Abstract
Chronic low back pain (CLBP) is a highly prevalent and disabling musculoskeletal pain condition among older adults. Transcutaneous electrical nerve stimulation (TENS) is commonly used to treat CLBP, however, TENS response for older adults compared to younger adults is untested. In a dose-response study stratified by age, sixty participants with axial CLBP (20 young, 20 middle-aged, 20 older) received four 20-minute sessions of high frequency, high intensity TENS over a two to three-week period in a laboratory-controlled setting. Experimental measures of pain sensitivity (mechanical pressure pain detection threshold, PPT) and central pain excitability (phasic heat temporal summation, TS; heat aftersensations, AS) were assessed before and after TENS. Episodic or immediate axial CLBP relief was assessed after TENS via measures of resting pain, movement-evoked-pain, and self-reported disability. Cumulative or prolonged axial CLBP relief was assessed by comparing daily pain report across sessions. Independent of age, individuals experienced episodic increase in PPT and reduction in AS following TENS application. Similarly, all groups, on average, experienced episodic axial CLBP relief via improved resting pain, movement-evoked pain, and disability report. Under this design, no cumulative effect was observed as daily pain did not improve for any age group across the four sessions. However, older adults received higher TENS amplitude across all sessions in achieving similar TENS responses to younger adults. These findings suggest that older adults experience similar episodic axial CLBP relief as younger individuals following high frequency, high intensity TENS when higher dosage parameters are used.
Keywords: Age, axial, low back pain, TENS
1. INTRODUCTION
Chronic low back pain (CLBP) is a prevalent condition among older adults and a major contributor to the exponential rise in pain management utilization.10,36,37,66 Pharmacologic treatment is commonly prescribed for CLBP, however, such methods hold higher health risks for older adults.3 Alternatively, transcutaneous electrical nerve stimulation (TENS) may be more suitable for older adults since it is a conservative, non-pharmacologic CLBP treatment. TENS has been extensively studied and quantification of past results are mixed.27,30,40,64 However, recent clinical research has advanced our understanding of TENS efficacy in important ways. Specifically, TENS effects appear to be more effective for movement-evoked versus resting pain and require a high intensity stimulus (i.e. strong, yet tolerable).39,47,52,62 However, the efficacy of such parameters based on age remains uninvestigated.
Mechanistic research has elucidated age-related changes in laboratory correlates of central pain excitability. Specifically, older adults are purported to have enhanced pain facilitation, whereby application of a repetitively delivered painful stimulus is perceived to be progressively more painful despite unchanging stimulus intensity.12,33 Older adults have also demonstrated attenuated pain inhibition such that pain reduction following a painful stimulus is either less than, or slower than, younger adults.13,31,49,65 Collectively, these findings suggest age-related neuroplastic changes which may reduce the capability for older adults to respond to pain relieving treatments like TENS, since the mechanism of action includes activation of the central descending inhibitory pain system.52,54,61
Therefore, our purpose was to test whether response to high frequency, high intensity TENS differed by age group among individuals with axial CLBP. This study had two principle aims. The first aim was to assess age differences in experimental pain response to TENS during rest. We hypothesized that older adults with axial CLBP would have decreased TENS response due to enhanced pain facilitation and reduced pain inhibition.32 The second aim was to assess age group differences in CLBP measures of TENS response- including resting pain, movement-evoked pain, and disability. Although studies have yet to compare TENS response for CLBP across age groups, we anticipated older adults to have reduced response based on age-related changes in the descending inhibitory pain system.32 Finally, this study had two exploratory aims. First was to assess daily pain across multiple TENS treatment sessions to ascertain whether TENS effects were cumulative under the current design. Next was to assess age group differences in TENS amplitude to provide preliminary indication of dosage and response by age group using a standard stimulus intensity instruction set. The overarching goal of this study was to determine age-specific TENS effects using both experimental pain and axial CLBP self-report measures, to provide novel information regarding TENS pain reduction capacity among older adults.
2. METHODS
2.1 Study Population
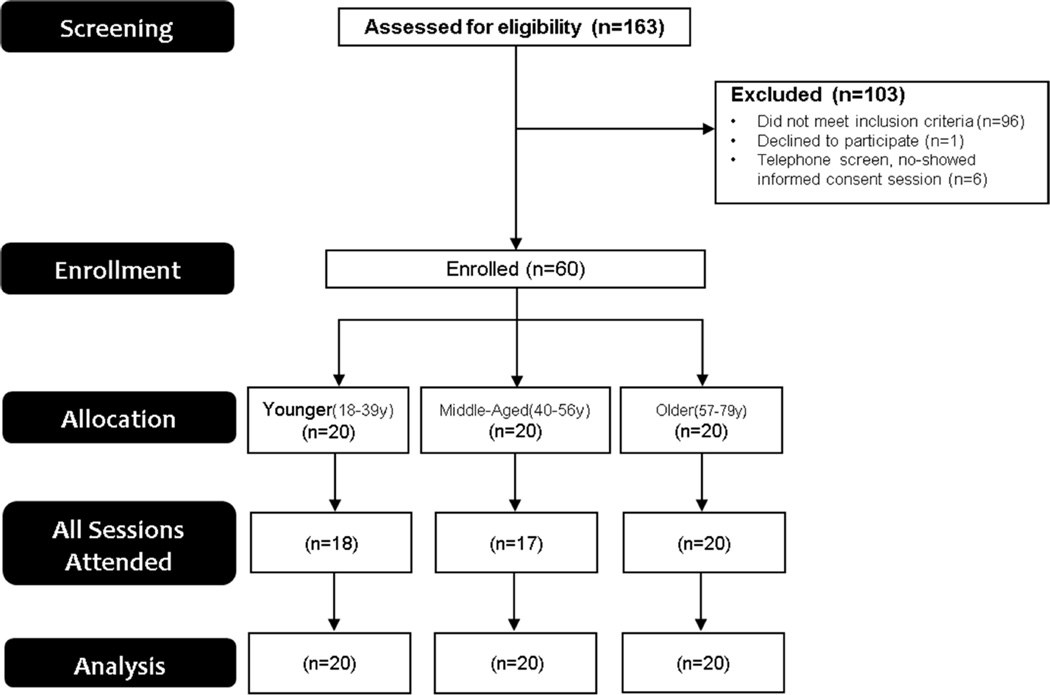
Simple purposive sampling stratified to a priori age group quotas was used to enroll screened participants with axial CLBP (Figure 1). Once adequate group sampling had occurred, enrollment to that particular age group ceased. All participants were not currently seeking care and were recruited from the community via printed advertisements. In addition, a community health program was utilized which linked research opportunities with prospective participants via social and media services. Participants were categorized based on the age groups: young (18–39 years old), middle-aged (40–56 years old), and older (57–79 years old). Age groups ranges were determined a priori based on previous research.31,48 This study was approved by the University of Florida Institutional Review Board and all participants provided written informed consent prior to enrollment.
Figure 1.
