Abstract
Helminth infections are known to induce modulation of both innate and adaptive immune responses in active and latent tuberculosis (TB). However, the role of helminth infections in modulating systemic cytokine responses in active and latent tuberculosis (LTB) is not known. To define the systemic cytokine levels in helminth-TB coinfection, we measured the circulating plasma levels of Type 1, Type 2, Type 17, other pro-inflammatory and regulatory cytokines in individuals with active TB (ATB) with or without coexistent Strongyloides stercoralis (Ss) infection by multiplex ELISA. Similarly, we also measured the same cytokine levels in individuals with LTB with or without concomitant Ss infection in a cross-sectional study. Our data reveal that individuals with ATB or LTB and coexistent Ss infection have significantly lower levels of Type 1 (IFNγ, TNFα and IL-2) and Type 17 (IL-17A and IL-17F) cytokines compared to those without Ss infection. In contrast, those with ATB and LTB with Ss infection have significantly higher levels of the regulatory cytokines (IL-10 and TGFβ), and those with LTB and Ss infection also have significantly higher levels of Type 2 cytokines (IL-4, IL-5 and IL-13) as well. Finally, those with LTB (but not ATB) exhibit significantly lower levels of other pro-inflammatory cytokines (IFNα, IFNβ, IL-6, IL-12 and GM-CSF). Our data therefore reveal a profound effect of Ss infection on the systemic cytokine responses in ATB and LTB and indicate that coincident helminth infections might influence pathogenesis of TB infection and disease.
Keywords: Tuberculosis, Strongyloides, Helminths, Cytokines
INTRODUCTION
Helminth infections and tuberculosis are both major public health problems worldwide with tuberculosis (TB) afflicting nearly 10 million new cases annually 1 and helminths infecting over 2 billion people 2. In addition, TB and helminth infections share considerable geographical overlap with both infections affecting mostly lower and some middle-income countries worldwide 3. Furthermore, the larvae of many intestinal helminths migrate through the lungs, thereby providing a biological pathway for these helminths to influence the host immune response to TB 3. Typically, TB manifests itself as a clinical spectrum ranging from asymptomatic, latent infection to clinically active pulmonary or extra-pulmonary disease. After initial infection, most individuals control bacterial replication and enter a period of infectious latency known as latent tuberculosis (LTB). Approximately, 5–10% of those with LTB progress to active tuberculosis (ATB) in their lifetime, a progression reflecting the failure of host immune responses in containing bacterial replication 1. While the adaptive immune system is pivotal in the pathogenesis of TB disease, it is also abundantly clear that systemic inflammatory and cytokine responses also significantly influence disease activity and severity 4, 5.
Helminth parasites are commonly characterized by their ability to establish chronic infections in humans, sometimes lasting decades. Although helminth infections are rarely lethal, they can contribute to morbidity in adults and impair physical and cognitive development in children 6. Among the helminth parasites, Strongyloides stercoralis (Ss) the causative agent of strongyloidiasis, infects ~50–100 million people worldwide 7, 8. Ss infection is often clinically asymptomatic and longstanding due, in large part, to the parasites’ autoinfective lifecycle and their ability to modulate or evade the host immune system 2, 9. Recent, epidemiological and experimental evidence provide evidence that helminths (both systemic and intestinal) have a negative regulatory role in the immune response to TB infection and disease, although no evidence exists for an impact on clinical outcomes 3, 10, 11.
Helminth infections are known to induce modulatation of T cell mediated immune responses to TB antigens in both LTB and ATB 12. However, their role in modulating systemic cytokine responses in coincident LTB and ATB has not been fully explored. We therefore hypothesized that the regulatory networks established during chronic Ss infection could potentially modulate the cytokine response to TB infection and disease. Thus, we examined the systemic levels of a variety of systemic cytokines, shown to influence either susceptibility or resistance to active disease in LTB or systemic or local pathology in ATB. We find that coexistent Ss infection has a major impact on the innate and adaptive cytokine responses in both LTB and ATB, with a much more pronounced effect on LTB.
MATERIALS AND METHODS
Ethics statement
All individuals were examined as part of a natural history study protocol approved by Institutional Review Boards of the National Institute of Allergy and Infectious Diseases (USA) and the National Institute for Research in Tuberculosis (India), and informed written consent was obtained from all participants.
Study population
We studied a group of 88 individuals with active pulmonary TB, 42 of whom were infected with S. stercoralis (hereafter ATB+Ss) infection and 46 of whom had active TB alone (ATB) (Table 1). We also studied another group of 88 individuals with latent TB, 44 of whom were infected with S. stercoralis (hereafter LTB+Ss) infection and 44 of whom had active TB alone (LTB). All the study individuals were recruited from patients and their relatives attending the outpatient clinic at the Stanley Medical Hospital, Chennai. This was a cross-sectional study and a convenient sampling methodology was used. Samples were collected at the time of diagnosis of ATB and LTB. ATB was diagnosed microbiologically on the basis of being culture positive for Mtb by solid cultures in LJ medium and all were sputum smear positive. LTB individuals were asymptomatic with positive Quantiferon Gold - in -tube tests and normal chest radiographs. Ss infection was diagnosed by the presence of IgG antibodies to the 31-kDa recombinant NIE antigen by the Luciferase Immunoprecipitation System Assay (LIPS), as described previously 13. This test has a been reported previously to be the most accurate serologic test for diagnosis of Ss infection 14. We used a cutoff of 10,000 light units as determined by positive and negative controls previously, which had used stool microscopy as the reference standard. All individuals were also negative for filarial infection by filarial antigen tests (ICT card test and TropBio ELISA) but stool microscopy for intestinal helminths was not done. All individuals were HIV negative (determined by rapid card test), non-diabetic (determined by fasting blood glucose) and anti-tuberculous and antihelmintic (self-reported) treatment naive. The two groups of active TB individuals did not differ significantly in bacillary burden (as estimated by smear grades at the time of diagnosis following Ziehl-Nielsen staining). Moreover, the individuals in this study were different from the group of individuals described in our previous studies 15, 16.
