Abstract
Background
Research grounded in behavioral economics has previously linked addictive behavior to disrupted decision-making and reward-processing, but these principles have not been examined in prescription opioid addiction, which is currently a major public health problem. This study examined whether pre-treatment drug reinforcement value predicted opioid use during outpatient treatment of prescription opioid addiction.
Methods
Secondary analyses examined participants with prescription opioid dependence who received 12 weeks of buprenorphine-naloxone and counseling in a multi-site clinical trial (N = 353). Baseline measures assessed opioid source and indices of drug reinforcement value, including the total amount and proportion of income spent on drugs. Weekly urine drug screens measured opioid use.
Results
Obtaining opioids from doctors was associated with lower pre-treatment drug spending, while obtaining opioids from dealers/patients was associated with greater spending. Controlling for demographics, opioid use history, and opioid source frequency, patients who spent a greater total amount (OR = 1.30, p < .001) and a greater proportion of their income on drugs (OR = 1.31, p < .001) were more likely to use opioids during treatment.
Conclusions
Individual differences in drug reinforcement value, as indicated by pre-treatment allocation of economic resources to drugs, reflects propensity for continued opioid use during treatment among individuals with prescription opioid addiction. Future studies should examine disrupted decision-making and reward-processing in prescription opioid users more directly and test whether reinforcer pathology can be remediated in this population.
Keywords: Prescription opioids, Behavioral economics, Buprenorphine-naloxone, Treatment outcome
1. Introduction
Prescription opioid addiction has become a significant public health problem and economic burden in the United States and in other developed nations (Birnbaum et al., 2011; Ling et al., 2011). Prescription opioids are currently the second-most commonly abused drug in the United States (SAMHSA, 2013b). Furthermore, among all drugs of abuse prescription opioid-related overdoses are currently the most common and had the greatest proportional increase in the last 15 years (Calcaterra et al., 2013; Jones et al., 2013). Rates of prescription opioid abuse and admissions for prescription opioid addiction treatment have also increased rapidly during this time period (SAMHSA, 2013a; Atluri et al., 2014; Compton and Volkow, 2006). These disturbing trends have prompted increased federal attention on prescription opioid addiction, including research aimed at developing effective treatments and understanding treatment response (Compton and Volkow, 2006; Manchikanti, 2006).
Among various theoretical models of addictive behavior, behavioral economics has rapidly developed as a conceptual framework for explaining maladaptive substance use. As a blend of behavior analysis and principles of economics, behavioral economics examines decision-making processes that govern the allocation of limited resources (e.g., time, money, effort) to competing goals under various constraints (Hursh, 1993). Individuals with addiction typically have dysfunction in these processes. Despite increased psychosocial and financial costs, these individuals continually expend greater amounts of resources to obtain and use drugs (Bickel et al., 2014a, 2010). Perhaps the most well-known marker of such dysfunction in addiction is delay discounting, as individuals with drug dependence typically exhibit irrational preferences for immediate vs. delayed rewards (Bickel et al., 2014b; MacKillop et al., 2011). Individuals with drug abuse or dependence also exhibit elevated drug demand, characterized by greater valuation of substances that persists despite the presence of situational factors that typically reduce consumption (Hursh et al., 2005). These include increased unit price or the presence of other reinforcers (Murphy and MacKillop, 2006; Murphy et al., 2009). Recent linkage to neural and genetic biomarkers has also demonstrated the potential importance of excessive delay discounting and elevated drug demand as phenotypic markers of addictive behavior (MacKillop, 2013; Mackillop et al., 2014).
In addition to these phenotypes of decision-making established primarily in laboratory research settings, behavioral economic theory has guided the development of ecologically-valid indices of drug reinforcement value in the natural environment. These metrics infer drug reinforcement value by quantifying the amount of actual resources, such as time or money, that one directs towards obtaining and using a substance. For example, a discretionary spending index that compares income allocated to alcohol vs. savings has predicted future alcohol relapse in abstinent drinkers (Tucker et al., 2009, 2006, 2002). Naturalistic cocaine purchase time has also predicted self-administration of cocaine in the laboratory (Greenwald and Steinmiller, 2014). Similar to delay discounting and demand, drug-seeking in the natural environment has been validated as a distinct phenotype with genetic underpinnings (Greenwald et al., 2013). These naturalistic metrics are promising potential markers of drug reinforcement value and poor treatment response in individuals with prescription opioid addiction, but have not been previously examined in this population.
In a previous multi-site treatment study for prescription opioid addiction, participants received enhanced or standard medical management and 12 weeks of open-label buprenorphine-naloxone (BUP–NLX). Endpoint abstinence was achieved by 49% of the sample, with no difference between psychosocial treatment conditions (Weiss et al., 2011). Older age, lifetime major depression, no history of non-oral use of opioids, and no previous opioid treatment predicted greater odds of endpoint abstinence (Dreifuss et al., 2013). Although markers of opioid use history and baseline dependence severity have predicted treatment outcome (Dreifuss et al., 2013; Hillhouse et al., 2013; Soyka et al., 2008), patients with similar levels of severity in opioid dependence or opioid use may be allocating vastly different levels of resources to obtain and use drugs. These individual differences in drug reinforcement value may be uniquely and incrementally predictive of treatment response. Furthermore, as opposed to relatively immutable demographic and historical factors, behavioral economic processes of decision-making can be altered through interventions (Koffarnus et al., 2013; Murphy et al., 2013, 2012). An examination of drug reinforcement value in prescription opioid users may therefore provide insight into malleable processes that could be used to bolster treatment effects, perhaps with interventions that increase the salience of substance-free rewards (Murphy et al., 2012).