Flow diagram of patient enrollment
2.2 Inclusion and Exclusion Criteria
Individuals were considered for study inclusion if experiencing axial CLBP for equal to or greater than three months, which also had to serve as their primary complaint. Additionally, average daily pain intensity was required to be equal to or greater than 40/100 at worst on a zero to 100 scale (0=‘no pain’; 100=‘ worst pain imaginable’) to ensure individuals were experiencing a moderate level of axial CLBP. Individuals were excluded for the following: 1) symptoms of lower extremity nerve root involvement such as motor weakness and sensory disturbance; 2) axial CLBP resulting from trauma (e.g. car accident, work accident, fall); 3) treatment for CLBP by any health care professional (e.g. MD, DC, PT) within the past month; 4) prior surgery for low back musculoskeletal pain; 5) opioid use; 6) comorbidities including uncontrolled hypertension, diabetic neuropathy, circulatory disorders interfering with activities of daily living, cardiac event history (e.g. myocardial infarction), or epilepsy; 7) implanted cardiac pacemaker; 8) psychiatric-related hospital admission within the past year; or 8) pregnancy. In addition, prospective participants were screened for cognitive impairment prior to the informed consent procedure, which was determined by a score lower than 23 on the Mini Mental State Examination (MMSE).15 Participants aged 70 years or older also underwent a brief neurological test to ensure intact sensory function.11
2.3 Procedures
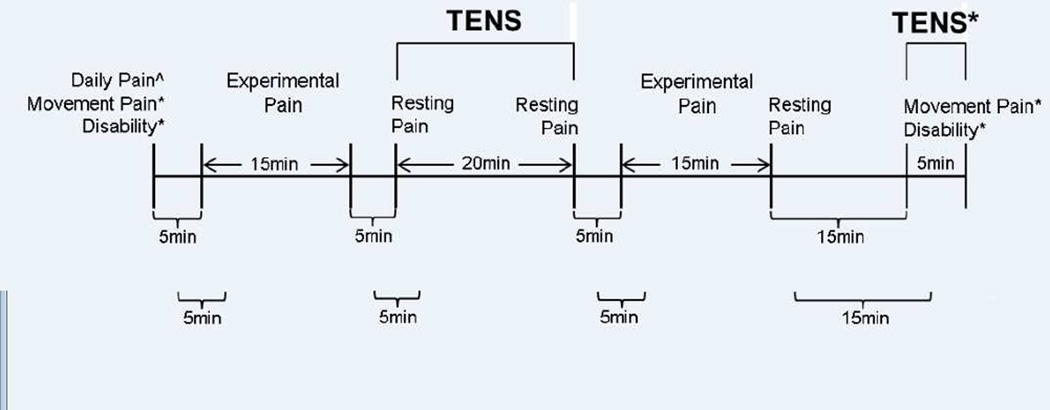
This was a dose-response design stratified by the aforementioned age groups of young, middle-aged, and older. The intent was to examine age group differences in TENS effects as provided clinically using the current dosing recommendations.52,61 Therefore, a placebo arm was not included in this analysis. Participants completed five experimental sessions over a three to four week period (TENS provided in visits 1 to 4), with up to two sessions completed per week. The weekly intervention frequency is similar to a seminal TENS efficacy trial performed among individuals with CLBP.38 Testing was conducted by the same experimenter in a single climate-controlled laboratory at the same time of day across all sessions. Participants completed intake questionnaires (demographic and pain) and were assessed for movement pain intensity and self-reported disability at the beginning of session 1. Study procedures are outlined in Figure 2. In each of the first four sessions, participants were assessed for resting and experimental pain response after a 20-minute application of TENS to the lumbar spine in a resting position. In addition, Movement-evoked pain and self-reported disability were assessed with and without TENS application at the end of the first session, only. Daily pain intensity was assessed at the beginning of all five sessions.
Figure 2.
Study Procedures. ^Assessed over 5 sessions; *Assessed at session 1, only.
2.4 Experimental Pain Response
Pressure pain detection threshold (PPT) assessed local pain sensitivity and was performed by applying 1 kilogram-force per centimeter-squared per second using a Wagner Force Ten FDX 25 Digital Force Gauge™ with a 1cm2 flat rubber tip (Wagner Instruments, Greenwich, CT). Experimenters were proficient with PPT testing through application in patient care and previous clinical trials. PPT sites were the bilateral posterior superior iliac spines (PSIS). Prior to testing, participants were educated on PPT which included visualization of the testing unit and its application. Upon testing, participants were instructed to indicate when pressure first became painful (first onset of pain) by verbalizing the word ‘pain.’ To improve testing precision, three trials were performed at each PSIS, with the last two ratings at each site averaged to calculate a PPT Score. PPT has been shown to have excellent test-retest reliability in axial regions.28 Further, prior research has determined higher PPT for older adults,18,32 as well as increased local PPT following TENS.8,45,62
Phasic heat temporal summation (TS) provided indication of central pain excitability changes67 and was measured using a 3cm2 thermode connected to a PATHWAY Model Contact Heat-Evoked Potential Stimulator (CHEPS). Five consecutive 50°C heat pulses at 0.33Hz inter-stimul us interval (ISI) were delivered to the plantar aspect of the right foot, posterior to the first metatarsophalangeal joint. Participants rated the second pain they felt after each heat pulse using the numeric pain rating scale (NPRS), with zero equal to “no pain” and “100” equal to “worst pain imaginable.” TS was determined using the established calculation of absolute difference between the fifth and first pain ratings.1,23,50 Individuals reporting 100/100 NPRS during TS were removed from analysis because 1) the extent of summation cannot be observed, and 2) ratings are more likely an indication of A-delta pain transmission (first pain). Previously, healthy older adults have shown enhanced TS compared to younger adults.12,33 Specific to TENS response, TS has not previously changed following TENS,62 although Liebano et al. found changes in temporal summation of pain to mechanical stimuli.35 Collectively, a paucity of studies have assessed temporal summation effects (either heat or mechanical) from TENS and, to our knowledge, none have assessed age differences and/or in an axial CLBP cohort.
Aftersensations (AS) is an additional measure of central pain excitability change which can be assessed using experimental pain models.22,67 Prolonged aftersensations have been observed among older adults49 and individuals with chronic pain conditions.56–58 Contact heat was delivered by a 3cm2 thermode connected to a PATHWAY Model Advanced Thermal Stimulator (ATS) (Medoc Advanced Medical Systems, Ramat Yishai, Israel). 15-second stimulus-response testing at the first session determined the tonic heat stimulus temperature (46°C, 47°C, or 48°C) corresponding to a pain rating of 50/100 NPRS, which was then used for all sessions. For AS, a 30-second tonic heat stimulus was applied to the plantar aspect of the right foot, posterior to where TS was assessed. Next, temperature was decreased at a rate of 10°C/second to neutral temperature (33°C), durin g which time individuals provided pain intensity (0–100 NPRS) every two seconds for a total of 10 seconds. The five corresponding pain ratings were then entered into an trapezoidal area-under-the-curve (AUC) formula43 to calculate AS.
2.5 Axial CLBP Measures
Episodic dependent measures included resting pain, movement-evoked pain, and self-reported disability, while the cumulative effects dependent measure was daily pain. Resting pain was CLBP pain intensity rated on a zero to 100 NPRS, which is a responsive measure in musculoskeletal pain assessment.9 Movement-evoked pain intensity was assessed using the Back Performance Scale (BPS).59 During the BPS, participants performed five functional tasks specific to the spine, including 1) grasping toes with fingertips in a sitting position; 2) forward bending from standing; 3) picking up paper from standing; 4) long-sitting from supine; and 5) lifting a 5kg box from floor to table repeatedly. Participants provided a pain intensity rating after each task (0–100 NPRS) and the five pain ratings were averaged to calculate movement-evoked pain intensity. Disability was the cumulative score of the BPS ranging from zero (no disability) to 15 (maximum disability).59 Score for each task ranged from zero to three, with zero indicating the ability to perform the task without difficulty and three indicating the inability or unwillingness to perform the task, or maximum difficulty with the task. Finally, daily pain intensity was assessed using the Brief Pain Inventory Short Form (BPI), which is a 10 point numeric scale measure of best and worst pain intensity over the previous 24 hours, average pain intensity, and present pain intensity.60 A mean of the four ratings served as the daily pain intensity score for each session.
