs) modeled on the Berlin Heart, including both blood and air fluid chambers. Results demonstrated that decreased residence time, presumed linked to thrombotic risk, could be achieved through automated shape optimization.[88–90] Additional developments in VAD design and simulations were recently summarized in Marsden et al.[91]
Three-dimensional (3D) printing and virtual device implantation have received recent attention as promising tools for surgical planning and device placement, particularly for repair of tetralogy of Fallot, pulmonary atresia and pulmonary collaterals (Figure 3).[92,93] Virtual implantation tools and 3D printing have successfully guided surgical placement of the SynCardia Total Artificial Heart, in at least 32 patients in six countries thus far.[94] Incorporation of 3D printing into surgical planning, compared to standard approaches, has demonstrated decreases in mean surgical case time (cut to close), operating room time (wheel in to wheel out), and 30-day readmission rate, in a sizable cohort of over 200 patients encompassing a range of CHD diagnoses. Marked reductions in surgical time, with averages up to 125 minutes, were observed for smaller subsets of patients, including those with transposition of the great arteries.[95] These tools also hold particular promise for improving patient, parent, and trainee education, as demonstrated in recent studies incorporating 3D printed models into clinical practice at Great Ormond Street and Phoenix Children’s Hospitals.[93,95,96]
FIGURE 3.
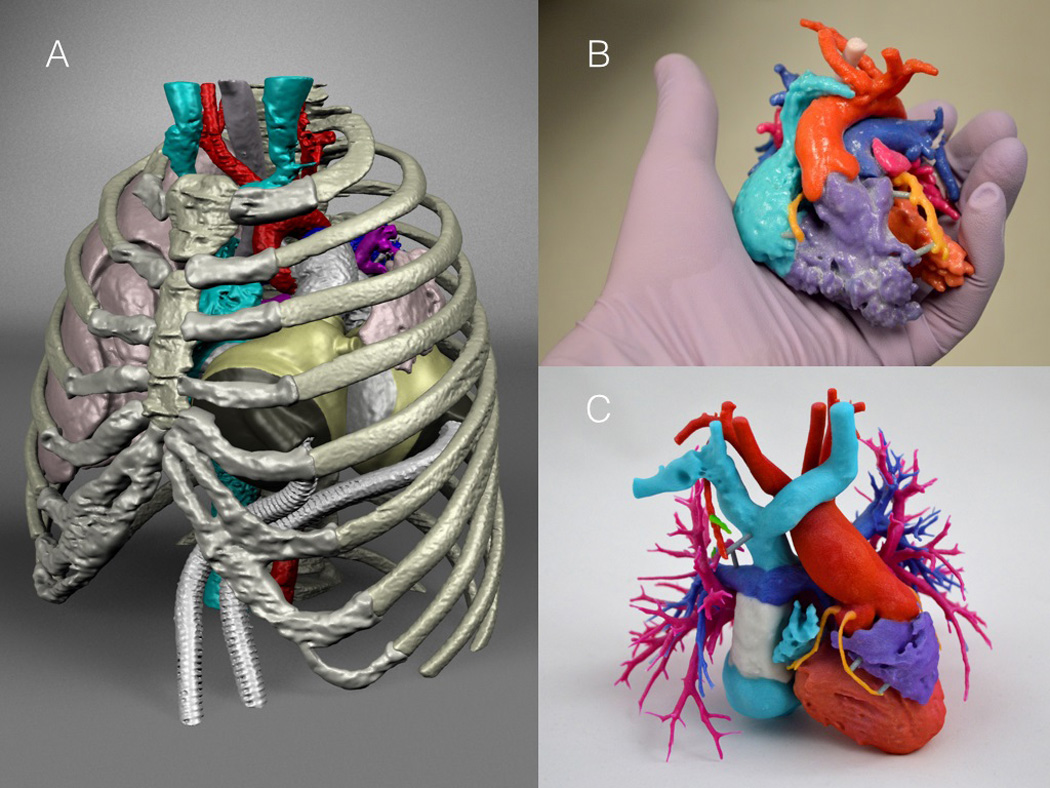
Illustration of (A) virtual implantation to aid in the placement of the SynCardia Total Artificial Heart, and 3D printed models to aid in surgical and treatment planning for (B) double outlet right ventricle and (C) the Fontan circulation. Courtesy of Dr. David Frakes.
FETAL CIRCULATION
Modeling of the fetal circulation is an area of particular promise for linking hemodynamics with mechanobiology and mechanisms of cardiac development and the origins of congenital heart disease. A recent collaborative study used CFD to quantify wall shear stress during zebrafish cardiac development at multiple time points post-fertilization.[97] CFD simulations were also used, together with an experimental platform, to quantify changes in pressure and flow distribution in the fetal circulation using a chick model of aortic arch obstruction.[98]
FUTURE OUTLOOK AND CONCLUSIONS
Computational modeling holds promise for increased clinical application in multiple congenital and pediatric heart diseases. Simulations can be used to augment clinical imaging, and to support clinical decisions in surgical and treatment planning, and device placement. Simulations can also provide a quantitative means to elucidate the relationship between hemodynamics and biological processes such as thrombosis, growth and remodeling, and mechanobiology. Development of new simulation tools should continue with an eye towards increased physiologic and biologic realism and increased clinical utility.
