Abstract
Coxiella burnetii utilizes a Type IV Secretion System (T4SS) to modify host endomembrane transport systems to form a unique lysosome-derived niche called the Coxiella-containing vacuole (CCV). Although the CCV has lysosomal properties, this organelle displays distinct characteristics such as homotypic fusion and a cholesterol enriched limiting membrane, in addition to robustly interacting with autophagosomes. This review describes recent advances in understanding CCV biogenesis and the mechanisms C. burnetii employs to maintain this unique compartment.
Keywords: Coxiella burnetii, autophagy, traffic, T4SS, Coxiella-containing vacuole
1. Introduction
Coxiella burnetii is a Gram-negative, intracellular, bacterial pathogen that causes the disease Q Fever [14]. Macrophages internalize C. burnetii, which results in the formation of a phagosome that matures canonically along the endocytic pathway. This compartment develops into a lysosome-derived organelle called the Coxiella-containing vacuole (CCV). The lumen of the mature CCV is acidic, contains active lysosomal proteases and the vacuole membrane contains lysosomal proteins such as the vacuolar ATPase, LAMP-1, and CD63 [19, 29].
Coxiella burnetii requires a Dot/Icm Type IVB Secretion System (T4SS) to replicate inside of the CCV. The C. burnetii T4SS translocates approximately 130 bacterial effector proteins across the CCV membrane and into the host cell [5, 6, 42, 25, 43]. Coxiella burnetii strains containing loss-of-function mutations in essential dot or icm genes cannot replicate inside host cells [1, 5]. It is assumed that some T4SS effectors modify traffic of host endomembrane transport vesicles because the CCV itself is a modified lysosome. Few C. burnetii T4SS effectors have been functionally connected to a host pathway, let alone assigned a specific biochemical activity [23, 27, 35, 22, 8]. Accordingly, many details of how C. burnetii alters membrane transport in the host to facilitate CCV biogenesis remain unclear.
Here, we review the contributions of host transport pathways including endocytosis, cholesterol transport, autophagy, and membrane fusion to CCV biogenesis, and C. burnetii T4SS effectors that modify these pathways (Fig. 1). We hope to underscore the unique nature of the CCV and how investigation of its specialized characteristics will improve our understanding of C. burnetii pathogenesis and fundamental cellular processes that control lysosome biogenesis.
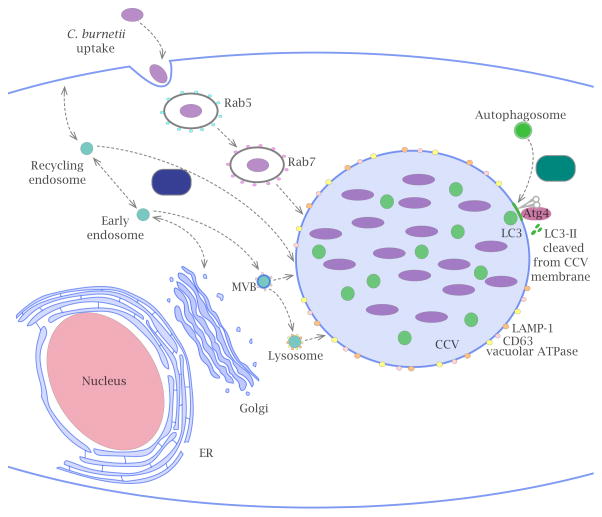
Figure 1. Coxiella burnetii transport and interactions with host endosomal organelles.
Upon uptake, C. burnetii resides in an endocytic vacuole that is transported to lysosomes by a process that requires the host endocytic machinery and Rab proteins (Rab5 and Rab7) that regulate early and late endosomal fusion events. Once in an acidified compartment, C. burnetii activates the Dot/Icm system and initiates translocation of an estimated 130 bacterial effector proteins into the host cell cytosol. The effector protein Cig2 promotes robust fusion of autophagosomes with the CCV, and locks the compartment in an autolysosomal state of maturation that is necessary for the process of CCV homotypic fusion. The interaction between the effector protein CvpA and the host clathrin adaptor AP-2 promotes interactions between the CCV and endocytic recycling vesicles. Cholesterol enrichment of the CCV is likely facilitated by fusion of cholesterol-rich organelles with the CCV, however, effectors that modulate cholesterol remain to be identified (compartments enriched in cholesterol are depicted in blue and include the plasma membrane, Golgi apparatus, ER, MVB, and CCV).