2.6 TENS Intervention
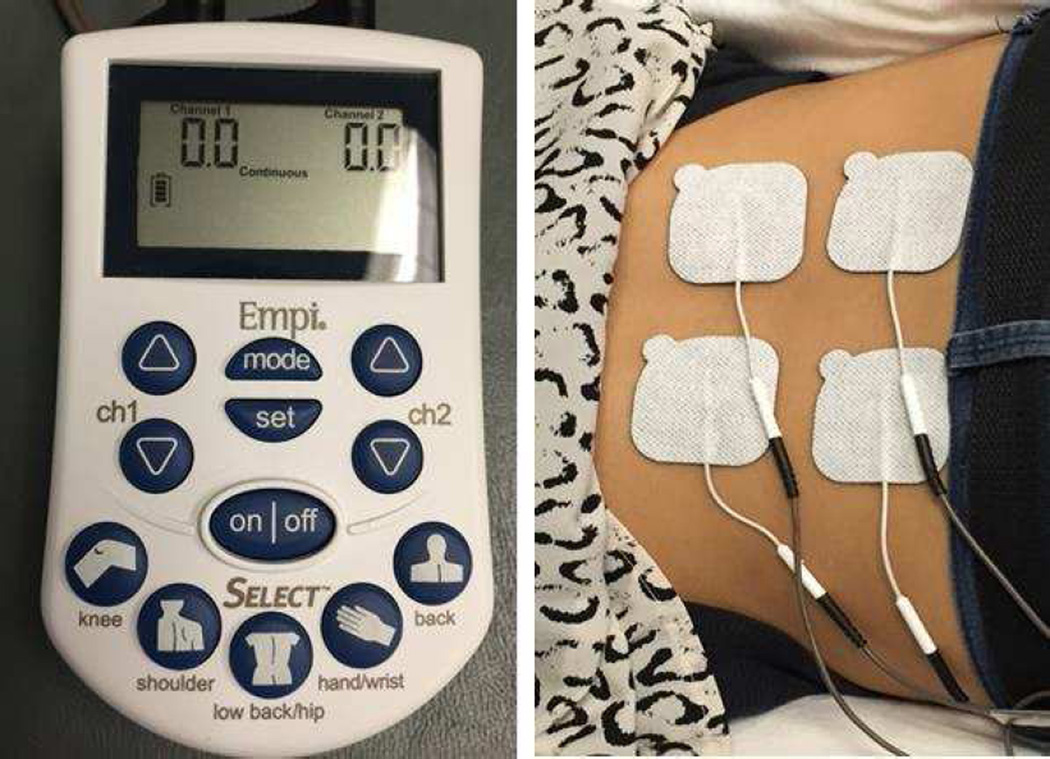
TENS was delivered using dual channel portable electrical stimulation units with two leads and four carbon-cloth electrodes (EMPI Select TENS™ Pain Management System, DJO Inc, St. Paul Minnesota). Units were calibrated by the manufacturer prior to delivery. TENS waveform was balanced asymmetrical at 125Hz frequency. Pulse duration was variable based on intensity, however ranged between 16 and 360 microseconds. To control for positional intolerance during the intervention, participants were given the option of being reclined, prone, or sidelying with appropriate pillow support. However, the selected body position for TENS was maintained across all sessions. Electrode placement paralleled clinical application, in that electrodes were immediately above and below the spinal level corresponding to pain complaint. However, we opted to use a crossed pattern of electrode placement whereby channel electrodes were on opposite sides (left/right) and level (high/low) of the spine (Figure 3). This was intended to align with a previous study by Vance et al, where similar electrode positions were used to intersect the knee joint in an osteoarthritic cohort.62
Figure 3.
TENS Unit and Setup. 4 carbon electrode pads are affixed to the patients low back region (right) in a crossed-electrode pattern, and connected via 2 leads to the EMPI TENS Select unit (left).
TENS application was not blinded based on age, as this was not feasible in the current research design. However, all TENS application was done in a standard manner and participants were blinded to channel intensity, which was set by a licensed physical therapist by increasing both channels simultaneously at a rate of 1mA/sec. Participants were instructed to verbalize when a “strong, but tolerable and not painful” stimulus was experienced, which should correspond with a 70/100 stimulus intensity (0 equal to “no sensation” and 100 equal to “intolerable sensation”). While 70/100 is an arbitrary intensity level, we aimed to ensure the stimulus was stronger than moderate though not intolerable. Further, a numeric anchor may improve homogeneity of stimulus perception above and beyond a strength descriptor. At the same time, an additional stop rule was in place to ensure TENS did not evoke a motor stimulus. Once channel intensity surpassed 15mA, the physical therapist palpated lumbar paraspinals in the region of the electrodes. If motor activation was detected, channel intensity was decreased by 10% as has been previously used in TENS effects studies.46,62 Once stimulus intensity was set, TENS remained on for 20 minutes.62
At the first session, TENS was also applied during the BPS to assess movement-evoked pain and self-reported disability response. TENS electrodes were reapplied as described above with participants in a standing position. Channel intensity was increased to the level corresponding to TENS during rest. Participants then performed the five tasks of the BPS and verbalized movement-evoked pain (0–100 NPRS) after each task, while a rater scored disability using the 0–15 BPS scale.
2.7 Statistical Analysis
Analyses were completed using IBM® SPSS® Statistics software, Version 21 (2012, IBM® Corp; Armonk, NY). Alpha level was set at p=.05 for all analyses. To our knowledge, a priori investigation of TENS effects by age group has not been performed and thus could not be powered upon. However, Larivière et al. observed attenuated experimental pain change for older adults compared to younger and middle adults in sample sizes of 20.31 Further, Marchand et al. observed greater TENS reduction for individuals with CLBP (n=14) versus placebo-TENS (n=12), albeit in controlled samples.38 Therefore, three age group samples of 20 individuals (n=60) were deemed adequate to observe changes in experimental and clinical pain following TENS, and by age group. Separate multivariate mixed-model ANOVAs were created to assess age-related change in experimental pain response across sessions for each test (PPT, TS, AS), with age group as the between-subject factor and time (pre- TENS, post-TENS) and session (n = 4) as the within subject factors. Box’s M and Levene’s tests examined homogeneity of variances and covariances across dependent variables. Mauchley’s test examined model sphericity, and Greenhouse-Geisser correction was used in presence of sphericity violation. Bonferonni test was used to assess pairwise comparisons as this is an established, conservative method of controlling for familywise error.
Similar multivariate mixed model ANOVAs tested age-response in resting pain, movement-evoked pain, and disability across sessions. For resting pain, age group was the between-subject factor, and time (pre-tens, post-TENS, 20 minutes post-TENS) the within-subjects factor. In addition, change in resting pain was examined across all sessions with session (n=4) the within-subject factor. Disability and movement-evoked pain were assessed using similar methods, albeit within a single session. Movement-evoked pain was the average pain intensity (NPRS) during performance of the five BPS tasks, while disability was the BPS composite score. For both models, age group was the between-subjects factor, and condition (BPS tasks with and without TENS) the within-subject score.
For exploratory analyses, cumulative daily pain change across sessions was assessed using BPI as the daily pain score. Group served as the between-subject factor, and session (n=5) served as the within-subject factor. Daily pain was collected on a fifth occasion after the final TENS session to determine cumulative effects beyond immediate effects from the fourth session. Assumption testing and pairwise comparisons for all models were similar to those used in experimental pain response models. For age group differences in TENS amplitude, Univariate ANOVA was performed for each session. Change in TENS amplitude across sessions was then examined using mixed-model ANOVA, with age group as between-group factor and session (n=4) as the within-group factor.
3. RESULTS
Sixty individuals were enrolled from September, 2013 until October, 2014 (Figure 1). Groups were similar in the proportion of females, income, and axial CLBP characteristics; though different in age (planned) and education (Table 1). In total, 92% (n=55) of participants completed all testing sessions, while 95% (n=57) completed four of the five sessions. Attrition was due to either not completing all sessions within the allotted 4 week timeframe (n=2) or being withdrawn due to non-compliance with attendance policy (n=3).
Table 1.
Intake Characteristics by Age Group
| FACTOR | YOUNG n=20 |
MIDDLE n=20 |
OLDER n=20 |
p | |||
|---|---|---|---|---|---|---|---|
| Age | 30.65 | (6.41) | 48.85 | (4.45) | 63.50 | (5.34) | <.001** |
| Female (%) | 75 | 65 | 65 | 0.735 | |||
| Education (%) | |||||||
| Less than High School Diploma | 10 | 20 | 20 | ||||
| High School Diploma | 10 | 45 | 20 | 0.020^ | |||
| College Attendance | 80 | 35 | 70 | ||||
| Income (%) | |||||||
| <$20,000 | 55 | 70 | 42 | ||||
| $20,001–$50,000 | 25 | 20 | 32 | 0.509 | |||
| >$50,000 | 20 | 10 | 26 | ||||
| Pain Relieving Medication (%) | 20 | 25 | 25 | 0.911 | |||
| Tobacco Use (%) | 25 | 45 | 25 | 0.292 | |||
| Caffeine Use (%) | 75 | 85 | 75 | 0.675 | |||
| Axial CLBP Measures | |||||||
| Pain Duration (Weeks) | 208.15 | (180.19) | 280.32 | (344.32) | 255.60 | (343.13) | 0.745 |
| Daily Pain Intensity (0–10) | 4.96 | (1.60) | 4.89 | (1.94) | 4.51 | (2.05) | 0.720 |
| Movement-Evoked Pain Intensity (0–100 | ) 39.52 | (22.68) | 39.90 | (25.24) | 33.15 | (27.57) | 0.639 |
| Disability (0–15) | 4.80 | (2.35) | 6.25 | (3.25) | 6.05 | (3.35) | 0.266 |
Different across all groups, significant at p<.001;
Different between younger and middle-aged groups, significant at p<.05;
Standard deviation reported in parentheses
3.1 Experimental Pain Response
Experimental pain response following TENS is outlined in Table 2. Independent of age group, PPT increased at the first (F1,57=12.51, p<.01), second (F1,54=9.49, p<.01), third (F1,54=4.24, p<.05), and fourth (F1,54=14.47, p<.001) sessions following TENS. The lone interaction for PPT was age group by time (pre-TENS, post-TENS) at the fourth session (F2,54=3.83, p<.05). Pairwise comparisons revealed that at this session, the larger rate of change occurred for older versus younger and middle-aged adults.
