Introduction
About one in forty adults in the general population has chronic neuropathic pain, making it the most frequent condition affecting the peripheral nervous system 57. Chronic neuropathic pain presents a heterogeneous burden with a large prevalence 14 in certain susceptible subpopulations, for example in people living with HIV 33. The HIV-related distal sensory neuropathy affects every third patient 33. CNP may result from diverse insults, including diabetes, HIV, trauma, and certain medications 96, 97. Chronic neuropathic pain remains under-diagnosed and difficult to treat 37. Regardless of etiology, chronic neuropathic pain persists despite attempts at management with opioids, NSAID, anticonvulsants (gabapentin), anti-inflammatory agents, antidepressants and complementary medicine approaches 37.
A recent systematic review concluded that cannabis is effective in selected neurological disorders, including multiple sclerosis, but did not address chronic neuropathic pain 55. Considering the recent wave of cannabis legalization83, the continued legal wrangling 69, its widespread medicinal and recreational use87, 98 and additional randomized controlled trials [RCT] published on cannabis recently, we performed a meta-analysis to investigate if inhaled cannabis alleviates chronic neuropathic pain 88, 92,9. Previous (systematic) reviews did not investigate inhaled cannabis for chronic neuropathic pain or were unable to synthesize all available data, did not include recently published RCTs and varied considerably in their inclusion criteria, study selection and data synthesis, leading to conflicting and outdated conclusions 15, 21, 26, 51, 55, 59-61, 67, 73, 83, 86, 99. As cannabis should undergo the same evidence-based review as other potent prescription medication 91, an update is needed 88, 92.
In cooperation with all primary study authors, we performed an individual patient data Bayesian meta-analysis of RCTs 77 (Supplementary Box 1: Bayesian approach to statistical inference). While classical meta-analysis pools aggregate data extracted from published study reports, individual patient data meta-analysis synthesizes the original data of the individual subjects obtained from the primary study authors 82. This often gives individual patient data meta-analysis more power82. We selected Bayesian evidence synthesis for the analysis, anticipating that incomplete outcome reporting, varied endpoints, limited availability of aggregate or individual patient data and diversity of study designs with varied statistical analysis approaches would pose a formidable challenge to classical (frequentist) methods of meta-analysis80. Classical meta-analysis may also underestimate the between-study-variability for small numbers of trials 27, 79 leading to inaccurate inferences, while Bayesian methods provide more robust estimates of between-study variance.
Objectives
We performed an individual patient data Bayesian responder meta-analysis to study if inhaled cannabis provides relief for chronic neuropathic pain.
Methods
We registered our protocol with PROSPERO6. We identified studies by a combination of electronic and manual searches (Figure 1) (Supplementary Appendix 1 (Search strategy). We followed the recommendations of the QUORUM and PRISMA statements 64 including the PRISMA checklist (Supplementary Appendix 2). We searched in Cochrane Central, PubMed, EMBASE and AMED without any language restriction with a combination of free text and controlled vocabulary, employing the highly sensitive search strategy 48. We conducted a hand search in the conference abstracts of the Conference on Retroviruses and Opportunistic Infections 2011, the International AIDS Conference and the World Congress of Pain 2010 and reference lists.
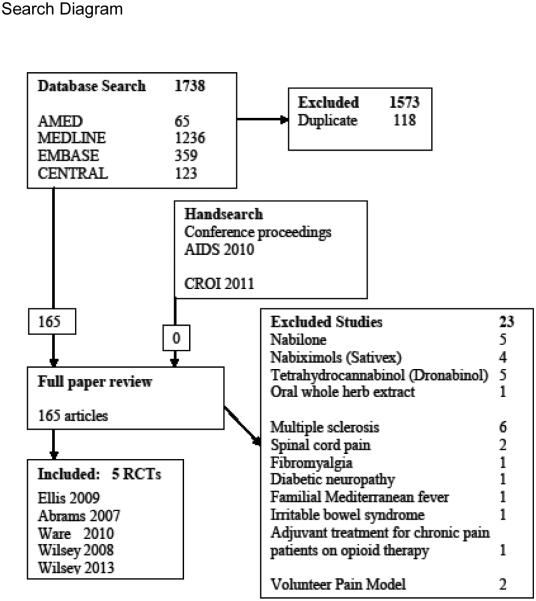
Figure 1.
The Quorum flow chart details our search in a diagram. We selected 165 articles for full review from 1738 hits in multiple electronic databases; five randomized trials (RCT) met the inclusion criteria. Excluded studies are double counted if they met more than one exclusion criterion, e.g. disease and mode of administration.
We considered RCTs investigating chronic painful neuropathy. We included diabetic, traumatic and HIV-related etiologies. We excluded multiple sclerosis, a central rather than a peripheral pain condition. The nature of the intervention likely interfered with effective participant blinding 4 and which was therefore not required for study inclusion. We only included studies comparing inhaled Cannabis Sativa to placebo, because inhaled whole herb cannabis differs significantly in composition, bioavailability, and pharmacodynamics from synthetic cannabinoids 76.
Three review authors (MHA, GC, KS) screened the citations using explicit criteria for study exclusion. Using a standard data collection form, two authors (MHA & GC) extracted the data independently, reconciling any differences by consensus. Study authors provided individual patient data 3, 35, 89, 93, 95. We recorded details of trial design, conflict of interests, sponsors, participant characteristics, interventions and outcome measures, inclusion and exclusion criteria, comorbidity and HIV status, cannabis provenience, dose and mode of administration. We extracted data on attrition and on adverse effects.
We compared the proportion of patients having a more than 30% clinical improvement in chronic neuropathic pain assessed with a continuous patient reported instrument (e.g., the Visual Analogue Scale) comparing baseline to post-treatment with inhaled cannabis. In essence, we dichotomized the outcome in a responder analysis, emerging as the preferred method for pain outcomes research 31, 36. We chose this patient centered concept of minimally clinically important difference (MCID) 63, because chronic neuropathic pain, our primary outcome, is patient reported and may have a skewed distribution, with no more than 40–60% of patients obtaining even partial relief of their pain 30 : a statistically significant change in the population mean of a continuous pain outcome may not correspond to a clinically meaningful improvement for many individual subjects 65. In other words, large studies may detect population differences too small for individual patients to appreciate. However, responder analysis converts continuous pain outcomes to dichotomous responder data allowing a more meaningful comparison between interventions 66, 78. By convention, we classified participants as “responder” if their pre- to post treatment reduction in the continuous spontaneous pain outcome (e.g. VAS score) was larger than 30% 31, 36.
Two authors (GC and MHA) independently assessed the risk of bias of included studies according to the Cochrane Collaboration 48 on the basis of a checklist of design components and contacted authors for missing information. We summarized this in a risk of bias graph (Figure 2: Summary of risk of bias graph) and provide detailed information in the supplement (Supplementary table 1: Details on methodological quality of included studies). This comprised randomization, allocation concealment, observer blinding, intention-to-treat analysis, selective reporting and conflict of interests. We achieved consensus by informal discussion. In inhaled cannabis interventions, blinding of patients and providers can be difficult and hence received less weight in the evaluation of performance bias, but not with regard to detection bias.
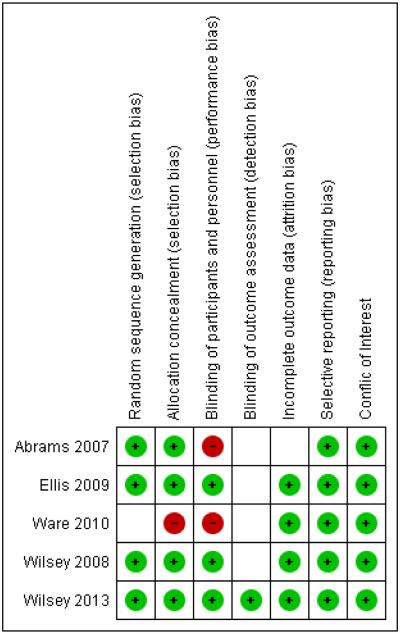
Figure 2.