Table 1.
Demographics and hematological parameters of the study population
| LTB | LTB+Ss | p value | ATB | ATB+Ss | p value | |
|---|---|---|---|---|---|---|
| n=44 | n=44 | n=46 | n=42 | |||
| Age | 35 (23–60) | 48 (28–64) | NS | 36 (18–65) | 42 (18–65) | NS |
| M/F | 21/23 | 20/24 | NS | 35/11 | 33/09 | NS |
| Smear grade: 1+/2+/3+ | nil | nil | NA | 25/10/11 | 20/18/4 | NS |
| NIE LIPS | Neg | Pos | NA | Neg | Pos | NA |
| WBC 103/uL | 7.56 (4–15.1) | 7.96 (5.6–17.7) | NS | 9.95 (4.3–20.3) | 9.78 (4.1–15) | NS |
| Hb g/dL | 13.1 (6–17.7) | 13.42 (9.1–17.6) | NS | 11.9 (7.4–19.6) | 11.5 (3.7–20.2) | NS |
| Neutrophil 103/uL | 3.87 (2.26– 8.26) | 4.02 (2.31– 7.56) | NS | 6.9 (3.65– 17.74) | 6.31 (2.14– 12.58) | NS |
| Lymphocytes 103/uL | 2.34 (1.11–4.61) | 2.54 (1.56–4.61) | NS | 1.7 (0.64–3.92) | 1.87 (1.03–3.81) | NS |
| Monocytes 103/uL | 0.51 (0.23–1.32) | 0.52 (0.27–1.06) | NS | 0.7 (0.18–1.33) | 0.74 (0.25–1.81) | NS |
| Eosinophils 103/uL | 0.48 (0.13–2.27) | 0.44 (0.16–1.95) | NS | 0.23 (0.06– 1.06) | 0.29 (0.04–4.34) | NS |
Values represent the geometric mean or median (and range) and the p values were calculated using the Mann-Whitney U test.
ELISA
Plasma cytokines were measured using a Bioplex multiplex cytokine assay system (Bio-Rad, Hercules, CA). The parameters analyzed were IFNγ, TNFα, IL-2, IL-17A, IL-4, IL-5, IL-10, IL-6, IL-12p70 and GM-CSF. Plasma levels of TGFβ, IL-1α, IL-1β (all R& D Systems); IL-17F (Biolegend); IL-22 (eBioscience); Type 1 interferons (IFNs) - IFNα (multiple subtypes) and IFNβ (PBL Interferon Source) were measured by ELISA. All samples were run in duplicates.
Statistical analysis
Data analyses were performed using GraphPad PRISM (GraphPad Software, Inc., San Diego, CA, USA). Geometric means (GM) were used for measurements of central tendency. Comparisons were made using either by Mann-Whitney U test for comparison between 2 groups or by Kruskal-Wallis test with Dunn’s multiple comparisons for multiple groups or by Student’s t-test and adjusted by Benjamin-Hochberg Procedure for the heatmaps. R software package was used to plot the heatmap for log2 transformed values of plasma levels.
RESULTS
Study population characteristics
The baseline characteristics including demographic and hematological features of the study population are shown in Table 1. As can be seen, compared to ATB individuals, ATB+Ss individuals exhibited no significant differences in age, gender, smear grades or hematological parameters. Similarly, compared to LTB individuals, LTB+Ss individuals exhibited no significant differences in age, gender or hematological parameters. Moreover, cytokine levels did not exhibit any correlation with age, gender or smear grades.