2. Role of the endocytic pathway in initiating CCV biogenesis
Genetic and chemical approaches demonstrate that initial transport of C. burnetii to the lysosome is mediated by host endocytic machinery. Mutants deficient in Dot/Icm transporter function are phagocytosed by host cells and transported to lysosomes but cannot form mature CCVs because translocation of T4SS effectors is blocked. These mutants survive in tight-fitting phagolysosomes for several days after infection [1, 28, 33, 5]. When expression of the icmDJB operon is induced one day after infection with a mutant having a transposon insertion in the icmD gene, the mutant regains the ability to create a vacuole that supports C. burnetii replication. This indicates that effector proteins delivered by the T4SS are not required for modulation of early endocytic processes that occur before bacteria are transported to a lysosomal compartment [1]. Similarly, inhibition of bacterial protein synthesis with chloramphenicol does not block C. burnetii transit to the lysosome but prevents translocation of effectors [18]. On the host side, inhibition of phagosome acidification with bafilomycin A1 also blocks bacterial replication and prevents Dot/Icm-mediated translocation of effectors [34]. Additionally, it takes several hours after infection until delivery of effectors into the host cell cytoplasm can be detected [34]. Multiple C. burnetii effectors produced constitutively from a plasmid have been analyzed for translocation kinetics, and translocation for all was shown to be dependent on endocytic transport and vacuole acidification. These data indicate that the host endocytic machinery delivers C. burnetii to an acidified organelle by a process that does not require bacterial effector proteins, and that the Dot/Icm system begins to deliver effector proteins into the host cell after C. burnetii senses the environment of a late-endocytic organelle,. Although these data do not rule out the possibility that there are specialized effectors that might be translocated before C. burnetii senses a late endocytic compartment, this seems unlikely as bacteria are not metabolically active in early endocytic compartments, which would limit their capacity to energize the T4SS.
Several members of the Rab GTPase family are required for transit of C. burnetii through the endocytic pathway. Rab GTPases associate with intracellular membranes and promote interactions between components of membrane transport machinery, such as SNARE proteins and coat components. Rab5 and Rab7 are essential regulators of membrane fusion with early and late endosomes, respectively. Rab5 associates with phagosomes containing C. burnetii within minutes of infection and promotes fusion with early endosomes. Next, Rab7 associates with the CCV to promote fusion with late endosomes and remains associated with the mature CCV [34]. Importantly, interfering with the function of these Rab proteins, either by production of dominant negative Rab5 or Rab7 or RNA silencing of Rab5 or Rab7, interferes with CCV maturation, and prevents C. burnetii replication [38]. In addition to Rab5 and Rab7, other Rab proteins that contribute to CCV maturation have been identified [2, 17, 36], but the mechanism by which these Rab proteins facilitate CCV biogenesis remains to be determined.
3. Novel features of the lysosome-derived CCV
3.1 Role of cholesterol in CCV biogenesis
Lysosomal membranes normally contain low levels of cholesterol, whereas, cholesterol is highly abundant on the membrane of the mature CCV [30, 36]. In uninfected cells, cholesterol regulates membrane fluidity and is enriched in the plasma membrane, Golgi apparatus, endoplasmic reticulum (ER) and multivesicular bodies (MVB) [30, 36]. Cholesterol also serves as an important regulator of membrane transport, and as such, is a potential contributor to CCV biogenesis. Drugs that block cholesterol synthesis inhibit C. burnetii entry and replication [16]. However, these inhibitors also affect synthesis of sterol intermediates that may be required for other processes. A mouse embryonic fibroblast cell line deficient for the last enzyme in the cholesterol biosynthesis pathway (DHCR24−/−) grown in cholesterol-depleted media was able to support replication of C. burnetii, although bacterial entry was negatively affected [26]. Additionally, C. burnetii expresses a sterol reductase that may affect cholesterol metabolism in the host [7, 15]. Thus, whether cholesterol enrichment on the CCV is important for infection or whether this is a secondary consequence of manipulation of other membrane transport processes remains an open question.