This summary of bias graph shows that the included studies were mostly of good quality in the domains of sequence generation, concealed allocation, incomplete outcome data and selective reporting and with regards to conflict of interest. However, the nature of the intervention likely interfered with effective blinding resulting possibly in high risk of performance bias in all studies and possibly detection bias due to a lack of blinding of outcome observers.
Our results are based on individual patient data obtained from primary authors who helped resolve data inconsistencies when evident. We estimated the content and the dose administered following published methods 11, 62 in cooperation with the primary study authors.
We compared the reported primary outcome with the planned primary outcome in the study protocols to assess reporting bias. We explored undue sponsor influence 48. We considered an examination of publication bias using graphical and statistical tests 32. We investigated study heterogeneity using a chi2 test and calculation of an I2 analogue Bayesian statistic 48.
Data synthesis, statistical model and sensitivity analysis
We performed full Bayesian probability modelling 23 of the population averaged subject specific effect 100 as detailed in the statistical supplement (Supplementary Appendix 3). We pooled treatment effects following a hierarchical random-effects Bayesian responder model. Kruschke provided an accessible introduction to Bayesian methods in health sciences 56. Ashby recently offered a chronological outline of applications in medicine 7, while Spiegelhalter compiled the first concise overview 80. Gelman described Bayesian hierarchical modelling approaches more formally 39. Supplementary boxes explain basic concepts of Bayesian inference (Supplementary Boxes 1 through 3). The prior for the between-study-variability (Cauchy) and the pooled effect estimate (normal distribution) was centered at zero with a standard deviation of 100; We preferred the Cauchy distribution over the closely related t-distribution, because the Cauchy is more robust in accommodating outliers 38, 39; these priors for our meta-analysis were uninformative and served to ensure computational convergence of the Markov chain Monte Carlo algorithm. Our priors were subsequently subjected to sensitivity analysis. Inference was implemented by employing a Gibbs sampling scheme to generate a computer simulation of a Monte Carlo sample from the posterior distribution in OpenBugs 58. Our OpenBugs program code is provided in Supplementary Appendix 4. We uploaded details on Monte Carlo Markov Chain convergence including graphs demonstrating mixing as supplementary material (Supplementary Figure 1: Traceplots and Supplementary Figure 2: Autocorrelation). Differences in the design and quality of studies were the focus of a sensitivity analysis. We tested the sensitivity of our results to our Bayesian model and its assumptions: We investigated our choice of prior and model parameters and reanalyzed the individual patient responder data (a) in a frequentist random effects meta-analysis and (b) controlling for cannabis dose as an explanatory variable of the between study variability in a meta-regression (methods and data not shown but available on request).
Reporting
We estimated the number needed to treat (NNT) and calculated the Bayes factor 40, compared to the classical p-values in supplementary Box 3. We provided forest plots for the individual trials broken down by dose for the period level data (Figure 3). The reported pooled Bayesian estimate is the population-averaged subject-specific odds ratio comparing inhaled cannabis versus placebo for chronic painful neuropathy and their 95% Bayesian credible intervals (CRI95%), displayed as a the standard diamond used for synthesized effects47.
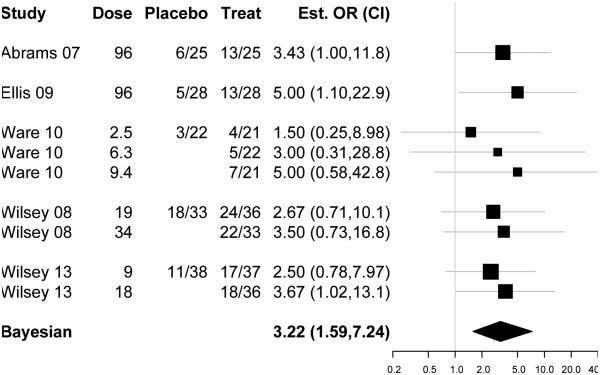
Figure 3.
The forest plot displays odds ratio (with the 95% credible interval indicated by horizontal bars on the log scale) to indicate their contribution to the Bayesian pooled effect estimate shown below as a diamond with the Bayesian 95% credible interval. The table on the left lists the raw responder data at the study level. For Ware 2010, Wilsey 2008 and Wilsey 2013, the responder data are broken down by dose, listing the number of observed responses for each crossover periods and the corresponding cannabis dose. The increased effect with increased cannabis content (evident in the period level data of Ware 2010, Wilsey 2008 and Wilsey 2013) is additional evidence in support of cannabis’s effect for chronic painful neuropathy.
Differences from the initial protocol
In our initial Prospero protocol registration we considered including all types of studies, populations and cannabis interventions. We intended to do a network analysis in one coherent Bayesian model. We found published aggregate data insufficient for evidence synthesis and therefore we decided to attempt an individual patient data meta-analysis, but limiting ourselves to only RCTs investigating inhaled cannabis and updated the protocol accordingly
Results
Our search (Figure 1) was completed in April 2014 and yielded 1738 references (1236 in Medline, 359 in Embase, 123 in Cochrane Central, and 65 in Amed) matching the predefined search parameters. We excluded 118 duplicates and 1573 references, in which we could clearly discern from the title and/or abstract that they were not randomized trials or did not investigate Cannabis for a painful condition. Our hand search yielded no additional references. Except for the five included studies 3, 35, 89, 93, 95, all of the remaining 163 publications studied different modes of cannabis administration or included participants with other painful conditions. No study investigated outcomes beyond two weeks. We summarized the characteristics of the five RCTs meeting our inclusion criteria (Table 1: Summary of included studies) and detailed their characteristics (Table 2: Detailed characteristics of included studies). We listed important excluded studies with reasons for their exclusion (Supplementary table 2: characteristics of excluded studies).
Table 1.
Summary of included studies
| Author | Abrams | Ellis | Ware | Wilsey | Wilsey |
|---|---|---|---|---|---|
| Year | 2007 | 2008 | 2010 | 2008 | 2013 |
| Neuropathy | HIV-DSPN | HIV-DSPN | post- traumatic |
sensory | mixed |
| Subjects | 50 | 34 | 23 | 38 | 39 |
| Allocation | randomized | ||||
| Intervention | Inhaled cannabis versus placebo | ||||
| Outcome | VAS | DDS | NRS | VAS | VAS |
| Follow up | 5 days | 2 weeks | 5-6 hours | ||
| Design | parallel | cross-over | |||
| Statistics | Mann- Whitney |
Wilcoxon Rank Sum |
General and linear mixed
models |
||
We summarize the five included randomized controlled clinical trials on inhaled cannabis for chronic neuropathic pain. The etiology of chronic neuropathic pain varied (including traumatic, central, diabetic and HIV-related). Study authors employed several patient reported pain outcome instruments: VAS (Visual Analogue Scale); NRS (Numerical Rating Scale); DDS (Descriptor Differential Scale).
Table 2.