Coexistent Ss infection is associated with diminished levels of Type 1 and Type 17 cytokines in ATB and LTB
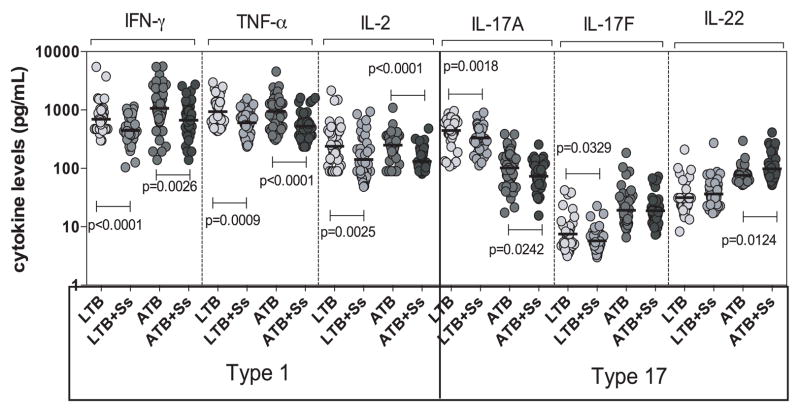
To determine the influence of coexistent Ss infection on Type 1 and Type 17 cytokines in ATB and LTB, we measured the circulating levels of IFNγ, TNFα, IL-2, IL-17A, IL-17F and IL-22 in those with ATB, ATB+Ss, LTB and LTB+Ss (Figure 1). As shown in Figure 1, the systemic levels of all three Type 1 cytokines – IFNγ (GM of 669.5 pg/ml in ABT+Ss versus 1066 pg/ml in ATB, p=0.0026), TNFα (GM of 528.6 pg/ml vs. 945.2 pg/ml, p<0.0001) and IL-2 (GM of 131.3 pg/ml vs. 239.6 pg/ml, p<0.0001) were significantly lower in ATB+Ss compared to ATB individuals. In addition, the systemic levels of IFNγ (GM of 444 pg/ml in LTB+Ss versus 693.9 pg/ml in LTB, p<0.0001), TNFα (GM of 605.5 pg/ml vs. 938.5 pg/ml, p=0.0009) and IL-2 (GM of 142.1 pg/ml vs. 238.1 pg/ml, p=0.0025) were significantly lower in LTB+Ss compared to LTB individuals. Similarly, the systemic levels of the prototypical Type 17 cytokine – IL-17A (GM of 73.3 pg/ml vs. 102.2 pg/ml, p=0.0242) was also significantly lower in ATB+Ss compared to ATB individuals. In contrast, the systemic levels of IL-22 was significantly higher in ATB compared to ATB+Ss individuals (GM of 97.9 pg/ml vs. 76.2 pg/ml, p=0.0124). In comparison to LTB individuals, LTB+Ss individuals exhibit significantly lower levels of both Th17 cytokines - IL-17A (GM of 333.3 pg/ml vs. 446 pg/ml, p=0.0018) and IL-17F (GM of 5.7 pg/ml vs. 7.5 pg/ml, p=0.0329). Thus, coexistent Ss infection is associated with down modulation of systemic Type 1 and Type 17 cytokines in both ATB and LTB.
Figure 1. Helminth infections are associated with diminished plasma levels of Type 1 and Type 17 cytokines in active and LTB.
The plasma levels of Type 1 (IFNγ, TNFα, IL-2) and Type 17 (IL-17A, IL-17F, IL-22) cytokines - were measured by multiplex ELISA in active pulmonary TB individuals with (ATB+Ss, n=42) or without Ss coinfection (ATB, n=46) and in latent - TB infected individuals with (LTB+Ss, n=44) or without Ss coinfection (LTB, n=44). The results are shown as scatterplots with each circle representing a single individual and the bar representing the GM. P values were calculated using the Kruskal-Wallis test with Dunn’s multiple comparisons.
Coexistent Ss infection is associated with elevated levels of Type 2 and/or regulatory cytokines in ATB and LTB
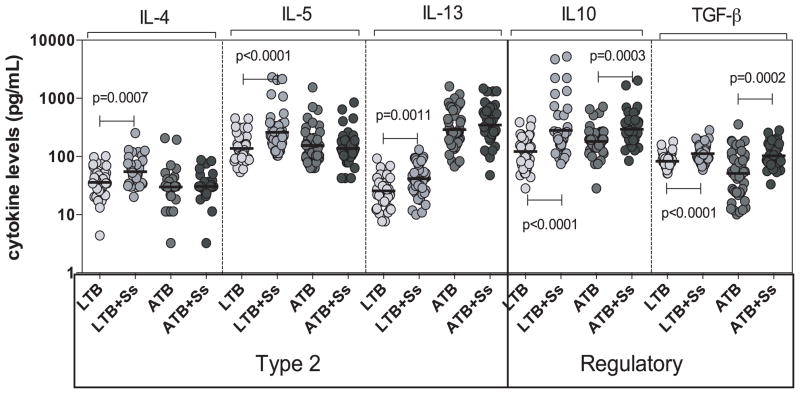
To determine the influence of coexistent Ss infection on Type 2 and regulatory cytokines in ATB and LTB, we measured the circulating levels of IL-4, IL-5 and IL-13 as well as IL-10 and TGFβ in ATB and ATB+Ss as well as LTB and LTB+Ss individuals (Figure 2). As shown in Figure 2, the systemic levels of all three Type 2 cytokines – IL-4 (GM of 54.5 pg/ml in LTB+Ss versus 34.9 pg/ml in LTB, p=0.0007), IL-5 (GM of 260.5 pg/ml vs. 137.4 pg/ml, p<0.0001) and IL-13 (GM of 41.7 pg/ml vs. 25.7 pg/ml, p=0.0011) were significantly higher in LTB+Ss compared to LTB individuals. No differences in the Type 2 cytokine levels was observed in ATB+Ss compared to ATB individuals. Similarly, the systemic levels of the regulatory cytokines IL-10 (GM of 280.6 pg/ml vs. 121.4 pg/ml, p<0.0001) and TGFβ (GM of 112.2 pg/ml vs. 82.9 pg/ml, p<0.0001) was also significantly higher in LTB+Ss compared to LTB individuals. In comparison to ATB individuals, ATB+Ss individuals exhibit significantly higher levels of both IL-10 (GM of 297.3 pg/ml vs. 181.5 pg/ml, p=0.0003) and TGFβ (GM of 102.7 pg/ml vs. 51.1 pg/ml, p=0.0002). Thus, coexistent Ss infection is associated with up regulation of systemic Type 2 and regulatory cytokines in LTB and regulatory cytokines alone in ATB.
Figure 2. Helminth infections are associated with elevated plasma levels of Type 2 and regulatory cytokines in active and LTB.
