Abstract
In 2001, a hybrid strain of Vibrio cholerae O1 El Tor that expresses a classical cholera toxin (CT) emerged and this hybrid variant rapidly replaced the previous El Tor strain around the world. The global emergence of this variant coincided with anecdotal reports that cholera patients were presenting with more severe dehydration and disease in many locations. We compared severity of disease in cholera patients from before and after emergence of the hybrid strain at a diarrheal hospital in Dhaka, Bangladesh. We did indeed find that cholera patients presented with more severe dehydration and severe disease in the latter period; however, this was also true for “all non-cholera patients” as well. In addition, in sub-analyses of patients who presented with rotavirus and enterotoxigenic E. coli (ETEC), we found similar results. Comparing the two periods for differences in patient characteristics, nutritional status, vaccination status and income, we were unable to detect a plausible cause for patients presenting with more severe disease in the latter period. Because we observed a shift in severity for both cholera and non-cholera, our results indicate that the altered El Tor strain cannot fully explain the differences in cholera severity before and after 2001
Keywords: Cholera, Vibrio cholerae, hybrid, watery diarrhea, severity
Introduction
Cholera is a life-threatening dehydrating diarrhea that results from ingestion of toxigenic serogroups of Vibrio cholerae. V. cholerae is differentiated serologically by the O antigen of its lipopolysaccharide (LPS); the vast majority of human cholera is caused either by the O1 or O139 serogroups that carry the genes for cholera toxin 1. The O1 serogroup of V. cholerae is further classified into two biotypes (classical and El Tor) and two major serotypes (Inaba and Ogawa) 2. Classical and El Tor strains differ from each other in both phenotypic and genetic characteristics, as well as by small differences in the type of cholera toxin produced. Although classical strains are thought to have caused the first six cholera pandemics between 1817 and 1923, and continued to cause endemic and epidemic disease after that, the El Tor biotype emerged in 1905 and is the causative agent for the current 7th cholera pandemic that started in 1961 and continues today. El Tor biotype strains have now fully replaced the earlier classical strains, and the last classical strain of V. cholerae O1 identified at the icddr,b was in 1992 1, 3, 4.
Cholera in Bangladesh occurs both as an endemic disease, with seasonal peaks before and after the monsoons, and in epidemics that often take place during or following frequent floods, droughts, and cyclones that occur in the country5. Several factors have been previously associated with a higher risk for cholera and cholera with severe dehydration, including periods of flooding, malnutrition, retinol deficiency, partial and pre-existing immunity, and the presence of blood group O and other genetic risks factors 5–12.
It has been suggested that cholera outbreaks have become more frequent and severe over the past 10–15 years 5, 11, 13, 14 with higher morbidity. One postulated explanation for the increasing severity of disease has been the emergence in the year 2001 of strains of V. cholerae O1 El Tor that have acquired cholera toxin genes of the classical biotype, but that are otherwise similar to previous 7th pandemic El Tor strains 14. These strains have been referred to as “altered El Tor biotype strains”15. The relationship between altered El Tor biotype strains and more severe disease has been proposed since cholera caused by classical strains of V. cholerae O1 was more often severe than that caused by El Tor strains when both biotypes co-circulated 2, 4, suggesting that the differences in cholera toxin genes might relate to disease severity. However, careful documentation that cholera is more severe in recent years than previously, and exploration of potential explanations, has not previously been undertaken to our knowledge. To address this, we used a surveillance system that is in place at the international centre for diarrheal disease and research (icddr,b) and that records causes of diarrhea seen, disease severity, and a variety of patient data in a systematic way. In Bangladesh, altered strains of El Tor V. cholerae O1, producing cholera toxin of the classical type, were first identified in 2001 15, 16 and molecular analysis of V. cholerae strains isolated from patients coming to the icddr,b since 2001 show that 100% of the strains have this hybrid or altered phenotype 17. We therefore choose as our study periods a four year interval after emergence of the hybrid strains (2005–2008) and a four year interval before the emergence of the altered El Tor strains (1997–2000). Each of these study periods also contained one major flood (1998 and 2007).
As such, our comparison allows examination of the potential role of the altered El Tor biotype strains in cholera severity. To see if any possible association was cholera-specific, we also performed similar analyses for “all non-cholera patients”, and sub-analyses for other common causes of watery diarrhea: rotavirus and enterotoxigenic E. coli (ETEC).
Materials and Methods
Study design
This study was conducted at the Clinical Research and Service Centre (CRSC) of the International Centre for Diarrheal Disease Research (icddr,b) in Dhaka, Bangladesh. The hospital cares for approximately 125,000 patients annually, including 10,000–20,000 cholera patients. A surveillance system exists at the icddr,b that systematically samples children and adults with diarrheal illnesses. Since 1996, every 50th patient has been enrolled into the systematic surveillance system. Patients entered into the surveillance system and their family members are interviewed by health workers who collect demographic, socioeconomic, and clinical data. A physician documents the clinical condition, including dehydration status as per WHO guidelines 18, 19, and a stool or rectal swab sample is collected for microbiologic evaluation. All demographic, microbiologic, treatment and outcome data are systematically recorded and entered into a database that was used for the present study. In the earlier time period of our severity study, 1997–2000, there were an estimated 9,940 patients entered into the surveillance system, whereas in the more recent time period studied, 2005–2008, 9,237 patients were entered.