Detailed characteristics of included studies
| Study ID | Year | Journal | Pubmed ID | Trial registry ID |
|---|---|---|---|---|
| Abrams 2007 | 2007 | Neurology | 17296917 | NCT00046722 |
| Population | 55 HIV + adults with symptomatic
HIV-DSPN and at least 30/100 VAS, on stable pain regimen for 8 weeks prior to enrolment, with prior experience of smoking cannabis randomized in 2 groups of size: 27/28. Our Bayesian analysis is based on 50 participants with one observation per patient, as provided by the primary study authors. Age (Experimental, Control): 50 years (SD ±6)), 47 (SD ± 7) Gender (male/female/other): Experimental 22/5/0 Control 26/2/0 |
|||
| Intervention |
Experimental: Patient
smoked one cigarette three times per day as tolerated Pre-rolled, whole herb Cannabis cigarettes were provided by NIDA and contained 3.56% delta-9-THC. Control: identical pre-rolled cigarettes with the active ingredient extracted. Dose estimate: 32 mg THC per session; 96 mg THC per day |
|||
| Primary Outcome | Daily pain diary recording the VAS
at 8am for average pain during the previous 24 hours. |
|||
| Study Methods | Randomized, double-blind (patient,
outcome assessor), parallel design, placebo controlled, single center (university) clinical trial in San Francisco, California, USA starting in 2003 |
|||
| Notes | Also published as an abstract at
the 2nd Annual meeting of the International Association for Cannabis as Medicine, 2005 Secondary outcomes: Acute analgesic effects: Long thermal stimulation Anti-hyperalgesic effects: Heat-capsaicin model, Profile of Mood States |
|||
| Study ID | Year | Journal | Pubmed ID | Trial registry ID |
| Ellis 2009 | 2008 | Neuropsychopharmacology | 18688212 | NCT00255580 |
| Population | 34 HIV + adults with symptomatic
HIV-DSPN and pain score >5/20 on DDS (Discriptor Differential Scale), most participants were previously exposed to potentially neurotoxic deoxy-nucleoside reverse transcriptase inhibitors; 16 started control/experimental, 18 started experimental/control. 28 participants with a total of 56 observed responses were included in the Bayesian analysis. Age (all) 49.1 years (SD ±6.9)), Gender (male/female/other): 33/1/0 |
|||
| Intervention |
Experimental: Patient
smoked cannabis, titrating dose up or down to effective pain control/ tolerable adverse effects, starting at 4 percent to range between 1 to 8 percent delta-9-tetrahydrocannabinol (THC) concentration by weight. Pre-rolled, whole herb Cannabis cigarettes were provided by the National Institute on Drug Abuse. Control: identical pre-rolled cigarettes with the active ingredient extracted. Dose estimate: average 96 mg THC per day |
|||
| Primary Outcome | Crossover difference in change of
DDS (Descriptor Differential Scale 0-20 scale “a ratio scale containing 24 words describing pain intensity and unpleasantness.”) comparing baseline to after treatment |
|||
| Study Methods | Randomized, double-blind (patient,
outcome assessor), cross-over design, placebo controlled, single center (university) clinical trial at the University of California, San Diego in 2006 |
|||
| Notes | Secondary outcomes: McGill
Questionaire, VAS, SIP (Sickness Impact Profile), BSI (Brief Symptom Inventory), UKU side effect rating, Highness/Sedation Scale, HIV load |
|||
| Study ID | Year | Journal | Pubmed ID | Trial registry ID |
| Ware 2010 | 2010 | Canadian Medical Association Journal |
20805210 | ISRCTN68314063 |
| Population | 23 adults with non HIV neuropathy
pain of at least 3 months duration caused by trauma or surgery defined by pain intensity score greater than 40/100 VAS, on a stable analgesic regimen, not having smoked cannabis in the preceding year. 23 participants with a total of 86 observed responses were included in the Bayesian analysis. Age (all): 45.4 years (SD ±12.3) Gender (male/female/other): 11/12/0 |
|||
| Intervention |
Experimental: NIDA
and Prairie plant systems prepared three different potencies of THC (2.5%, 6%, 9.4%) from whole herb in gelatine capsules inhaled through pipe. Control: Ethanolic extraction was used to prepare the placebo. Dose estimate: 0, 1.625, 3.9 and 5.85 mg/day (average) THC per period |
|||
| Primary Outcome | Average Daily Pain Intensity on
the 11-item numeric rating scale (NRS) average over five treatment days (least pain value, average pain value and worst pain value) during four consecutive crossover periods of 14 days each (five treatment days and 9 washout days afterwards) |
|||
| Study Methods | Randomized, double-blind (patient,
outcome assessor), 4 period crossover Latin square design, placebo controlled, single center (university) clinical trial in McGill University, Montreal, Canada, starting in 2003 |
|||
| Notes | The linear model did not consider
inter participant effect. Secondary outcomes: Pain Quality: McGill Questionaire, Sleep (Leeds Sleep Evaluation Questionaire), Mood Effects: short-form Profile of Mood States, Quality of Life: EQ-5D health outcomes |
|||
| Study ID | Year | Journal | Pubmed ID | Trial registry ID |
| Wilsey 2008 | 2008 | The Journal of Pain | 18403272 | NCT00254761 |
| Population | 38 adults with non HIV neuropathy
(complex regional pain syndrome (CRPS type I), spinal cord injury, peripheral neuropathy, or nerve injury) with prior cannabis experience and a VAS > 30/100. 38 participants with 102 observed responses were included in the Bayesian analysis. Age (all): 46 years (range 21-71) Gender (male/female/other): 20/18/0 |
|||
| Intervention |
Experimental
Participants inhaled a total of 9 standardized cued-puff.
Cannabis was harvested from whole plant and rolled into cigarettes at the U of Mississippi under supervision of NIDA ranging in strength from 0%, 3.5% to 7%. Control: Placebo Cigarettes were made from whole plant with extraction of Cannabis. Dose estimate: 0 Placebo, 19.25 (low dose, range 7-30.45), 34.3 (high dose, range 18.9-60.9) mg THC/day (Session) |
|||
| Primary Outcome | VAS measuring spontaneous pain
relief; time effects were studied with a linear model. |
|||
| Study Methods | Randomized, double-blind (patient,
outcome assessor), parallel design, placebo controlled, single center (university) clinical trial at UC Davis Medical Center Sacramento, California started in November 2003 |
|||
| Notes | Secondary outcomes: pain
unpleasantness (VAS), heat pain threshold, Neuropathic Pain Scale, Neurocognitive assessment and plasma Cannabis concentration |
|||
| Study ID | Year | Journal | Pubmed ID | Trial registry ID |
| Wilsey 2013 | 2013 | The Journal of Pain | 23237736 | NCT01037088 |
| Population | 39 adults with non HIV neuropathy
due to reflex sympathetic dystrophy, peripheral neuropathy, post-herpetic neuralgia, post-stroke pain, multiple sclerosis or spinal cord injury with previous cannabis exposure (16 current, 23 ex-users) and a VAS pain intensity greater than 30/100. 39 participants with 111 observed responses were included in the Bayesian analysis. Age (all): 50 years (SD ±11)) Gender (male/female/other): 28/11/0 |
|||
| Intervention |
Experimental:
Participants used a volcano vaporizer under the flexible-dose
design of Wilsey 2008. The minimum and maximum cumulative doses for each visit were 8 and 12 puffs. Cannabis was harvested at the University of Mississippi under the supervision of NIDA. Control: Placebo was made from whole plant with removal of cannabinoids. Dose estimate: Maximum of 0, 10.32, 28 mg THC/day (Session), presuming they were administered the entire 800 mg dose. |
|||
| Primary Outcome | VAS before and after consuming vaporized cannabis. | |||
| Study Methods | Randomized, double-blind (patient,
outcome assessor), crossover design, placebo controlled, single center (university) clinical trial at the University of California, Davis, started December 2009 |
|||
| Notes | Secondary outcomes: Patient Global
Impression of Change (PGIC); Neuropathic Pain Scale (NPS); WAIS-III, Hopkins Verbal Learning Test (revised), Grooved Pegboard Test |
|||
We provide detailed characteristics of the five included randomized controlled clinical trials investigating smoked or inhaled cannabis for painful neuropathy. Two trials recruited patients with HIV related Distal Sensory Polyneuropathy (HIV DSPN), three included participants with neuropathies of other etiologies. (VAS Visual Analogue Scale; SD Standard deviation; NA not available; NIDA US National Institute of Drug Abuse)
Descriptive characteristics of included studies and participants
178 middle aged participants, (approximately equal numbers of men and women) with painful neuropathy of at least three months duration (pain scores at least about 3/10), were enrolled in five RCTs executed across North America. Two trials recruited only HIV+ individuals with HIV-related chronic painful neuropathy 1, 2, 34; sexual orientation and transgender data were not reported. Three trials recruited patients with neuropathy secondary to trauma 88, spinal cord injury, diabetes mellitus and complex regional pain syndrome 92, 94. Psychiatric disease, substance abuse and significant cardiopulmonary disease were explicit exclusion criteria. While prior cannabis experience was a prerequisite for inclusion for some studies 1, 2, 92, 94, current use was an exclusion criterion in all. Prescribed opioid use was not specified among the inclusion or exclusion criteria.