The plasma levels of Type 2 (IL-4, IL-5, IL-13) and regulatory (IL-10, TGFβ) cytokines were measured by multiplex ELISA in active pulmonary TB individuals with (ATB+Ss, n=42) or without Ss coinfection (ATB, n=46) and in latent-TB infected individuals with (LTB+Ss, n=44) or without Ss coinfection (LTB, n=44). The results are shown as scatterplots with each circle representing a single individual and the bar representing the GM. P values were calculated using the Kruskal-Wallis test with Dunn’s multiple comparisons.
Coexistent Ss infection is associated with diminished levels of pro-inflammatory cytokines in ATB and LTB
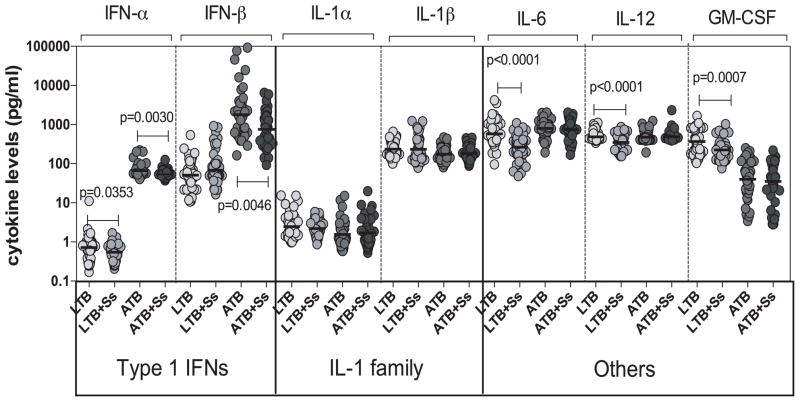
To determine the influence of coexistent Ss infection on other pro-inflammatory cytokines in ATB and LTB, we measured the circulating levels of IFNα, IFNβ, IL-1α, IL-1β, IL-6, IL-12 and GM-CSF in ATB and ATB+Ss as well as LTB and LTB+Ss individuals (Figure 3). As shown in Figure 3, the systemic levels of Type 1 IFNs - IFNα (GM of 53.7 pg/ml in ATB+Ss versus 66.3 pg/ml in ATB, p=0.0030) and IFNβ (GM of 755.5 pg/ml vs. 1781 pg/ml, p=0.0046) were significantly lower in ATB+Ss compared to ATB individuals. In addition, as shown in Figure 3, the systemic levels of IL-6 (GM of 266 pg/ml vs. 576 pg/ml, p<0.0001), IL-12 (GM of 350.3 pg/ml vs. 487.7 pg/ml, p<0.0001) and GM-CSF (GM of 224.2 pg/ml vs. 370.2 pg/ml, p=0.0007) were all significantly lower in LTB+Ss compared to LTB individuals. Thus, coexistent Ss infection is associated with down modulation of systemic pro-inflammatory cytokines in both ATB and LTB.
Figure 3. Helminth infections are associated with diminished plasma levels of pro-inflammatory cytokines in LTB.
The plasma levels of Type 1 IFNs (IFNα, IFNβ), IL-1 family (IL-1α, IL-1β) and other pro-inflammatory (IL-6, IL-12, GM-CSF) cytokines were measured by ELISA in active pulmonary TB individuals with (ATB+Ss, n=42) or without Ss coinfection (ATB, n=46) and in latent-TB infected individuals with (LTB+Ss, n=44) or without Ss coinfection (LTB, n=44). The results are shown as scatterplots with each circle representing a single individual and the bar representing the GM. P values were calculated using the Kruskal-Wallis test with Dunn’s multiple comparisons.
Elevated circulating levels of IFNα, IFNβ, IFNγ but decreased levels of IL-1α and IL-1β in ATB
Elevated circulating levels of certain cytokines (such as Type 1 IFNs and IFNγ) combined with decreased levels of certain cytokines (IL-1 family) are known to reflect disease activity and/or severity in pulmonary TB 5, 17. To verify these data in our study groups comprising of only individuals with TB and no helminth infection, we examined the circulating levels of Type 1 (IFNγ, TNFα, IL-2), Type 2 (IL-4, IL-5, IL-13), Type 17 (IL-17A, IL-17F, IL-22), regulatory (IL-10, TGFβ), Type 1 IFNs (IFNα, IFNβ), IL-1 family (IL-1α, IL-1β) and other (IL-6, IL-12, GM-CSF) cytokines in ATB (n=46) and compared them to those in LTB individuals (n=44). As shown in Table 2, ATB individuals exhibited significantly higher levels of IFNγ, IL-17F, IL-22, IL-13, IL-10, IFNα, IFNβ and IL-6 in comparison to LTB individuals. In contrast, ATB individuals exhibited significantly lower levels of IL-17A, IL-1α, IL-1β and GM-CSF in comparison to LTB individuals. Hence, our data thus confirms previous reports and adds new data to the finding that the balance among different cytokine families might reflect disease activity in ATB.
Table 2.