3.2 Role of autophagy in CCV biogenesis
The CCV displays high levels of microtubule-associated protein 1A/1B-light chain 3 (LC3), which is a protein that is covalently attached to the limiting membranes of autophagosomes. Autophagy is an ancient and conserved process used by eukaryotic cells to envelope cytosolic proteins or damaged organelles in a double-membrane bound structure called an autophagosome. Autophagosomes fuse with lysosomes to deliver captured cargo into lysosomes for hydrolytic degradation [10]. Fusion of autophagosomes with lysosomes generates a hybrid organelle called an autolysosome.
When LC3 is synthesized it exists as a soluble protein called LC3-I. Induction of autophagy leads to proteolytic processing of the C-terminus of LC3 and subsequent conjugation of LC3 to phosphatidylethanolamine on expanding phagophore membranes. The lipidated form of LC3 is called LC3-II. Because autophagosomes are double membrane structures, lipidated LC3 will be present both on the cytosolic surface of the autophagosome and also on the luminal face of the internal membrane [9]. Upon fusion with lysosomes, the cellular protease Atg4 deconjugates LC3-II from the cytosolic surface of the autolysosome, but the lipidated LC3 contained in the lumen of autophagic bodies will persist in the autolysosome until this structure is degraded by lysosomal enzymes [31, 13]. Thus, autophagosomes and autolysosomes can be identified by microscopy through visualization of LC3, and autophagy can be measured by immunoblot detection of the ratio of LC3-I to LC3-II [21].
When the protein GFP-LC3 was overproduced in CHO cells infected with C. burnetii it was found to colocalize with the CCV [32, 40, 39], which suggested that the CCV has characteristics of an autolysosome. Because overexpression of GFP-LC3 can result in aberrant localization [39], and the quenching of fluorescence that will occur when GFP fusion proteins are present in acidified organelles prevents detection of luminal GFP-LC3 [2, 44], additional studies were conducted to characterize interactions between CCVs and host autophagosomes Studies using cells producing RFP-LC3, which is not sensitive to low pH environments, confirmed LC3 localizes to the CCV membrane and showed that LC3 is also contained in the CCV lumen [37]. Importantly, recent data examining the localization of endogenous LC3 showed clearly the accumulation of LC3 in the CCV lumen, but endogenous levels of LC3 were not detected on the CCV membrane [3]. This supports a model in which autophagosomes fuse with the CCV, which delivers autophagic bodies containing LC3 into the lumen of the CCV. Although overproduced GFP-LC3 can be detected on the CCV membrane, the inability to detect endogenous LC3 on the CCV membrane suggests that after fusion the lipidated LC3 on the cytosolic face of the membrane is rapidly deconjugated. The observation that LC3 continues to accumulate in the CCV as this organelle expands during C. burnetii intracellular replication indicates constitutive fusion of autophagosomes with the CCV, which essentially locks the CCV in an autolysosomal state of maturation.
Initial studies examining GFP-LC3 localization suggested that fusion of autophagosomes with the CCV precedes fusion with lysosomes and is important for biogenesis of a CCV that supports C. burnetii replication [11]. Recent studies have shown efficient replication of C. burnetii in cells where genes encoding proteins essential for autophagy were silenced and that mutant C. burnetii that reside in LC3-negative vacuoles do not display intracellular growth defects [33]. Thus, host autophagy does not appear to be essential for formation of a CCV that supports C. burnetii replication.
3.3 CCV homotypic fusion
When a single host cell independently internalizes multiple C. burnetii, the vacuoles in which bacteria reside will usually fuse with each other to form a single CCV. This process is called homotypic fusion. How and why homotypic fusion occurs remains unknown. Recent data indicate that CCV homotypic fusion requires both proteins from the host and bacterial effectors. In the host, a group of proteins that directly mediates vesicle fusion events, called SNAREs, are candidates for proteins that mediate CCV homotypic fusion. At least one SNARE, VAMP-7, localizes to the CCV when it is overexpressed, and siRNA knockdown or expression of truncated versions of VAMP-7 reduces CCV fusogenicity [2, 36]. A genome-wide siRNA screen identified a SNARE-like protein called Syntaxin-17 as being important for homotypic fusion of CCVs [30, 33]. Because Syntaxin-17 has an important role in the biogenesis of autophagosomes and the fusion of autophagosomes with lysosomes [4], the role of autophagy in CCV homotypic fusion was further investigated. Indeed, silencing of essential host autophagy genes such as Atg5 resulted in defects in CCV homotypic fusion [12, 20, 30], which indicates that autophagy plays an important role in the homotypic fusion process.