Characteristics of interventions and comparators
All studies investigated inhaled cannabis. The five studies used different doses, estimated as detailed in the Supplementary Table 3. All five studies used whole Cannabis plant provided by the US National Institute of Drug Abuse (NIDA). Three studies administered cannabis as pre-rolled cigarettes 1, 2, 34, 94, one through a Volcano vaporizer 92 and one as gelatin capsules smoked through a pipe at home 88. All five studies used identical looking placebo as comparator. Concomitant non-study analgesics were permitted and continued in both arms.
Clinical outcomes and adverse effects
All five RCTs reported as primary outcomes continuous patient-reported spontaneous pain intensity scales. We report the study level observed odds ratio (with 95% credible interval indicted by vertical bars on the log scale) as a measure of their contribution to the Bayesian pooled effect estimate (shown as a diamond with 95% credible intervals below) (Figure 3: Forest Plot). The breakdown of responder data by dose suggested an increased effect with increased cannabis content.
Withdrawals due to adverse effects were rare: One case of serious adverse effects leading to withdrawal occurred in the placebo group, e.g. a case of psychosis and two others in active treatment groups (e.g., hypertension and increased pain). Subjective side effects included anxiety, disorientation, and difficulty to concentrate, headache, dry eyes, burning sensation, dizziness and numbness and were reported as being mild. Wilsey reported short-term declines in attention, psychomotor performance and learning and memory in the highest dose (7% tetrahydrocannabinol) group. Memory impairment was also seen in the placebo group and at lower doses, albeit at lower levels 94. Statistically significant physiological changes (like increases in heart rate) were observed in one 34, but not in another study 88. Reports of euphoria or “high” were rare 88. Psychoactive effects (like feeling “high”) were statistically significantly associated with treatment allocation in two studies 2, 34 and increased in frequency with increasing dose 88, 94; they were mostly mild. The included studies followed patients only for days to weeks and hence did not report long term adverse effects.
Study Design
All five studies were randomized, placebo controlled and double-blind, four used a cross-over 34, 88, 92, 94, one study a parallel design 1. Duration of follow-up varied from hours 92, 94, to days 2, 34 or weeks 88.
Risk of bias in included studies
We characterized the risk of bias of included studies (Figure 2: Summary of risk of bias graph; Supplementary table 1: Details on methodological quality of included studies). Randomization and allocation concealment were well described and suggested a low risk of bias. Ineffective participant blinding might have possibly resulted in performance bias in all studies; placebo effects are likely, where participants guessed their allocation, possibly leading them to overestimate the effect of inhaled cannabis on pain. Blinding of outcome observer was well described in one study92, and the use of patient diaries as outcome instrument led us to estimate the risk of detection bias as unclear in the remaining studies. Incomplete outcome data were well described in all studies and are detailed in Table 2. Withdrawals potentially related to treatment effects lead to high risk of bias in one study 88, but did not seem to be associated with group allocation in all others 2, 34, 92, 94. All included trials reported their primary outcome as specified in the protocol. We investigated publication bias in a funnel plot proposed by Egger 32, because with fewer studies than ten studies, the power of the tests is insufficient to distinguish chance from real asymmetry 48. Studies only received public funding; all authors provided detailed conflicts of interest statements.
Evidence synthesis of effects
Based on data from 178 patients with 405 total observed responses, we estimated the odds ratio for a more than 30% reduction in pain scores in response to inhaled cannabis versus placebo for chronic painful neuropathy as 3.2 with a Bayesian credible interval (subsequently denoted with the subscript CRI95%) [1.59, 7.24]CRI 95%, and the NNT as 5.55 [3.35, 13.7]CRI 95%. We estimated the posterior probability of effect of Cannabis for chronic painful neuropathy to be 99.7% and the Bayes factor as 332 (Figure 3: Forest plot of cannabis effects on chronic painful neuropathy). The Bayesian analogue i-square statistic was 0. The posterior probability that the between-study variability in effects was greater than what would be expected by chance is 0.45. Effects seemed to increase with tetrahydrocannabinol (THC) content supporting cannabis’s effect for chronic painful neuropathy as seen in the forest plot (Figure 3: Forest plot of cannabis effects on chronic painful neuropathy). Specifically, the increased effect with increased cannabis content (evident in the period level data of Ware 2010, Wilsey 2008 and Wilsey 2013) is additional evidence consistent with cannabis’s effect for chronic painful neuropathy. However, a meta-regression of cannabis dose (data not shown, but available on request) did not change our estimates or inferences. The aggregate and individual data on adverse effects were too sparse to be pooled. Model convergence is documented in the supplementary material (Supplementary Figure 1: Traceplots and Supplementary Figure 2: Autocorrelation).
Sensitivity analysis
When we performed a sensitivity analysis (available on request) with regards to differences in the quality of studies, we found effect estimate and credible intervals to be robust regarding the inclusion or exclusion of any single study. Our inferences were rather insensitive to priors (between study variance) in our Bayesian model (Supplementary Box 2: Informed versus neutral priors). Reanalyzing the data in a frequentist random effects meta-analysis did not change the results.
Discussion
Our evidence synthesis of individual patient data from 178 participants with 405 observations in five RCTs with a follow up ranging from days to weeks (Figure 3: Forest plot), provides evidence that inhaled cannabis results in short term reductions in chronic neuropathic pain for one in every five to six patients treated (NNT 5.6 with a Bayesian 95% credible interval ranging between 3.4 and 14); based on the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) definition of at least moderate benefit 31, inhaled cannabis improved pain by an odds ratios of 3.2 (Bayesian 95% credible interval of [1.6, 7.2]CRI 95% (Figure 3: Forest plot). The Bayes factor is 332 corresponding to a posterior probability of effect of 99.7%.
We infer that this effect applies equally across chronic painful neuropathies of different etiologies (e.g. diabetic and traumatic chronic painful neuropathy or HIV related distal sensory neuropathy). The effects are remarkably homogenous across studies (Bayesian I2 analogue = 0%) (Table 1). Dose dependency further supports the notion of cannabis effect on neuropathy (Figure 3: Forest plot) (Supplementary Table 3: Cannabis dosing). Our results (NNT 5.6 [3.4, 14]CRI95%) suggest that inhaled cannabis may be about as potent as gabapentin (Cochrane review update: NNT 5.9 (4.6 to 8.3)CI 95% for diabetic neuropathy.(Moore 2014). The NNT of inhaled cannabis could potentially rival currently available therapeutics for chronic neuropathic pain90, whose NNT typically range well above 8, if there is any evidence at all17, 24, 28. However, we caution that our findings await confirmation in long-term pragmatic community based trials. Our findings are remarkable considering the dearth of effective treatment options for chronic painful neuropathies or chronic pain in general 54.
