Abstract
Background
Cardiovascular disease (CVD) is of increasing concern among breast cancer survivors. However the burden of this comorbidity in this group relative to the general population, and its temporal pattern, remains unknown.
Methods
We compared deaths due to CVD in a population-based sample of 1,413 women with incident breast cancer diagnosed in 1996-1997, and 1,411 age-matched women without breast cancer. Date and cause of death through December 31, 2009 were assessed through the National Death Index and covariate data was gathered through structured interviews and medical record abstraction. Hazard ratios and 95% confidence intervals (CI) were calculated using Cox regression for overall mortality (HR) and CVD-specific death (cause-specific HR). Subdistribution hazard ratios (sHR) for CVD death were estimated from the Fine-Gray model.
Results
Risk of death was greater among breast cancer survivors compared to women without breast cancer [HR: 1.8 (1.5, 2.1)]. An increase in CVD-related death among breast cancer survivors was evident only 7 years after diagnosis [years 0-7, cause-specific HR: 0.80 (0.53, 1.2), subdistribution HR: 0.59 (0.40, 0.87)]; years 7+, cause-specific HR: 1.8 (1.3, 2.5), subdistribution HR: 1.9 (1.4, 2.7); p-interaction: 0.001]. An increase in CVD-related mortality was observed among breast cancer survivors receiving chemotherapy.
Conclusions
Breast cancer survivors are at greater risk for CVD-related mortality compared to women without breast cancer and this increase in risk is manifest approximately 7 years after diagnosis. Efforts should be made to identify risk factors and interventions that can be employed during this brief window to reduce the excess burden of CVD in this vulnerable population.
Keywords: cardiovascular disease, mortality, breast cancer, cancer survivorship, competing risks
Introduction
Breast cancer mortality among American women has decreased by more than 2% per year in the last decade1, with nearly 90% of breast cancer patients currently surviving 5 years past their date of diagnosis.2 In the United States there are nearly 3 million female breast cancer survivors3 many of whom die from conditions unrelated to their malignancy.4,5 This trend becomes more pronounced as age at diagnosis increases6 and among those with early stage disease.4,6
Cardiovascular disease (CVD) is an important public health issue; among the general U.S. population it represents the leading cause of death among both men and women7 and is an issue of growing concern among cancer survivors.8-11 For breast cancer survivors specifically, CVD represents the greatest single non-cancer cause of death, accounting for approximately 35% of non-breast cancer mortality for survivors 50 years of age and older.6 However, our current understanding of the etiology of CVD among breast cancer survivors is limited.10,12 Previous studies have examined risk factors for CVD-related mortality within cohorts of cancer survivors only, mostly focusing on breast cancer treatment.10,13 Although an increased risk of CVD has been documented in clinical studies, there have been few reports of the magnitude of this comorbidity in population-based cohorts of breast cancer survivors. Furthermore, the extent to which breast cancer survivors experience CVD related outcomes relative to women without breast cancer, and the time when any excess risk of CVD becomes manifest, has not been formally evaluated previously. This ambiguity limits the ability for clinicians to identify and provide accurate recommendations for CVD risk reduction strategies specific to breast cancer survivors, particularly with regard to the timing of the interventions.
The objective of this study was to estimate the relative burden of death due to CVD among a population-based sample of breast cancer survivors compared to an age-matched sample of women without breast cancer. We also explored whether rates of CVD death varied over time or according to cancer-related treatments.
Methods
This follow-up study uses resources from the population-based Long Island Breast Cancer Study Project (LIBCSP),14 which was approved by the Institutional Review Board of participating institutions.
Study Population
Women with breast cancer were English-speaking adults diagnosed with a first primary in situ or invasive breast cancer between August 1, 1996-July 31, 1997, and residents of Nassau or Suffolk counties, NY. Potentially eligible women with breast cancer were identified through all hospitals in the study catchment area, and patients’ physicians were contacted to confirm the diagnosis and obtain permission to contact the women. Women without breast cancer were residents of the same two counties in NY, frequency matched to the expected distribution of survivors in 5-year age groups, and identified using random digit dialing for those under 65 years of age, and from Health Care Finance Rosters for those age 65 or over. Baseline interview respondents included 1,508 women with (82.1% of eligible cases) and 1,556 without breast cancer (62.7% of eligible controls). Women ranged from 20 to 98 years of age at baseline, and 94% were white and 4% were African American. At baseline, 1,414 breast cancer survivors agreed to be re-contacted, and 1,033 ultimately participated in the follow-up interview.
Outcome Assessment
The National Death Index,15 was used to establish vital status for all LIBCSP participants through 2009. We considered death from any cause (n=712; 268 women without breast cancer and 444 breast cancer survivors), as well as death from CVD (n=300; 129 and 171, respectively) if any of the following cardiovascular disease-related International Classification of Disease codes were listed as the primary cause of death: 394.9, 402.9, 410, 414.0, 427.5, I10, I11.9, I21.9, I25.1, I25.4 and I46.9. These codes were selected to include common cardiovascular disease outcomes, including myocardial infarction, cardiac arrest, hypertensive heart disease, coronary artery aneurysm and dissection. Women without a death record match in the NDI database were deemed alive as of December 31, 2009. Follow-up time ranged from 0.2 to 13.5 years.