Cytokine levels in LTB and ATB individuals
| Cytokines | LTB (n=44) | ATB (n=46) | p value |
|---|---|---|---|
| IFN-γ | 693.9 (300.66–5486.29) | 1066 (140.08–5574.88) | 0.0007 |
| TNF-α | 938.5 (437.56–2960.7) | 945.2 (307.32–4537.08) | NS |
| IL-2 | 238.1 (88.66–2150.32) | 249.6 (89.6–1094.62) | NS |
| IL-17A | 446 (108.56–955.3) | 102.2 (17.49–388.6) | <0.0001 |
| IL-17F | 7.505 (3.15–42.21) | 19.06 (6.56–184.55) | <0.0001 |
| IL-22 | 31.79 (8.36–211.38) | 76.24 (51.8–295.8) | <0.0001 |
| IL-4 | 35.9 (4.38–100.92) | 29.89 (3.24–206.55) | NS |
| IL-5 | 137.4 (53.78–452.71) | 154.1 (63.23–1542.03) | NS |
| IL-13 | 25.67 (7.64–92.23) | 289.3 (67.52–1604.89) | <0.0001 |
| IL-10 | 121.4 (28.29–421.08) | 181.5 (28.29–716.57) | 0.0022 |
| TGF-β | 82.89 (51.02–180.02) | 51.14 (10.12–357.36) | NS |
| IFN-α | 0.7166 (0.17–11.12) | 66.25 (40.34–219.32) | <0.0001 |
| IFN-β | 50.85 (10.71–537.68) | 1781 (162.89–92354.94) | <0.0001 |
| IL-1α | 2.458 (0.97–15.44) | 1.559 (0.58–15.01) | 0.0027 |
| IL-1β | 235.7 (100.39–654.02) | 174.1 (80.64–470.37) | 0.0039 |
| IL-6 | 576 (96.37–4196.09) | 799.8 (193.81–2019.38) | 0.0062 |
| IL-12 | 487.7 (341.68–1112.30) | 476.7 (193.81–1233.11) | NS |
| GM-CSF | 370.2 (104.08–1671.36) | 39.9 (3.40–250.45) | <0.0001 |
Values represent geometric mean with 95% confidence interval and p values were calculated using the Mann-Whitney U test.
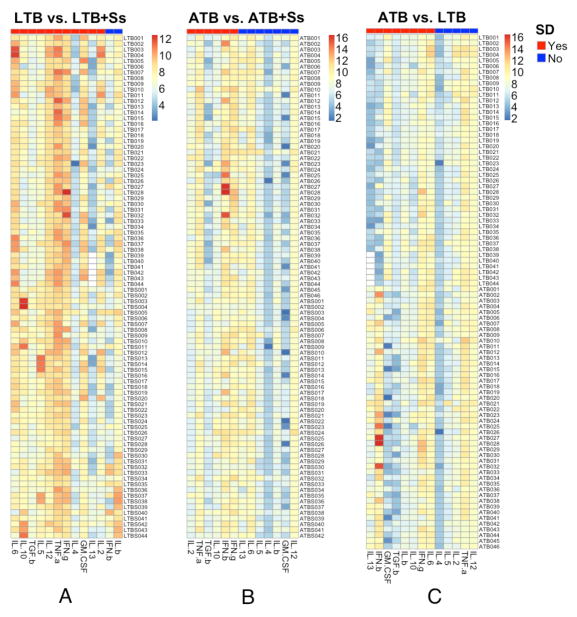
The heatmap reveals trends of cytokine modulation in helminth - TB coinfection
To visualize the trends in the modulation of systemic cytokines in Ss - TB coinfection and disease, heatmaps were plotted for the various cytokines in LTB versus LTB+Ss; ATB versus ATB+Ss and LTB versus ATB and compared the differentially expressed cytokines following adjustment for multiple comparisons. As shown in Figure 4A, the cytokines that are differentially expressed in LTB+Ss and LTB groups are IL-6, IL-10, TGFβ, IL-5, IL-12, TNFα, IL-4, GM-CSF, IL-13 and IL-2. Similarly, as shown in Figure 4B, the cytokines that are differentially expressed in ATB+Ss and ATB groups are IL-2, TNFα, TGFβ, IL-10 and IFNβ. Finally, the cytokines that are differentially expressed in ATB and LTB groups are IL-13, IFNβ, GM-CSF, TGFβ, IL-1β, IL-10, IFNγ and IL-6 (Figure 4C). Thus, heatmaps reveal clear trends in the modulation of systemic cytokines in Ss-TB coinfection.
Figure 4. Heatmaps depicting the trends in the modulation of cytokines in Ss-TB coinfection and in LTB versus ATB.
Heatmaps of log2 transformed plasma levels for LTB vs. LTB+Ss, ATB vs. ATB+Ss, and ATB vs. LTB were shown in panel A, B, C respectively, in which each row stands for a sample and each column stands for a cytokine. The annotation bar indicates if there is a significant difference (SD) between two groups or not for each cytokine. The p value of each cytokine was calculated by Student’s t-test and adjusted by Benjamini-Hochberg Procedure.
DISCUSSION
Numerous studies in humans as well as in animal models clearly suggest that helminth infections or their products can engender protection from a variety of inflammatory diseases such as allergic disease, autoimmune disease and inflammatory bowel disease 18, 19. The characteristic ability of helminths to express and secrete immunoregulatory molecules to suppress anti-helminth and pathological responses at multiple levels renders helminths with the ability to also modulate host pathology during other chronic infections 18, 20. Typically associated with classical Th2 responses, helminth infections are also known inducers of a variety of regulatory pathways including regulatory T and B cells, alternatively activated macrophages and other accessory cells to suppress host - protective (and possibly pathological) pro-inflammatory responses 2. Moreover, experimental infection with helminths or treatment with helminth products have recently been shown to exert beneficial effects on inflammatory bowel disease and other inflammatory disorders 21, 22. We and others have previously shown that helminth infections can modulate both the innate (TLR responses) and adaptive (T cell responses) arms of the immune system in active and LTB in an antigen-specific manner 15, 23, 24. In this study, we sought to elucidate the systemic effects (if any) of a chronic helminth infection on the systemic cytokine response that is characteristic of ATB and LTB. S. stercoralis infection is known to overlap geographically with M. tuberculosis 25 and, more specifically, Ss infection in the mouse has been shown to impair immune responses to TB infection 3 and to alter the expression of biomarkers associated with pathogenesis in ATB 16. Finally, Ss larvae migrate from the skin to the intestine via the lungs and hence have the potential to modulate responses in TB. Therefore, we elected to examine the interaction of Ss and M. tuberculosis at the systemic level in both active and latent infection.