This study is an initial investigation of behavioral economic predictors of opioid use during prescription opioid addiction treatment. We examined the total amount and proportion of income allocated to drugs prior to treatment in the aforementioned multisite clinical trial (Weiss et al., 2011). We hypothesized that individuals who spent greater total amounts and a greater proportion of their income on drugs prior to treatment would be more likely to continue using opioids during treatment. Considering that prescription opioid users obtain opioids from a variety of sources and the purchase price from illicit sources tends to be higher (Cicero et al., 2008; Mars et al., 2014), we expected that individuals who frequently obtained opioids from illicit sources would spend greater amounts of money on drugs. We also considered that this effect could confound the expected relationship between drug spending and within-treatment opioid use, because frequently obtaining opioids from illicit sources might correspond to both greater spending and greater severity of dependence. Therefore we controlled for prescription opioid source variables and measures of opioid dependence severity in our analyses of opioid use outcomes, to examine whether the hypothesized association between baseline drug reinforcement value and opioid use during treatment was independent of these potential confounds.
2. Methods
2.1. Study design
This study involved secondary analyses of Phase 2 of the Prescription Opioid Addiction Treatment Study (POATS), a multi-site, adaptive, randomized clinical trial of psychosocial treatment with adjunctive open-label BUP–NLX for prescription opioid addiction (Weiss et al., 2011, 2010). All POATS participants were initially randomized to standard or enhanced medical management and entered a 4-week BUP–NLX detoxification (Phase 1). Those who failed to sustain abstinence during the detoxification and an 8-week follow-up (93% of the full sample) were eligible to enter Phase 2. In Phase 2, hereafter referred to as the “treatment phase”, participants were re-randomized to psychosocial treatment condition, received 12 weeks of BUP–NLX maintenance, and attended the clinic weekly for physician appointments, urine drug screens, and completion of other study measures. The primary POATS report revealed no significant main effects of enhanced psychosocial treatment on achievement of endpoint abstinence in Phase 1 or Phase 2 (Weiss et al., 2011).
2.2. Study sample
All POATS participants were at least 18 years old, met DSM-IV criteria for current prescription opioid dependence, were physiologically dependent on opioids, were cleared by their prescribing physician if receiving prescription opioids for pain, agreed to birth control if female, and had no unstable medical or psychiatric conditions. Key exclusion criteria included use of heroin on ≥ 4 days in the past month, any lifetime injection of heroin, or physiological dependence on alcohol, sedatives, or stimulants. The full inclusion/exclusion criteria can be found in previous reports (Weiss et al., 2011, 2010). The current study included all Phase 2 participants with full data on baseline covariates and predictors (n = 353), with seven participants from the original Phase 2 sample excluded due to missing baseline information. General clinical and demographic characteristics of the sample are displayed in Table 1. Analyses revealed no significant differences between this subsample and the full POATS Phase 2 sample.
Table 1.
Summary statistics of study variables for participants receiving treatment for rescription opioid dependence (N = 353).
| Variable | % (n) or M (SD) |
|---|---|
| Gender: % (n) male | 58% (204) |
| Race: % (n) white | 90% (319) |
| Years of education: M (SD) | 12.9 (2.1) |
| Marital status: % (n) currently married | 26% (93) |
| Major depression: % (n) with lifetime MDD | 34.3 (121) |
| Days of prescription opioid use in past 30 days: M (SD) | 27.9 (3.6) |
| Heroin history: % (n) ever used | 26% (91) |
| Prescription opioid route history: % (n) ever used non-orally | 85% (300) |
| Prescription opioid treatment history: % (n) ever received | 35% (123) |
| Number of prescription opioid dependence criteria: M (SD) | 6.4 (0.88) |
| Total money ($) spent on drugs in last month: M (SD) | 1,013.4 (1,243.7) |
| Proportion of income spent on drugs in last month: M (SD) | 141.6 (283.6) |
| Prescription opioid source in last 6 months: | |
| Doctor for legitimate pain/medical problem: % (n) with any | 59% (207) |
| Dealer or patient that sells their medication: % (n) with any | 93% (328) |
2.3. Study measures
2.3.1. Opioid Use
Opioid use during treatment was measured via urine drug screens (UDS) obtained at each weekly study visit, which tested for prescription analgesics, illicit opioids, and methadone. Weekly UDS results were coded as positive or negative for any opioids, which provided a weekly dichotomous and biologically-confirmed measure of opioid use. Participants provided a total of 4,006 UDS during treatment, with a mean of 10.4 (SD = 2.8) each, 29% of which were positive for opioids.
2.3.2. Pre-treatment drug spending
At baseline of Phase 1 the Addiction Severity Index (ASI)–Lite (Cacciola et al., 2007) captured variables related to total income and drug spending. A single item from the ASI-Lite drug section measured total amount of money spent on drugs in the last 30 days, hereafter referred to as “total drug spending”. We created a “proportion drug spending” variable by dividing total drug spending by total income, which was captured by summing responses on the income source items in the ASI-Lite Employment section. Total drug spending and proportion drug spending were used as separate predictors, as these variables were significantly correlated but not completely redundant (r = .24, R2 = .06). Both variables were extremely positively skewed (see Table 1) and were thus natural log-transformed prior to analyses.