Covariates
After signed informed consent was obtained, all participants completed the in-person baseline interview, which occurred within approximately 3 months of diagnosis for women with breast cancer. The baseline interview included assessment of history of comorbidities, menopausal status, education, income, first course of treatment for the first primary breast cancer diagnosis, and other factors. Only women with breast cancer were asked to complete the telephone follow-up interview, which occurred approximately 5 years later, to obtain more detailed information on full course of treatment for the first primary breast cancer.
Medical records were reviewed for information on tumor characteristics and treatment for 1,402 women with breast cancer who signed a medical record release form at baseline, and, again, for 598 women with breast cancer who signed a medical record release at the follow-up interview. Treatment data from the medical record agreed strongly with the data from the follow-up questionnaire16 (kappa coefficients: radiation therapy κ=0.97, chemotherapy κ=0.96 and hormone therapy κ=0.92), and thus the self-reported data are used in these analyses. Data on tumor size was collected from the New York State Cancer Registry.
Statistical Analysis
All statistical analyses were conducted using Stata 13 (College Station, TX). For overall mortality, Kaplan-Meier failure curves were calculated and Cox proportional hazards regression17 was used to estimate adjusted hazard ratios (HR) and 95% confidence intervals (CI). For CVD mortality we conducted a competing risks analysis18-20 because other causes of death (e.g. breast cancer) may remove women from the risk set before the event of interest (CVD mortality) is observed. For CVD-related death we estimated the competing risks analog to the Kaplan-Meier failure function, the cumulative incidence function.21 For the competing risks analysis we estimated two measures of association: the cause-specific hazard ratio and the subdistribution hazard ratio.19 The cause-specific hazard ratio represents the rate at which women with breast cancer experience CVD-related deaths relative to women without breast cancer among those still alive;22,23 this was estimated from a Cox model with all non-CVD causes of death treated as censored. The subdistribution hazard ratios indicate the relative difference in the cumulative incidence of CVD deaths between women with and without breast cancer,18,19 and were estimated using the Fine-Gray model.22 The subdistribution hazard ratio accounts for the influence of other causes of death (e.g. breast cancer) that would render subjects incapable of experiencing the death of interest (CVD), and therefore reflects the relative risk of experiencing a CVD-related death.22,23
Confounders included variables that may affect both breast cancer incidence and cardiovascular disease mortality: age (at reference date=date of diagnosis for women with breast cancer or date of identification for women without breast cancer; restricted cubic spline with 5 knots at equally spaced percentiles), menopausal status (pre-/post-menopausal), previous use of hormone replacement therapy (HRT, yes/no), cigarette smoking history (never, passive exposure only, active smoker only, both active smoker and passive exposure), average lifetime alcohol intake (non-drinkers, <15 g/day, 15-30 g/day, 30 or more g/day), body mass index (BMI, kg/m2) the year before reference date and self-reported history of the following comorbidities at reference date: diabetes, dyslipidemia, myocardial infarction, hypertension, or stroke. All confounders were ascertained relative to the study participant’s reference date. We additionally considered adjustment for additional factors that could be related to access to care, health care utilization and health behavior (income and education) and cardiovascular disease risk (cholesterol-lowering medications and aspirin use), but adjustment for these factors did not materially change the estimates and thus we did not include them in the final models. The proportional hazards assumption was assessed using a Wald test for the interaction between follow-up time and each covariate. Violations of this assumption were noted for history of myocardial infarction and hypertension for overall mortality, and for history of diabetes for CVD mortality, therefore the final respective models included interactions between these terms and follow-up time. Breast cancer status also violated this assumption in all models, and so the overall association is interpreted as an average effect over the entire follow-up period;23 we additionally report this association separated by duration of follow-up, described below.
We evaluated the potential for the association with breast cancer status to vary over time by exploring interactions with follow-up time categorized as before and after 6 years of follow-up (e.g. follow-up time ≤ 6 years vs. > 6 years) for overall mortality, and 7 years for CVD mortality, determined to be the most meaningful cutpoints based on empirical results and visual inspection of Kaplan-Meier failure curves and cumulative incidence function plots. Significance in this interaction was determined at the 5% level. We additionally explored heterogeneity of the association among breast cancer survivors by classifying them according to treatment (chemotherapy, hormone therapy, radiation therapy). Small numbers for these subgroup analyses precluded categorization of breast cancer survivors into more detailed treatment types or combinations.
Final Analytic Sample Size
Small amounts of missing data were noted for baseline menopausal status (30 cases/63 controls), history of HRT (4 cases/1 control), smoking (37 cases/42 controls), BMI (17 cases/28 controls), or any CVD-related comorbidity (9 cases/13 controls) yielding a sample of 1,413 women with, and 1,411 women without, breast cancer for the primary analyses.
Results
At the reference date the mean age of women in our sample was 57 years for women without breast cancer and 59 years for women with breast cancer. In our population-based sample of 1,413 women diagnosed with a first primary breast cancer in 1996-1997 the number of deaths through 2009 was higher for all-cause and CVD-related mortality (415 and 133, respectively) than among the 1,411 age-matched population-based women without breast cancer (242 and 105, respectively) (Table 1). When considering the six pre-existing CVD-related risk factors (history of myocardial infarction, history of stroke, pre-existing diabetes, hypertension or high cholesterol, or BMI>=30 kg/m2), we noted that the average number of risk factors was slightly higher among women with breast cancer (mean: 1.03) than among women without breast cancer (0.97).
Table 1.