Our review enhances the existing literature on treatment for chronic neuropathic pain
Our evidence synthesis contradicts, updates or complements the finding of several older and recent reviews on cannabis, by providing a meta-analysis for chronic neuropathic pain 15, 16, 94, by updating evidence 15, 16, 17, 18 or broadening the scope. We were able to include recent RCTs, not published or accessible to the previous reviews by Campbell, Phillips, Iskedjian, Lutge, or Lynch 22, 51, 59, 60, 73. Compared to previous studies72, our individual patient data meta-analysis and the inclusion of additional and recent clinical trials [which] augmented the power to detect an effect, if it existed and amplified the confidence in the pooled effect estimate (NNT = 5.6) by shrinking the 95% credible interval to 3, 4, 14 our posterior probability of the short term effects of inhaled cannabis is now very high (99.7%). Our analysis complements the recent evidence synthesis of cannabis for certain other neurological conditions by the American Academy of Neurology, which did not investigate cannabis for chronic neuropathic pain 54 and supports another narrative review (published after submission of this manuscript) which concluded, “Use of marijuana for chronic pain, neuropathic pain, and spasticity due to multiple sclerosis is supported by high-quality evidence.” with an individual patient data meta-analysis 49
Strength
Individual patient data meta-analysis increased the power of our meta-analysis
We performed an individual patient meta-analysis. Unlike conventional meta-analysis based on published aggregate data, individual patient data meta-analysis synthesizes the individual participants’ original data obtained from the included studies’ principal investigators 47. Individual patient data meta-analysis is arguably the gold-standard of evidence synthesis 77, 82, not just because it allows for detailed data checking, but because meta-analysis is often not feasible using only summary data. Synthesizing the diversity of reported outcomes of studies on inhaled cannabis for chronic painful neuropathy was a significant challenge for previous reviews. [15,16,17,18] In our review insufficient published outcome data and variations in design and outcome reporting would have led to the exclusion of relevant trials, because the published aggregate data lacked the necessary detail for pooling in a meta-analysis 5, 92, 95.
The individual patient data meta-analysis and the inclusion of additional recently published RCTs increased the power of our evidence synthesis and greatly increased the confidence in the effect of inhaled cannabis for chronic neuropathy compared to previous reviews. 50, 59, 60, 72; The Bayesian posterior probability of more than 99.7% indicates the very high likelihood that inhaled cannabis is effective in the short term for one in five or six patients with chronic neuropathic pain (Supplementary Box 1: Bayesian approach to statistical inference), (unlike the classical p-value which indicates how unlikely the observed outcomes data are, given a Null-hypothesis of no effect). To our knowledge, this is the first Bayesian individual patient data meta-analysis in medicine [Asby 2006].
The observed short term effect of inhaled cannabis is meaningful for one in five or six patients with chronic neuropathy
Our responder analysis is showing a statistically significant and minimal clinically important difference for one in five to six patients, an effect measure easily understood by patients, payers and providers alike 63. Responder analysis has been advocated for patient reported outcomes in chronic pain trials to distinguish a minimal but statistically significant difference between groups on a population basis from a clinically meaningful effect for the individual participant 31, 65, 66. Our cutoff for a meaningful response (>30%) is (1) grounded in what patients themselves judge as important improvement 36 and (2) based on expert consensus [IMMPACT] 63. Based solely on frequentist hypothesis testing, responder analysis may miss the goal, while losing power 78. Our individual patient data Bayesian meta-analysis allowed us to calculate a posterior probability of effect larger than 99.7%.
Limitations
Effects are consistent across different etiologies and populations
We pooled data from populations with chronic painful neuropathy of different etiologies and in different populations. We included HIV-related distal sensory polyneuropathy, post traumatic, complex regional pain syndrome, peripheral and diabetic peripheral neuropathy and patients with and without previous exposure to cannabis. Similar approaches were also taken by authors of previous reviews on cannabis for chronic painful neuropathy 15, 16, 17, 18. Evidence synthesis across distinct, but closely related painful neuropathies, is reasonable because their clinical course and pathological mechanism are considered similar and receive uniform treatment recommendations 10, 68; Indeed, the “etiological factors responsible for driving the mechanisms are not disease specific” and “disease diagnosis is not helpful in selecting the optimal pain therapy” 96, 97. Even if the absence of evidence for heterogeneity constitutes no evidence for clinical homogeneity 48, the consistency and uniformity of the effect of inhaled cannabis on chronic neuropathic pain across different etiologies and populations, further enhances our confidence in the generalizability of our findings 53. Yet, our meta-analysis can only be as strong as the underlying data (Table 1 and Table 2) and the methodological quality (Figure 2: Summary of bias graph; Supplemental Table 1: Details on methodological quality); the small number of included studies, their small number of participants and shortcomings in allocation concealment46 and attrition (Table 2: Detailed characteristics of included studies) limit our ability to draw firm conclusions. The small numbers of studies found in each subgroup precluded a formal study of publication bias: A graphical analysis or the test proposed by Egger 1997 should at least include 10 studies because with fewer studies the power of the tests is insufficient to distinguish chance from real asymmetry (Higgins JPT, 2011). We find that the use of an active placebo to mimic the psychotropic effects of experimental treatments, while improving blinding, does not necessarily improve the evidence regarding effectiveness in a pragmatic clinical setting, but acknowledge the risk of performance bias76. Also meta-analyses of sparse data can be unstable42, 71; however, our evidence synthesis is based on individual patient data from all included trials, the best available source of evidence, short of a large RCT 48, 82.
Cannabis dose and mode of administration may influence pain relief
Estimating bioavailable cannabis is difficult. Many factors influence the amount of THC per cigarette, particularly whether the material is dry or freshly picked (Supplementary Table 3: Cannabis dosing in included studies). The dose delivered likely differs from what is actually ingested 75; we validated our dose estimates with primary authors on the included studies. In the forest plot of the raw responder data, higher dose seems to be associated with stronger effect (Figure 3: Forest plot). Our sensitivity analysis controlling for cannabis dose only marginally improved the precision (data not shown); hence, at the individual patient level, the dose differences didn’t explain the differences in effect. This may effectively reflect the individual dose titration.
Importantly, we cannot comment on long-term adverse effects because available trials followed their patients to a maximum of two weeks4. Recently, several authors have raised concerns about driving while intoxicated, withdrawal, addiction, adverse cardiovascular, pulmonary and cognitive effects, especially in the developing brain, although several of these misgivings remain contentious13, 18, 19, 29, 41, 44, 52, 74, 84, 91, 98. Extrapolating from recreational use is problematic and the risk-benefit balance differs when pain is medically intractable. Clearly, we need to learn more about benefits and risk associated with long-term cannabis use.
Our Bayesian meta-analysis is robust to parameter choices and model assumptions
Bayesian methods are sometimes critiqued for their presumed subjectivity, but the short term effects of inhaled cannabis for about one in five patients with chronic neuropathic pain are robust and independent of our mode of evidence synthesis. Our assumptions are modeled explicitly and tested 16: Priors for our meta-analysis were uninformative in order to minimize subjectivity and just served to ensure computational convergence. As detailed in the results and illustrated in supplementary Box 2, when subjected to sensitivity analysis our findings were robust to the choice of parameters and models. Unsurprisingly, running a frequentist analysis resulted in similar estimates, except that our credible intervals of our Bayesian estimate were more conservative because they were based on more cautious between study variance estimates. Obviously, the Bayesian approach provides a posterior probability (99.7%) [for the short term benefits of inhaled cannabis for about one in five patients with chronic neuropathy], an inference not possible in the frequentist paradigm 80. The result of any meta-analysis will critically depend on this estimate of the between study variance. Our between-study-variability estimation was more conservative than the classical random effects approach promoted by the Cochrane Collaboration 48, which itself is more conservative than the often employed fixed effects model. Indeed the continued debate on fixed versus random effects models, concerns about assumptions and underestimation of between-study-variability 27 demonstrate that the classical “frequentist” statistical approach is also not free of subjectivity 85. The use of subjective model parameters choices, destroys the illusion of objectivity in “frequentist” as well as in meta-analysis 85. Our Bayesian approach transparently included any subjective choice explicitly in our model and subjected all to sensitivity analysis 25, 71.