Infection with M. tuberculosis typically results in either resistance to infection or development of latent infection 1. A small percentage (5–10%) of individuals with latent infection usually progress to active pulmonary disease. The factors that promote acquisition of infection and/or promote development of disease are still poorly understood. Moreover, the correlates of protective immunity to both ATB and LTB remains to be well defined 26. While CD4+ T cells and the production of key cytokines - IFNγ and TNFα have been clearly associated with immune mediated protection against TB infection in animal models and humans 1, 27, the role of other cytokine networks and their effects on this response still lack clarity. However, evidence from animal models supports a role for Type 1 and Type 17 cytokines in protection 27, 28 and a role for Type 2 and regulatory cytokines in either susceptibility to disease or enhanced disease severity 29,30. In addition, the interplay between other pro-inflammatory cytokines, most notably Type 1 interferons and cytokines of the IL-1 family are also thought to play a pivotal role in immunity to TB 31.
Our study reveals the following salient features in terms of the plasma cytokine responses in ATB or LTB individuals with or without coexisting Ss infection. First, Type 1 cytokines, normally associated with protective immunity in both LTB and active pulmonary TB 1, including IFNγ, TNFα and IL-2, are clearly shown to be down-modulated in helminth co-infected individuals. This is the most direct associative evidence that coexistent helminth infection can indeed profoundly alter the protective immune response in TB infection and disease, at least at the systemic level. Second, Type 17 cytokines, especially IL-17A is also down modulated in helminth/TB co-infected individuals. Among the Type 17 cytokines, only IL-17A has been shown to clearly exert protective immunity against TB infection in mice 32. The role of IL-17F and IL-22 in TB infection remains poorly explored. Our data would therefore suggest that while the prototypical Th17 cytokine (IL-17A) follows the same pattern as Type 1 cytokines, IL-22 and to a lesser extent IL-17F do not. These findings suggest that a more detailed investigation into the roles of IL-17F and IL-22 need to be performed in animal models of TB infection. Third, our data reveal that Type 2 cytokines reveal a far more profound effect on latent infection than on active infection in the presence of helminth coinfection. This would suggest that Type 2 cytokines, acting through a variety of mechanisms, such as inhibition of protective Type 1/Type 17 responses, induction of alternative activation of macrophages, abrogation of host protective autophagy and other intracellular protective responses, could facilitate the development of reactivation of active disease in LTB, as has been previously reported 30. Fourth, our data shows an important association of increase in regulatory cytokines with the presence of helminth co-infection in both active and LTB, thereby suggesting that both IL-10 and TGFβ play an important role in balancing the immune responses to TB, as previously postulated 29. Fifth, our data show that a panel of pro-inflammatory cytokines, including IFNα, IL-6, IL-12 and GM-CSF are predominantly modulated by coinfection only in LTB individuals. This might be reflective of the fact that coincident helminth infections are unable to exert significant modulatory effects in the face of an overwhelmingly inflammatory response induced by TB disease per se 4 or due to differences in intensity or severity of Ss infection between the groups.
Finally, our study also offers novel insights into the differences in plasma cytokines between active and LTB. In comparison to LTB individuals, active TB patients exhibit increased concentrations of cytokines known to promote disease pathology (such as Type 1 IFNs, IFNγ and IL-6) as well as cytokines that clearly function by down modulating protective cytokine responses (such as IL-10 and IL-13). In contrast, active TB individuals exhibit significantly lower concentration of host protective cytokines (such as IL-1α, IL-1β, IL-17 and GM-CSF). These data suggest that apart from the conventionally explored Type 1 cytokines, others such as IL-1 family of cytokines, IL-17 and GM-CSF might be associated with protection from development of active disease. Indeed, recent evidence from murine models does implicate a critical role for all of the above cytokines in host protection against TB 32–34. In addition, independent analysis using heat map analysis also confirms the trends observed in the modulation of systemic cytokines in helminth-TB coinfection and the separation of LTB versus ATB. Although NIE ELISA has been reported as the current best indicator recommended for these types of studies, it still cannot conclusively discern between current, recent or past resolved infection. Moreover, Ss can often be asymptomatic, and there are no current methods for reliably indicating severity. Therefore, it is not possible to rule out differences in intensity or severity of infection between the different groups. It is also difficult to exclude any effect of other common helminth coinfections for which screening was not performed. Regardless, the consistency of the data demonstrates significant trends corresponding with Ss antibody presence that logically make sense alongside other emerging investigations in this area.