Microbiological evaluation
As part of the surveillance system, stool or rectal swab specimens were evaluated for enteric pathogens including Vibrio cholerae, enterotoxinogenic Escherichia coli (ETEC), Salmonella spp., Shigella spp., Campylobacter jejuni, and rotavirus, using standard techniques previously described 20–23. For isolation of V. cholerae, specimens were cultured on taurocholate-tellurite-gelatin agar plates. Specimens were also enriched in alkaline peptone water for 4 hours and then cultured 24. Specific monoclonal antibodies were used on colonies of V. cholerae to detect V. cholerae O1 and O139 serogroups, as well as to differentiate between Ogawa and Inaba serotypes of V. cholerae O123, 25, 26. Every 100th strain of V. cholerae O1 was typed by PCR to distinguish the altered El Tor variant from the original El Tor biotype; quantification of cholera toxin production was not carried out 15. For detection of ETEC, specimens were cultured overnight on MacConkey agar plates 22, 27, six freshly isolated lactose-fermenting E. coli colonies were isolated, and genes for heat-labile or heat-stable enterotoxin were detected by PCR, as previously described 28. We also examined stool samples by direct microscopy to detect enteric parasites 7.
Assessing clinical severity
As part of the surveillance system at the icddr,b hospital, the assessment of dehydration status of the patient on presentation was determined by the Dhaka method based on WHO criteria ref. Assessment was based on clinical evaluation and examination of the level of consciousness, eyes, and tongue; the presence of thirst; skin-pinch, and radial pulse of the patient by trained health personnel. Criteria for assessing hydration status were consistent throughout the study periods.
The nutritional status of children was expressed in terms of the number of standard deviations of an anthropometric index, such as height-for-age, weight-for-age, or weight-for-height, expressed as a “Z” score in relation to the new World Health Organization growth standards 29. The anthropometric measurements used were HAZ (“height-for-age z-score”), WAZ (‘weight-for-age z score”), and WHZ (“weight-for-height z-score”). We considered Z scores of < −2 SD as a moderate to severe degree of being stunted, underweight or wasted, respectively. A Z score of < −3 SD was considered evidence of severe malnutrition in all categories.
Vaccination status of children
The icddr,b surveillance system records the clinical history, including immunization status of children. In the case of children, their parents or female caregivers are interviewed, and a trained research assistant collects this information using a pre-defined and pre-tested surveillance questionnaire and entered in to electronic database. From this database, information on 669 children less than 2 years of age in the two time periods(1997–2000 vs. 2005–2008) attending the hospital during was extracted. The vaccination status was recorded; for this mostly parents/female caregivers were interviewed and, in about 2% of cases, EPI health cards were reviewed.
Statistical analysis
Statistical analyses were performed using Statistical Package for Social Sciences (SPSS, Chicago, IL) Version 12.0. Differences in proportion of cases were assessed by Pearson’s X2 analysis. Strength of associations was made by calculation of the odds ratio (OR) with 95% confidence intervals (CIs) using Epi Info version 3.4 (Epi Info 2002, CDC). Comparison between two means was performed using the independent sample t test; when data were not normally distributed, the Mann-Whitney U test was used. Statistical significance was defined as a two-tailed p < 0.05.
Results
Patients entered into the hospital surveillance system and comparison of pathogens isolated between the two time periods
We examined hospital surveillance data of the icddr,b from a four year period before the first isolation of altered El Tor variant strains of V. cholerae O1 (1997–2000) and compared that to data from a four year period after that, during which 100% of isolates of V. cholerae O1 were of the altered El Tor variant (2005–2009) (Table 1). Flooding is a regular occurrence in Dhaka, and we have previously shown that cholera burden and severity increases during flooding [15]. We choose our two periods to match number of years, including a single flood, and pre- and post-emergence of the hybrid strain of V. cholerae. Overall, the proportion of pathogens was comparable in the two periods, with a slight decrease in proportion of cholera patients in the second period (28% versus 25%). Serotypes of cholera routinely fluctuate and Ogawa strains predominated in the earlier period, but Ogawa and Inaba strains were more equally represented in the later time period. Although V. cholerae O139 strains were present in the earlier time period, they were not detected in the later time period. The second most common pathogen isolated in both periods was rotavirus (24% and 22%, respectively). The rate of isolation of several other diarrheal pathogens from patients decreased slightly between the two time periods, including for ETEC, rotavirus, Shigella spp., Salmonella spp, and E. histolytica (Table 1). In the subset of children under 5 years of age (data not shown), the frequency of detection of rotavirus increased a small but significant amount between the two time periods (39% versus 43%; p < 0.001).
Table 1.