4. Coxiella burnetii T4SS effectors regulate host membrane traffic to promote CCV biogenesis
Coxiella burnetii mutants have been used to identify which bacterial effectors modulate membrane transport. Screening of a transposon insertion library of individual C. burnetii mutants revealed a role for the effector Cig2 (also known as CvpB or Cbu0021) in promoting homotypic fusion of the CCV [20, 12]. Cells infected with cig2 mutants contain multiple CCVs, which results from a defect in homotypic fusion. Importantly, the cig2 mutant phenotype was complemented by expression of plasmid-encoded Cig2, which indicated that this defect in homotypic fusion resulted from the loss of Cig2 function [33]. Cig2 mutants were also reported to reside in tight fitting lysosomal compartments [33], which further suggests this effector is involved in biogenesis of the mature CCV. Given that the CCV displays features of an autolysosome and that host autophagy was found to be important for CCV homotypic fusion, vacuoles containing cig2 mutants were analyzed for LC3 accumulation. These data revealed that LC3 does not accumulate in vacuoles formed by cig2 mutants. Thus, the Cig2 protein is essential for promoting constitutive fusion of autophagosomes with the CCV to maintain this organelle in an autolysosomal stage of maturation, and this process is important for the highly fusogenic properties of the CCV. Additionally, when HeLa cells ectopically producing GFP-Cig2 are infection the GFP-Cig2 protein is observed on the CCV membrane, which suggests that Cig2 may play a direct role in modulating membrane transport at the CCV [33]. How Cig2 promotes fusion of autophagosomes with the CCV remains unknown, as this protein does not display homology to any other proteins and no clear enzymatic activities have been detected [28, 43].
In addition to Cig2, recent data indicate that the effector CvpA actively regulates host membrane transport. A C. burnetii cvpA mutant was found to have an intracellular replication defect, which indicates that CvpA plays a role in formation of the mature CCV. Importantly, plasmid-encoded CvpA complemented the intracellular replication defect displayed by the cvpA mutant. CvpA contains multiple eukaryotic-like endocytic sorting motifs, which are important for promoting CCV biogenesis. These motifs function in directing CvpA to endocytic compartments and promote interactions with host proteins involved in clathrin-mediated endocytic transport. A recent model suggests that CvpA facilitates CCV interactions with recycling endosomes, and this interaction is important for biogenesis of a CCV that supports C. burnetii replication [24].
CvpA and Cig2 are the only T4SS effectors that have been linked to cellular membrane transport pathways based on the phenotype of specific loss-of-function effector mutants, Because effector proteins delivered by T4SS secretion systems are present in the host cell in small amounts it is usually difficult to localize endogenously translocated effectors during infection. Thus, several localization studies have relied on ectopic expression of effectors upon transfection of host cells with plasmid-encoded alleles in conjunction with C. burnetii infection. Using this approach, ectopically produced GFP-tagged effector proteins CvpC, CvpD, and CvpE were localized to the CCV membrane, CpeB localized to LC3 positive structures, CpeC localized with ubiquitin-rich structures, CpeD localized to the ER, Cbu0077 localized to lysosomes and CCVs, Cbu0635 localized to the Golgi, and Cbu1532 and Cbu1825 localized to mitochondria [33]. It is unclear if these studies reflect the true locations of these effectors because overproduction of GPF-tagged effectors can result in mislocalization, which is in part because the biophysical constraints that occur during translocation into the host cytosol by the T4SS are not duplicated when proteins are ectopically synthesized by the host transcription and translation machineries. Thus, further studies are required to validate the subcellular organelles targeted by these C. burnetii effector proteins.