2.3.3. Prescription opioid source
The Pain and Opiate Analgesic Use History (Weiss et al., 2010) was administered at Phase 1 baseline and captured participants' self-reported frequency of obtaining prescription opioids from various sources in the past six months, on a scale of 0 (“Never”) to 5 (“Always”). The two variables used in this study were frequency of obtaining opioids from doctors for legitimate medical treatment (M = 1.36, SD = 1.54) and frequency of obtaining opioids from a dealer or a patient selling their medication, by using the maximum frequency score across dealers or patients (M = 3.76, SD = 1.41).
2.3.4. Baseline demographics and clinical covariates
We included demographic and clinical covariates previously associated with opioid use in this sample (Dreifuss et al., 2013) or other opioid treatment studies. All variables were assessed at Phase 1 baseline, with descriptive statistics provided in Table 1. Demographics included gender, race, marital status, and years of education as assessed with the ASI-Lite. The baseline Pain and Opiate Analgesic Use History captured several markers of opioid dependence severity including previous heroin use, history of non-oral prescription opioid use, history of opioid dependence treatment, and pre-treatment prescription opioid use (days in the past month using prescription opioids). The Composite International Diagnostic Interview (Robins et al., 1988) assessed number of prescription opioid dependence criteria and lifetime major depressive disorder.
2.4. Statistical analyses
2.4.1. Preliminary analyses
Preliminary analyses examined descriptive statistics of the two drug spending variables and opioid source variables. Given the significant positive skew in both drug spending variables we conducted natural-log transformations to reduce the potential for extreme observations to disproportionately influence model estimation. We then examined Pearson correlations between log-transformed drug spending variables and prescription opioid source variables. Pearson and point-biserial correlations examined associations between drug-spending variables and markers of opioid dependence severity, to determine whether drug spending might provide unique information and predictive validity over these traditional baseline predictors.
2.4.2. Prediction of weekly opioid use during treatment
Repeated measures of weekly opioid use were dichotomous variables nested within individuals and were analyzed with multilevel logistic regression models, which included random intercepts for person and random slopes for linear time. All covariates and predictors were time-invariant and estimated with fixed effects, along with the fixed effects of linear and quadratic time. Resulting models estimated the probability of opioid use in a given week of the treatment phase as a function of time, covariates, and predictors, while also accounting for the person-level clustering of observations and individual differences in the rate of change in opioid use. All available opioid UDS measures were included via maximum-likelihood (ML) estimation, a preferred method when longitudinal data is considered missing-at-random (Schafer and Graham, 2002). Because individuals with any missing opioid use data were less likely to have major depression (40% vs. 27%, χ2 (1) = 6.72, p < .01), major depression was included as a covariate in all models. Preliminary models examined covariate effects, and significant covariates (p < .05) were retained for separate models that tested total drug spending or proportion drug spending as predictors of weekly opioid use. Final models incorporated prescription opioid source frequency variables as covariates. All analyses were conducted in Stata 13.0 (StataCorp., 2013).
3. Results
3.1. Descriptive statistics
Summary statistics for all baseline variables are provided in Table 1. At the mean level participants spent $1,013.40 (Mdn = $600) on drugs in the month prior to study entry, ranging from $0 to $10,000. The mean proportion drug spending was 141.63% and ranged from 0 to 2,000%, with the median (50%) indicating that half of the sample had spent at least 50% of their past-month income on drugs in the month prior to entering the trial. Natural-log transformation improved the skew of total drug spending (M = 6.11, Mdn = 6.40, SD = 1.76) and proportion drug spending (M = 3.87, Mdn = 3.93, SD = 1.58). The average score for legitimate doctor obtaining (M = 1.36) was between “Rarely” and “Sometimes”, while the average score for obtaining opioids from dealers or other patients (M = 3.76) was closer to “Most of the time”. Just over half of the sample (53%) reported obtaining prescription opioids from both of these sources at least once in the last six months.
3.2.Association between drug spending and opioid source
Frequency of obtaining opioids from doctors was significantly and negatively correlated with total drug spending (r = −.36) and proportion drug spending (r = −.25), while frequency of obtaining opioids from dealers/patient sellers was positively correlated with total (r = .53) and proportion drug spending (r = .45). These results indicated that patients who spent a greater amount and proportion of their income on drugs prior to treatment tended to obtain opioid medications from doctors less frequently and dealers/patients more frequently.
3.3. Associations between drug spending and opioid use/dependence severity
Pearson and point-biserial correlations between total drug spending, proportion drug spending, and markers of opioid use and dependence severity are displayed in Table 2. Both total drug spending and proportion drug spending were significantly and positively correlated with lifetime heroin use, previous non-oral use of prescription opioids, and number of prescription opioid dependence criteria. Proportion drug spending was also significantly correlated with previous opioid treatment. The shared variance between spending variables and severity did not exceed 7% for any measure. While larger spenders did tend to have more severe opioid dependence, the small amount of overlapping variance suggests the spending variables represent distinct and unique information from these markers of dependence severity.