Total patients admitted to the icddr,b hospital and included in the surveillance system during the two periods studied, and pathogens identified
| Total patients in surveillance system | 1997–2000 N=9940 |
2005–2008 N=9237 |
P value |
|---|---|---|---|
| Pathogens isolated | No. (% of total) | No. (% of total) | |
| V. cholerae (total) | 2758 (28.0) | 2314(25.0) | 0.16 |
| V. cholerae O1* | 2266 (23.0) | 2314 (25.0) | <0.001 |
| Ogawa | 1940 (20.0) | 1336 (14.0) | <0.001 |
| Inaba | 326 (3.3) | 978 (11.0) | <0,001 |
| V. cholerae O139 | 492 (4.9) | 0 (0) | |
| Rotavirus | 2361 (24.0) | 2009 (22.0) | <0.001 |
| ETEC | 1243 (13.0) | 617 (6.7) | <0.001 |
| Shigella | 549 (5.5) | 300 (3.0) | <0.001 |
| Salmonella | 136 (1.4) | 96 (1.0) | 0.03 |
| Giardia | 178 (1.8) | 157 (1.7) | 0.23 |
| E. histolytica | 125 (1.3) | 80 (0.9) | <0.001 |
| Others | 1840 (19.0) | 2017 (21.0) | <0.001 |
From 2001 onwards, all V. cholerae O1 strains isolated at the icddr,b were of the altered El Tor biotype; prior to that, all were of the original El Tor biotype.
Comparison of disease severity in patients with cholera and other pathogens between the two time periods
The age and gender of patients with cholera was not significantly different between the two time periods (Table 2A). Cholera patients admitted in the later time period had evidence of more severe disease on admission, as reflected by a larger percentage having more than 6 stools a day, more patients having severe dehydration on presentation, more patients requiring intravenous fluids, and patients staying slightly longer in the hospital before being discharged home. The increased severity of diarrheal disease was seen in both adults and children less than 5 years of age.
Table 2A.
Severity of disease in patients admitted with cholera to the icddr,b hospital during the two periods studied
| All cholera patients | |||
|---|---|---|---|
| 1997–2000 N = 2758 (%) |
2005–2008 N = 2314 (%) |
p value | |
| Severe Dehydration | 1315 (48) | 1635 (71) | <0.001 |
| IV fluids required | 1877 (68) | 1851 (80) | <0.001 |
| >6 stools/day | 2531 (92) | 2189 (95) | <0.001 |
| >10 vomit/day | 426 (15) | 380 (16) | 0.344 |
| ¥Length of hospital stay in hours | 24 | 27 | < 0.001 |
| Fever >37.7 °C | 55 (2.0) | 15 (0.7) | <0.001 |
| Age yrs (Median) | 21 | 20 | |
| Sex- Male | 1521 (55) | 1314 (57) | 0.242 |
Confidence intervals (CI) and p values were derived from χ2 (chi-square) tests.
Mann-Whitney U test, for continuous variables
We similarly assessed severity of disease for all non-cholera patients seen in the two time periods (7182 for the period 1997–2000 and 6923 for the period 2005–2008; Table 2B), as well as in subsets of patients presenting with ETEC (1243 for the period 1997–2000 and 617 for the period 2005–2008), and for patients presenting with rotavirus (2361 for the period 1997–2000 and 2009 for the period 2005–2008) (data not shown). Importantly, we found the “non-cholera patients” as a group also presented with more severe dehydration and more severe disease in the later period (Fig 1). In addition, in sub-analyses of patients who presented with rotavirus and enterotoxigenic E. coli (ETEC), other common causes of dehydrating diarrhea in Bangladesh, we found similar results: patients with these pathogens also presented with more severe disease and severe dehydration in the later than the earlier time-period (data not shown).
Table 2B.
Severity of disease in patients admitted with non-cholera to icddr,b hospital during the two periods
| All Non Cholera patients | |||
|---|---|---|---|
| 1997–2000 N = 7182 (%) |
2005–2008 N = 6923 (%) |
p value | |
| Severe Dehydration | 554 (8) | 1347 (20) | <0.001 |
| IV fluids required | 1047 (15) | 1728 (26) | <0.001 |
| >6 stools/day | 6315 (89) | 6287 (92) | <0.001 |
| >10 vomit/day | 415 (6) | 443 (6) | 0.344 |
| ¥Length of hospital stay in hours | 8 | 10 | <0.001 |
| Fever >37.7 °C | 576 (8) | 347 (5) | <0.001 |
| Age years (Median) | 21 | 20 | |
| Sex- Male | 4247 (60) | 4112 (60) | 0.242 |
Confidence intervals (CI) and p values were derived from χ2 (chi-square) tests.
Mann-Whitney U test, for continuous variables
Figure 1.