5. Conclusion
It is clear that C. burnetii has evolved sophisticated strategies to survive and replicate inside the hostile environment of a lysosome-derived organelle and that this requires bacterial manipulation of host membrane transport pathways. Recent studies show that infection of host cells deficient for either clathrin-mediated endosomal recycling or autophagy result in strong, visible, vacuolar phenotypes that can be linked to specific T4SS effectors. This highlights that this pathogen has extensive “knowledge” of host cell biology. There remain many unanswered questions as to the exact contributions of bacterial effectors and host pathways toward CCV biogenesis. Although it is clear that CCV homotypic fusion requires the effector Cig2 and autophagy, how Cig2-mediated subversion of autophagy promotes survival and replication of C. burnetii in animals remains to be determined. Because regulatory mechanisms that control the dynamics of fusion of autophagosomes and lysosomes are poorly understood, understanding the function of Cig2 may provide important insight into the molecular processes that govern biogenesis and maintenance of autolysosomes. Thus, future investigations that focus on specific host factors within trafficking pathways that modify the CCV, and how effectors of the T4SS modify these host factors, promise to reveal not only more about the unique nature of the CCV, but also about yet undiscovered host cell biological processes.
Acknowledgments
This work was supported by NIH Grant R01 AI114760 (CRR) and a National Science Foundation Graduate Research Fellowship (LJK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beare PA, Gilk SD, Larson CL, Hill J, Stead CM, Omsland A, et al. Dot/Icm type IVB secretion system requirements for Coxiella burnetii growth in human macrophages. MBio. 2011;2:e00175–11. doi: 10.1128/mBio.00175-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beron W, Gutierrez MG, Rabinovitch M, Colombo MI. Coxiella burnetii localizes in a Rab7-labeled compartment with autophagic characteristics. Infect Immun. 2002;70:5816–21. doi: 10.1128/IAI.70.10.5816-5821.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campbell TN, Choy FYM. The effect of pH on green fluorescent protein: a brief review. Mol Biol Today. 2001;2 [Google Scholar]
- 4.Campoy EM, Mansilla ME, Colombo MI. Endocytic SNAREs are involved in optimal Coxiella burnetii vacuole development. Cell Microbiol. 2013;15:922–41. doi: 10.1111/cmi.12087. [DOI] [PubMed] [Google Scholar]
- 5.Carey KL, Newton HJ, Luhrmann A, Roy CR. The Coxiella burnetii Dot/Icm system delivers a unique repertoire of type IV effectors into host cells and is required for intracellular replication. PLoS Pathog. 2011;7:e1002056. doi: 10.1371/journal.ppat.1002056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen C, Banga S, Mertens K, Weber MM, Gorbaslieva I, Tan Y, et al. Large-scale identification and translocation of type IV secretion substrates by Coxiella burnetii. Proc Natl Acad Sci U S A. 2010;107:21755–60. doi: 10.1073/pnas.1010485107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Czyz DM, Potluri LP, Jain-Gupta N, Riley SP, Martinez JJ, Steck TL, et al. Host-directed antimicrobial drugs with broad-spectrum efficacy against intracellular bacterial pathogens. MBio. 2014;5:e01534–14. doi: 10.1128/mBio.01534-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eckart RA, Bisle S, Schulze-Luehrmann J, Wittmann I, Jantsch J, Schmid B, et al. Antiapoptotic activity of Coxiella burnetii effector protein AnkG is controlled by p32-dependent trafficking. Infect Immun. 2014;82:2763–71. doi: 10.1128/IAI.01204-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilk SD, Beare PA, Heinzen RA. Coxiella burnetii expresses a functional Delta24 sterol reductase. J Bacteriol. 2010;192:6154–9. doi: 10.1128/JB.00818-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilk SD, Cockrell DC, Luterbach C, Hansen B, Knodler LA, Ibarra JA, et al. Bacterial colonization of host cells in the absence of cholesterol. PLoS Pathog. 2013;9:e1003107. doi: 10.1371/journal.ppat.1003107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gutierrez MG, Vazquez CL, Munafo DB, Zoppino FC, Beron W, Rabinovitch M, et al. Autophagy induction favours the generation and maturation of the Coxiella-replicative vacuoles. Cell Microbiol. 2005;7:981–93. doi: 10.1111/j.1462-5822.2005.00527.x. [DOI] [PubMed] [Google Scholar]