Table 2.
Experimental pain response following TENS, by session
| Session 1 Mean Δ (95%CI) |
p | Session 2 Mean Δ (95%CI) |
p | Session 3 Mean Δ (95%CI) |
p | Session 4 Mean Δ (95%CI) |
p | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PPT | Young | 0.46 | (0.08,0.84) | 0.34 | (−0.05,0.74) | 0.21 | (−0.14,0.56) | 0.19 | (−0.19,0.57) | ||||
| Middle | 0.19 | (−0.19,0.57) | 0.436 | 0.14 | (−0.22,0.50) | 0.362 | 0.08 | (−0.27,0.42) | 0.588 | 0.20 | (−0.16,0.56) | 0.028* | |
| Older | 0.52 | (0.14,0.90) | 0.51 | (0.15,0.87) | 0.32 | (−0.01,0.66) | 0.82 | (0.45,1.20) | |||||
| Total | 0.39 | (0.17,0.61) | 0.001 | 0.33 | (0.12,0.55) | 0.003 | 0.20 | (0.01,0.40) | 0.044 | 0.41 | (0.19,0.62) | <0.001 | |
| TS | Young | 4.76 | (−3.99,13.52) | −2.77 | (−11.30,5.77) | 0.17 | (−6.80,7.14) | 0.13 | (−5.57,5.82) | ||||
| Middle | 6.44 | (−2.10,14.95) | 0.571 | 0.32 | (−7.75,8.38) | 0.870 | −3.35 | (−3.82,10.52) | 0.463 | −3.74 | (−8.97,1.49) | 0.556 | |
| Older | 0.45 | (−7.62,8.52) | −0.94 | (−9.23,7.34) | −2.79 | (−3.99,9.57) | 0.83 | (−4.54,6.21) | |||||
| Total | 3.89 | (−0.99,8.76) | 0.116 | −1.13 | (−5.92,3.66) | 0.638 | −0.13 | (−4.16,3.90) | 0.948 | −0.93 | (−4.07,2.21) | 0.427 | |
| AS | Young | −27.14 | (−0.96,−53.32) | −33.12 | (−6.58,−59.66) | −14.97 | (0.26,−30.20) | −8.81 | (7.94,−25.56) | ||||
| Middle | −29.70 | (−4.86,−54.54) | 0.925 | −39.73 | (−15.26,−64.19) | 0.600 | −7.08 | (8.15,−22.31) | 0.562 | 0.58 | (16.88,−15.72) | 0.112 | |
| Older | −34.20 | (−9.31,−58.99) | −22.35 | (−4.86,−54.54) | −3.93 | (18.37,−10.52) | −24.14 | (−7.39,−40.88) | |||||
| Total | −30.33 | (−15.73,−44.93) | <0.001 | −31.73 | (−17.20,−46.27) | <0.001 | −8.66 | (−0.02,−17.31) | 0.049 | −10.78 | (−1.21,−20.37) | 0.028 | |
Mean Δ, mean change after TENS (95% CI of change reported in parentheses); p Age groups, significance value of age by time interaction; p Total, significance of time main effect (before and after TENS);
PPT change in older group only.
Independent of age group, AS decreased at the first (F1,55=17.33, p<.001), second (F1,54=19.16, p<.001), third (F1,53=4.04, p<.05), and fourth (F1,52=5.10, p<.05) sessions. The lone interaction for AS was time by session (F3,47=5.10, p<.05). Pairwise comparisons revealed that AS response was higher in the first session compared to the third session. TS was not found to change within or across sessions, and/or across age groups following TENS (p>.05).
3.2 Axial CLBP Response
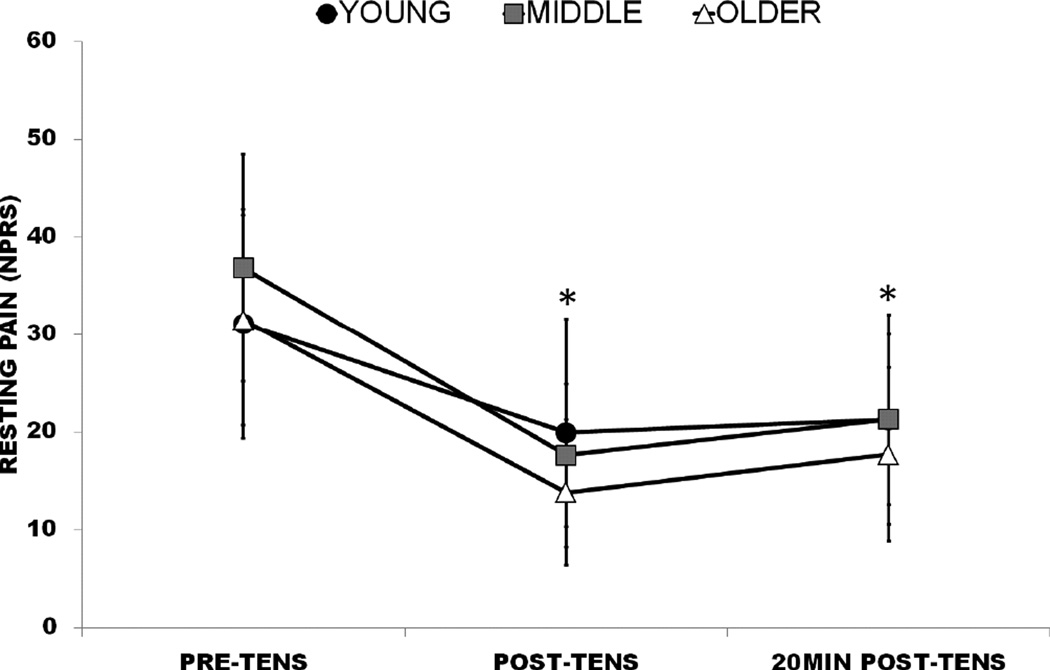
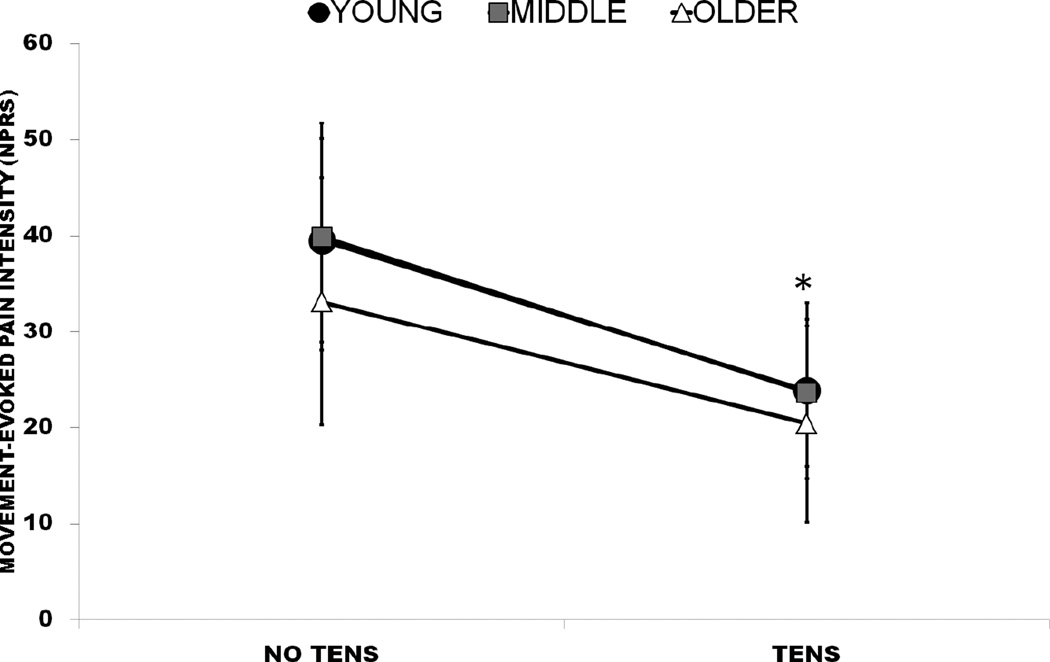
Age group interactions did not exist for resting pain, movement-evoked pain, or disability (p>.05). Resting pain changed for all groups during the first (F2,56=22.24, p<.001), second (F2,52=18.96, p<.001), third (F2,53=18.60, p<.001), and fourth (F2,53=18.20, p<.001) sessions. Pairwise comparisons revealed post-TENS resting pain and 20min post-TENS resting pain to be lower than pre-TENS resting pain at all sessions, although not significantly different from each other (Figure 4). Movement-evoked pain (F1,54=35.81, p<.001; Figure 5) and disability (F1,54=18.90, p<.001) also improved with TENS during BPS tasks compared to without. Daily pain was not observed to change across sessions either by age or for the entire axial CLBP cohort (p>.05).