Table 2.
Pearson and point biserial correlations between pre-treatment drug spending variables and markers of prescription opioid use and dependence severity.
| Total drug spending | Proportion drug spending | |||
|---|---|---|---|---|
|
|
|
|||
| r | R2 | r | R2 | |
| Number of days using prescription opioids | .10 | .01 | .02 | <.01 |
| Lifetime heroin usea | .14** | .02 | .20*** | .04 |
| Prior opioid treatmenta | .09 | .01 | .12* | .01 |
| Previous non-oral usea | .22** | .05 | .27*** | .07 |
| Number of opioid dependence criteria | .17** | .03 | .21*** | .04 |
Dichotomous variable.
p < .05.
p < .01.
p < .001.
3.4. Predicting opioid use during treatment
Weekly opioid UDS results were modeled as outcomes in multilevel logistic regression, with the initial covariate model including time (linear and quadratic), demographics, and clinical covariates (major depression and opioid dependence severity variables), with random intercepts (person) and slopes (time) accounting for individual heterogeneity in the initial level and change over time in opioid use. Linear and quadratic time, education, major depression, and several opioid dependence history variables predicted opioid use during treatment (see Table 3). The probability of opioid use decreased non-linearly from baseline to the end of treatment, with lower education, no major depression, greater pre-treatment opioid use, prior heroin use, and prior non-oral use of prescription opioids predicting greater probability of opioid use during treatment.
Table 3.
Results of multilevel logistic regression models predicting opioid use during treatment from time, covariates, drug spending variables, and source of prescription opioids.
| Predictors/covariates | Total drug spending | Proportion drug spending | ||
|---|---|---|---|---|
|
|
|
|||
| Model 1 OR (SE) | Model 2 OR (SE) | Model 1 OR (SE) | Model 2 OR (SE) | |
| Week | 0.52 (0.02)*** | 0.52 (0.02)*** | 0.52 (0.02)*** | 0.52 (0.02)*** |
| Week2 | 1.04 (0.004)*** | 1.04 (0.004)*** | 1.04 (0.004)*** | 1.04 (0.004)*** |
| Years of education | 1.07 (0.05) | 1.05 (0.05) | 1.10 (0.05)* | 1.09 (0.05)* |
| Major depression (lifetime) | 0.80 (0.16) | 0.80 (0.16) | 0.73 (0.15) | 0.73 (0.15) |
| Pre-treatment prescription opioid use | 1.03 (0.03) | 1.03 (0.03) | 1.04 (0.03) | 1.04 (0.03) |
| Heroin use | 2.00 (0.44)*** | 2.06 (0.45)*** | 1.88 (0.41)** | 1.92 (0.42)** |
| Non-oral use of prescription opioids | 0.66 (0.19) | 0.63 (0.18) | 0.68 (0.20) | 0.67 (0.20) |
| Drug spending (total or proportion) | 1.27 (0.08)*** | 1.30 (0.09)*** | 1.31 (0.08)*** | 1.31 (0.08)*** |
| Doctor obtaining frequency score | 0.94 (0.07) | 0.92 (0.06) | ||
| Dealer/patient obtaining score | 0.90 (0.08) | 0.94 (0.08) | ||
p < .05,
p < .01,
p < .001.
The two baseline drug spending variables were then incorporated into separate models as predictors, controlling for these significant covariates. As shown in Table 3, participants with greater total drug spending were significantly more likely to use opioids during the 12-week treatment phase (OR = 1.27, p < .001, 95% CI [1.13, 1.43]). As shown in Figure 1, the covariate-adjusted probability of opioid use during treatment increased as a function of log-transformed total drug spending. To assist in interpretation of this association, the average model-adjusted probabilities were plotted across quintile groups of the original total spending values (see Figure 1). This significant association persisted when controlling for the non-significant effects of doctor opioid source frequency (OR = 0.94, p = .39) and dealer/patient source frequency (OR =0.90, p = .26). Overall, these results indicated that participants with greater total pre-treatment spending on drugs were more likely to use opioids during treatment, regardless of their demographics, severity of opioid use and dependence, or source of prescription opioids.
Figure 1.
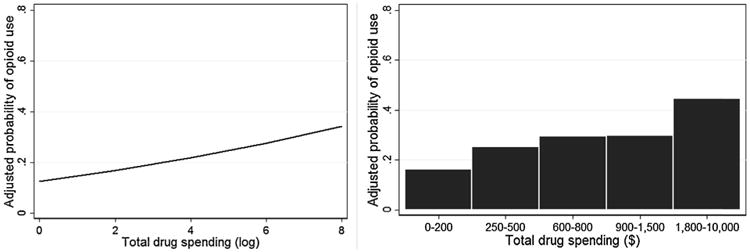
Association between total drug spending in the month prior to treatment and adjusted probability of opioid use during treatment.
A separate model examined log-transformed proportion drug spending as a predictor of opioid use, with similar findings. As shown in Figure 2, spending a greater proportion of one's past-month income on drugs predicted greater probability of opioid use during treatment (OR = 1.31, p < .001, 95% CI [1.15, 1.49]). A similar pattern was observed when viewing the average model-adjusted probability of opioid use over quintile groups of the original proportion drug spending values (Figure 2). Proportion drug spending continued to predict opioid use when controlling for doctor source frequency (OR = 0.94, p = .39, 95% CI [0.82, 1.08]) and dealer/patient source frequency, which did not predict opioid use outcomes. Overall, participants who spent a greater proportion of their pre-treatment income on drugs were more likely to use opioids during treatment, regardless of demographics, severity of opioid use or dependence, or source of prescription opioids prior to treatment.