Recommendations for future research
We lack long-term pragmatic clinical trials to determine if cannabis’s effects on chronic painful neuropathy are sustained and durable, if cannabis use is feasible in the community given the associated stigma 1, 20, if cannabis can be safely prescribed in vulnerable and young populations 29, 84 and if long-term adverse effects outweigh the benefits of inhaled cannabis 13, 44, 52, 74, 84. While the cost of inhaled cannabis is likely to be low, medicinal cannabis continues to be controversial, (indeed illegal in many jurisdictions) and patients may vary in their preferences to inhale cannabis, especially as long as it remains stigmatized. We need to investigate if individual titration allows for the best balance of beneficial to adverse effects. Cannabis effects for other conditions should equally be explored in publicly-funded rigorous randomized clinical trials. Solid clinical evidence may facilitate selective prescribing, prevent misuse and reduce opioid related harms 45.
Conclusions
Our individual patient data meta-analysis suggests that inhaled cannabis results in short term benefits for chronic neuropathic pain with a NNT of 5.6 [3.4, 13] CRI95% (Figure 3: Forrest Plot). We lack evidence regarding sustained long-term benefits and risks in the community setting. The small number of included studies and participants (Table 1: Summary of included studies) and risk of detection and performance bias weaken our ability to draw firm conclusions (Figure 2: Summary of bias graph). Our responder analysis compared the proportion of patients with an at least 30% reduction in chronic pain as minimally clinically important difference, a meaningful improvement at the individual patient level for about one in every five to six patients treated 30, 63. This effect in chronic painful neuropathy is consistent across diverse etiologies, all hitherto resistant to available treatments (Table 1: Summary of included studies). To our knowledge, ours is the first Bayesian individual patient data meta-analysis. The Bayesian modelling approach may be flexibly extended to other fields and questions where variance in outcome reporting hampers the classical approach to meta-analysis 7, 8, 85.
Supplementary Material
Highlights.
Inhaled cannabis appears to provide short term relief from chronic neuropathic pain for one in five to six patients treated.
Our novel Bayesian hierarchical model allowed the synthesis of all available patient data from five RCT with disparate design and outcome reporting.
Pragmatic long term studies are needed to confirm the safety and effectiveness of inhaled cannabis for chronic neuropathic pain in the community.
Acknowledgements
We gratefully acknowledge the assistance of Maud Dupuy and Kirti Dasu.
Sources of support:
This work was supported by the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH), through CTSA grant numbers UL1TR000086, TL1RR000087, and KL2TR000088), the Center for Drug Evaluation and Research (CDER) through grant number R01-AT005824 and in part by Grant 5R01AT5824 from the National Center for Complementary and Alternative Medicine (NCCAM). Supported by the University of California Center for Medicinal Cannabis Research and NIH Grant 5-MO1-RR00083. Its contents are solely the responsibility of the authors and do not necessary represent the official view of the NCCAM, NCRR or NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
The authors have no conflicts of interest.
References
- 1.Abrams DI. Medical marijuana: tribulations and trials. J Psychoactive Drugs. 1998;30:163–169. doi: 10.1080/02791072.1998.10399686. [DOI] [PubMed] [Google Scholar]
- 2.Abrams DI, Jay CA, Shade SB, Vizoso H, Reda H, Press S, Kelly ME, Rowbotham MC, Petersen KL. Cannabis in painful HIV-associated sensory neuropathy: a randomized placebo-controlled trial. Neurology. 2007;68:515–521. doi: 10.1212/01.wnl.0000253187.66183.9c. [DOI] [PubMed] [Google Scholar]
- 3.Abrams DI, Jay CA, Shade SB, Vizoso H, Reda H, Press S, Kelly ME, Rowbotham MC, Petersen KL. Cannabis in painful HIV-associated sensory neuropathy: A randomized placebo-controlled trial. Neurology. 2007;68(67):515–521. doi: 10.1212/01.wnl.0000253187.66183.9c. 2007. [DOI] [PubMed] [Google Scholar]
- 4.Andreae MH, Andreae DA. Regional anaesthesia to prevent chronic pain after surgery: a Cochrane systematic review and meta-analysis. Br J Anaesth. 2013;111:711–720. doi: 10.1093/bja/aet213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andreae MH, Carter G, Johnson M, Sacks H. Bayesian methods improve evidence synthesis for complementary and alternative medicine: Cannabis for painful HIV-related distal sensory polyneuropathy (HIV-DSPN) Regional Anesthesia and Pain Medicine. 2011;36:E178. [Google Scholar]
- 6.Andreae MH, Sacks H, Indyk D, Carter G, Johnson M. Cannabis for HIV related chronic neuropathy. Available at: http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42011001182.
- 7.Ashby D. Bayesian statistics in medicine: a 25 year review. Stat Med. 2006;25:3589–3631. doi: 10.1002/sim.2672. [DOI] [PubMed] [Google Scholar]
- 8.Ashby D, Smith AF. Evidence-based medicine as Bayesian decision-making. Stat Med. 2000;19:3291–3305. doi: 10.1002/1097-0258(20001215)19:23<3291::aid-sim627>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 9.Ashton JC, Milligan ED. Cannabinoids for the treatment of neuropathic pain: clinical evidence. Current Opinion in Investigational Drugs. 2008;9:65–75. [Review] [79 refs] [PubMed] [Google Scholar]
- 10.Attal N, Cruccu G, Baron R, Haanpaa M, Hansson P, Jensen TS, Nurmikko T. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol. 2010;17:1113–e1188. doi: 10.1111/j.1468-1331.2010.02999.x. [DOI] [PubMed] [Google Scholar]
- 11.Azorlosa JL, Heishman SJ, Stitzer ML, Mahaffey JM. Marijuana smoking: effect of varying delta 9-tetrahydrocannabinol content and number of puffs. J Pharmacol Exp Ther. 1992;261:114–122. [PubMed] [Google Scholar]
- 12.Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, Vist GE, Falck-Ytter Y, Meerpohl J, Norris S, Guyatt GH. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64:401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 13.Barber PA, Roberts S, Spriggs DA, Anderson NE. Adverse cardiovascular, cerebrovascular, and peripheral vascular effects of marijuana: what cardiologists need to know. Am J Cardiol. 2014;113:1086. doi: 10.1016/j.amjcard.2014.01.400. [DOI] [PubMed] [Google Scholar]
- 14.Baron R, Binder A, Wasner G. Neuropathic pain: Diagnosis, pathophysiological mechanisms, and treatment. The Lancet Neurology. 2010;9(8):807–819. doi: 10.1016/S1474-4422(10)70143-5. 2010. [DOI] [PubMed] [Google Scholar]
- 15.Beard S, Hunn A, Wight J. Treatments for spasticity and pain in multiple sclerosis: a systematic review. Health technology assessment (Winchester, England) 2003;7(40):iii–111. doi: 10.3310/hta7400. ix-x, 41. 2003. [DOI] [PubMed] [Google Scholar]
- 16.Best N, Ashby D, Dunstan F, Foreman D, McIntosh N. A Bayesian approach to complex clinical diagnoses: a case-study in child abuse. Journal of the Royal Statistical Society: Series A (Statistics in Society) 2013;176:53–96. [Google Scholar]