Baseline characteristics, and vital status as of December 31, 2009, among a population-based sample of 1,413 breast cancer survivors and 1,411 women without breast cancer frequency matched by age. Long Island Breast Cancer Study Project, 1996-2009.
| Women without Breast Cancer (n=1,411) |
Breast Cancer Survivors (n=1,413) |
|
|---|---|---|
| Deaths from any cause | 242 | 415 |
| Deaths from CVDa | 105 | 133 |
| Deaths from Breast Cancera | 2 | 135 |
| Menopausal status | ||
| Premenopausal, No. (%) | 485 (34) | 466 (33) |
| Postmenopausal, No. (%) | 926 (66) | 947 (67) |
| Ever used hormone replacement therapy |
||
| No | 1,073 (76) | 1,033 (73) |
| Yes | 338 (24) | 408 (27) |
| Smoking | ||
| Never | 177 (13) | 161 (11) |
| Passive exposure only | 465 (33) | 464 (33) |
| Ever active smoker only | 132 (9.4) | 133 (9.4) |
| Both active and passive | 637 (45) | 655 (46) |
| Alcohol intake, Lifetime average g/day |
||
| Non-drinker | 551 (39) | 550 (39) |
| 0-15 | 672 (48) | 651 (46) |
| 15-30 | 107 (7.6) | 142 (10) |
| >=30 | 81 (5.7) | 70 (4.9) |
| BMIb (kg/m2) in year before reference dateb |
||
| <18.5 | 31 (2.2) | 25 (1.8) |
| 18.5-24.9 | 662 (47) | 629 (44) |
| 25.0-29.9 | 415 (29) | 450 (32) |
| ≥30.0 | 303 (22) | 309 (22) |
| Hypertension | ||
| No | 946 (67) | 925 (65) |
| Yes | 465 (33) | 488 (34) |
| Diabetes | ||
| No | 1,313 (93) | 1,285 (91) |
| Yes | 98 (6.9) | 128 (9.1) |
| High Cholesterol | ||
| No | 980 (69) | 988 (70) |
| Yes | 431 (30) | 425 (30) |
| Myocardial Infarction | ||
| No | 1,367 (97) | 1,356 (96) |
| Yes | 44 (3.1) | 57 (4.0) |
| Stroke | ||
| No | 1,387 (98) | 1,372 (97) |
| Yes | 24 (1.7) | 41 (2.9) |
| Chemotherapy | ||
| No | N/A | 561 (58) |
| Yes | N/A | 400 (42) |
| Radiation Therapy | ||
| No | N/A | 375 (39) |
| Yes | N/A | 590 (61) |
| Hormone Therapy | ||
| No | N/A | 369 (39) |
| Yes | N/A | 580 (61) |
| Tumor size | ||
| <2cm | N/A | 781 (76) |
| >=2cm | N/A | 248 (24) |
| Missing | N/A | 479 |
CVD: cardiovascular disease. According to primary cause of death field from National Death Index data.
BMI: body mass index.
As shown in Figure 1, risk of death from all causes was greater among breast cancer survivors compared to the population-based sample without breast cancer [adjusted HR (95% CI): 1.8 (1.5, 2.1)]. Breast cancer was also associated with greater rate of CVD-related death across the entire follow-up [adjusted cause-specific HR: 1.3 (1.0, 1.7); subdistribution HR: 1.2 (0.89, 1.5)].
Figure 1.
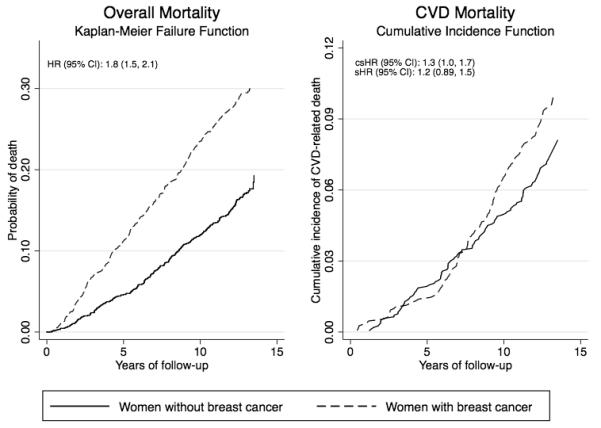
Unadjusted Kaplan-Meier failure curves and adjusted hazard ratios (HR) for overall mortality (first panel) and cumulative incidence function, cause-specific HR (csHR) and subdistribution HR (sHR) for CVD-related mortality (second panel) among a population-based sample of breast cancer survivors and age-matched women without breast cancer. The Long Island Breast Cancer Study, 1996-2009.
*HRs adjusted for age at reference date (age at diagnosis for breast cancer survivors and date of identification for women without breast cancer), menopausal status, previous use of hormone replacement therapy, smoking history, average lifetime alcohol intake, body mass index the year before reference date, income, education, and history of the following cardiovascular disease risk factors: diabetes, myocardial infarction, hypertension, dyslipidemia or stroke. Model for overall mortality included interaction between follow-up time and history of hypertension and myocardial infarction and models for CVD mortality included interaction between follow-up time and history of diabetes.