Figure 4.
Resting pain rating change with TENS by age group, sessions collapsed; *, Significant difference from Pre-TENS. Error bars indicate 95% confidence interval (CI).
Figure 5.
Movement-evoked pain ratings and concurrent TENS application by age group; *, Significant difference from No TENS. Error bars indicate 95% confidence interval (CI).
3.3 TENS Amplitude
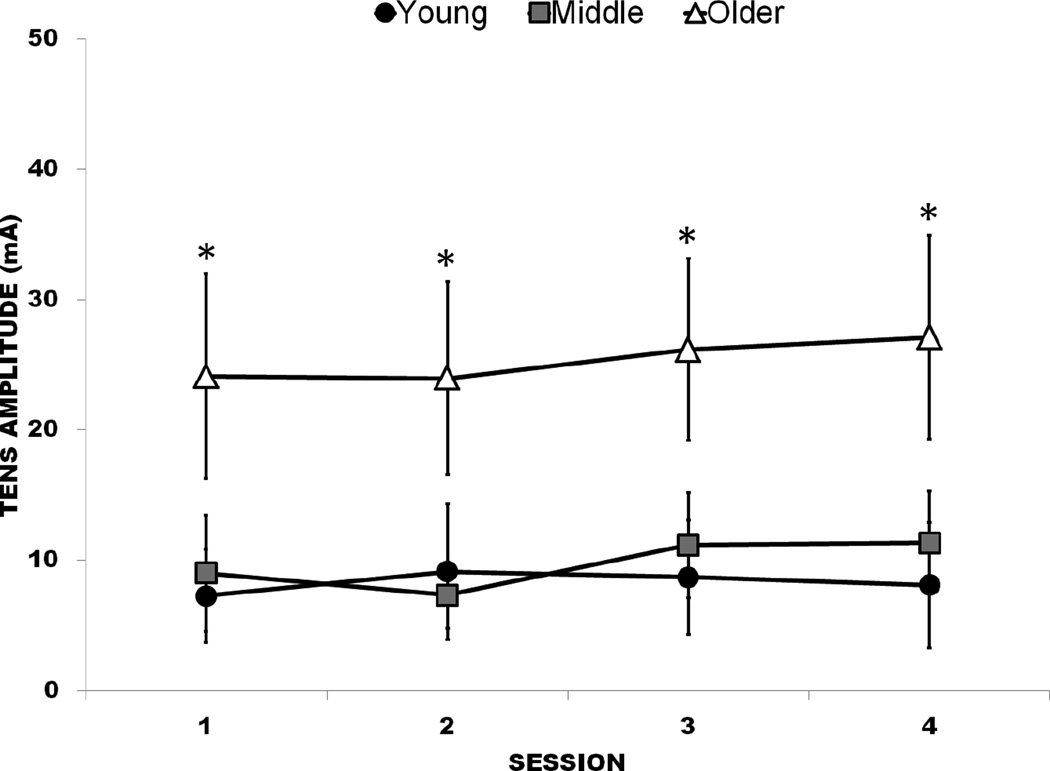
Older adults were observed to have higher TENS amplitude compared to younger and middle-aged adults at the first (F2,59=11.89, p<.001), second (F2,56=12.71, p<.001), third (F2,56=13.67, p<.001), and fourth (F2,56=13.76, p<.001) sessions. TENS amplitude did not change across sessions, either by group or for the entire CLBP sample (p>.05; Figure 6). Collapsed across sessions, older adults had a mean of 25.3mA (SD=14.1) compared to 7.9 mA (SD=8.4) and 9.6mA (SD=6.8) for younger and middle-aged adults, respectively.
Figure 6.
TENS amplitude across all sessions, by age group. Error bars indicate 95% confidence interval (CI). *, Significant difference in older group compared to middle-aged and younger groups
4. DISCUSSION
In the current study, we found TENS evoked similar changes in experimental pain responses across all age groups, suggesting improved pain sensitivity and reduced central pain excitability. Similarly, older adults showed similar episodic improvements in resting pain, movement-evoked pain, and disability compared to younger cohorts. Therefore, age-related neuroplastic changes to the pain system may not be severe enough to render non-pharmacological pain modulation obsolete. However, older adults demonstrated higher TENS amplitude in conjunction with these effects, which may be indication of dosage required to reach pain inhibition potential. Further, since higher dosage may be needed for older adults to achieve similar TENS response as younger adults, the former may be routinely under-dosed when perception based instructions of ‘strong but tolerable’ are not used to set TENS amplitude.
Independent of age, pain sensitivity (PPT) reduction with TENS corroborates a wealth of previous research.6,8,35,39,42,45,46 By comparison, the observed changes in central pain excitability with TENS are less understood. Dailey et al. reported that TENS restored conditioned pain modulation (CPM) in individuals with fibromyalgia, which suggests normalization of pain inhibitory function.8 To our knowledge, the present study was the first to examine TENS effects on AS, and our findings align with the Dailey et al. study. However, we observed minimal effects on TS (another measure of central pain excitability), which was also reported in a previous study of individuals knee osteoarthritis.62 The authors of that study proposed that heat pain stimulus response may reflect cutaneous hyperalgesia which could have limited effects from TENS. In contrast, mechanical TS has reduced with TENS, implying either potential differential effects from deep-tissue hyperalgesia (as previously suggested46,62) or a larger activation of A-delta fibers versus c-fibers. Nevertheless, current and previous findings imply some capacity for TENS to influence laboratory correlates of central excitability, which align with previous mechanistic work examining descending (top-down) TENS mechanisms in animals. Sluka et al. and others have found blocking activation of opioid receptors in the rostroventral medulla (RVM) inhibits TENS response.29,53 Similarly, TENS response has been tied to synaptic transmission in the midbrain periaqueductal gray area, as well as opioid, muscarinic, and GABA receptor activation in the spinal cord.61
Age-related TENS amplitude differences likely occurred from older adults having a higher TENS perception threshold. TENS mechanisms are purported to induce bottom-up response by activating large diameter afferent fibers,34,44 and work in both animals and humans has determined multiple physiologic changes with age – including loss of these large fibers.18 Further, higher TENS perception among older adults is in line with experimental pain testing showing overall age-related pain threshold increase.18,32 However, current findings are preliminary and do not indicate a direct relationship between TENS amplitude and either experimental and/or axial CLBP.
A second, though less likely explanation for age-related TENS amplitude differences was altered perceptions of the instruction set “strong, yet tolerable and not painful.” Older adults have shown difficulty with certain rating scales, particularly related to sensory interpretation.16,17 However, older adults reportedly do well with descriptive scales (i.e. McGill Pain Questionnaire).16,17 Further, scale comprehension was likely enhanced by including a numeric anchor (70/100 stimulus intensity). This scale is similar to the NPRS for pain intensity, which is a valid tool for use amongst older adults.26 In addition, TENS instruction was provided over multiple sessions rather than a single session. If age group differences in TENS perception were the product of learning effect, amplitude would have regressed towards the mean over time. However, TENS amplitude differences remained constant over time for all age groups. Nevertheless, future psychometric investigation will confirm the appropriateness of this instruction set in older adults. Until such time, TENS dosing should remain perception based – as lower intensity or set-dosing parameters may run the risk of under-dosing older adults.