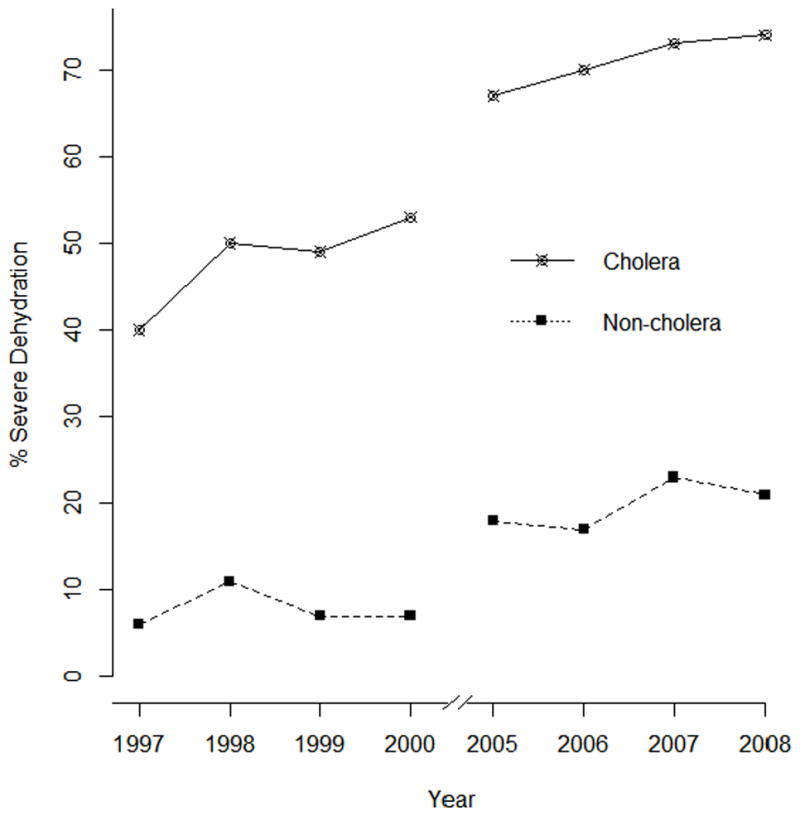
Severity of disease in cholera and non-cholera patients admitted to the icddr,b hospital by year
Correlation of cholera severity with malnutrition in children
The severity of cholera related more to indices of weight than height, and was even more prominent in undernourished and wasted patients in the later time period (64% vs. 71%; 64% vs. 75%) (Supplemental Table 1A). Malnourished patients admitted with diarrhea due to pathogens other than V. cholerae also had more frequent severe dehydration, similar to those patients with cholera (Supplemental Table 1B).
Changes in nutritional and vaccination status of children between the two time periods
We compared malnutrition indicators as well as vaccination status for children between the two time periods (Supplemental Table 2A). Despite the increasing severity of cholera seen in the later time period Children were less malnourished in the later period (severely stunted 23% vs. 14%; severely undernourished 31% vs. 25%; p=0.03-<0.001), and more likely to have received childhood vaccines (DPT 69% vs. 81%; Polio 69% vs. 81%; p=0.001-<0.001) (Table 2A),. This finding was also observed in the “non-cholera” cohort (Supplemental Table 2B). A similar finding was observed in the rotavirus cohort but we did not detect a trend toward improved nutritional status in the ETEC cohort (data not shown).
Changes in health seeking behavior of patients with cholera between the two time periods
Another possibility for the observation of more severe dehydration in patients presenting with cholera in the later time period might be a change in health seeking behavior, such as individuals self-treating less severe disease at home with antibiotics and ORS and coming to care later if that failed; a change in the socioeconomic background of the patient that could influence a range of variables related to disease severity; or perhaps needing to travel longer distances to reach care in the later period, leading patients to arrive more severely ill. Although there was a slight but significant increase in use of ORS at home before presentation in the population overall, there was no difference in the use of ORS in children under 5 who also presented with increased cholera severity in the later time period (Supplemental Table 3A). Children in the later period took antibiotics at home before presentation less frequently, although there was no difference in the population overall. There were also no differences in patients having diarrhea at home for more than a day before presentation, and no difference in the mean distance to the hospital between the two time periods. Finally, patients in the later period were less likely to come from a poorer family, consistent with the overall improvement in economic conditions in Bangladesh over the 12 year period of the study. Comparative analyses of the non-cholera cohort overall, and the subsets with rotavirus and ETEC specifically, also showed increased use of ORS and decreased use of home antibiotics in the later period (Supplemental Table 3B). Non-cholera patients, overall, and rotavirus diarrheal patients traveled farther in the later period, although ETEC patients traveled a shorter distance.