CVD-related mortality among the breast cancer survivors appeared to increase sharply after approximately year 5. Analyses that considered heterogeneity of these relationships over the follow-up showed a significant difference in the association between mortality and breast cancer status over time (Table 2). As expected, within the first 6 years the overall mortality rate was much greater among breast cancer survivors than women without breast cancer [within years 0-6, HR: 2.2 (1.7, 2.8); after year 6, HR: 1.5 (1.3, 1.9), p-interaction: 0.04], largely driven by the greater number of breast cancer-related deaths among survivors in the years immediately following diagnosis. However, the rate of CVD death was greater among longer-term breast cancer survivors compared to the women without breast cancer after 7 years of follow-up [csHR: 1.8 (1.3, 2.5)]. In contrast, there was a trend towards an inverse association of breast cancer status with CVD-related death within the first 7 years [cause-specific HR: 0.80 (0.53, 1.2), p-interaction: 0.004]. After year 7, breast cancer survivors had nearly twice the risk of CVD mortality compared to those without breast cancer [sHR: 1.9 (1.4, 2.7)], while the risk of CVD death was lower among breast cancer survivors in the early years [years 0-7, subdistribution HR: 0.59 (0.40, 0.87), p-interaction <0.001]. This reduction in incident CVD mortality among survivors in the short term most likely reflects the influence of the major competing risk in this group (breast cancer-related death).
Table 2.
Overall and CVD mortality among a population-based sample of breast cancer survivors and women without breast cancer stratified by time since the beginning of follow-up. The Long Island Breast Cancer Study Project, 1996-2009.
| Overall Mortality | |||
|---|---|---|---|
| HRa (95% CI) | |||
| Time since beginning of follow-up |
Women without Breast Cancer (235 deaths) |
Breast Cancer Survivors (407 deaths) |
pb |
| 0-6 years | 1.0 | 2.2 (1.7, 2.8) | 0.04 |
| >6 years | 1.0 | 1.5 (1.3, 1.9) | |
| CVD Mortality | |||
|
Time since beginning
of follow-up |
Women without
Breast Cancer (114 deaths) |
Breast Cancer
Survivors (155 deaths) |
|
|
| |||
| Cause-specific HRa,c (95% CI) | pb | ||
| 0-7 years | 1.0 | 0.80 (0.53, 1.2) | 0.004 |
| >7 years | 1.0 | 1.8 (1.3, 2.5) | |
| Subdistribution HRa,d (95% CI) | pb | ||
| 0-7 years | 1.0 | 0.59 (0.40, 0.87) | <0.001 |
| >7 years | 1.0 | 1.9 (1.4, 2.7) | |
Models adjusted for age at reference date (date of diagnosis for breast cancer survivors and date of identification for women without breast cancer), menopausal status, previous use of hormone replacement therapy, smoking history, average lifetime alcohol intake, body mass index the year before reference date, income, education, and history of the following cardiovascular disease risk factors: diabetes, myocardial infarction, hypertension, dyslipidemia or stroke. Model for overall mortality included interaction between follow-up time and history of hypertension and myocardial infarction and models for CVD mortality included interaction between follow-up time and history of diabetes.
P-value for likelihood ratio test of interaction between breast cancer status and time.
Cause-specific hazard ratio estimates relative rate of CVD mortality between women with and without breast cancer, not accounting for competing causes of death.
Subdistribution hazard ratio reflects association between breast cancer diagnosis and incidence of CVD death.
The CVD-specific mortality rate was elevated among those who received chemotherapy [cause-specific HR: 1.7 (1.1, 2.6)] but not those not receiving chemotherapy [cause-specific HR: 1.1 (0.82, 1.6)] (Table 3). Similar increases were observed regardless of receipt of hormone therapy, while a more pronounced, but non-significant increase was seen among those not receiving radiation therapy [without radiation therapy cause-specific HR: 1.5 (1.0, 2.2); with radiation therapy cause-specific HR: 1.2 (0.82, 1.7)]. Associations with the cumulative incidence of CVD death (sHRs) followed similar patterns, but were attenuated. Given that adjuvant chemotherapy is associated with early menopause, which may impact CVD risk, we conducted an exploratory analysis where we examined the relationship with chemotherapy treatment among women who were pre-menopausal at diagnosis (n=466 women with breast cancer and 485 women without) and found more pronounced associations with chemotherapy in this subgroup than we observed in the overall population [cause-specific HR: 2.8 (1.3, 6.3), sHR: 2.5 (1.1, 5.7) among women receiving chemotherapy vs. women without breast cancer; cause-specific HR: 1.4 (0.43, 4.3),subdistribution HR: 1.4 (0.43, 4.2) among women not receiving chemotherapy vs. women without breast cancer] (data not in table).
Table 3.
CVD mortality by treatment regimen for a population-based sample of breast cancer survivors and women without breast cancer. Long Island Breast Cancer Study Project, 1996-2009.