Figure 2.
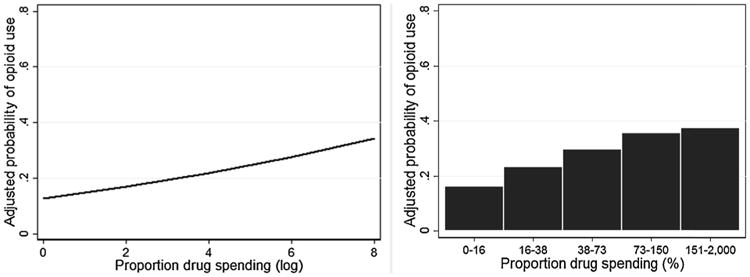
Association between proportion of past-month income spent on drugs in the month prior to treatment and adjusted probability of opioid use during treatment.
4. Discussion
In this initial investigation of behavioral economics in prescription opioid addiction, greater levels of pre-treatment drug reinforcement value predicted biologically-verified opioid use during treatment. Participants who spent a greater total amount or greater proportion of their income on drugs were more likely to use opioids during 12 weeks of open-label BUP–NLX and standard or enhanced counseling. These effects were independent of other clinical and demographic covariates that previously predicted endpoint abstinence in this sample, including previous opioid dependence treatment, use of opioids via non-oral routes, or frequency of opioid use immediately prior to treatment. As continued opioid use during BUP–NLX maintenance is one marker of poor treatment progress, our findings suggest excessive drug reinforcement value is a risk factor for poor treatment response in prescription opioid users.
Drug dependence is associated with excessive discounting of delayed rewards and elevated drug demand, which have been collectively referred to as “reinforcer pathology” (Bickel et al., 2014a). While primarily established in animal and human laboratory settings, extensions of these principles to naturalistic and clinical environments have found that excessive delay discounting predicts poor treatment response (MacKillop and Kahler, 2009; Washio et al., 2011). Furthermore, abstinent alcoholics who allocated greater levels of discretionary income to alcohol were more likely to relapse in the future (Tucker et al., 2009, 2006, 2002). Allocation of income to drugs is a putative marker of in-vivo drug reinforcement value, because any income allocated to drugs is not available for other immediate or delayed non-drug reinforcers, such as food, drug-free activities, or savings (Roddy and Greenwald, 2009; Tucker et al., 2002). Despite the uniformly high frequency of opioid use and high levels of severity in our sample, individuals displayed large variability in the amount and proportion of their income they allocated to obtaining drugs prior to treatment. These individual differences may represent deficits in decision-making that impede on the shift to non-drug forms of reinforcement during treatment of prescription opioid addiction.
While reinforcer pathology has been implicated in many other forms of addictive behavior (Greenwald and Hursh, 2006; Greenwald and Steinmiller, 2014), to our knowledge this study is the first extension of behavioral economic principles to prescription opioid addiction. In addition to validating a novel predictor of clinical outcomes in this population, the current study support the use of behavioral economic principles to investigate prescription opioid addiction in more rigorous experimental designs. Behavioral economic studies informed by basic science in decision-making have produced key advances in the understanding of addictive behavior, including the recognition of delay discounting and drug demand as putative intermediate phenotypes with neural and genetic underpinnings (MacKillop, 2013; Mackillop et al., 2014). More direct investigations of behavioral economics in prescription opioid users may be warranted, given the mounting evidence in support of reinforcer pathology as a trans-disease phenotype of addiction and other behavioral disorders (Bickel et al., 2014b).
Prescription opioid addiction is somewhat unique in the variety of potential sources for obtaining drugs, which include legitimate medical treatment and diversion through illicit channels (Cicero et al., 2008). We expected that frequent obtaining of illicit opioids would correspond to greater drug spending, because diverted prescription opioids have been described as more costly (Mars et al., 2014). Our findings supported this hypothesis, as greater total amount and proportion of income spent on drugs corresponded with higher frequency scores for obtaining from doctors and lower frequency scores for obtaining from dealers/patients. Patients who obtain opioids legitimately from doctors appear to have a reduced burden for opioid-seeking, possibly due to their legitimized access and reduced out-of-pocket cost through prescription reimbursement. We also considered that dependence severity and illicit source frequency could potentially confound the association between spending and treatment outcome. Greater spending could simply reflect more severe dependence and a shift to illegitimate sources of opioids. However, our findings suggest that regardless of opioid source frequency and several markers of opioid dependence severity previously associated with poor treatment outcome, excessive pre-treatment drug reinforcement value is a marker of poor treatment prognosis.