Discussion
Our data confirm earlier reports that cholera patients seen in more recent years have an increased severity of diarrheal disease, with higher numbers of stools on presentation, more severe dehydration, longer hospital stay, and requirement for more IV fluids, compared to patients seen prior to 2000 5, 14, 15, 32. Importantly, we also found that “all non-cholera” diarrheal patients and patients with diarrhea caused by rotavirus or ETEC were also more likely to present with more severe diseases in the later period compared to the earlier period. In order to try to understand this phenomenon, we analyzed a number of possible contributors but were unable to identify an obvious correlate. In the later period we found better nutritional status, better vaccination rates (suggesting improved engagement with the health care system), improved socio-economic status, improved use of ORS, similar duration of illness, and similar distance traveled to seek care consistent with the overall improvement of health indicators seen in Bangladesh in recent years 31. As such, we cannot at present explain the observation that all diarrheal patients presenting for care at the icddr,b seem to be presenting with more severe disease. We observed a shift in severity for both cholera and non-cholera, our results indicate that the altered El Tor strain cannot fully explain the differences in cholera severity before and after 2001”. Cholera severity has been associated with a range of host and environmental factors, including timely use of rehydration, access to care, pre-existing immunity, blood group, and other host genetic factors [6,7]. Severe dehydration from cholera is seen more commonly in children who are malnourished 30. Previous studies suggest a possible synergy between the depressed absorption of carbohydrate, protein and fat by intestinal mucosal cells, lower lactase activity and increased secretion produced by cholera toxin [41, 42] might also contribute to increased severity of cholera. The relative prevalence of the two serotypes of V. cholerae O1, Ogawa and Inaba, fluctuates from season to season and from one year to another 33, and usually one or the other of the serotypes is responsible for the majority of cases in any geographic area 34, 35. In our study, there was a difference in the relative predominance of the two serotypes: V. cholerae O1 Ogawa strains predominated in the earlier period, but the serotypes were more evenly distributed in the later time period. We have previously observed that cholera caused by the Ogawa serotype is associated with more severe dehydration 36. Since Ogawa strains were less predominant in the later period, this difference in circulating serotypes does not appear to be a major determinant of the increased severity seen.
The biotype (classical versus El Tor) of V. cholerae can also affect disease severity, with cholera caused by classical biotype organisms being considered more severe [2.3]. There are a number of genotypic and phenotypic differences between the biotypes [39], so the reason for this altered severity is not understood; however, it is possible that the differences between the classical and El Tor cholera toxin molecules themselves may contribute. Indeed, this is the reason that has been proposed to explain the more severe disease seen with hybrid strains of El Tor V. cholerae expressing classical-type cholera toxin (CT). Of note, to our knowledge, there is a paucity of experimental data to support this hypothesis.
The reason that classical cholera toxin might cause more severe disease compared to El Tor cholera toxin is uncertain. The B subunit of classical type toxin differs from the El Tor CTB at the 18th and 47th positions of amino acid residues (classical CT: His-18 and Thr-47; and El Tor CT: Tyr-18, Ile-47); the B subunit mediates attachment to the ganglioside GM1 on eukaryotic cell surfaces. The A-subunit of the El Tor and classical CT toxins are identical in amino acid sequence 37. In vitro, CT expression by El Tor strains is characteristically assessed using AKI media, growth at 37°C and in relative anaerobiosis; under these conditions, El Tor altered variant strains produce 20 times more CT than prototypic El Tor strains, an amount equivalent to that produced by classical strains 38, 39. Classical strains of V. cholerae are associated with more fluid accumulation in the rabbit ileal loop model than prototypic El Tor strains, but altered El Tor strains cause fluid secretion equivalent to that classical strains 39. We are not aware of data on the effect of altered El Tor CT in CHO cells or cyclic AMP (cAMP) induction in vitro. As such, whether hybrid strains of El Tor V. cholerae expressing classical CT can cause more severe disease in humans is possible but uncertain, and the data we present in this report would suggest that increasing severity in cholera patients cannot be ascribed solely to emergence of the hybrid organisms.
Our study has a number of significant limitations, and could not address a number of potential influences. Our study is a retrospective analysis of surveillance data, and we cannot exclude a shift in reporting bias between the two periods, although staff at the icddr,b adhere to standard clinical operating procedures that characterize assessment of dehydration by WHO criteria, and microbiologic analysis did not change during the study period. In addition, changes in population not discernible in our analysis are possible, although the catchment area of the icddr,b has remained constant over the two periods studied, and there has been no large in or out migration during the study periods. Our study could also not exclude the possibility that the increasing severity might be related to differing levels of pre-existing immunity against cholera in the population; however, the percentage of patients presenting with cholera in both periods was the same (suggesting a similar population burden during the two periods). Similarly, in other studies by our team, we found no change in the baseline Vibriocidal antibody titer of cholera patients presenting to the icddr,b during these periods, an observation consistent with a stable burden of cholera in the community 23, 40, 41. Blood group and other host factors can also affect cholera severity, and these data are not collected as part of the surveillance system, so we cannot comment on their potential impact, although no major population change occurred between the two time periods. We also did not directly compare the full microbiologic characteristics, including full genomic sequences of the circulating strains during the two periods, although previous analysis has shown that the older and hybrid strains are largely identical except for differences in cholera toxin 3, 4.
In summary, although our data cannot exclude the possibility that the recently emerged variant El Tor V. cholerae O1 can cause more severe disease, our results suggest that other yet undefined factors may be driving the observed shift in when and how patients with cholera and other dehydrating diarrheal illnesses present in Dhaka, Bangladesh.
Supplementary Material
Patients after emergence of hybrid strain presented with severe Cholera
Findings were also true for rotavirus and enterotoxigenic E. coli diarrhoea.
Nutrition, vaccination status, income were not affecting factor for severe disease
Severity was more in malnourished children pre and post emergence of hybrid strain.