Our findings have major implications for the design of studies exploring vaccine induced immune responses in individuals from geographical areas of helminth co-endemicity 3. Understanding the balance between pro- and anti- inflammatory cytokines is crucial for developing more effective measures of combating TB infection and disease, and the lack of knowledge of the mechanisms that mediate protection and pathogenesis is a major hurdle in improving vaccination and therapeutic strategies 4. Clearly, coexistent helminth infection can significantly skew the systemic immune response in TB infections or disease and alter the immune response to TB antigens. Our data add another layer of complexity the understanding of chronic co-infections and suggest that the presence of a coexistent chronic infection could also have a bystander effect on the disease manifestations in TB. While our study is clearly preliminary and needs to be confirmed in a much larger setting with additional examination of local cytokine responses -- and while longitudinal studies examining the effect of anthelmintic treatment on systemic and antigen - specific cytokine responses in TB needs to be examined -- our data clearly delineate the major regulatory effects that helminth infections can exert on the immune response to third party infections and suggest additional targets to direct the host response in order to combat tuberculosis.
Acknowledgments
We thank Dr. Satiswaran and Prabbu Balakrishnan for valuable assistance in collecting the clinical data for this study. We thank Kadar Moideen, M. Saravanan and R. Anuradha for technical assistance and the Department of Bacteriology, NIRT for bacterial cultures. We thank the staff of the Department of Clinical Research, NIRT, Department of Epidemiology, NIRT, and Government Stanley Hospital, Chennai, for valuable assistance in recruiting the patients for this study.
Footnotes
Conflict of interest: None reported.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.O’Garra A, Redford PS, McNab FW, Bloom CI, Wilkinson RJ, Berry MP. The immune response in tuberculosis. Annu Rev Immunol. 2013;31:475–527. doi: 10.1146/annurev-immunol-032712-095939. [DOI] [PubMed] [Google Scholar]
- 2.Allen JE, Maizels RM. Diversity and dialogue in immunity to helminths. Nat Rev Immunol. 2011;11:375–88. doi: 10.1038/nri2992. [DOI] [PubMed] [Google Scholar]
- 3.Salgame P, Yap GS, Gause WC. Effect of helminth-induced immunity on infections with microbial pathogens. Nat Immunol. 2013;14:1118–26. doi: 10.1038/ni.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Etna MP, Giacomini E, Severa M, Coccia EM. Pro- and anti-inflammatory cytokines in tuberculosis: a two-edged sword in TB pathogenesis. Semin Immunol. 2014;26:543–51. doi: 10.1016/j.smim.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 5.Wallis RS, Kim P, Cole S, Hanna D, Andrade BB, Maeurer M, Schito M, Zumla A. Tuberculosis biomarkers discovery: developments, needs, and challenges. Lancet Infect Dis. 2013;13:362–72. doi: 10.1016/S1473-3099(13)70034-3. [DOI] [PubMed] [Google Scholar]
- 6.King CH. Health metrics for helminthic infections. Adv Parasitol. 2010;73:51–69. doi: 10.1016/S0065-308X(10)73003-7. [DOI] [PubMed] [Google Scholar]
- 7.Babu S, Nutman TB. Immunology of lymphatic filariasis. Parasite Immunol. 2013 doi: 10.1111/pim.12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonne-Annee S, Hess JA, Abraham D. Innate and adaptive immunity to the nematode Strongyloides stercoralis in a mouse model. Immunol Res. 2011;51:205–14. doi: 10.1007/s12026-011-8258-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maizels RM, Yazdanbakhsh M. Immune regulation by helminth parasites: cellular and molecular mechanisms. Nat Rev Immunol. 2003;3:733–44. doi: 10.1038/nri1183. [DOI] [PubMed] [Google Scholar]
- 10.Chatterjee S, Kolappan C, Subramani R, Gopi PG, Chandrasekaran V, Fay MP, Babu S, Kumaraswami V, Nutman TB. Incidence of active pulmonary tuberculosis in patients with coincident filarial and/or intestinal helminth infections followed longitudinally in South India. PLoS One. 2014;9:e94603. doi: 10.1371/journal.pone.0094603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chatterjee S, Nutman TB. Helminth-induced immune regulation: implications for immune responses to tuberculosis. PLoS Pathog. 2015;11:e1004582. doi: 10.1371/journal.ppat.1004582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Metenou S, Babu S, Nutman TB. Impact of filarial infections on coincident intracellular pathogens: Mycobacterium tuberculosis and Plasmodium falciparum. Curr Opin HIV AIDS. 2012;7:231–8. doi: 10.1097/COH.0b013e3283522c3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramanathan R, Burbelo PD, Groot S, Iadarola MJ, Neva FA, Nutman TB. A luciferase immunoprecipitation systems assay enhances the sensitivity and specificity of diagnosis of Strongyloides stercoralis infection. J Infect Dis. 2008;198:444–51. doi: 10.1086/589718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bisoffi Z, Buonfrate D, Sequi M, Mejia R, Cimino RO, Krolewiecki AJ, Albonico M, Gobbo M, Bonafini S, Angheben A, Requena-Mendez A, Munoz J, Nutman TB. Diagnostic accuracy of five serologic tests for Strongyloides stercoralis infection. PLoS Negl Trop Dis. 2014;8:e2640. doi: 10.1371/journal.pntd.0002640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.George PJ, Anuradha R, Kumar NP, Sridhar R, Banurekha VV, Nutman TB, Babu S. Helminth infections coincident with active pulmonary tuberculosis inhibit mono- and multifunctional CD4+ and CD8+ T cell responses in a process dependent on IL-10. PLoS Pathog. 