As highlighted in the studies reviewed here, research directions have now begun to move away from mere technical demonstration of tools and application in case studies, towards higher impact clinical applications and larger clinical studies. It is becoming more apparent that if modeling tools are to become commonplace in the clinic, well powered clinical trials demonstrating impact on patient outcomes from clinical use of CFD will be essential.
While there has been an abundance of work on single ventricle physiology, there have been relatively fewer studies in other areas of pediatric cardiology, despite larger patient populations. We see particular promise for clinical impact from modeling Tetralogy of Fallot and associated pulmonary artery stenoses and collaterals, Alagille syndrome, and coarctation of the aorta. In addition, simulations can fill the missing gaps between hemodynamic forces and mechanobiological response. There is particular promise for high impact in pulmonary hypertension, and fetal development. Finally, there is a continued need for development of pediatric-appropriate devices, and simulations, together with optimization, offer a means to accelerate designs at lower cost and lower risk.
KEY FINDINGS.
Computational modeling has led to clinical translation of novel surgical methods for single ventricle palliation.
Future simulation studies should move beyond single ventricle physiology into other areas of pediatric and congenital heart disease.
Simulation methodologies should be expanded to incorporate greater physiologic and biological realism.
Future clinical acceptance will be driven by increased validation, quantifying confidence in simulation predictions, and demonstration of improved clinical outcomes in clinical trials incorporating simulations.
ACKNOWLEDGEMENTS
This work was supported by the Leducq Foundation as part of a Transatlantic Network of Excellence for Cardiovascular Research, a Burroughs Wellcome Fund Career award at the Scientific Interface, NSF CAREER OCI-1150184, and the Vera Moulton Wall Center, Stanford University. We also acknowledge the open source SimVascular project at www.simvascular.org.
LIST OF ABBREVIATIONS
- ABG
Assisted bidirectional Glenn
- CFD
Computational fluid dynamics
- G&R
Growth and remodeling
- KD
Kawasaki Disease
- LPN
Lumped parameter network
- MRI
Magnetic resonance imaging
- PIV
Particle image velocimetry
- TCPC
Total cavopulmonary connection
- ToF
Tetralogy of Fallot
- UQ
Uncertainty quantification
- VAD
ventricular assist device
Footnotes
CONFLICTS OF INTEREST:
There are no conflicts of interest.
REFERECES
- 1.Dubini G, deLeval MR, Pietrabissa R, Montevecchi FM, Fumero R. A numerical fluid mechanical study of repaired congenital heart defects. Application to the total cavopulmonary connection. Journal of Biomechanics. 1996;29:111–121. doi: 10.1016/0021-9290(95)00021-6. [DOI] [PubMed] [Google Scholar]
- 2.deLeval MR, Dubini G, Migliavacca F, Jalali H, Camporini G, Redington A, Pietrabissa R. Use of computational fluid dynamics in the design of surgical procedures: Application to the study of competitive flows in cavopulmonary connections. Journal of Thoracic and Cardiovascular Surgery. 1996;111:502–510. doi: 10.1016/s0022-5223(96)70302-1. [DOI] [PubMed] [Google Scholar]
- 3.Wilson N. Mechanical Engineering. Stanford University; 2002. Geometric Algorithms and Software Architecture for Computational Prototyping: Applications in Vascular Surgery and MEMS. [Google Scholar]
- 4.Long CC, Hsu MC, Bazilevs Y, Feinstein JA, Marsden AL. Fluid-structure interaction simulations of the Fontan procedure using variable wall properties. International Journal for Numerical Methods in Biomedical Engineering. 2012;28:513–527. doi: 10.1002/cnm.1485. [DOI] [PubMed] [Google Scholar]
- 5.Bazilevs Y, Calo VM, Zhang Y, Hughes TJR. Isogeometric fluid-structure interaction analysis with applications to arterial blood flow. Computational Mechanics. 2006;38:310–322. [Google Scholar]
- 6.Figueroa CA, Vignon-Clementel IE, Jansen KE, Hughes TJR, Taylor CA. A coupled momentum method for modeling blood flow in three-dimensional deformable arteries. Computer Methods in Applied Mechanics and Engineering. 2006;195:5685–5706. [Google Scholar]
- 7.Migliavacca F, Balossino R, Pennati G, Dubini G, Hsia TY, de Leval MR, Bove EL. Multiscale modelling in biofluidynamics: Application to reconstructive paediatric cardiac surgery. Journal of Biomechanics. 2006;39:1010–1020. doi: 10.1016/j.jbiomech.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 8.Bove E, Migliavacca F, de Leval M, Balossino R, Pennati G, Lloyd T, Khambadkone S, Hsia T, Dubini G. Use of mathematic modeling to compare and predict hemodynamic effects of the modified Blalock-Taussig and right ventricle-pulmonary artery shunts for hypoplastic left heart syndrome. Journal of Thoracic and Cardiovascular Surgery. 2008:312–320. doi: 10.1016/j.jtcvs.2007.04.078. [DOI] [PubMed] [Google Scholar]