- 12.Hamasaki M, Furuta N, Matsuda A, Nezu A, Yamamoto A, Fujita N, et al. Autophagosomes form at ER-mitochondria contact sites. Nature. 2013;495:389–93. doi: 10.1038/nature11910. [DOI] [PubMed] [Google Scholar]
- 13.He C, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet. 2009;43:67–93. doi: 10.1146/annurev-genet-102808-114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Honarmand H. Q Fever: An Old but Still a Poorly Understood Disease. Interdiscip Perspect Infect Dis. 2012;2012:8. doi: 10.1155/2012/131932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Howe D, Heinzen RA. Replication of Coxiella burnetii is inhibited in CHO K-1 cells treated with inhibitors of cholesterol metabolism. Ann N Y Acad Sci. 2005;1063:123–9. doi: 10.1196/annals.1355.020. [DOI] [PubMed] [Google Scholar]
- 16.Howe D, Heinzen RA. Coxiella burnetii inhabits a cholesterol-rich vacuole and influences cellular cholesterol metabolism. Cell Microbiol. 2006;8:496–507. doi: 10.1111/j.1462-5822.2005.00641.x. [DOI] [PubMed] [Google Scholar]
- 17.Howe D, Heinzen RA. Fractionation of the Coxiella burnetii parasitophorous vacuole. Methods Mol Biol. 2008;445:389–406. doi: 10.1007/978-1-59745-157-4_25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Howe D, Melnicakova J, Barak I, Heinzen RA. Maturation of the Coxiella burnetii parasitophorous vacuole requires bacterial protein synthesis but not replication. Cell Microbiol. 2003;5:469–80. doi: 10.1046/j.1462-5822.2003.00293.x. [DOI] [PubMed] [Google Scholar]
- 19.Howe D, Shannon JG, Winfree S, Dorward DW, Heinzen RA. Coxiella burnetii phase I and II variants replicate with similar kinetics in degradative phagolysosome-like compartments of human macrophages. Infect Immun. 2010;78:3465–74. doi: 10.1128/IAI.00406-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Itakura E, Kishi-Itakura C, Mizushima N. The hairpin-type tail-anchored SNARE syntaxin 17 targets to autophagosomes for fusion with endosomes/lysosomes. Cell. 2012;151:1256–69. doi: 10.1016/j.cell.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 21.Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO. 2000;19:5720–8. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klingenbeck L, Eckart RA, Berens C, Luhrmann A. The Coxiella burnetii type IV secretion system substrate CaeB inhibits intrinsic apoptosis at the mitochondrial level. Cell Microbiol. 2013;15:675–87. doi: 10.1111/cmi.12066. [DOI] [PubMed] [Google Scholar]
- 23.Larson CL, Beare PA, Howe D, Heinzen RA. Coxiella burnetii effector protein subverts clathrin-mediated vesicular trafficking for pathogen vacuole biogenesis. Proc Natl Acad Sci U S A. 2013;110:E4770–9. doi: 10.1073/pnas.1309195110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larson CL, Beare PA, Voth DE, Howe D, Cockrell DC, Bastidas RJ, et al. Coxiella burnetii effector proteins that localize to the parasitophorous vacuole membrane promote intracellular replication. Infect Immun. 2015;83:661–70. doi: 10.1128/IAI.02763-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lifshitz Z, Burstein D, Peeri M, Zusman T, Schwartz K, Shuman HA, et al. Computational modeling and experimental validation of the Legionella and Coxiella virulence-related type-IVB secretion signal. Proc Natl Acad Sci U S A. 2013;110:E707–E15. doi: 10.1073/pnas.1215278110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lippincott-Schwartz J, Phair RD. Lipids and cholesterol as regulators of traffic in the endomembrane system. Annu Rev Biophys. 2010;39:559–78. doi: 10.1146/annurev.biophys.093008.131357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luhrmann A, Nogueira C, Carey KL, Roy CR. Inhibition of pathogen-induced apoptosis by a Coxiella burnetii type IV effector protein. Proc Natl Acad Sci U S A. 2010;107:18997–9001. doi: 10.1073/pnas.1004380107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martinez E, Cantet F, Fava L, Norville I, Bonazzi M. Identification of OmpA, a Coxiella burnetii protein involved in host cell invasion, by multi-phenotypic high-content screening. PLoS Pathog. 