| Cause-specific HRa,b (95% CI) | |||
|---|---|---|---|
| Treatment | Women without Breast Cancer |
Breast Cancer Survivors without Treatment |
Breast Cancer Survivors with Treatment |
| Chemotherapy | 1.0 | 1.1 (0.82, 1.6) | 1.7 (1.1, 2.6) |
| Hormone Therapy | 1.0 | 1.3 (0.82, 1.9) | 1.3 (0.93, 1.8) |
| Radiation Therapy | 1.0 | 1.5 (1.0, 2.2) | 1.2 (0.82, 1.7) |
| Subdistribution HRa,c (95% CI) | |||
| Treatment |
Women without
Breast Cancer |
Breast Cancer
Survivors without Treatment |
Breast Cancer
Survivors with Treatment |
|
| |||
| Chemotherapy | 1.0 | 1.1 (0.77, 1.5) | 1.4 (0.95, 2.2) |
| Hormone Therapy | 1.0 | 1.2 (0.77, 1.8) | 1.2 (0.86, 1.7) |
| Radiation Therapy | 1.0 | 1.3 (0.89, 2.0) | 1.1 (0.77, 1.6) |
Models adjusted for age at reference date (date of diagnosis for breast cancer survivors and date of identification for women without breast cancer), menopausal status, previous use of hormone replacement therapy, smoking history, average lifetime alcohol intake, body mass index the year before reference date, income, education, and history of the following cardiovascular disease risk factors: diabetes, myocardial infarction, hypertension, dyslipidemia or stroke. Models also included interaction between follow-up time and history of diabetes.
Cause-specific hazard ratio estimates relative rate of CVD mortality between women with and without breast cancer, not accounting for competing causes of death.
Subdistribution hazard ratio reflects association between breast cancer diagnosis and incidence of CVD death.
Discussion
We are the first, to our knowledge, to determine that death due to CVD is higher among a population-based cohort of breast cancer survivors than a comparable group of women without breast cancer. We were also able to identify at which point after diagnosis—approximately year 7—CVD becomes a disproportionate cause of death for cancer survivors. The increase in CVD-related mortality among breast cancer survivors, compared to other women, appeared to be strongest among survivors who received chemotherapy for their first primary breast cancer. Our finding that breast cancer survivors are at greater risk for CVD than women without breast cancer has important clinical implications for these women who are already older at diagnosis, and the long-term survivors who continue to age.
The most noteworthy finding of our study is that the increased risk of CVD-related death among breast cancer survivors does not become apparent until several years after diagnosis. The near-null cause-specific HR for CVD mortality observed within the first 7 years reflects the fact that near diagnosis the rate of CVD mortality is similar between breast cancer survivors and women without breast cancer, however the significant inverse subdistribution HR suggests that the cumulative incidence of CVD-related death is lower among breast cancer survivors during this time since breast cancer survivors are more likely to experience the competing risk of breast cancer-related death. However, this dynamic appears to change around year 7, when not only does the influence of the competing risk decrease, but the association of breast cancer diagnosis with rate of CVD death increases sharply. These two factors combine to yield a nearly twofold increase in the incidence of CVD for these long-term survivors (shown by the subdistribution HR in years 7+). The current paradigm that suggests survivorship 5 years after diagnosis is a meaningful threshold for cancer survivors24 does not account for the substantial increase in risk of non-cancer related death that occurs after this point. Instead, it over-emphasizes improvements in short-term survival attributable to improvements in treatment, while ignoring the sizeable late effects of such treatment on risk of potentially fatal comorbidities, in particular cardiovascular disease.
Several potential mechanisms could play a role in these associations. Cancer survivors have been reported to have a higher prevalence of CVD risk factors compared to the general population,25 although this difference was not pronounced in our cohort when we compared the average number of risk factors between women in these two groups. Additionally, the cardiotoxic effects of breast cancer treatments, specifically radiotherapy and chemotherapy, are well documented.10,13,26 Anthracycline-related cardiotoxicity is especially established,26,27 however this agent is predominantly related to congestive heart failure and cardiomyopathy,26,27 which were not included in our definition of CVD-related death. Therefore, the observed increase in CVD-related deaths among women receiving chemotherapy could be attributed to a number of other agents with effects that act on the cardiovascular system.26 In addition to any direct effects of chemotherapy on CVD risk, there may be indirect mechanisms linked with early menopause,28 which is associated with unfavorable changes in lipid levels29 and hemostasis factors30 that may increase risk of cardiovascular events.31 This is consistent with our observation of a more pronounced association for chemotherapy treatment among pre-menopausal women. Our overall finding of an increased risk of CVD-related death among breast cancer patients who underwent chemotherapy is consistent with these previous studies, yet we are the first to quantify the magnitude of this increase compared to women without breast cancer. We did note a modest decrease in the rate of CVD-related death among women with breast cancer in the first several years after diagnosis, which could be consistent with a previously noted reduction in death due to myocardial infarction associated with tamoxifen.32 However, a limited number of events in the years shortly after diagnosis limited our ability to examine treatment-by-time interactions in this analysis.
Although our finding of no increase in CVD mortality among those receiving radiation therapy was in agreement with some previous studies,33,34 this result lies in contrast to others.9,35-39 Radiation therapy, in particular to the left side of the chest wall, may increase CVD risk through injury to cardiac muscle or the surrounding vasculature.40 However, several recent studies have failed to observe a difference in cardiac events between women receiving radiation therapy for left- vs. right-sided tumors.34,42,43 A potential explanation for these recent null findings, including ours, is that current radiotherapy techniques are more targeted and less cardiotoxic than older regimens.41,42 It is also possible that the severity or form of radiation-induced CVD is less fatal than chemotherapy-induced disease, as our outcome was mortality rather than incident disease. Alternatively, radiation-induced CVD could take longer to manifest than we have available follow-up, as studies have found an increase in risk of morbidity or mortality more than 15 years36 to 20 years37,39 after treatment, a time period several years past what we had available. Other reports have found a synergistic effect between radiotherapy and other systemic therapies13 that we did not have the study power to examine here.