- 9.Min J, Berman D, Shaw L, Mauri L, Koo BK, Erglis A, Leipsic J. Fractional Flow Reserved Derived From Computed Tomographic Angiography (FFRCT) for Intermediate Severity Coronary Lesions: Results from the DeFACTO Trial (Determination of Fractional Flow Reserve by Anatomic Computed TOmographic Angiography) Journal of the American College of Cardiology. 2012;60:B6–B6. [Google Scholar]
- 10.Nakazato R, Park H-B, Berman DS, Gransar H, Koo B-K, Erglis A, Lin FY, Dunning AM, Budoff MJ, Malpeso J, et al. Noninvasive Fractional Flow Reserve Derived From Computed Tomography Angiography for Coronary Lesions of Intermediate Stenosis Severity Results From the DeFACTO Study. Circulation-Cardiovascular Imaging. 2013;6:881–889. doi: 10.1161/CIRCIMAGING.113.000297. [DOI] [PubMed] [Google Scholar]
- 11.Taylor CA, Fonte TA, Min JK. Computational Fluid Dynamics Applied to Cardiac Computed Tomography for Noninvasive Quantification of Fractional Flow Reserve Scientific Basis. Journal of the American College of Cardiology. 2013;61:2233–2241. doi: 10.1016/j.jacc.2012.11.083. [DOI] [PubMed] [Google Scholar]
- 12.Sankaran S, Moghadam ME, Kahn AM, Tseng EE, Guccione JM, Marsden AL. Patient-Specific Multiscale Modeling of Blood Flow for Coronary Artery Bypass Graft Surgery. Annals of Biomedical Engineering. 2012;40:2228–2242. doi: 10.1007/s10439-012-0579-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim HJ, Vignon-Clementel IE, Coogan JS, Figueroa CA, Jansen KE, Taylor CA. Patient-Specific Modeling of Blood Flow and Pressure in Human Coronary Arteries. Annals of Biomedical Engineering. 2010;38:3195–3209. doi: 10.1007/s10439-010-0083-6. [DOI] [PubMed] [Google Scholar]
- 14.Smith NP, Pullan AJ, Hunter PJ. Generation of an anatomically based geometric coronary model. Annals of Biomedical Engineering. 2000;28:14–25. doi: 10.1114/1.250. [DOI] [PubMed] [Google Scholar]
- 15.Nordsletten DA, Niederer SA, Nash MP, Hunter PJ, Smith NP. Coupling multi-physics models to cardiac mechanics. Progress in Biophysics & Molecular Biology. 2011;104:77–88. doi: 10.1016/j.pbiomolbio.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Chung B, Cebral JR. CFD for Evaluation and Treatment Planning of Aneurysms: Review of Proposed Clinical Uses and Their Challenges. Annals of Biomedical Engineering. 2015;43:122–138. doi: 10.1007/s10439-014-1093-6. [DOI] [PubMed] [Google Scholar]
- 17.Mut F, Raschi M, Scrivano E, Bleise C, Chudyk J, Ceratto R, Lylyk P, Cebral JR. Association between hemodynamic conditions and occlusion times after flow diversion in cerebral aneurysms. Journal of Neurointerventional Surgery. 2015;7:286–290. doi: 10.1136/neurintsurg-2013-011080. [DOI] [PubMed] [Google Scholar]
- 18.Les AS, Shadden SC, Figueroa CA, Park JM, Tedesco MM, Herfkens RJ, Dalman RL, Taylor CA. Quantification of Hemodynamics in Abdominal Aortic Aneurysms During Rest and Exercise Using Magnetic Resonance Imaging and Computational Fluid Dynamics. Annals of Biomedical Engineering. 2010;38:1288–1313. doi: 10.1007/s10439-010-9949-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hossain SS, Zhang Y, Fu X, Brunner G, Singh J, Hughes TJR, Shah D, Decuzzi P. Magnetic resonance imaging-based computational modelling of blood flow and nanomedicine deposition in patients with peripheral arterial disease. Journal of the Royal Society, Interface / the Royal Society. 2015;12 doi: 10.1098/rsif.2015.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arbia G, Corsini C, Moghadam ME, Marsden AL, Migliavacca F, Pennati G, Hsia T-Y, Vignon-Clementel IE Modeling Congenital Hearts A. Numerical blood flow simulation in surgical corrections: what do we need for an accurate analysis? Journal of Surgical Research. 2014;186:44–55. doi: 10.1016/j.jss.2013.07.037. [DOI] [PubMed] [Google Scholar]
- 21.Kung EO, Les AS, Figueroa CA, Medina F, Arcaute K, Wicker RB, McConnell MV, Taylor CA. In Vitro Validation of Finite Element Analysis of Blood Flow in Deformable Models. Annals of Biomedical Engineering. 2011;39:1947–1960. doi: 10.1007/s10439-011-0284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kung EO, Les AS, Medina F, Wicker RB, McConnell MV, Taylor CA. In Vitro Validation of Finite-Element Model of AAA Hemodynamics Incorporating Realistic Outlet Boundary Conditions. Journal of Biomechanical Engineering-Transactions of the Asme. 2011;133 doi: 10.1115/1.4003526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiulli JA, Conover TA, Corsini C, Schievano S, Hsia TY, Figliola RS. Design of an experimental mock circulatory system for the Fontan circulation; ASME Summer Bioengineering Conference; 2010. [Google Scholar]