While an assumption of this study is that drug spending reflects a unique domain of drug reinforcement value, an alternative view is that greater spending predicted opioid use because it is a proxy for dependence severity. To the extent possible given the available data, our findings did not support this alternative view. Drug spending did correlate significantly with markers of opioid use and dependence severity, but the amount of overlapping variance was small. Furthermore, drug spending predicted opioid use during treatment when controlling for all other markers of opioid dependence severity. These findings replicate those of previous studies of alcohol where discretionary spending predicted future alcohol problems independently of initial alcohol dependence severity and drinking intensity (Tucker et al., 2006, 2002). In contrast to dependence severity, drug-seeking behaviors (including monetary investment) have been described as an intermediate phenotype, and this hypothesis was supported by findings that the BDNF Val66Met genotype predicted spending but not consumption in heroin users (Greenwald et al., 2013). Future studies of prescription opioid users should examine opioid use with greater specificity, such as with combined measures of frequency and quantity of use, and conduct direct assessments of delay discounting and opioid demand. This would allow a more explicit test of relationships between reinforcer pathology, dependence severity, and treatment outcome. In reality drug reinforcement value and dependence severity are likely mutually reinforcing processes, as the progression from infrequent use to habitual use and dependence is marked by excessive reliance on drugs for reinforcement and devaluation of non-drug rewards (Bickel et al., 2014a).
Our findings must be interpreted in light of several limitations. Most importantly, this study was a secondary analysis of a clinical trial for prescription opioid addiction, in which we examined hypotheses not incorporated into the original protocol. These findings, while novel, should be considered as preliminary and warranting of confirmation through a prospectively designed study. Limitations of the drug-related spending and income variables in this study should be noted, including the consideration that key variables were captured with the ASI-Lite, which is primarily intended as a multidimensional measure of addiction severity. Another caveat was our assumption that self-reported drug spending was primarily allocated to prescription opioids, when the observed item refers generally to “drugs” without specifying drug type. Our assumption was supported by low rates of other drug use in this sample, but using drug-specific measures as in previous naturalistic studies of other substances (Greenwald and Steinmiller, 2014; Tucker et al., 2002) would allow us to examine opioid-related spending with greater accuracy. While we controlled for several indicators of opioid dependence severity, other important markers, such as quantity of opioid use, could not be examined. Furthermore, this study only examined Phase 2 of POATS, thereby limiting the sample to patients that had failed a previous short-term BUP–NLX detoxification and were retained for the Phase 2 intervention. The sample was also predominantly Caucasian and relatively well-educated. While these demographics reflect emerging trends in opioid dependence in the United States (Cicero et al., 2014), further confirmation is necessary before extending these findings to more general populations.
In summary, patients with prescription opioid addiction who spent a larger total amount and proportion of their income on drugs prior to treatment were more likely to use opioids during 12 weeks of treatment, despite a robust intervention comprised of BUP–NLX, weekly physician appointments, and standard or enhanced medical management. The intensity of this intervention was apparently insufficient to sustain abstinence in patients with excessive levels of drug reinforcement value. In these patients it may be necessary to implement other forms of opioid maintenance or provide treatments that focus more explicitly on enhancing reinforcement from non-drug sources of reward to sustain reductions in opioid use.
Acknowledgments
The NIDA CTN publications committee has reviewed and approved this manuscript
Role of Funding Source. The analysis, interpretation, and preparation of this study was supported by National Institute on Drug Abuse (NIDA) grants 5T32 DA026400, 5R01 DA030577, 5R01 DA035054, and 3U10DA01304. The design, conduct, data collection, and management of the original clinical trial providing the data for this study was conducted in the NIDA Clinical Trials Network (CTN) and was supported by NIDA CTN grants 2U10DA015831, 2U10DA013045, 2U10DA015815, 2U10DA013727, 2U10DA020036, 2U10DA013035, 2U10DA013714, and 5U10DA013732. The sponsor of the original clinical, the NIDA Center for the CTN, collaborated in the design and conduct of the original trial but was not involved in the conceptualization of this study.
Footnotes
Contributors: Dr. Worley conceptualized the study, conducted the statistical analyses, and drafted the manuscript. Dr. Shoptaw and Dr. Bickel provided mentoring in study conceptualization and edited the manuscript. Dr. Ling edited the manuscript and was Co-Principal Investigator for the clinical trial that generated the data utilized in this manuscript.
Conflict of interest: Dr. Shoptaw has received clinical research supplies from Pfizer, Inc., and from Medicinova, Inc.