The TENS parameters used in this study demonstrated immediate (episodic) effects on pain, which may have important clinical implications for older adults. First, pharmacologic pain treatments are also episodic – yet less ideal for older adults due to increased health risks and/or potential mismanagement of care.5,41,55 If future research determines that TENS episodic efficacy is similar to pharmacologic treatment, it may prove a viable, lower risk alternative for axial CLBP. Second, episodic TENS effects would have limited utility in clinical care, while wider TENS access (home, community use) would potentially be more effective for axial CLBP as individuals could use ‘as needed’ (PRN). A study by Chesterton et al. found limited added effects of home TENS in conjunction with primary care for tennis elbow.7 However, to our knowledge, research has not examined such an age-based model for CLBP. Compliance, safety and outcomes among older adults require future trial assessment, though the utility and feasibility of TENS outside clinical care has already been proposed.52 Further, future research should consider TENS efficacy and effectiveness for outcomes outside of pain that are specific to older adults or important to individuals. For example, a recent qualitative study by Gladwell et al. found factors such as distraction and relief of tension and spasm to be important factors for TENS use among individuals with chronic musculoskeletal pain.20
In contrast to previous studies, we did not observe cumulative effects using the current design of up to twice a week for 4 visits. A paucity of studies exists showing cumulative TENS effects among CLBP participants, though an elegant study by Marchand et al. observed cumulative pain reduction among CLBP participants receiving TENS twice a week.38 However, the duration of TENS treatment was much longer than the current study - 10 weeks or 20 sessions. A more recent study by Facci et al. found cumulative pain reduction for CLBP following 10 TENS sessions within a 2 week period.14 Based on these findings, potential for cumulative pain reduction using either increased frequency or a longer duration cannot be discounted. Still, such treatment periods are outside the feasibility of most current supervised care models. Therefore, the potential for cumulative TENS effects with greater frequency and/or duration is further argument for translation of TENS access to home and community settings.
4.1 Strengths and Limitations
There are strengths to our study, most notably a design specific to examining age-related differences in TENS response. Often, age is include as a covariate without considering comorbidities, cognition, or the age-appropriateness of measures. Moreover, studies often include only a small age range, whereas we included even distribution across the lifespan (i.e. age 20 to 74 years). Multiple axial CLBP measures were used, and we were able to examine episodic versus cumulative TENS effects. Finally, this study included an element of stimulus response allowing for age group comparisons of TENS amplitude.
There are also limitations to this study. We utilized similar TENS intervention methods as employed in previous studies,46,62 however, did not include a placebo arm. Therefore, we cannot discern active TENS effects from non-specific effects of treatment (e.g. expectation, natural history) and this will be a high priority for future investigations using this age-based design. Second, pulse duration was variable rather than fixed, so intensity cannot be considered solely a product of increasing amplitude. However, previous animal work has downplayed pulse duration in analgesic TENS effects,21 and variable pulse duration has been used previously to examine human pain sensitivity response to TENS.39 Third, movement-evoked pain and disability was assessed at only one session so as to limit number of TENS applications per session and over time. However, this precludes our assessment of change in movement-evoked pain and disability over time, which should be examined in future studies. Finally, despite adequate sample size to observe large TENS effects, the study may have been underpowered to identify smaller effects. This should be considered for powering future studies.
4.2 Future Directions
While not uniform,8 the majority of research shows active TENS to reduce pain above and beyond placebo TENS.4,35,46,62 However, both expectation analgesia and TENS analgesia have overlapping mechanisms as opioid receptors are activated in multiple cortical and subcortical regions.63,68 Since age-related pain neuroplasticity affects the same regions,18,19 age-related change in expectation versus TENS analgesia should be determined by implementing a placebo arm to the current design. Future studies should also examine age group differences in a more comprehensive examination of TENS dosing and response, either by within-group randomization of TENS amplitude or a standard amplitude across age group. In addition, while lumbar patho-anatomical changes increase with age, pathology has not been strongly associated with pain severity or found to predict pain recovery.2,24,25,51 However, use of imaging in future studies will elucidate whether interactions exist between pathology and TENS response among older adults.
Clinically, prospective studies will determine the TENS effectiveness on axial CLBP among older adults in clinical settings, including as a preventative measure to chronicity and/or maintenance of function and quality of life. Finally, we indicate how a specific intervention fared across age groups, which is a novel design for assessing clinical treatment efficacy. This model may help to determine age-specific efficacy for other commonly utilized conservative treatments like spinal-manipulative therapy, strength training exercise, and acupuncture. Moreover, comparison of age-specific efficacy across treatments will help to identify those which provide the greatest treatment response for older adults.
5. CONCLUSIONS
With TENS, older adults with axial CLBP experienced episodic reduction of resting pain, movement-evoked pain, and improved function during spine-specific tasks. Pain reduction for all measures was similar to younger and middle-aged adults when dosing was based on perceived stimulation tolerance. These findings may be related to pain sensitivity and/or central pain excitability, as both measures improved with TENS and older adults demonstrated higher amplitude in conjunction with experiencing effects similar to younger individuals. Future studies will expand upon these findings to determine age group differences in expectation versus active TENS response, associations between experimental pain and axial CLBP report changes from TENS, and age group differences in (and effects from) TENS dosing.
Perspective.
This study examined age group differences in experimental and axial CLBP response to TENS, delivered under the current recommended parameters of strong, but tolerable amplitude. Older adults had comparable TENS response though at higher TENS amplitude than younger adults, which may have important mechanistic and clinical implications.
Highlights.
Age Group Comparisons of TENS Response among Individuals with Chronic Axial Low Back Pain
Age differences in TENS response were examined in a chronic low back pain cohort.
Independent of age, pain sensitivity and central excitability improved after TENS.
Independent of age, resting pain, movement pain, and disability improved after TENS.
Older adults demonstrated similar TENS response as younger and middle-aged adults.
Older adults had higher TENS amplitude than younger and middle-aged adults.
Acknowledgments
We thank Trevor Lentz and Lindsay Orr for their contributions toward study experimentation, and Jackie Ellison and LePaige Godfrey from UF HealthStreet for their contributions towards study recruitment.
This study was supported by funding from National Institutes of Health and National Center for Research Resources CTSA grant (UL1 TR000064 and TL1TR000066), and the University of Florida Department of Physical Therapy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
All authors are listed and that all have contributed substantially to the manuscript. No conflicts of interest exist for any of the authors. No role was played by sponsors in the design, methods, subject recruitment, data collection, analysis, or preparation of this paper.
REFERENCES
- 1.Anderson RJ, Craggs JG, Bialosky JE, Bishop MD, George SZ, Staud R, Robinson ME. Temporal summation of second pain: variability in responses to a fixed protocol. Eur J Pain. 2013;17:67–74. doi: 10.1002/j.1532-2149.2012.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balagué F, Mannion AF, Pellisé F, Cedraschi C. Non-specific low back pain. Lancet. 2012;379:482–491. doi: 10.1016/S0140-6736(11)60610-7. [DOI] [PubMed] [Google Scholar]