Severe dehydrating cholera in later time period may affect health seeking behavior
Acknowledgments
This research was supported by core grants to the icddr,b. icddr,b is thankful to the Government of Australia, Bangladesh, Canada, Sweden and UK for providing core/unrestricted support. Additionally, the study was supported by the following grants: U01 AI058935 (S.B.C. and E.T.R); RO3 AI063079 (FQ); AI106878 (E.T.R.); and the Swedish Agency for Research and Economic Cooperation (FQ and A-M S) (Sida-SAREC; Grant INT-ICDDR,B-HN-01-AV). F.C. was a previous recipient of Fogarty/Ellison Fellowship in Global Health awarded by the Fogarty International Center at the National Institutes of Health (D43 TW005572).
Footnotes
Ethical statement
The surveillance system and data analyses were approved by the Ethical Review Committee of the icddr,b.
Conflict of interest statement
There is no conflict of interest of the authors.
Author Contributions
FC, ET, FQ, AF and SC contributed to the study design. FC, FQ, AF, AIK contributed to the implementation and supervision of the study. FC, AK, FQ, ET and AF analyzed the data and took responsibility for the accuracy of the data analysis. All authors participated in the writing of the manuscript and had access to the data in the study. All authors saw and approved the final version of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Harris JB, LaRocque RC, Qadri F, Ryan ET, Calderwood SB. Cholera. Lancet. 2012;379:2466–76. doi: 10.1016/S0140-6736(12)60436-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaper JB, Morris JG, Jr, Levine MM. Cholera. Clin Microbiol Rev. 1995;8:48–86. doi: 10.1128/cmr.8.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alam M, Islam MT, Rashed SM, Johura FT, Bhuiyan NA, Delgado G, Morales R, Mendez JL, Navarro A, Watanabe H, Hasan NA, Colwell RR, Cravioto A. Vibrio cholerae classical biotype strains reveal distinct signatures in Mexico. J Clin Microbiol. 2012;50:2212–6. doi: 10.1128/JCM.00189-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Faruque SM, Abdul Alim AR, Rahman MM, Siddique AK, Sack RB, Albert MJ. Clonal relationships among classical Vibrio cholerae O1 strains isolated between 1961 and 1992 in Bangladesh. J Clin Microbiol. 1993;31:2513–6. doi: 10.1128/jcm.31.9.2513-2516.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harris AM, Chowdhury F, Begum YA, Khan AI, Faruque AS, Svennerholm AM, Harris JB, Ryan ET, Cravioto A, Calderwood SB, Qadri F. Shifting prevalence of major diarrheal pathogens in patients seeking hospital care during floods in 1998, 2004, and 2007 in Dhaka, Bangladesh. Am J Trop Med Hyg. 2008;79:708–14. [PMC free article] [PubMed] [Google Scholar]
- 6.Harris JB, Khan AI, LaRocque RC, Dorer DJ, Chowdhury F, Faruque AS, Sack DA, Ryan ET, Qadri F, Calderwood SB. Blood group, immunity, and risk of infection with Vibrio cholerae in an area of endemicity. Infect Immun. 2005;73:7422–7. doi: 10.1128/IAI.73.11.7422-7427.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harris JB, Podolsky MJ, Bhuiyan TR, Chowdhury F, Khan AI, Larocque RC, Logvinenko T, Kendall J, Faruque AS, Nagler CR, Ryan ET, Qadri F, Calderwood SB. Immunologic Responses to Vibrio cholerae in Patients Co-Infected with Intestinal Parasites in Bangladesh. PLoS Negl Trop Dis. 2009;3:e403. doi: 10.1371/journal.pntd.0000403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karlsson EK, Harris JB, Tabrizi S, Rahman A, Shlyakhter I, Patterson N, O’Dushlaine C, Schaffner SF, Gupta S, Chowdhury F, Sheikh A, Shin OS, Ellis C, Becker CE, Stuart LM, Calderwood SB, Ryan ET, Qadri F, Sabeti PC, Larocque RC. Natural selection in a bangladeshi population from the cholera-endemic ganges river delta. Sci Transl Med. 5:192ra86. doi: 10.1126/scitranslmed.3006338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palmer DL, Koster FT, Alam AK, Islam MR. Nutritional status: a determinant of severity of diarrhea in patients with cholera. J Infect Dis. 1976;134:8–14. doi: 10.1093/infdis/134.1.8. [DOI] [PubMed] [Google Scholar]
- 10.Harris AM, Bhuiyan MS, Chowdhury F, Khan AI, Hossain A, Kendall EA, Rahman A, LaRocque RC, Wrammert J, Ryan ET, Qadri F, Calderwood SB, Harris JB. Antigen-specific memory B-cell responses to Vibrio cholerae O1 infection in Bangladesh. Infect Immun. 2009;77:3850–6. doi: 10.1128/IAI.00369-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.CDC. Centers for Disease Control and Prevention. Update: cholera outbreak—Haiti, 2010. MMWR Morb Mortal Wkly Rep. 2010:1473–9. [PubMed] [Google Scholar]