- 24.Moghadam ME, Vignon-Clementel IE, Figliola R, Marsden AL Modeling Congenital Hearts A. A modular numerical method for implicit 0D/3D coupling in cardiovascular finite element simulations. Journal of Computational Physics. 2013;244:63–79. [Google Scholar]
- 25.Moghadam ME, Bazilevs Y, Hsia T-Y, Vignon-Clementel IE, Marsden AL Modeling Congenital Hearts A. A comparison of outlet boundary treatments for prevention of backflow divergence with relevance to blood flow simulations. Computational Mechanics. 2011;48:277–291. [Google Scholar]
- 26.Esmaily-Moghadam M, Bazilevs Y, Marsden AL. A new preconditioning technique for implicitly coupled multidomain simulations with applications to hemodynamics. Computational Mechanics. 2013;52:1141–1152. [Google Scholar]
- 27.Esmaily-Moghadam M, Marsden AL. Multiscale Modeling of Cardiovascular Flows for Clinical Decision Support. Applied Mechanics Reviews. 2015;67:030804. [Google Scholar]
- 28.Sankaran S, Marsden A. The impact of uncertainty on shape optimization of idealized bypass graft models in unsteady flow. Physics of Fluids. 2010;22 [Google Scholar]
- 29.Sankaran S, Marsden A. A Stochastic Collocation Method for Uncertainty Quantification and Propagation in Cardiovascular Simulations. Journal of Biomechanical Engineering-Transactions of the Asme. 2011;133 doi: 10.1115/1.4003259. [DOI] [PubMed] [Google Scholar]
- 30.Pant S, Fabreges B, Gerbeau JF, Vignon-Clementel IE. A Multiscale Filtering-Based Parameter Estimation Method for Patient-Specific Coarctation Simulations in Rest and Exercise; Statistical Atlases and Computational Models of the Heart. Imaging and Modelling Challenges. 4th International Workshop, STACOM 2013, Held in Conjunction with MICCAI 2013. Revised Selected Papers: LNCS 8330; 2014. pp. 102–109. [Google Scholar]
- 31.Schiavazzi DE, Baretta A, Marsden AL, Hsia TY, Pennati G. Parameter estimation in single-ventricle lumped circulation models under uncertainty. PART 2: application to Norwood patients. IEEE Transactions on Biomedical Engineering. 2015 doi: 10.1002/cnm.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schiavazzi DE, Baretta A, Pennati G, Hsia TY, Marsden AL. Parameter estimation in single-ventricle lumped circulation models under uncertainty. PART I: methodology and application to synthetic patient data. IEEE Transactions on Biomedical Engineering. 2015 doi: 10.1002/cnm.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moireau P, Bertoglio C, Xiao N, Figueroa CA, Taylor CA, Chapelle D, Gerbeau JF. Sequential identification of boundary support parameters in a fluid-structure vascular model using patient image data. Biomechanics and Modeling in Mechanobiology. 2013;12:475–496. doi: 10.1007/s10237-012-0418-3. [DOI] [PubMed] [Google Scholar]
- 34.Schiavazzi DE, Arbia G, Baker C, Hlavacek AM, Hsia TY, Marsden AL, Vignon-Clementel IE Investigators MoCHAM. Uncertainty quantification in pediatric virtual surgery hemodynamics predictions. International Journal of Numerical Methods in Biomedical Engineering. 2015 doi: 10.1002/cnm.2737. [DOI] [PubMed] [Google Scholar]
- 35.Ramachandra AB, Sankaran S, Humphrey JD, Marsden AL. Computational Simulation of the Adaptive Capacity of Vein Grafts in Response to Increased Pressure. Journal of Biomechanical Engineering-Transactions of the Asme. 2015;137 doi: 10.1115/1.4029021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Figueroa CA, Baek S, Taylor CA, Humphrey JD. A computational framework for fluid-solid-growth modeling in cardiovascular simulations. Computer Methods in Applied Mechanics and Engineering. 2009;198:3583–3602. doi: 10.1016/j.cma.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sankaran S, Humphrey JD, Marsden AL. An efficient framework for optimization and parameter sensitivity analysis in arterial growth and remodeling computations. Computer Methods in Applied Mechanics and Engineering. 2013;256:200–210. doi: 10.1016/j.cma.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Naito Y, Imai Y, Shin'oka T, Kashiwagi J, Aoki M, Watanabe M, Matsumura G, Kosaka Y, Konuma T, Hibino N, et al. Successful clinical application of tissue-engineered graft for extracardiac Fontan operation. Journal of Thoracic and Cardiovascular Surgery. 2003;125:419–420. doi: 10.1067/mtc.2003.134. [DOI] [PubMed] [Google Scholar]