- 3.Barber JB, Gibson SJ. Treatment of Chronic Non-Malignant Pain in the Elderly. Drug Safety. 2009;32:457–474. doi: 10.2165/00002018-200932060-00003. [DOI] [PubMed] [Google Scholar]
- 4.Barker R, Lang T, Steinlechner B, Mora B, Heigel P, Gauss N, Zimpfer M, Kober A. Transcutaneous electrical nerve stimulation as prehospital emergency interventional care: treating acute pelvic pain in young women. Neuromodulation. 2006;9:136–142. doi: 10.1111/j.1525-1403.2006.00053.x. [DOI] [PubMed] [Google Scholar]
- 5.Buckeridge D, Huang A, Hanley J, Kelome A, Reidel K, Verma A, Winslade N, Tamblyn R. Risk of Injury Associated with Opioid Use in Older Adults. Journal of the American Geriatrics Society. 2010;58:1664–1670. doi: 10.1111/j.1532-5415.2010.03015.x. [DOI] [PubMed] [Google Scholar]
- 6.Chesterton LS, Foster NE, Wright CC, Baxter GD, Barlas P. Effects of TENS frequency, intensity and stimulation site parameter manipulation on pressure pain thresholds in healthy human subjects. Pain. 2003;106:73–80. doi: 10.1016/s0304-3959(03)00292-6. [DOI] [PubMed] [Google Scholar]
- 7.Chesterton LS, Lewis AM, Sim J, Mallen CD, Mason EE, Hay EM, van der Windt DA. Transcutaneous electrical nerve stimulation as adjunct to primary care management for tennis elbow: pragmatic randomised controlled trial (TATE trial) BMJ. 2013;347:f5160. doi: 10.1136/bmj.f5160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dailey DL, Rakel BA, Vance CGT, Liebano RE, Amrit AS, Bush HM, Lee KS, Lee JE, Sluka KA. Transcutaneous electrical nerve stimulation reduces pain, fatigue and hyperalgesia while restoring central inhibition in primary fibromyalgia. Pain. 2013;154:2554–2562. doi: 10.1016/j.pain.2013.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dannecker EA, George SZ, Robinson ME. Influence and stability of pain scale anchors for an investigation of cold pressor pain tolerance. J Pain. 2007;8:476–482. doi: 10.1016/j.jpain.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deyo RA, Mirza SK, Martin BI, Kreuter W, Goodman DC, Jarvik JG. Trends, Major Medical Complications, and Charges Associated With Surgery for Lumbar Spinal Stenosis in Older Adults. JAMA. 2010;303:1259–1265. doi: 10.1001/jama.2010.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dutton M. Orthopaedic Examination, Evaluation, and Intervention. 2nd Edition. McGraw-Hill Medical; 2008. 2nd ed. [Google Scholar]
- 12.Edwards RR, Fillingim RB. Effects of age on temporal summation and habituation of thermal pain: clinical relevance in healthy older and younger adults. J Pain. 2001;2:307–317. doi: 10.1054/jpai.2001.25525. [DOI] [PubMed] [Google Scholar]
- 13.Edwards RR, Fillingim RB, Ness TJ. Age-related differences in endogenous pain modulation: a comparison of diffuse noxious inhibitory controls in healthy older and younger adults. Pain. 2003;101:155–165. doi: 10.1016/s0304-3959(02)00324-x. [DOI] [PubMed] [Google Scholar]
- 14.Facci LM, Nowotny JP, Tormem F, Trevisani VFM. Effects of transcutaneous electrical nerve stimulation (TENS) and interferential currents (IFC) in patients with nonspecific chronic low back pain: randomized clinical trial. Sao Paulo Med J. 2011;129:206–216. doi: 10.1590/S1516-31802011000400003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 16.Gagliese L. Pain and aging: the emergence of a new subfield of pain research. J Pain. 2009;10:343–353. doi: 10.1016/j.jpain.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 17.Gagliese L, Katz J. Age differences in postoperative pain are scale dependent: a comparison of measures of pain intensity and quality in younger and older surgical patients. Pain. 2003;103:11–20. doi: 10.1016/s0304-3959(02)00327-5. [DOI] [PubMed] [Google Scholar]
- 18.Gibson SJ, Farrell M. A review of age differences in the neurophysiology of nociception and the perceptual experience of pain. Clin J Pain. 2004;20:227–239. doi: 10.1097/00002508-200407000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Gibson SJ, Weiner DK. Pain in older persons. Seattle: IASP Press; 2005. [Google Scholar]
- 20.Gladwell PW, Badlan K, Cramp F, Palmer S. Direct and Indirect Benefits Reported by Users of Transcutaneous Electrical Nerve Stimulation for Chronic Musculoskeletal Pain: Qualitative Exploration Using Patient Interviews. Phys Ther. 2015 doi: 10.2522/ptj.20140120. [DOI] [PubMed] [Google Scholar]
- 21.Gopalkrishnan P, Sluka KA. Effect of varying frequency, intensity, and pulse duration of transcutaneous electrical nerve stimulation on primary hyperalgesia in inflamed rats. Archives of Physical Medicine and Rehabilitation. 2000;81:984–990. doi: 10.1053/apmr.2000.5576. [DOI] [PubMed] [Google Scholar]
- 22.Gottrup H, Kristensen AD, Bach FW, Jensen TS. Aftersensations in experimental and clinical hypersensitivity. Pain. 2003;103:57–64. doi: 10.1016/s0304-3959(02)00415-3. [DOI] [PubMed] [Google Scholar]
- 23.Granot M, Granovsky Y, Sprecher E, Nir R-R, Yarnitsky D. Contact heat-evoked temporal summation: tonic versus repetitive-phasic stimulation. Pain. 2006;122:295–305. doi: 10.1016/j.pain.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 24.Haig AJ, Geisser ME, Tong HC, Yamakawa KSJ, Quint DJ, Hoff JT, Chiodo A, Miner JA, Phalke VV. Electromyographic and magnetic resonance imaging to predict lumbar stenosis, low-back pain, and no back symptoms. J Bone Joint Surg Am. 2007;89:358–366. doi: 10.2106/JBJS.E.00704. [DOI] [PubMed] [Google Scholar]
- 25.Haig AJ, Tong HC, Yamakawa KSJ, Parres C, Quint DJ, Chiodo A, Miner JA, Phalke VC, Hoff JT, Geisser ME. Predictors of pain and function in persons with spinal stenosis, low back pain, and no back pain. Spine. 2006;31:2950–2957. doi: 10.1097/01.brs.0000247791.97032.1e. [DOI] [PubMed] [Google Scholar]
- 26.Hjermstad MJ, Fayers PM, Haugen DF, Caraceni A, Hanks GW, Loge JH, Fainsinger R, Aass N, Kaasa S. Studies comparing Numerical Rating Scales, Verbal Rating Scales, and Visual Analogue Scales for assessment of pain intensity in adults: a systematic literature review. J Pain Symptom Manage. 2011;41:1073–1093. doi: 10.1016/j.jpainsymman.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 27.Johnson MI, Walsh DM. Pain: Continued uncertainty of TENS’ effectiveness for pain relief. Nat Rev Rheumatol. 2010;6:314–316. doi: 10.1038/nrrheum.2010.77. [DOI] [PubMed] [Google Scholar]
- 28.Jones DH, Kilgour RD, Comtois AS. Test-retest reliability of pressure pain threshold measurements of the upper limb and torso in young healthy women. J Pain. 2007;8:650–656. doi: 10.1016/j.jpain.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 29.Kalra A, Urban MO, Sluka KA. Blockade of opioid receptors in rostral ventral medulla prevents antihyperalgesia produced by transcutaneous electrical nerve stimulation (TENS) J Pharmacol Exp Ther. 2001;298:257–263. [PubMed] [Google Scholar]
- 30.Khadilkar A, Odebiyi DO, Brosseau L, Wells GA. Transcutaneous electrical nerve stimulation (TENS) versus placebo for chronic low-back pain. In: The Cochrane Collaboration, editor. Cochrane Database of Systematic Reviews [Internet] Chichester, UK: John Wiley & Sons, Ltd; 2008. [cited 2014 Oct 13]. Available from: http://summaries.cochrane.org/CD003008/BACK_transcutaneous-electrical-nerve-stimulation-tens-versus-placebo-for-chronic-low-back-pain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Larivière M, Goffaux P, Marchand S, Julien N. Changes in pain perception and descending inhibitory controls start at middle age in healthy adults. Clin J Pain. 2007;23:506–510. doi: 10.1097/AJP.0b013e31806a23e8. [DOI] [PubMed] [Google Scholar]
- 32.Lautenbacher S. Experimental Approaches in the Study of Pain in the Elderly. Pain Medicine. 2012;13:S44–S50. doi: 10.1111/j.1526-4637.2012.01326.x. [DOI] [PubMed] [Google Scholar]
- 33.Lautenbacher S, Kunz M, Strate P, Nielsen J, Arendt-Nielsen L. Age effects on pain thresholds, temporal summation and spatial summation of heat and pressure pain. Pain. 2005;115:410–418. doi: 10.1016/j.pain.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 34.Levin MF, Hui-Chan CW. Conventional and acupuncture-like transcutaneous electrical nerve stimulation excite similar afferent fibers. Arch Phys Med Rehabil. 1993;74:54–60. [PubMed] [Google Scholar]
- 35.Liebano RE, Rakel B, Vance CGT, Walsh DM, Sluka KA. An investigation of the development of analgesic tolerance to TENS in humans. Pain. 2011;152:335–342. doi: 10.1016/j.pain.2010.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manchikanti L, Falco FJE, Singh V, Pampati V, Parr AT, Benyamin RM, Fellows B, Hirsch JA. Utilization of interventional techniques in managing chronic pain in the Medicare population: analysis of growth patterns from 2000 to 2011. Pain Physician. 2012;15:E969–E982. [PubMed] [Google Scholar]