Dr. Bickel is affiliated with HealthSim, LLC. Dr. Ling has served as consultant to Reckitt Benckiser and Titan Pharmaceuticals, and has received unrestricted educational and research grants through UCLA from Reckitt Benckiser Pharmaceuticals.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Atluri S, Sudarshan G, Manchikanti L. Assessment of the trends in medical use and misuse of opioid analgesics from 2004 to 2011. Pain Phys. 2014;17:E119–E128. [PubMed] [Google Scholar]
- Bickel WK, Johnson MW, Koffarnus MN, Mackillop J, Murphy JG. The behavioral economics of substance use disorders: reinforcement pathologies and their repair. Annu Rev Clin Psychol. 2014a;10:641–677. doi: 10.1146/annurev-clinpsy-032813-153724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Koffarnus MN, Moody L, Wilson AG. The behavioral- and neuro-economic process of temporal discounting: A candidate behavioral marker of addiction. Neuropharmacology. 2014b;76:518–527. doi: 10.1016/j.neuropharm.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Yi R, Mueller ET, Jones BA, Christensen DR. The behavioral economics of drug dependence: towards the consilience of economics and behavioral neuroscience. Curr Top Behav Neurosci. 2010;3:319–341. doi: 10.1007/7854_2009_22. [DOI] [PubMed] [Google Scholar]
- Birnbaum HG, White AG, Schiller M, Waldman T, Cleveland JM, Roland CL. Societal costs of prescription opioid abuse, dependence, and misuse in the United States. Pain Med. 2011;12:657–667. doi: 10.1111/j.1526-4637.2011.01075.x. [DOI] [PubMed] [Google Scholar]
- Cacciola JS, Alterman AI, McLellan AI, Lin YT, Lynch KG. Initial evidence for the reliability and validity of a “Lite” version of the Addiction Severity Index. Drug Alcohol Depend. 2007;87:297–302. doi: 10.1016/j.drugalcdep.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Calcaterra S, Glanz J, Binswanger IA. National trends in pharmaceutical opioid related overdose deaths compared to other substance related overdose deaths: 1999-2009. Drug Alcohol Depend. 2013;131:263–270. doi: 10.1016/j.drugalcdep.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicero TJ, Ellis MS, Surratt HL, Kurtz SP. The changing face of heroin use in the United States: a retrospective analysis of the past 50 years. JAMA Psychiatry. 2014;71:821–826. doi: 10.1001/jamapsychiatry.2014.366. [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Shores CN, Paradis AG, Ellis MS. Source of drugs for prescription opioid analgesic abusers: a role for the Internet? Pain Med. 2008;9:718–723. doi: 10.1111/j.1526-4637.2007.00323.x. [DOI] [PubMed] [Google Scholar]
- Compton WM, Volkow ND. Major increases in opioid analgesic abuse in the United States: concerns and strategies. Drug Alcohol Depend. 2006;81:103–107. doi: 10.1016/j.drugalcdep.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Dreifuss JA, Griffin ML, Frost K, Fitzmaurice GM, Potter JS, Fiellin DA, Selzer J, Hatch-Maillette M, Sonne SC, Weiss RD. Patient characteristics associated with buprenorphine/naloxone treatment outcome for prescription opioid dependence: results from a multisite study. Drug Alcohol Depend. 2013;131:112–118. doi: 10.1016/j.drugalcdep.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald MK, Steinmiller CL. Cocaine behavioral economics: from the naturalistic environment to the controlled laboratory setting. Drug Alcohol Depend. 2014;141:27–33. doi: 10.1016/j.drugalcdep.2014.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald MK, Steinmiller CL, Sliwerska E, Lundahl L, Burmeister M. BDNF Val(66)Met genotype is associated with drug-seeking phenotypes in heroin-dependent individuals: a pilot study. Addict Biol. 2013;18:836–845. doi: 10.1111/j.1369-1600.2011.00431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillhouse M, Canamar CP, Ling W. Predictors of outcome after short-term stabilization with buprenorphine. J Subst Abuse Treat. 2013;44:336–342. doi: 10.1016/j.jsat.2012.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh SR. Behavioral economics of drug self-administration: an introduction. Drug Alcohol Depend. 1993;33:165–172. doi: 10.1016/0376-8716(93)90058-x. [DOI] [PubMed] [Google Scholar]
- Hursh SR, Galuska CM, Winger G, Woods JH. The economics of drug abuse: a quantitative assessment of drug demand. Mol Interv. 2005;5:20–28. doi: 10.1124/mi.5.1.6. [DOI] [PubMed] [Google Scholar]
- Jones CM, Mack KA, Paulozzi LJ. Pharmaceutical overdose deaths, United States, 2010. JAMA. 2013;309:657–659. doi: 10.1001/jama.2013.272. [DOI] [PubMed] [Google Scholar]
- Koffarnus MN, Jarmolowicz DP, Mueller ET, Bickel WK. Changing delay discounting in the light of the competing neurobehavioral decision systems theory: a review. J Exp Anal Behav. 2013;99:32–57. doi: 10.1002/jeab.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling W, Mooney L, Hillhouse M. Prescription opioid abuse, pain and addiction: clinical issues and implications. Drug Alcohol Rev. 2011;30:300–305. doi: 10.1111/j.1465-3362.2010.00271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKillop J. Integrating behavioral economics and behavioral genetics: delayed reward discounting as an endophenotype for addictive disorders. J Exp Anal Behav. 2013;99:14–31. doi: 10.1002/jeab.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackillop J, Amlung MT, Acker J, Gray JC, Brown CL, Murphy JG, Ray LA, Sweet LH. The neuroeconomics of alcohol demand: an initial investigation of the neural correlates of alcohol cost-benefit decision making in heavy drinking men. Neuropsychopharmacology. 