- 39.Patterson JT, Gilliland T, Maxfield MW, Church S, Naito Y, Shinoka T, Breuer CK. Tissue-engineered vascular grafts for use in the treatment of congenital heart disease: from the bench to the clinic and back again. Regenerative Medicine. 2012;7:409–419. doi: 10.2217/rme.12.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu ZL, Chen N, Shadden SC, Marsden JE, Kamocka MM, Rosen ED, Alber M. Study of blood flow impact on growth of thrombi using a multiscale model. Soft Matter. 2009;5:769–779. [Google Scholar]
- 41.Leiderman K, Fogelson AL. Grow with the flow: a spatial-temporal model of platelet deposition and blood coagulation under flow. Mathematical Medicine and Biology-a Journal of the Ima. 2011;28:47–84. doi: 10.1093/imammb/dqq005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marsden AL. Simulation based planning of surgical interventions in pediatric cardiology. Physics of Fluids. 2013;25 doi: 10.1063/1.4825031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Biglino G, Giardini A, Hsia T-Y, Figliola R, Taylor AM, Schievano S, Group MC. Modeling single ventricle physiology: review of engineering tools to study first stage palliation of hypoplastic left heart syndrome. Frontiers in pediatrics. 2013;1:31–31. doi: 10.3389/fped.2013.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Zélicourt DA, Marsden A, Fogel MA, Yoganathan A. Imaging and patient-specific simulations for the Fontan surgery: Current methodologies and clinical applications. Progress in Pediatric Cardiology. 2010;30:31–44. doi: 10.1016/j.ppedcard.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feinstein JA, Benson DW, Dubin AM, Cohen MS, Maxey DM, Mahle WT, Pahl E, Villafane J, Bhatt AB, Peng LF, et al. Hypoplastic Left Heart Syndrome Current Considerations and Expectations. Journal of the American College of Cardiology. 2012;59:S1–S42. doi: 10.1016/j.jacc.2011.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goldberg DJ, Shaddy RE, Ravishankar C, Rychik J. The failing Fontan: etiology, diagnosis and management. Expert review of cardiovascular therapy. 2011;9:785–793. doi: 10.1586/erc.11.75. [DOI] [PubMed] [Google Scholar]
- 47.Hsia T-Y, Cosentino D, Corsini C, Pennati G, Dubini G, Migliavacca F Modeling Congenital H. Use of Mathematical Modeling to Compare and Predict Hemodynamic Effects Between Hybrid and Surgical Norwood Palliations for Hypoplastic Left Heart Syndrome. Circulation. 2011;124:S204–S210. doi: 10.1161/CIRCULATIONAHA.110.010769. [DOI] [PubMed] [Google Scholar]
- 48.Esmaily-Moghadam M, Hsia T-Y, Marsden AL, Investigators M. The assisted bidirectional Glenn: A novel surgical approach for first-stage single-ventricle heart palliation. Journal of Thoracic and Cardiovascular Surgery. 2015;149:699–705. doi: 10.1016/j.jtcvs.2014.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou J, Esmaily-Moghadam M, Conover T, Hsia T-Y, Marsden AL, Figliola R. In vitro experimental assessment of the assisted bidirectional Glenn procedure for stage one single ventricle repair. doi: 10.1007/s13239-015-0232-z. submitted. [DOI] [PubMed] [Google Scholar]
- 50.Schiavazzi DE, Kung EO, Marsden AL, Baker C, Pennati G, Hsia T-Y, Hlavacek A, Dorfman AL Modeling Congenital Hearts A. Hemodynamic effects of left pulmonary artery stenosis after superior cavopulmonary connection: A patient-specific multiscale modeling study. Journal of Thoracic and Cardiovascular Surgery. 2015;149:689-+. doi: 10.1016/j.jtcvs.2014.12.040. [DOI] [PubMed] [Google Scholar]
- 51.DeCampli WM. If only Poiseuille had had a computer. Journal of Thoracic and Cardiovascular Surgery. 2015;149:697–698. doi: 10.1016/j.jtcvs.2014.09.024. [DOI] [PubMed] [Google Scholar]
- 52.Kung E, Baretta A, Baker C, Arbia G, Biglino G, Corsini C, Schievano S, Vignon-Clementel IE, Dubini G, Pennati G, et al. Predictive modeling of the virtual Hemi-Fontan operation for second stage single ventricle palliation: Two patient-specific cases. Journal of biomechanics. 2013;46:423–429. doi: 10.1016/j.jbiomech.2012.10.023. [DOI] [PubMed] [Google Scholar]
- 53.Marsden AL, Bernstein AJ, Reddy VM, Shadden SC, Spilker RL, Chan FP, Taylor CA, Feinstein JA. Evaluation of a novel Y-shaped extracardiac Fontan baffle using computational fluid dynamics. Journal of Thoracic and Cardiovascular Surgery. 2009;137 doi: 10.1016/j.jtcvs.2008.06.043. 394-U187. [DOI] [PubMed] [Google Scholar]
- 54.Soerensen DD, Pekkan K, de Zelicourt D, Sharma S, Kanter K, Fogel M, Yoganathan AP. Introduction of a new optimized total cavopulmonary connection. Annals of Thoracic Surgery. 2007;83:2182–2190. doi: 10.1016/j.athoracsur.2006.12.079. [DOI] [PubMed] [Google Scholar]