- 12.Ali A, Chen Y, Johnson JA, Redden E, Mayette Y, Rashid MH, Stine OC, Morris JG., Jr Recent clonal origin of cholera in Haiti. Emerg Infect Dis. 2011;17:699–701. doi: 10.3201/eid1704.101973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwartz BSHJ, Khan AI, Larocque RC, Sack DA, Malek MA, Faruque AS, Qadri F, Calderwood SB, Luby SP, Ryan ET. Diarrheal epidemics in Dhaka, Bangladesh, during three consecutive floods: 1988, 1998, and 2004. Am J Trop Med Hyg. 2006;74:1067–73. [PMC free article] [PubMed] [Google Scholar]
- 14.Siddique AK, Nair GB, Alam M, Sack DA, Huq A, Nizam A, Longini IM, Jr, Qadri F, Faruque SM, Colwell RR, Ahmed S, Iqbal A, Bhuiyan NA, Sack RB. El Tor cholera with severe disease: a new threat to Asia and beyond. Epidemiol Infect. 2010;138:347–52. doi: 10.1017/S0950268809990550. [DOI] [PubMed] [Google Scholar]
- 15.Nair GB, Qadri F, Holmgren J, Svennerholm AM, Safa A, Bhuiyan NA, Ahmad QS, Faruque SM, Faruque AS, Takeda Y, Sack DA. Cholera due to altered El Tor strains of Vibrio cholerae O1 in Bangladesh. J Clin Microbiol. 2006;44:4211–3. doi: 10.1128/JCM.01304-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alam M, Islam A, Bhuiyan NA, Rahim N, Hossain A, Khan GY, Ahmed D, Watanabe H, Izumiya H, Faruque AS, Akanda AS, Islam S, Sack RB, Huq A, Colwell RR, Cravioto A. Clonal transmission, dual peak, and off-season cholera in Bangladesh. Infect Ecol Epidemiol. 2011;1:1–13. doi: 10.3402/iee.v1i0.7273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rashed SM, Mannan SB, Johura FT, Islam MT, Sadique A, Watanabe H, Sack RB, Huq A, Colwell RR, Cravioto A, Alam M. Genetic characteristics of drug-resistant Vibrio cholerae O1 causing endemic cholera in Dhaka, 2006–2011. J Med Microbiol. 61:1736–45. doi: 10.1099/jmm.0.049635-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stoll BJ, Glass RI, Huq MI, Khan MU, Holt JE, Banu H. Surveillance of patients attending a diarrhoeal disease hospital in Bangladesh. Br Med J (Clin Res Ed) 1982;285:1185–8. doi: 10.1136/bmj.285.6349.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.WHO. Treatment of diarrhoea: a manual for physicians anal other senior health workers. World Health Organization; 1990. [Google Scholar]
- 20.WHO. Manual for laboratory investigations of acute enteric infections. 1987. pp. 9–20. [Google Scholar]
- 21.Unicomb LE, Kilgore PE, Faruque SG, Hamadani JD, Fuchs GJ, Albert MJ, Glass RI. Anticipating rotavirus vaccines: hospital-based surveillance for rotavirus diarrhea and estimates of disease burden in Bangladesh. Pediatr Infect Dis J. 1997;16:947–51. doi: 10.1097/00006454-199710000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Qadri F, Das SK, Faruque AS, Fuchs GJ, Albert MJ, Sack RB, Svennerholm AM. Prevalence of toxin types and colonization factors in enterotoxigenic Escherichia coli isolated during a 2-year period from diarrheal patients in Bangladesh. J Clin Microbiol. 2000;38:27–31. doi: 10.1128/jcm.38.1.27-31.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qadri F, Wenneras C, Albert MJ, Hossain J, Mannoor K, Begum YA, Mohi G, Salam MA, Sack RB, Svennerholm AM. Comparison of immune responses in patients infected with Vibrio cholerae O139 and O1. Infect Immun. 1997;65:3571–6. doi: 10.1128/iai.65.9.3571-3576.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lesmana M, Rockhill RC, Sutanti D, Sutomo A. An evaluation of alkaline peptone water for enrichment of Vibrio cholerae in feces. Southeast Asian J Trop Med Public Health. 1985;16:265–7. [PubMed] [Google Scholar]
- 25.Colwell RR, Hasan JA, Huq A, Loomis L, Siebeling RJ, Torres M, Galvez S, Islam S, Tamplin MT, Bernstein D. Development and evaluation of a rapid, simple, sensitive, monoclonal antibody-based co-agglutination test for direct detection of Vibrio cholerae 01. FEMS Microbiol Lett. 1992;76:215–9. doi: 10.1016/0378-1097(92)90339-p. [DOI] [PubMed] [Google Scholar]