2014;39:1988–1995. doi: 10.1038/npp.2014.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKillop J, Amlung MT, Few LR, Ray LA, Sweet LH, Munafo MR. Delayed reward discounting and addictive behavior: a meta-analysis. Psychopharmacology (Berl) 2011;216:305–321. doi: 10.1007/s00213-011-2229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKillop J, Kahler CW. Delayed reward discounting predicts treatment response for heavy drinkers receiving smoking cessation treatment. Drug Alcohol Depend. 2009;104:197–203. doi: 10.1016/j.drugalcdep.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manchikanti L. Prescription drug abuse: what is being done to address this new drug epidemic? Testimony before the Subcommittee on Criminal Justice, Drug Policy and Human Resources. Pain Physician. 2006;9:287–321. [PubMed] [Google Scholar]
- Mars SG, Bourgois P, Karandinos G, Montero F, Ciccarone D. Every ‘never’ I ever said came true”: transitions from opioid pills to heroin injecting. Int J Drug Policy. 2014;25:257–266. doi: 10.1016/j.drugpo.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy JG, Correia CJ, Dennhardt AA. Behavioral economic factors in addictive processes. In: Miller PM, editor. Principles of Addiction. Vol. 1. Elsevier; Amsterdam: 2013. pp. 249–257. [Google Scholar]
- Murphy JG, Dennhardt AA, Skidmore JR, Borsari B, Barnett NP, Colby SM, Martens MP. A randomized controlled trial of a behavioral economic supplement to brief motivational interventions for college drinking. J Consult Clin Psychol. 2012;80:876–886. doi: 10.1037/a0028763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy JG, MacKillop J. Relative reinforcing efficacy of alcohol among college student drinkers. Exp Clin Psychopharmacol. 2006;14:219–227. doi: 10.1037/1064-1297.14.2.219. [DOI] [PubMed] [Google Scholar]
- Murphy JG, MacKillop J, Skidmore JR, Pederson AA. Reliability and validity of a demand curve measure of alcohol reinforcement. Exp Clin Psychopharmacol. 2009;17:396–404. doi: 10.1037/a0017684. [DOI] [PubMed] [Google Scholar]
- Robins LN, Wing J, Wittchen HU, Helzer JE, Babor TF, Burke J, Farmer A, Jablenski A, Pickens R, Regier DA, et al. The Composite International Diagnostic Interview: an epidemiologic instrument suitable for use in conjunction with different diagnostic systems and in different cultures. Arch Gen Psychiatry. 1988;45:1069–1077. doi: 10.1001/archpsyc.1988.01800360017003. [DOI] [PubMed] [Google Scholar]
- Roddy J, Greenwald M. An economic analysis of income and expenditures by heroin-using research volunteers. Subst Use Misuse. 2009;44:1503–1518. doi: 10.1080/10826080802487309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer JL, Graham JW. Missing data: our view of the state of the art. Psychol Methods. 2002;7:147–177. [PubMed] [Google Scholar]
- Soyka M, Zingg C, Koller G, Kuefner H. Retention rate and substance use in methadone and buprenorphine maintenance therapy and predictors of outcome: results from a randomized study. Int J Neuropsychopharmacol. 2008;11:641–653. doi: 10.1017/S146114570700836X. [DOI] [PubMed] [Google Scholar]
- StataCorp. Stata: Release. Vol. 13. StataCorp LP; College Station, TX: 2013. [Google Scholar]
- SAMHSA. Drug Abuse Warning Network, 2011: National Estimates of Drug-Related Emergency Department Visits. Substance Abuse and Mental Health Services Administration; Rockville, MD: 2013a. [PubMed] [Google Scholar]
- SAMHSA. HSDUH Series H-46, HHS Publication No (SMA) 13–4795. Rockville, MD: 2013b. Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings. [Google Scholar]
- Tucker JA, Roth DL, Vignolo MJ, Westfall AO. A behavioral economic reward index predicts drinking resolutions: moderation revisited and compared with other outcomes. J Consult Clin Psychol. 2009;77:219–228. doi: 10.1037/a0014968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker JA, Vuchinich RE, Black BC, Rippens PD. Significance of a behavioral economic index of reward value in predicting drinking problem resolution. J Consult Clin Psychol. 2006;74:317–326. doi: 10.1037/0022-006X.74.2.317. [DOI] [PubMed] [Google Scholar]
- Tucker JA, Vuchinich RE, Rippens PD. Predicting natural resolution of alcohol-related problems: a prospective behavioral economic analysis. Exp Clin Psychopharmacol. 2002;10:248–257. doi: 10.1037//1064-1297.10.3.248. [DOI] [PubMed] [Google Scholar]
- Washio Y, Higgins ST, Heil SH, McKerchar TL, Badger GJ, Skelly JM, Dantona RL. Delay discounting is associated with treatment response among cocaine-dependent outpatients. Exp Clin Psychopharmacol. 2011;19:243–248. doi: 10.1037/a0023617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RD, Potter JS, Fiellin DA, Byrne M, Connery HS, Dickinson W, Gardin J, Griffin ML, Gourevitch MN, Haller DL, Hasson AL, Huang Z, Jacobs P, Kosinski AS, Lindblad R, McCance-Katz EF, Provost SE, Selzer J, Somoza EC, Sonne SC, Ling W. Adjunctive counseling during brief and extended buprenorphine-naloxone treatment for prescription opioid dependence: a 2-phase randomized controlled trial. Arch Gen Psychiatry. 2011;68:1238–1246. doi: 10.1001/archgenpsychiatry.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RD, Potter JS, Provost SE, Huang Z, Jacobs P, Hasson A, Lindblad R, Connery HS, Prather K, Ling W. A multi-site, two-phase, Prescription Opioid Addiction Treatment Study (POATS): rationale, design, and methodology. Contemp Clin Trials. 2010;31:189–199. doi: 10.1016/j.cct.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]