- 55.Martin MH, Feinstein JA, Chan FP, Marsden AL, Yang W, Reddy VM. Technical feasibility and intermediate outcomes of using a handcrafted, area-preserving, bifurcated Y-graft modification of the Fontan procedure. Journal of Thoracic and Cardiovascular Surgery. 2015;149:U239–U381. doi: 10.1016/j.jtcvs.2014.08.058. [DOI] [PubMed] [Google Scholar]
- 56.Kanter KR, Haggerty CM, Restrepo M, de Zelicourt DA, Rossignac J, Parks WJ, Yoganathan AP. Preliminary clinical experience with a bifurcated Y-graft Fontan procedure-A feasibility study. Journal of Thoracic and Cardiovascular Surgery. 2012;144:383–389. doi: 10.1016/j.jtcvs.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang W, Chan FP, Reddy VM, Marsden AL, Feinstein JA. Flow simulations and validation for the first cohort of patients undergoing the Y-graft Fontan procedure. Journal of Thoracic and Cardiovascular Surgery. 2015;149:247–255. doi: 10.1016/j.jtcvs.2014.08.069. [DOI] [PubMed] [Google Scholar]
- 58.Haggerty CM, Kanter KR, Restrepo M, de Zelicourt DA, Parks WJ, Rossignac J, Fogel MA, Yoganathan AP. Simulating hemodynamics of the Fontan Y-graft based on patient-specific in vivo connections. Journal of Thoracic and Cardiovascular Surgery. 2013;145:663–670. doi: 10.1016/j.jtcvs.2012.03.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baretta A, Corsini C, Yang W, Vignon-Clementel IE, Marsden AL, Feinstein JA, Hsia TY, Dubini G, Migliavacca F, Pennati G, et al. Virtual surgeries in patients with congenital heart disease: a multi-scale modelling test case. Philosophical Transactions of the Royal Society a-Mathematical Physical and Engineering Sciences. 2011;369:4316–4330. doi: 10.1098/rsta.2011.0130. [DOI] [PubMed] [Google Scholar]
- 60.Khiabani RH, Whitehead KK, Han D, Restrepo M, Tang E, Bethel J, Paridon SM, Fogel MA, Yoganathan AP. Exercise capacity in single-ventricle patients after Fontan correlates with haemodynamic energy loss in TCPC. Heart. 2015;101:139–143. doi: 10.1136/heartjnl-2014-306337. [DOI] [PubMed] [Google Scholar]
- 61.Haggerty CM, Whitehead KK, Bethel J, Fogel MA, Yoganathan AP. Relationship of Single Ventricle Filling and Preload to Total Cavopulmonary Connection Hemodynamics. Annals of Thoracic Surgery. 2015;99:911–917. doi: 10.1016/j.athoracsur.2014.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kung E, Marsden A, Baker C, Giardini A, Figliola R, Hsia TY. Does TCPC power loss really affect exercise capacity? Heart. 2015;101 doi: 10.1136/heartjnl-2014-307379. [DOI] [PubMed] [Google Scholar]
- 63.Delorme Y, Anupindi K, Kerlo AE, Shetty D, Rodefeld M, Chen J, Frankel S. Large eddy simulation of powered Fontan hemodynamics. Journal of Biomechanics. 2013;46:408–422. doi: 10.1016/j.jbiomech.2012.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kerlo A-EM, Delorme YT, Xu D, Frankel SH, Giridharan GA, Rodefeld MD, Chen J. Experimental characterization of powered Fontan hemodynamics in an idealized total cavopulmonary connection model. Experiments in Fluids. 2013;54 [Google Scholar]
- 65.Giridharan GA, Koenig SC, Kennington J, Sobieski MA, Chen J, Frankel SH, Rodefeld MD. Performance evaluation of a pediatric viscous impeller pump for Fontan cavopulmonary assist. Journal of Thoracic and Cardiovascular Surgery. 2013;145:249–257. doi: 10.1016/j.jtcvs.2012.01.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Giridharan GA, Ising M, Sobieski MA, Koenig SC, Chen J, Frankel S, Rodefeld MD. Cavopulmonary Assist for the Failing Fontan Circulation: Impact of Ventricular Function on Mechanical Support Strategy. Asaio Journal. 2014;60:707–715. doi: 10.1097/MAT.0000000000000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vukicevic M, Conover T, Jaeggli M, Zhou J, Pennati G, Hsia TY, Figliola RS. Control of Respiration-Driven Retrograde Flow in the Subdiaphragmatic Venous Return of the Fontan Circulation. Asaio Journal. 2014;60:391–399. doi: 10.1097/MAT.0000000000000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Das A, Banerjee RK, Gottliebson WM. Right Ventricular Inefficiency in Repaired Tetralogy of Fallot: Proof of Concept for Energy Calculations From Cardiac MRI Data. Annals of Biomedical Engineering. 2010;38:3674–3687. doi: 10.1007/s10439-010-0107-2. [DOI] [PubMed] [Google Scholar]
- 69.Das A, Gottliebson WM, Karve M, Banerjee R. Comparison of Hemodynamic Endpoints between Normal Subject and Tetralogy Patient Using Womersley Velocity Profile and MR Based Flow Measurements. Molecular & Cellular Biomechanics. 2011;8:21–42. [PubMed] [Google Scholar]