A major strength of our study includes the innovative focus on a population-based sample of women with and without breast cancer. We are the first, to our knowledge, to compare risk for non-cancer mortality between a cohort of breast cancer survivors and a sample of women without breast cancer from the same source population. This study design offers a unique opportunity to identify differences in the risk of health outcomes between breast cancer survivors and a comparable group of women without breast cancer. Nearly all of the previous epidemiologic studies of cancer survivors have been limited to identification of risk factors among survivors alone. Our novel design allows us to identify unique patterns of risk in this subgroup, in particular the time at which risk between these two groups diverges. Our findings suggest that breast cancer survivors should be closely monitored for development of cardiovascular abnormalities and CVD risk factors beginning in the years immediately following diagnosis and treatment. Importantly, our population-based design strengthens the interpretation of our findings—since the study participants with and without breast cancer were both representative of the same source population from which they are drawn.
Although this study has noteworthy strengths, our conclusions must be interpreted in light of potential limitations. The women from this cohort are predominantly white, and these findings may not be generalizable to other racial and ethnic groups. Although we controlled for pre-existing comorbid conditions that may be related to CVD-mortality (and potentially breast cancer treatment choices), there is the potential for residual confounding by the degree to which the comorbid conditions are controlled or confounding by indication for analysis of treatment-specific effects. Data on cause of death was determined through the National Death Index (NDI), a reliable source for determination of all-cause mortality.15 The NDI uses death certificate information to establish cause of death, which is a potential source of misclassification. However, our conclusions that the rate of overall mortality between those with and without breast cancer tended to converge over time, while the CVD-related mortality rate was initially identical, then diverged, suggests that classification of death was accurate, or at least that any misclassification would be nondifferential. Such misclassifiation would result in either bias toward the null, or in certain circumstances, no bias.44 Another potential concern is our use of self-reported data for pre-existing CVD-related comorbidities. Yet self-reported comorbidities such as those considered here have been demonstrated to be associated with mortality in similar cohorts.45,46 Additionally, given the size of our study, we were only able to evaluate treatment modalities broadly and not by specific agents. Subsequent studies would be improved by a larger sample size, which would facilitate more detailed analyses, such as death from specific CVD-related conditions, or due to specific chemotherapy agents or combinations of treatments.
Our results show that years after diagnosis and treatment long-term breast cancer survivors are at increased risk of death due to cardiovascular disease compared to women from the general population. In future, larger studies are needed to confirm our findings and identify the forms of CVD that are most common in this group, as well as including incident CVD as well as CVD-related mortality. Identification of potential mechanisms through which the breast cancer survivorship experience influences CVD etiology will help identify potential targets for intervention in this high-risk population.
Acknowledgements
none
Financial support: This work was supported in part by the: National Institutes of Health (UO1CA/ES66572, P30ES10126, CA120780, K12CA120780); Susan G. Komen for the Cure (KG101573); and the American Institute for Cancer Research.
Footnotes
The authors report no financial or non-financial conflicts of interest.
References
- 1.American Cancer Society . Breast Cancer Facts and Figures. American Cancer Society; Atlanta: 2007-2008. [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 3.de Moor JS, Mariotto AB, Parry C, Alfano CM, Padgett L, Kent EE, Forsythe L, Scoppa S, Hachey M, Rowland JH. Cancer survivors in the United States: prevalence across the survivorship trajectory and implications for care. Cancer Epidemiol Biomarkers Prev. 2013;22(4):561–70. doi: 10.1158/1055-9965.EPI-12-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanrahan EO, Gonzalez-Angulo AM, Giordano SH, Rouzier R, Broglio KR, Hortobagyi GN, Valero V. Overall survival and cause-specific mortality of patients with stage T1a,bN0M0 breast carcinoma. J Clin Oncol. 2007;25(31):4952–60. doi: 10.1200/JCO.2006.08.0499. [DOI] [PubMed] [Google Scholar]