- 26.Rahman M, Sack DA, Mahmood S, Hossain A. Rapid diagnosis of cholera by coagglutination test using 4-h fecal enrichment cultures. J Clin Microbiol. 1987;25:2204–6. doi: 10.1128/jcm.25.11.2204-2206.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sjoling A, Wiklund G, Savarino SJ, Cohen DI, Svennerholm AM. Comparative analyses of phenotypic and genotypic methods for detection of enterotoxigenic Escherichia coli toxins and colonization factors. J Clin Microbiol. 2007;45:3295–301. doi: 10.1128/JCM.00471-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodas C, Iniguez V, Qadri F, Wiklund G, Svennerholm AM, Sjoling A. Development of multiplex PCR assays for detection of enterotoxigenic Escherichia coli colonization factors and toxins. J Clin Microbiol. 2009;47:1218–20. doi: 10.1128/JCM.00316-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.WHO. Multi-centre Growth Reference Study Group. WHO Child Growth Standards: methods and development: length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age. WHO; 2006. [Google Scholar]
- 30.Brown KH. Diarrhea and Malnutrition. The Journal of Nutrition. 2003;133:328S–32S. doi: 10.1093/jn/133.1.328S. [DOI] [PubMed] [Google Scholar]
- 31.WFP. Children’s Nutritional Status. World Food Programme; 2012. [Google Scholar]
- 32.Chatterjee S, Patra T, Ghosh K, Raychoudhuri A, Pazhani GP, Das M, Sarkar B, Bhadra RK, Mukhopadhyay AK, Takeda Y, Nair GB, Ramamurthy T, Nandy RK. Vibrio cholerae O1 clinical strains isolated in 1992 in Kolkata with progenitor traits of the 2004 Mozambique variant. J Med Microbiol. 2009;58:239–47. doi: 10.1099/jmm.0.003780-0. [DOI] [PubMed] [Google Scholar]
- 33.Longini IM, Jr, Yunus M, Zaman K, Siddique AK, Sack RB, Nizam A. Epidemic and endemic cholera trends over a 33-year period in Bangladesh. J Infect Dis. 2002;186:246–51. doi: 10.1086/341206. [DOI] [PubMed] [Google Scholar]
- 34.Barua D. History of cholera. New York: Plenum Press; 1992. pp. 1–35. [Google Scholar]
- 35.Pollitzer RSS, Burrows W. Cholera. Monogr Ser World Health Organ. 1959;58:1001–19. [PubMed] [Google Scholar]
- 36.Khan AI, Chowdhury F, Harris JB, Larocque RC, Faruque AS, Ryan ET, Calderwood SB, Qadri F. Comparison of clinical features and immunological parameters of patients with dehydrating diarrhoea infected with Inaba or Ogawa serotypes of Vibrio cholerae O1. Scand J Infect Dis. 42:48–56. doi: 10.3109/00365540903289688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanchez J, Holmgren J. Cholera toxin - a foe & a friend. Indian J Med Res. 133:153–63. [PMC free article] [PubMed] [Google Scholar]
- 38.Son MS, Megli CJ, Kovacikova G, Qadri F, Taylor RK. Characterization of Vibrio cholerae O1 El Tor biotype variant clinical isolates from Bangladesh and Haiti, including a molecular genetic analysis of virulence genes. J Clin Microbiol. 49:3739–49. doi: 10.1128/JCM.01286-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ghosh-Banerjee J, Senoh M, Takahashi T, Hamabata T, Barman S, Koley H, Mukhopadhyay AK, Ramamurthy T, Chatterjee S, Asakura M, Yamasaki S, Nair GB, Takeda Y. Cholera toxin production by the El Tor variant of Vibrio cholerae O1 compared to prototype El Tor and classical biotypes. J Clin Microbiol. 48:4283–6. doi: 10.1128/JCM.00799-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qadri F, Mohi G, Hossain J, Azim T, Khan AM, Salam MA, Sack RB, Albert MJ, Svennerholm AM. Comparison of the vibriocidal antibody response in cholera due to Vibrio cholerae O139 Bengal with the response in cholera due to Vibrio cholerae O1. Clin Diagn Lab Immunol. 1995;2:685–8. doi: 10.1128/cdli.2.6.685-688.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qadri F, Ryan ET, Faruque AS, Ahmed F, Khan AI, Islam MM, Akramuzzaman SM, Sack DA, Calderwood SB. Antigen-specific immunoglobulin A antibodies secreted from circulating B cells are an effective marker for recent local immune responses in patients with cholera: comparison to antibody-secreting cell responses and other immunological markers. Infect Immun. 2003;71:4808–14. doi: 10.1128/IAI.71.8.4808-4814.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rahim MA, editor. Management of diarrhea at ICDDR,B hospital. ICDDR,B. Glimpse [Internet] 2009 Jun;31 Available from: http://www.icddrb.org/publications/cat_view/52-publications/10078-glimpse?orderby=dmdate_published&ascdesc=DESC&start=10. [Google Scholar]
- 43.James WP. Intestinal absorption in protein-calorie malnutrition. Lancet. 1968;1:333–335. doi: 10.1016/s0140-6736(68)90797-6. [DOI] [PubMed] [Google Scholar]
- 44.Holemans K, Lambrechts A. Nitrogen metabolism and fat absorption in malnutrition and in kwashi-orkor. Journal of Nutrition. 1955;56:477–494. doi: 10.1093/jn/56.4.477. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