- 70.Fogel MA, Sundareswaran KS, de Zelicourt D, Dasi LP, Pawlowski T, Rome J, Yoganathan AP. Power loss and right ventricular efficiency in patients after tetralogy of Fallot repair with pulmonary insufficiency: Clinical implications. Journal of Thoracic and Cardiovascular Surgery. 2012;143:1279–1285. doi: 10.1016/j.jtcvs.2011.10.066. [DOI] [PubMed] [Google Scholar]
- 71.Leonardi B, Taylor AM, Mansi T, Voigt I, Sermesant M, Pennec X, Ayache N, Boudjemline Y, Pongiglione G. Computational modelling of the right ventricle in repaired tetralogy of Fallot: can it provide insight into patient treatment? European Heart Journal-Cardiovascular Imaging. 2013;14:381–386. doi: 10.1093/ehjci/jes239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rao AS, Menon PG. Presurgical planning using image-based in silico anatomical and functional characterization of Tetralogy of Fallot with associated anomalies. Interactive Cardiovascular and Thoracic Surgery. 2015;20:149–156. doi: 10.1093/icvts/ivu368. [DOI] [PubMed] [Google Scholar]
- 73.Yang C, Tang D, Geva T, Rathod R, Yamauchi H, Gooty V, Tang A, Gaudette G, Billiar KL, Kural MH, et al. Using contracting band to improve right ventricle ejection fraction for patients with repaired tetralogy of Fallot: A modeling study using patient-specific CMR-based 2-layer anisotropic models of human right and left ventricles. Journal of Thoracic and Cardiovascular Surgery. 2013;145 doi: 10.1016/j.jtcvs.2012.03.009. 285-+ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kheyfets VOR, Smith L, Schroeder T, Mueller T, Murali J, Lasorda S, Zikos D, Spotti A, Reilly J, Finol JJ, A E. Patient-specific computational modeling of blood flow in the pulmonary arterial circulation. Computer Methods and Programs in Biomedicine. 2015 doi: 10.1016/j.cmpb.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tang BT, Pickard SS, Chan FP, Tsao PS, Taylor CA, Feinstein JA. Wall shear stress is decreased in the pulmonary arteries of patients with pulmonary arterial hypertension: An image-based, computational fluid dynamics study. Pulmonary circulation. 2012;2:470–476. doi: 10.4103/2045-8932.105035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sengupta D, Kahn AM, Burns JC, Sankaran S, Shadden SC, Marsden AL. Image-based modeling of hemodynamics in coronary artery aneurysms caused by Kawasaki disease. Biomechanics and Modeling in Mechanobiology. 2012;11:915–932. doi: 10.1007/s10237-011-0361-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sengupta D, Kahn AM, Kung E, Moghadam ME, Shirinsky O, Lyskina GA, Burns JC, Marsden AL. Thrombotic risk stratification using computational modeling in patients with coronary artery aneurysms following Kawasaki disease. Biomechanics and Modeling in Mechanobiology. 2014;13:1261–1276. doi: 10.1007/s10237-014-0570-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rodo X, Curcoll R, Robinson M, Ballester J, Burns JC, Cayan DR, Lipkin WI, Williams BL, Couto-Rodriguez M, Nakamura Y, et al. Tropospheric winds from northeastern China carry the etiologic agent of Kawasaki disease from its source to Japan. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:7952–7957. doi: 10.1073/pnas.1400380111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Menon A, Eddinger TJ, Wang HF, Wendell DC, Toth JM, LaDisa JF. Altered hemodynamics, endothelial function, and protein expression occur with aortic coarctation and persist after repair. American Journal of Physiology-Heart and Circulatory Physiology. 2012;303:H1304–H1318. doi: 10.1152/ajpheart.00420.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Coogan JS, Chan FP, LaDisa JF, Taylor CA, Hanley FL, Feinstein JA. Computational fluid dynamic simulations for determination of ventricular workload in aortic arch obstructions. Journal of Thoracic and Cardiovascular Surgery. 2013;145:U489–U535. doi: 10.1016/j.jtcvs.2012.03.051. [DOI] [PubMed] [Google Scholar]
- 81.Goubergrits L, Riesenkampff E, Yevtushenko P, Schaller J, Kertzscher U, Hennemuth A, Berger F, Schubert S, Kuehne T. MRI-Based Computational Fluid Dynamics for Diagnosis and Treatment Prediction: Clinical Validation Study in Patients With Coarctation of Aorta. Journal of Magnetic Resonance Imaging. 2015;41:909–916. doi: 10.1002/jmri.24639. [DOI] [PubMed] [Google Scholar]
- 82.Szopos M, Poussineau N, Maday Y, Canniffe C, Celermajer DS, Bonnet D, Ou P. Computational modeling of blood flow in the aorta-insights into eccentric dilatation of the ascending aorta after surgery for coarctation. Journal of Thoracic and Cardiovascular Surgery. 2014;148:1572–1582. doi: 10.1016/j.jtcvs.2013.11.055. [DOI] [PubMed] [Google Scholar]