- 5.Chapman JA, Meng D, Shepherd L, Parulekar W, Ingle JN, Muss HB, Palmer M, Yu C, Goss PE. Competing causes of death from a randomized trial of extended adjuvant endocrine therapy for breast cancer. J Natl Cancer Inst. 2008;100(4):252–60. doi: 10.1093/jnci/djn014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schairer C, Mink PJ, Carroll L, Devesa SS. Probabilities of death from breast cancer and other causes among female breast cancer patients. J Natl Cancer Inst. 2004;96(17):1311–21. doi: 10.1093/jnci/djh253. [DOI] [PubMed] [Google Scholar]
- 7.Murphy SL, Xu J, Kochanek KD. Deaths: Final Data for 2010. National Vital Statistics Reports. 2013;61(4) [PubMed] [Google Scholar]
- 8.Mann DL, Krone RJ. Cardiac disease in cancer patients: an overview. Prog Cardiovasc Dis. 2010;53(2):80–7. doi: 10.1016/j.pcad.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Hooning MJ, Botma A, Aleman BM, Baaijens MH, Bartelink H, Klijn JG, Taylor CW, van Leeuwen FE. Long-term risk of cardiovascular disease in 10-year survivors of breast cancer. J Natl Cancer Inst. 2007;99(5):365–75. doi: 10.1093/jnci/djk064. [DOI] [PubMed] [Google Scholar]
- 10.Carver JR, Shapiro CL, Ng A, Jacobs L, Schwartz C, Virgo KS, Hagerty KL, Somerfield MR, Vaughn DJ. American Society of Clinical Oncology clinical evidence review on the ongoing care of adult cancer survivors: cardiac and pulmonary late effects. J Clin Oncol. 2007;25(25):3991–4008. doi: 10.1200/JCO.2007.10.9777. [DOI] [PubMed] [Google Scholar]
- 11.Hooning MJ, Aleman BM, van Rosmalen AJ, Kuenen MA, Klijn JG, van Leeuwen FE. Cause-specific mortality in long-term survivors of breast cancer: A 25-year follow-up study. Int J Radiat Oncol Biol Phys. 2006;64(4):1081–91. doi: 10.1016/j.ijrobp.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 12.Giordano SH, Hortobagyi GN. Time to remove the subspecialty blinders: breast cancer does not exist in isolation. J Natl Cancer Inst. 2008;100(4):230–1. doi: 10.1093/jnci/djn015. [DOI] [PubMed] [Google Scholar]
- 13.Chargari C, Kirov KM, Bollet MA, Magne N, Vedrine L, Cremades S, Beuzeboc P, Fourquet A, Kirova YM. Cardiac toxicity in breast cancer patients: from a fractional point of view to a global assessment. Cancer Treat Rev. 2011;37(4):321–30. doi: 10.1016/j.ctrv.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 14.Gammon MD, Neugut AI, Santella RM, Teitelbaum SL, Britton JA, Terry MB, Eng SM, Wolff MS, Stellman SD, Kabat GC, Levin B, Bradlow HL, Hatch M, Beyea J, Camann D, Trent M, Senie RT, Garbowski GC, Maffeo C, Montalvan P, Berkowitz GS, Kemeny M, Citron M, Schnabe F, Schuss A, Hajdu S, Vincguerra V, Collman GW, Obrams GI. The Long Island Breast Cancer Study Project: description of a multi-institutional collaboration to identify environmental risk factors for breast cancer. Breast Cancer Res Treat. 2002;74(3):235–54. doi: 10.1023/a:1016387020854. [DOI] [PubMed] [Google Scholar]
- 15.Cowper DC, Kubal JD, Maynard C, Hynes DM. A primer and comparative review of major US mortality databases. Ann Epidemiol. 2002;12(7):462–8. doi: 10.1016/s1047-2797(01)00285-x. [DOI] [PubMed] [Google Scholar]
- 16.Cleveland RJ, Eng SM, Abrahamson PE, Britton JA, Teitelbaum SL, Neugut AI, Gammon MD. Weight gain prior to diagnosis and survival from breast cancer. Cancer Epidemiol Biomarkers Prev. 2007;16(9):1803–11. doi: 10.1158/1055-9965.EPI-06-0889. [DOI] [PubMed] [Google Scholar]
- 17.Collett D. Modelling survival data in medical research. 2nd ed Chapman & Hall/CRC Press LLC; Boca Raton: 2003. [Google Scholar]
- 18.Lau B, Cole SR, Gange SJ. Competing risk regression models for epidemiologic data. American journal of epidemiology. 2009;170(2):244–56. doi: 10.1093/aje/kwp107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Latouche A, Allignol A, Beyersmann J, Labopin M, Fine JP. A competing risks analysis should report results on all cause-specific hazards and cumulative incidence functions. Journal of clinical epidemiology. 2013;66(6):648–53. doi: 10.1016/j.jclinepi.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 20.Andersen PK, Geskus RB, de Witte T, Putter H. Competing risks in epidemiology: possibilities and pitfalls. International journal of epidemiology. 2012;41(3):861–70. doi: 10.1093/ije/dyr213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marubini E. Statistics in practice. John Wiley & Sons; Chichester ;New York: 1995. Analysing survival data from clinical trials and observational studies. [Google Scholar]
- 22.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association. 1999;94(446):496–509. [Google Scholar]
- 23.Hernan MA. The hazards of hazard ratios. Epidemiology. 2010;21(1):13–5. doi: 10.1097/EDE.0b013e3181c1ea43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Welch HG, Schwartz LM, Woloshin S. Are increasing 5-year survival rates evidence of success against cancer? JAMA. 2000;283(22):2975–8. doi: 10.1001/jama.283.22.2975. [DOI] [PubMed] [Google Scholar]
- 25.Weaver KE, Foraker RE, Alfano CM, Rowland JH, Arora NK, Bellizzi KM, Hamilton AS, Oakley-Girvan I, Keel G, Aziz NM. Cardiovascular risk factors among long-term survivors of breast, prostate, colorectal, and gynecologic cancers: a gap in survivorship care? J Cancer Surviv. 2013;7(2):253–61. doi: 10.1007/s11764-013-0267-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Accordino MK, Neugut AI, Hershman DL. Cardiac effects of anticancer therapy in the elderly. J Clin Oncol. 2014;32(24):2654–61. doi: 10.1200/JCO.2013.55.0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doyle JJ, Neugut AI, Jacobson JS, Grann VR, Hershman DL. Chemotherapy and cardiotoxicity in older breast cancer patients: a population-based study. J Clin Oncol. 2005;23(34):8597–605. doi: 10.1200/JCO.2005.02.5841. [DOI] [PubMed] [Google Scholar]