- 37.Manchikanti L, Singh V, Pampati V, Smith HS, Hirsch JA. Analysis of growth of interventional techniques in managing chronic pain in the Medicare population: a 10-year evaluation from 1997 to 2006. Pain Physician. 2009;12:9–34. [PubMed] [Google Scholar]
- 38.Marchand S, Charest J, Li J, Chenard JR, Lavignolle B, Laurencelle L. Is TENS purely a placebo effect? A controlled study on chronic low back pain. Pain. 1993;54:99–106. doi: 10.1016/0304-3959(93)90104-W. [DOI] [PubMed] [Google Scholar]
- 39.Moran F, Leonard T, Hawthorne S, Hughes CM, McCrum-Gardner E, Johnson MI, Rakel BA, Sluka KA, Walsh DM. Hypoalgesia in response to transcutaneous electrical nerve stimulation (TENS) depends on stimulation intensity. J Pain. 2011;12:929–935. doi: 10.1016/j.jpain.2011.02.352. [DOI] [PubMed] [Google Scholar]
- 40.Nnoaham KE, Kumbang J. Transcutaneous electrical nerve stimulation (TENS) for chronic pain. Cochrane Database Syst Rev. 2008 doi: 10.1002/14651858.CD003222.pub2. CD003222. [DOI] [PubMed] [Google Scholar]
- 41.Pahor M, Guralnik JM, Wan JY, Ferrucci L, Penninx BW, Lyles A, Ling S, Fried LP. Lower body osteoarticular pain and dose of analgesic medications in older disabled women: the Women’s Health and Aging Study. Am J Public Health. 1999;89:930–934. doi: 10.2105/ajph.89.6.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pantaleão MA, Laurino MF, Gallego NLG, Cabral CMN, Rakel B, Vance C, Sluka KA, Walsh DM, Liebano RE. Adjusting pulse amplitude during transcutaneous electrical nerve stimulation (TENS) application produces greater hypoalgesia. J Pain. 2011;12:581–590. doi: 10.1016/j.jpain.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 43.Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- 44.Radhakrishnan R, Sluka KA. Deep tissue afferents, but not cutaneous afferents, mediate transcutaneous electrical nerve stimulation-Induced antihyperalgesia. J Pain. 2005;6:673–680. doi: 10.1016/j.jpain.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 45.Rakel BA, Zimmerman MB, Geasland K, Embree J, Clark CR, Noiseux NO, Callaghan JJ, Herr K, Walsh D, Sluka KA. Transcutaneous electrical nerve stimulation for the control of pain during rehabilitation after total knee arthroplasty: A randomized, blinded, placebo-controlled trial. PAIN®. 2014;155:2599–2611. doi: 10.1016/j.pain.2014.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rakel B, Cooper N, Adams HJ, Messer BR, Frey Law LA, Dannen DR, Miller CA, Polehna AC, Ruggle RC, Vance CGT, Walsh DM, Sluka KA. A new transient sham TENS device allows for investigator blinding while delivering a true placebo treatment. J Pain. 2010;11:230–238. doi: 10.1016/j.jpain.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rakel B, Frantz R. Effectiveness of transcutaneous electrical nerve stimulation on postoperative pain with movement. J Pain. 2003;4:455–464. doi: 10.1067/s1526-5900(03)00780-6. [DOI] [PubMed] [Google Scholar]
- 48.Riley JL, 3rd, Cruz-Almeida Y, Glover TL, King CD, Goodin BR, Sibille KT, Bartley EJ, Herbert MS, Sotolongo A, Fessler BJ, Redden DT, Staud R, Bradley LA, Fillingim RB. Age and race effects on pain sensitivity and modulation among middle-aged and older adults. J Pain. 2014;15:272–282. doi: 10.1016/j.jpain.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Riley JL, 3rd, King CD, Wong F, Fillingim RB, Mauderli AP. Lack of endogenous modulation and reduced decay of prolonged heat pain in older adults. Pain. 2010;150:153–160. doi: 10.1016/j.pain.2010.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Robinson ME, Wise EA, Gagnon C, Fillingim RB, Price DD. Influences of gender role and anxiety on sex differences in temporal summation of pain. J Pain. 2004;5:77–82. doi: 10.1016/j.jpain.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 51.Scheele J, Enthoven WTM, Bierma-Zeinstra SMA, Peul WC, van Tulder MW, Bohnen AM, Berger MY, Koes BW, Luijsterburg PAJ. Course and prognosis of older back pain patients in general practice: a prospective cohort study. Pain. 2013;154:951–957. doi: 10.1016/j.pain.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 52.Sluka KA, Bjordal JM, Marchand S, Rakel BA. What makes transcutaneous electrical nerve stimulation work? Making sense of the mixed results in the clinical literature. Phys Ther. 2013;93:1397–1402. doi: 10.2522/ptj.20120281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sluka KA, Deacon M, Stibal A, Strissel S, Terpstra A. Spinal blockade of opioid receptors prevents the analgesia produced by TENS in arthritic rats. J Pharmacol Exp Ther. 1999;289:840–846. [PubMed] [Google Scholar]
- 54.Sluka KA, Walsh D. Transcutaneous electrical nerve stimulation: basic science mechanisms and clinical effectiveness. J Pain. 2003;4:109–121. doi: 10.1054/jpai.2003.434. [DOI] [PubMed] [Google Scholar]
- 55.Spitz A, Moore AA, Papaleontiou M, Granieri E, Turner BJ, Reid MC. Primary care providers’ perspective on prescribing opioids to older adults with chronic non-cancer pain: A qualitative study. BMC Geriatrics. 2011;11:35. doi: 10.1186/1471-2318-11-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Staud R, Koo E, Robinson ME, Price DD. Spatial summation of mechanically evoked muscle pain and painful aftersensations in normal subjects and fibromyalgia patients. Pain. 2007;130:177–187. doi: 10.1016/j.pain.2007.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Staud R, Vierck CJ, Robinson ME, Price DD. Overall fibromyalgia pain is predicted by ratings of local pain and pain-related negative affect--possible role of peripheral tissues. Rheumatology (Oxford) 2006;45:1409–1415. doi: 10.1093/rheumatology/kel121. [DOI] [PubMed] [Google Scholar]
- 58.Staud R, Weyl EE, Riley JL, 3rd, Fillingim RB. Slow temporal summation of pain for assessment of central pain sensitivity and clinical pain of fibromyalgia patients. PLoS ONE. 2014;9:e89086. doi: 10.1371/journal.pone.0089086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Strand LI, Moe-Nilssen R, Ljunggren AE. Back Performance Scale for the assessment of mobility-related activities in people with back pain. Phys Ther. 2002;82:1213–1223. [PubMed] [Google Scholar]
- 60.Tan G, Jensen MP, Thornby JI, Shanti BF. Validation of the Brief Pain Inventory for chronic nonmalignant pain. J Pain. 2004;5:133–137. doi: 10.1016/j.jpain.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 61.Vance CGT, Dailey DL, Rakel BA, Sluka KA. Using TENS for pain control: the state of the evidence. Pain Manag. 2014;4:197–209. doi: 10.2217/pmt.14.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vance CGT, Rakel BA, Blodgett NP, de Santana JM, Amendola A, Zimmerman MB, Walsh DM, Sluka KA. Effects of Transcutaneous Electrical Nerve Stimulation on Pain, Pain Sensitivity, and Function in Patients With Knee Osteoarthritis: A Randomized Controlled Trial. Physical Therapy [Internet] 2012 doi: 10.2522/ptj.20110183. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22466027. [DOI] [PMC free article] [PubMed]
- 63.Wager TD, Scott DJ, Zubieta J-K. Placebo effects on human mu-opioid activity during pain. Proc Natl Acad Sci USA. 2007;104:11056–11061. doi: 10.1073/pnas.0702413104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Walsh DM, Howe TE, Johnson MI, Moran F, Sluka KA. The Cochrane Collaboration. Transcutaneous electrical nerve stimulation for acute pain. In: Walsh DM, editor. Cochrane Database of Systematic Reviews [Internet] Chichester, UK: John Wiley & Sons, Ltd; 2009. [cited 2013 May 13]. Available from: http://summaries.cochrane.org/CD006142/effectiveness-of-transcutaneous-electrical-nerve-stimulation-tens-as-a-sole-treatment-for-acute-pain-in-adults. [DOI] [PubMed] [Google Scholar]
- 65.Washington LL, Gibson SJ, Helme RD. Age-related differences in the endogenous analgesic response to repeated cold water immersion in human volunteers. Pain. 2000;89:89–96. doi: 10.1016/S0304-3959(00)00352-3. [DOI] [PubMed] [Google Scholar]
- 66.Weiner DK, Kim Y-S, Bonino P, Wang T. Low Back Pain in Older Adults: Are We Utilizing Healthcare Resources Wisely? Pain Medicine. 2006;7:143–150. doi: 10.1111/j.1526-4637.2006.00112.x. [DOI] [PubMed] [Google Scholar]
- 67.Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152:S2–S15. doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zubieta J-K, Bueller JA, Jackson LR, Scott DJ, Xu Y, Koeppe RA, Nichols TE, Stohler CS. Placebo effects mediated by endogenous opioid activity on mu-opioid receptors. J Neurosci. 2005;25:7754–7762. doi: 10.1523/JNEUROSCI.0439-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]