- 17.Birse F, Derry S, Moore RA. Phenytoin for neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2012;5:CD009485. doi: 10.1002/14651858.CD009485.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bostwick JM. Blurred boundaries: the therapeutics and politics of medical marijuana. Mayo Clin Proc. 2012;87:172–186. doi: 10.1016/j.mayocp.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bostwick JM, Reisfield GM, DuPont RL. Clinical decisions. Medicinal use of marijuana. New England Journal of Medicine. 2013;368:866–868. doi: 10.1056/NEJMclde1300970. [DOI] [PubMed] [Google Scholar]
- 20.Bottorff JL, Bissell LJ, Balneaves LG, Oliffe JL, Capler NR, Buxton J. Perceptions of cannabis as a stigmatized medicine: a qualitative descriptive study. Harm Reduct J. 2013;10:2. doi: 10.1186/1477-7517-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Campbell FA, Tramer MR, Carroll D, Reynolds DJ, Moore RA, McQuay HJ. Are cannabinoids an effective and safe treatment option in the management of pain? A qualitative systematic review. BMJ. 7303;323:13–16. doi: 10.1136/bmj.323.7303.13. [Review] [18 refs] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Campbell FA, Tramer MR, Carroll D, Reynolds DJM, Moore RA, McQuay HJ. Are cannabinoids an effective and safe treatment option in the management of pain? A qualitative systematic review. British Medical Journal. 2001;323(7303):7313–7316. doi: 10.1136/bmj.323.7303.13. 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carlin JB. Meta-analysis for 2 × 2 tables: a Bayesian approach. Stat Med. 1992;11:141–158. doi: 10.1002/sim.4780110202. [DOI] [PubMed] [Google Scholar]
- 24.Chaparro LE, Wiffen PJ, Moore RA, Gilron I. Combination pharmacotherapy for the treatment of neuropathic pain in adults. Cochrane Database Syst Rev. 2012;7:CD008943. doi: 10.1002/14651858.CD008943.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen HW. P values: use and misuse in medical literature. Am J Hypertens. 2011;24:18–23. doi: 10.1038/ajh.2010.205. [DOI] [PubMed] [Google Scholar]
- 26.Colombo B, Annovazzi POL, Comi G. Medications for neuropathic pain: Current trends. Neurological Sciences. 2006;27 doi: 10.1007/s10072-006-0598-7. SUPPL. [DOI] [PubMed] [Google Scholar]
- 27.Cornell JE, Mulrow CD, Localio R, Stack CB, Meibohm AR, Guallar E, Goodman SN. Random-Effects Meta-analysis of Inconsistent Effects: A Time for Change. Ann Intern Med. 2014;160 doi: 10.7326/M13-2886. [DOI] [PubMed] [Google Scholar]
- 28.Corrigan R, Derry S, Wiffen PJ, Moore RA. Clonazepam for neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2012;5:CD009486. doi: 10.1002/14651858.CD009486.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DuPont RL, Lieberman JA. Young brains on drugs. Science. 2014;344:557. doi: 10.1126/science.1254989. [DOI] [PubMed] [Google Scholar]
- 30.Dworkin RH, O'Connor AB, Backonja M, Farrar JT, Finnerup NB, Jensen TS, Kalso EA, Loeser JD, Miaskowski C, Nurmikko TJ, Portenoy RK, Rice AS, Stacey BR, Treede RD, Turk DC, Wallace MS. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain. 2007;132:237–251. doi: 10.1016/j.pain.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 31.Dworkin RH, Turk DC, McDermott MP, Peirce-Sandner S, Burke LB, Cowan P, Farrar JT, Hertz S, Raja SN, Rappaport BA, Rauschkolb C, Sampaio C. Interpreting the clinical importance of group differences in chronic pain clinical trials: IMMPACT recommendations. Pain. 2009;146:238–244. doi: 10.1016/j.pain.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 32.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ellis RJ, Rosario D, Clifford DB, McArthur JC, Simpson D, Alexander T, Gelman BB, Vaida F, Collier A, Marra CM, Ances B, Atkinson JH, Dworkin RH, Morgello S, Grant I. Continued high prevalence and adverse clinical impact of human immunodeficiency virus-associated sensory neuropathy in the era of combination antiretroviral therapy: the CHARTER Study. Arch Neurol. 2010;67:552–558. doi: 10.1001/archneurol.2010.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ellis RJ, Toperoff W, Vaida F, van den Brande G, Gonzales J, Gouaux B, Bentley H, Atkinson JH. Smoked medicinal cannabis for neuropathic pain in HIV: a randomized, crossover clinical trial. Neuropsychopharmacology. 2009;34:672–680. doi: 10.1038/npp.2008.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ellis RJ, Toperoff W, Vaida F, Van Den Brande G, Gonzales J, Gouaux B, Bentley H, Atkinson JH. Smoked medicinal cannabis for neuropathic pain in HIV: A randomized, crossover clinical trial. Neuropsychopharmacology. 2009;34(33):672–680. doi: 10.1038/npp.2008.120. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Farrar JT, Troxel AB, Stott C, Duncombe P, Jensen MP. Validity, reliability, and clinical importance of change in a 0-10 numeric rating scale measure of spasticity: a post hoc analysis of a randomized, double-blind, placebo-controlled trial. Clin Ther. 2008;30:974–985. doi: 10.1016/j.clinthera.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 37.Finnerup NB, Sindrup SH, Jensen TS. The evidence for pharmacological treatment of neuropathic pain. Pain. 2010;150:573–581. doi: 10.1016/j.pain.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 38.Gelman A. Prior distributions for variance parameters in hierarchical models (comment on article by Browne and Draper) Bayesian analysis. 2006;1:515–534. [Google Scholar]
- 39.Gelman A, Carlin JB, Stern HS, Dunson DB, Vehtari A, Rubin DB. Bayesian data analysis. CRC press; 2013. [Google Scholar]
- 40.Goodman SN. Introduction to Bayesian methods I: measuring the strength of evidence. Clin Trials. 2005;2:282–290. doi: 10.1191/1740774505cn098oa. discussion 301-284, 364-278. [DOI] [PubMed] [Google Scholar]
- 41.Greydanus DE, Hawver EK, Greydanus MM, Merrick J. Marijuana: current concepts(dagger) Front Public Health. 2013;1:42. doi: 10.3389/fpubh.2013.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gunnell D, Saperia J, Ashby D. Selective serotonin reuptake inhibitors (SSRIs) and suicide in adults: meta-analysis of drug company data from placebo controlled, randomised controlled trials submitted to the MHRA's safety review. BMJ. 2005;330:385. doi: 10.1136/bmj.330.7488.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Ytter Y, Schunemann HJ. What is "quality of evidence" and why is it important to clinicians? BMJ. 2008;336:995–998. doi: 10.1136/bmj.39490.551019.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hashibe M, Straif K, Tashkin DP, Morgenstern H, Greenland S, Zhang ZF. Epidemiologic review of marijuana use and cancer risk. Alcohol. 2005;35:265–275. doi: 10.1016/j.alcohol.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 45.Hayes MJ, Brown MS. Legalization of medical marijuana and incidence of opioid mortality. JAMA Intern Med. 2014;174:1673–1674. doi: 10.1001/jamainternmed.2014.2716. [DOI] [PubMed] [Google Scholar]
- 46.Hewitt C, Hahn S, Torgerson DJ, Watson J, Bland JM. Adequacy and reporting of allocation concealment: review of recent trials published in four general medical journals. BMJ. 2005;330:1057–1058. doi: 10.1136/bmj.38413.576713.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Higgins JPT, Green S, Cochrane Collaboration . Cochrane handbook for systematic reviews of interventions. Wiley-Blackwell; Chichester, England ; Hoboken, NJ: 2008. [Google Scholar]
- 48.Higgins JPT GS: Cochrane Handbook for Systematic Reviews of Interventions 5.1.0. Available at: www.cochrane-handbook.org.