- 28.Walshe JM, Denduluri N, Swain SM. Amenorrhea in premenopausal women after adjuvant chemotherapy for breast cancer. J Clin Oncol. 2006;24(36):5769–79. doi: 10.1200/JCO.2006.07.2793. [DOI] [PubMed] [Google Scholar]
- 29.Matthews KA, Meilahn E, Kuller LH, Kelsey SF, Caggiula AW, Wing RR. Menopause and risk factors for coronary heart disease. N Engl J Med. 1989;321(10):641–6. doi: 10.1056/NEJM198909073211004. [DOI] [PubMed] [Google Scholar]
- 30.Meilahn EN. Hemostatic factors and risk of cardiovascular disease in women. An overview. Arch Pathol Lab Med. 1992;116(12):1313–7. [PubMed] [Google Scholar]
- 31.Barrett-Connor E, Bush TL. Estrogen and coronary heart disease in women. JAMA. 1991;265(14):1861–7. [PubMed] [Google Scholar]
- 32.Braithwaite RS, Chlebowski RT, Lau J, George S, Hess R, Col NF. Meta-analysis of vascular and neoplastic events associated with tamoxifen. J Gen Intern Med. 2003;18(11):937–47. doi: 10.1046/j.1525-1497.2003.20724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang W, O’Connell D, Stuart K, Boyages J. Analysis of 10-year cause-specific mortality of patients with breast cancer treated in New South Wales in 1995. J Med Imaging Radiat Oncol. 2011;55(5):516–25. doi: 10.1111/j.1754-9485.2011.02304.x. [DOI] [PubMed] [Google Scholar]
- 34.Doyle JJ, Neugut AI, Jacobson JS, Wang J, McBride R, Grann A, Grann VR, Hershman D. Radiation therapy, cardiac risk factors, and cardiac toxicity in early-stage breast cancer patients. Int J Radiat Oncol Biol Phys. 2007;68(1):82–93. doi: 10.1016/j.ijrobp.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 35.Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Bronnum D, Correa C, Cutter D, Gagliardi G, Gigante B, Jensen MB, Nisbet A, Peto R, Rahimi K, Taylor C, Hall P. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368(11):987–98. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 36.Roychoudhuri R, Robinson D, Putcha V, Cuzick J, Darby S, Moller H. Increased cardiovascular mortality more than fifteen years after radiotherapy for breast cancer: a population-based study. BMC Cancer. 2007;7:9. doi: 10.1186/1471-2407-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harris EE, Correa C, Hwang WT, Liao J, Litt HI, Ferrari VA, Solin LJ. Late cardiac mortality and morbidity in early-stage breast cancer patients after breast-conservation treatment. J Clin Oncol. 2006;24(25):4100–6. doi: 10.1200/JCO.2005.05.1037. [DOI] [PubMed] [Google Scholar]
- 38.Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans E, Godwin J, Gray R, Hicks C, James S, MacKinnon E, McGale P, McHugh T, Peto R, Taylor C, Wang Y. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366(9503):2087–106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 39.Boukheris H, Rubino C, Arriagada R, Delaloge S, Koscielny S, Giardini M, Le MG, De Vathaire F. Long-term mortality in a cohort study of 6,800 French breast cancer patients treated between 1954 and 1983. Acta Oncol. 2008;47(6):1122–32. doi: 10.1080/02841860802167482. [DOI] [PubMed] [Google Scholar]
- 40.Senkus-Konefka E, Jassem J. Cardiovascular effects of breast cancer radiotherapy. Cancer Treat Rev. 2007;33(6):578–93. doi: 10.1016/j.ctrv.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 41.Harris EE. Cardiac mortality and morbidity after breast cancer treatment. Cancer Control. 2008;15(2):120–9. doi: 10.1177/107327480801500204. [DOI] [PubMed] [Google Scholar]
- 42.Nixon AJ, Manola J, Gelman R, Bornstein B, Abner A, Hetelekidis S, Recht A, Harris JR. No long-term increase in cardiac-related mortality after breast-conserving surgery and radiation therapy using modern techniques. J Clin Oncol. 1998;16(4):1374–9. doi: 10.1200/JCO.1998.16.4.1374. [DOI] [PubMed] [Google Scholar]
- 43.Patt DA, Goodwin JS, Kuo YF, Freeman JL, Zhang DD, Buchholz TA, Hortobagyi GN, Giordano SH. Cardiac morbidity of adjuvant radiotherapy for breast cancer. J Clin Oncol. 2005;23(30):7475–82. doi: 10.1200/JCO.2005.13.755. [DOI] [PubMed] [Google Scholar]
- 44.Rothman KJ, Greenland S, Lash TL. Modern epidemiology. 3rd ed Wolters Kluwer Health/Lippincott Williams & Wilkins; Philadelphia: 2008. [Google Scholar]
- 45.Patterson RE, Flatt SW, Saquib N, Rock CL, Caan BJ, Parker BA, Laughlin GA, Erickson K, Thomson CA, Bardwell WA, Hajek RA, Pierce JP. Medical comorbidities predict mortality in women with a history of early stage breast cancer. Breast Cancer Res Treat. 2010;122(3):859–65. doi: 10.1007/s10549-010-0732-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nechuta S, Lu W, Zheng Y, Cai H, Bao PP, Gu K, Zheng W, Shu XO. Comorbidities and breast cancer survival: a report from the Shanghai Breast Cancer Survival Study. Breast Cancer Res Treat. 2013;139(1):227–35. doi: 10.1007/s10549-013-2521-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