2014;10:e1004013. doi: 10.1371/journal.ppat.1004013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maurin M, Benoliel AM, Bongrand P, Raoult D. Phagolysosomes of Coxiella burnetii-infected cell lines maintain an acidic pH during persistent infection. Infect Immun. 1992;60:5013–16. doi: 10.1128/iai.60.12.5013-5016.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McDonough JA, Newton HJ, Klum S, Swiss R, Agaisse H, Roy CR. Host pathways important for Coxiella burnetii infection revealed by genome-wide RNA interference screening. MBio. 2013;4:e00606–12. doi: 10.1128/mBio.00606-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mizushima N. Autophagy: process and function. Genes Dev. 2007;21:2861–73. doi: 10.1101/gad.1599207. [DOI] [PubMed] [Google Scholar]
- 32.Mizushima N, Yoshimorim T, Levine B. Methods in Mammalian Autophagy Research. Cell. 2010;140:313–26. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Newton HJ, Kohler LJ, McDonough JA, Temoche-Diaz M, Crabill E, Hartland EL, et al. A screen of Coxiella burnetii mutants reveals important roles for Dot/Icm effectors and host autophagy in vacuole biogenesis. PLoS Pathog. 2014;10:e1004286. doi: 10.1371/journal.ppat.1004286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Newton HJ, McDonough JA, Roy CR. Effector Protein Translocation by the Coxiella burnetii Dot/Icm Type IV Secretion System Requires Endocytic Maturation of the Pathogen-Occupied Vacuole. PLoS ONE. 2013;8:e54566. doi: 10.1371/journal.pone.0054566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pan X, Luhrmann A, Satoh A, Laskowski-Arce MA, Roy CR. Ankyrin repeat proteins comprise a diverse family of bacterial type IV effectors. Science. 2008;320:1651–4. doi: 10.1126/science.1158160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Romano PS, Gutierrez MG, Beron W, Rabinovitch M, Colombo MI. The autophagic pathway is actively modulated by phase II Coxiella burnetii to efficiently replicate in the host cell. Cell Microbiol. 2007;9:891–909. doi: 10.1111/j.1462-5822.2006.00838.x. [DOI] [PubMed] [Google Scholar]
- 37.Stadler C, Rexhepaj E, Singan VR, Murphy RF, Pepperkok R, Uhlen M, et al. Immunofluorescence and fluorescent-protein tagging show high correlation for protein localization in mammalian cells. Nat Methods. 2013;10:315–23. doi: 10.1038/nmeth.2377. [DOI] [PubMed] [Google Scholar]
- 38.Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10:513–25. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- 39.Tanida I, Fau UT, Kominami E. LC3 and autophagy. Methods Mol Biol. 2008:77–88. doi: 10.1007/978-1-59745-157-4_4. [DOI] [PubMed] [Google Scholar]
- 40.Tanida I, Minematsu-Ikeguchi N, Ueno T, Kominami E. Lysosomal turnover, but not a cellular level, of endogenous LC3 is a marker for autophagy. Autophagy. 2005;1:84–91. doi: 10.4161/auto.1.2.1697. [DOI] [PubMed] [Google Scholar]
- 41.Voth DE, Beare PA, Howe D, Sharma UM, Samoilis G, Cockrell DC, et al. The Coxiella burnetii cryptic plasmid is enriched in genes encoding type IV secretion system substrates. J Bacteriol. 2011;193:1493–503. doi: 10.1128/JB.01359-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Voth DE, Howe D, Beare PA, Vogel JP, Unsworth N, Samuel JE, et al. The Coxiella burnetii ankyrin repeat domain-containing protein family is heterogeneous, with C-terminal truncations that influence Dot/Icm-mediated secretion. J Bacteriol. 2009;191:4232–42. doi: 10.1128/JB.01656-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weber MM, Chen C, Rowin K, Mertens K, Galvan G, Zhi H, et al. Identification of Coxiella burnetii type IV secretion substrates required for intracellular replciation an dCoxiella-containing vacuole formation. J Bacteriol. 2013;195:3914–24. doi: 10.1128/JB.00071-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Winchell CG, Graham JG, Kurten RC, Voth DE. Coxiella burnetii type IV secretion-dependent recruitment of macrophage autophagosomes. Infect Immun. 2014;82:2229–38. doi: 10.1128/IAI.01236-13. [DOI] [PMC free article] [PubMed] [Google Scholar]