- 83.Szeto K, Pastuszko P, del Alamo JC, Lasheras J, Nigam V. Bicuspid aortic valves experience increased strain as compared to tricuspid aortic valves. World journal for pediatric & congenital heart surgery. 2013;4:362–366. doi: 10.1177/2150135113501901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Capelli C, Taylor AM, Migliavacca F, Bonhoeffer P, Schievano S. Patient-specific reconstructed anatomies and computer simulations are fundamental for selecting medical device treatment: application to a new percutaneous pulmonary valve. Philosophical Transactions of the Royal Society a-Mathematical Physical and Engineering Sciences. 2010;368:3027–3038. doi: 10.1098/rsta.2010.0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kheradvar A, Groves EM, Dasi LP, Alavi SH, Tranquillo R, Grande-Allen KJ, Simmons CA, Griffith B, Falahatpisheh A, Goergen CJ, et al. Emerging Trends in Heart Valve Engineering: Part I. Solutions for Future. Annals of Biomedical Engineering. 2015;43:833–843. doi: 10.1007/s10439-014-1209-z. [DOI] [PubMed] [Google Scholar]
- 86.Kheradvar A, Groves EM, Goergen CJ, Alavi SH, Tranquillo R, Simmons CA, Dasi LP, Grande-Allen KJ, Mofrad MRK, Falahatpisheh A, et al. Emerging Trends in Heart Valve Engineering: Part II. Novel and Standard Technologies for Aortic Valve Replacement. Annals of Biomedical Engineering. 2015;43:844–857. doi: 10.1007/s10439-014-1191-5. [DOI] [PubMed] [Google Scholar]
- 87.Kheradvar A, Groves EM, Simmons CA, Griffith B, Alavi SH, Tranquillo R, Dasi LP, Falahatpisheh A, Grande-Allen KJ, Goergen CJ, et al. Emerging Trends in Heart Valve Engineering: Part III. Novel Technologies for Mitral Valve Repair and Replacement. Annals of Biomedical Engineering. 2015;43:858–870. doi: 10.1007/s10439-014-1129-y. [DOI] [PubMed] [Google Scholar]
- 88.Long CC, Marsden AL, Bazilevs Y. Fluid-structure interaction simulation of pulsatile ventricular assist devices. Computational Mechanics. 2013;52:971–981. [Google Scholar]
- 89.Long CC, Esmaily-Moghadam M, Marsden AL, Bazilevs Y. Computation of residence time in the simulation of pulsatile ventricular assist devices. Computational Mechanics. 2014;54:911–919. [Google Scholar]
- 90.Long CC, Marsden AL, Bazilevs Y. Shape optimization of pulsatile ventricular assist devices using FSI to minimize thrombotic risk. Computational Mechanics. 2014;54:921–932. [Google Scholar]
- 91.Marsden AL, Bazilevs Y, Long CC, Behr M. Recent advances in computational methodology for simulation of mechanical circulatory assist devices. Wiley Interdisciplinary Reviews-Systems Biology and Medicine. 2014;6:169–188. doi: 10.1002/wsbm.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ryan JR, Moe TG, Richardson R, Frakes DH, Nigro JJ, Pophal S. A Novel Approach to Neonatal Management of Tetralogy of Fallot, With Pulmonary Atresia, and Multiple Aortopulmonary Collaterals. Jacc-Cardiovascular Imaging. 2015;8:103–104. doi: 10.1016/j.jcmg.2014.04.030. [DOI] [PubMed] [Google Scholar]
- 93.Ejaz F, Ryan J, Henriksen M, Stomski L, Feith M, Osborn M, Pophal S, Richardson R, Frakes D. Color-coded patient-specific physical models of congenital heart disease. Rapid Prototyping Journal. 2014;20:336–343. [Google Scholar]
- 94.Park SS, Sanders DB, Smith BP, Ryan J, Plasencia J, Osborn MB, Wellnitz CM, Southard RN, Pierce CN, Arabia FA, et al. Total artificial heart in the pediatric patient with biventricular heart failure. Perfusion-Uk. 2014;29:82–88. doi: 10.1177/0267659113496580. [DOI] [PubMed] [Google Scholar]
- 95.Ryan JR. Biomedical Engineering. Arizona State University; 2015. Three-Dimensional Printing and Computational Visualization for Surgical Planning and Medical Education. [Google Scholar]
- 96.Biglino G, Capelli C, Wray J, Schievano S, Leaver L-K, Khambadkone S, Giardini A, Derrick G, Jones A, Taylor AM. 3D-manufactured patient-specific models of congenital heart defects for communication in clinical practice: feasibility and acceptability. BMJ open. 2015;5:e007165–e007165. doi: 10.1136/bmjopen-2014-007165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lee J, Moghadam ME, Kung E, Cao H, Beebe T, Miller Y, Roman BL, Lien CL, Chi NC, Marsden AL, et al. Moving Domain Computational Fluid Dynamics to Interface with an Embryonic Model of Cardiac Morphogenesis. Plos One. 2013;8 doi: 10.1371/journal.pone.0072924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lindsey SE, Menon PG, Kowalski WJ, Shekhar A, Yalcin HC, Nishimura N, Schaffer CB, Butcher JT, Pekkan K. Growth and hemodynamics after early embryonic aortic arch occlusion. Biomechanics and Modeling in Mechanobiolgy. 2014 doi: 10.1007/s10237-014-0633-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