2014;10:e1004375. doi: 10.1371/journal.ppat.1004375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.George PJ, Kumar NP, Sridhar R, Hanna LE, Nair D, Banurekha VV, Nutman TB, Babu S. Coincident helminth infection modulates systemic inflammation and immune activation in active pulmonary tuberculosis. PLoS Negl Trop Dis. 2014;8:e3289. doi: 10.1371/journal.pntd.0003289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walzl G, Ronacher K, Hanekom W, Scriba TJ, Zumla A. Immunological biomarkers of tuberculosis. Nat Rev Immunol. 2011;11:343–54. doi: 10.1038/nri2960. [DOI] [PubMed] [Google Scholar]
- 18.Harnett W, Harnett MM. Helminth-derived immunomodulators: can understanding the worm produce the pill? Nat Rev Immunol. 2010;10:278–84. doi: 10.1038/nri2730. [DOI] [PubMed] [Google Scholar]
- 19.van Riet E, Hartgers FC, Yazdanbakhsh M. Chronic helminth infections induce immunomodulation: consequences and mechanisms. Immunobiology. 2007;212:475–90. doi: 10.1016/j.imbio.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 20.Finlay CM, Walsh KP, Mills KH. Induction of regulatory cells by helminth parasites: exploitation for the treatment of inflammatory diseases. Immunol Rev. 2014;259:206–30. doi: 10.1111/imr.12164. [DOI] [PubMed] [Google Scholar]
- 21.Elliott DE, Weinstock JV. Helminthic therapy: using worms to treat immune-mediated disease. Adv Exp Med Biol. 2009;666:157–66. doi: 10.1007/978-1-4419-1601-3_12. [DOI] [PubMed] [Google Scholar]
- 22.Elliott DE, Weinstock JV. Where are we on worms? Curr Opin Gastroenterol. 2012;28:551–6. doi: 10.1097/MOG.0b013e3283572f73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Babu S, Bhat SQ, Kumar NP, Jayantasri S, Rukmani S, Kumaran P, Gopi PG, Kolappan C, Kumaraswami V, Nutman TB. Human Type 1 and 17 Responses in Latent Tuberculosis Are Modulated by Coincident Filarial Infection through Cytotoxic T Lymphocyte Antigen-4 and Programmed Death-1. J Infect Dis. 2009;200:288–98. doi: 10.1086/599797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.George PJ, Anuradha R, Kumaran PP, Chandrasekaran V, Nutman TB, Babu S. Modulation of mycobacterial-specific Th1 and Th17 cells in latent tuberculosis by coincident hookworm infection. J Immunol. 2013;190:5161–8. doi: 10.4049/jimmunol.1203311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lipner EM, Gopi PG, Subramani R, Kolappan C, Sadacharam K, Kumaran P, Prevots DR, Narayanan PR, Nutman TB, Kumaraswami V. Coincident filarial, intestinal helminth, and mycobacterial infection: helminths fail to influence tuberculin reactivity, but BCG influences hookworm prevalence. Am J Trop Med Hyg. 2006;74:841–7. [PubMed] [Google Scholar]
- 26.Nunes-Alves C, Booty MG, Carpenter SM, Jayaraman P, Rothchild AC, Behar SM. In search of a new paradigm for protective immunity to TB. Nat Rev Microbiol. 2014;12:289–99. doi: 10.1038/nrmicro3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cooper AM. Cell-mediated immune responses in tuberculosis. Annu Rev Immunol. 2009;27:393–422. doi: 10.1146/annurev.immunol.021908.132703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khader SA, Cooper AM. IL-23 and IL-17 in tuberculosis. Cytokine. 2008;41:79–83. doi: 10.1016/j.cyto.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ellner JJ. Immunoregulation in TB: observations and implications. Clin Transl Sci. 2010;3:23–8. doi: 10.1111/j.1752-8062.2010.00180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rook GA. Th2 cytokines in susceptibility to tuberculosis. Curr Mol Med. 2007;7:327–37. doi: 10.2174/156652407780598557. [DOI] [PubMed] [Google Scholar]
- 31.Mayer-Barber KD, Andrade BB, Oland SD, Amaral EP, Barber DL, Gonzales J, Derrick SC, Shi R, Kumar NP, Wei W, Yuan X, Zhang G, Cai Y, Babu S, Catalfamo M, Salazar AM, Via LE, Barry CE, 3rd, Sher A. Host-directed therapy of tuberculosis based on interleukin-1 and type I interferon crosstalk. Nature. 2014;511:99–103. doi: 10.1038/nature13489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gopal R, Monin L, Slight S, Uche U, Blanchard E, Fallert Junecko BA, Ramos-Payan R, Stallings CL, Reinhart TA, Kolls JK, Kaushal D, Nagarajan U, Rangel-Moreno J, Khader SA. Unexpected role for IL-17 in protective immunity against hypervirulent Mycobacterium tuberculosis HN878 infection. PLoS Pathog. 2014;10:e1004099. doi: 10.1371/journal.ppat.1004099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mayer-Barber KD, Andrade BB, Barber DL, Hieny S, Feng CG, Caspar P, Oland S, Gordon S, Sher A. Innate and adaptive interferons suppress IL-1alpha and IL-1beta production by distinct pulmonary myeloid subsets during Mycobacterium tuberculosis infection. Immunity. 2011;35:1023–34. doi: 10.1016/j.immuni.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rothchild AC, Jayaraman P, Nunes-Alves C, Behar SM. iNKT cell production of GM-CSF controls Mycobacterium tuberculosis. PLoS Pathog. 2014;10:e1003805. doi: 10.1371/journal.ppat.1003805. [DOI] [PMC free article] [PubMed] [Google Scholar]