- 49.Hill KP. Medical Marijuana for Treatment of Chronic Pain and Other Medical and Psychiatric Problems: A Clinical Review. JAMA. 2015;313:2474–2483. doi: 10.1001/jama.2015.6199. [DOI] [PubMed] [Google Scholar]
- 50.Iskedjian M, Bereza B, Gordon A, Piwko C, Einarson TR. Meta-analysis of cannabis based treatments for neuropathic and multiple sclerosis-related pain. Curr Med Res Opin. 2007;23:17–24. doi: 10.1185/030079906x158066. [DOI] [PubMed] [Google Scholar]
- 51.Iskedjian M, Bereza B, Gordon A, Piwko C, Einarson TR. Meta-analysis of cannabis based treatments for neuropathic and multiple sclerosis-related pain. Current Medical Research and Opinion. 2007;23(21):17–24. doi: 10.1185/030079906x158066. 2007. [DOI] [PubMed] [Google Scholar]
- 52.Joshi M, Joshi A, Bartter T. Marijuana and lung diseases. Curr Opin Pulm Med. 2014;20:173–179. doi: 10.1097/MCP.0000000000000026. [DOI] [PubMed] [Google Scholar]
- 53.Kish L. Statistical design for research. Wiley-Interscience; Hoboken, N.J.: 2004. Representation, Randomization, and Realism; pp. xxii–267. [Google Scholar]
- 54.Kissin I. Long-term opioid treatment of chronic nonmalignant pain: unproven efficacy and neglected safety? J Pain Res. 2013;6:513–529. doi: 10.2147/JPR.S47182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Koppel BS, Brust JC, Fife T, Bronstein J, Youssof S, Gronseth G, Gloss D. Systematic review: efficacy and safety of medical marijuana in selected neurologic disorders: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2014;82:1556–1563. doi: 10.1212/WNL.0000000000000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kruschke JK. Bayesian estimation supersedes the t test. J Exp Psychol Gen. 2013;142:573–603. doi: 10.1037/a0029146. [DOI] [PubMed] [Google Scholar]
- 57.Leger JM. Diagnosis of chronic neuropathy. J Neurol. 1999;246:156–161. doi: 10.1007/s004150050326. [DOI] [PubMed] [Google Scholar]
- 58.Lunn D, Thomas A, Best N, Spiegelhalter D. WinBUGS - A Bayesian modelling framework: Concepts, structure, and extensibility. Statistics and Computing. 2000;10:325–337. [Google Scholar]
- 59.Lutge EE, Gray A, Siegfried N. The medical use of cannabis for reducing morbidity and mortality in patients with HIV/AIDS. Cochrane Database Syst Rev. 2013;4:CD005175. doi: 10.1002/14651858.CD005175.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lynch ME, Campbell F. Cannabinoids for treatment of chronic non-cancer pain; a systematic review of randomized trials. Br J Clin Pharmacol. 2011;72:735–744. doi: 10.1111/j.1365-2125.2011.03970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Martin-Sanchez E, Furukawa TA, Taylor J, Martin JLR. Systematic review and meta-analysis of cannabis treatment for chronic pain. Pain Medicine. 2009;10(18):1353–1368. doi: 10.1111/j.1526-4637.2009.00703.x. 2009. [DOI] [PubMed] [Google Scholar]
- 62.McGilveray IJ. Pharmacokinetics of cannabinoids. Pain Res Manag. 2005;10(Suppl A):15A–22A. doi: 10.1155/2005/242516. [DOI] [PubMed] [Google Scholar]
- 63.McGlothlin AE, Lewis RJ. Minimal clinically important difference: defining what really matters to patients. JAMA. 2014;312:1342–1343. doi: 10.1001/jama.2014.13128. [DOI] [PubMed] [Google Scholar]
- 64.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 65.Moore RA, Derry S, Wiffen PJ. Challenges in design and interpretation of chronic pain trials. Br J Anaesth. 2013;111:38–45. doi: 10.1093/bja/aet126. [DOI] [PubMed] [Google Scholar]
- 66.Moore RA, Moore OA, Derry S, Peloso PM, Gammaitoni AR, Wang H. Responder analysis for pain relief and numbers needed to treat in a meta-analysis of etoricoxib osteoarthritis trials: bridging a gap between clinical trials and clinical practice. Ann Rheum Dis. 2010;69:374–379. doi: 10.1136/ard.2009.107805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Namaka M, Leong C, Grossberndt A, Klowak M, Turcotte D, Esfahani F, Gomori A, Intrater H. A treatment algorithm for neuropathic pain: An update. Consultant Pharmacist. 2009;24(12):885–902. doi: 10.4140/tcp.n.2009.885. 2009. [DOI] [PubMed] [Google Scholar]
- 68.O'Connor AB, Dworkin RH. Treatment of neuropathic pain: an overview of recent guidelines. Am J Med. 2009;122:S22–32. doi: 10.1016/j.amjmed.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 69.Okie S. Medical marijuana and the Supreme Court. New England Journal of Medicine. 2005;353(357):648–651. doi: 10.1056/NEJMp058165. 2005. [DOI] [PubMed] [Google Scholar]
- 70.Pacher P. Towards the use of non-psychoactive cannabinoids for prostate cancer. Br J Pharmacol. 2013;168:76–78. doi: 10.1111/j.1476-5381.2012.02121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pereira TV, Ioannidis JP. Statistically significant meta-analyses of clinical trials have modest credibility and inflated effects. J Clin Epidemiol. 2011;64:1060–1069. doi: 10.1016/j.jclinepi.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 72.Phillips TJ, Cherry CL, Cox S, Marshall SJ, Rice AS. Pharmacological treatment of painful HIV-associated sensory neuropathy: a systematic review and meta-analysis of randomised controlled trials. PLoS One. 2010;5:e14433. doi: 10.1371/journal.pone.0014433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Phillips TJ, Cherry CL, Cox S, Marshall SJ, Rice AS. Pharmacological treatment of painful HIV-associated sensory neuropathy: a systematic review and meta-analysis of randomised controlled trials. PLoS One. 2010;5(12):e14433. doi: 10.1371/journal.pone.0014433. 12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Preuss UW, Watzke AB, Zimmermann J, Wong JW, Schmidt CO. Cannabis withdrawal severity and short-term course among cannabis-dependent adolescent and young adult inpatients. Drug Alcohol Depend. 2010;106:133–141. doi: 10.1016/j.drugalcdep.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 75.Ramaekers J, Kauert G, Theunissen E. Up in Smoke: Comparability of THC Dosing across Performance Studies. Neuropsychopharmacology. 2006;31:2800. [Google Scholar]
- 76.Rintala DH, Holmes SA, Courtade D, Fiess RN, Tastard LV, Loubser PG. Comparison of the effectiveness of amitriptyline and gabapentin on chronic neuropathic pain in persons with spinal cord injury. Arch Phys Med Rehabil. 2007;88:1547–1560. doi: 10.1016/j.apmr.2007.07.038. [DOI] [PubMed] [Google Scholar]
- 77.Simmonds MC, Higgins JP, Stewart LA, Tierney JF, Clarke MJ, Thompson SG. Meta-analysis of individual patient data from randomized trials: a review of methods used in practice. Clin Trials. 2005;2:209–217. doi: 10.1191/1740774505cn087oa. [DOI] [PubMed] [Google Scholar]
- 78.Snapinn SM, Jiang Q. Responder analyses and the assessment of a clinically relevant treatment effect. Trials. 2007;8:31. doi: 10.1186/1745-6215-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Song F, Clark A, Bachmann MO, Maas J. Simulation evaluation of statistical properties of methods for indirect and mixed treatment comparisons. BMC Med Res Methodol. 2012;12:138. doi: 10.1186/1471-2288-12-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Spiegelhalter DJ, Abrams KR, Myles JP. Bayesian approaches to clinical trials and health care evaluation. Wiley; Chichester ; Hoboken, NJ: 2004. [Google Scholar]
- 81.Starowicz K, Makuch W, Korostynski M, Malek N, Slezak M, Zychowska M, Petrosino S, De Petrocellis L, Cristino L, Przewlocka B, Di Marzo V. Full inhibition of spinal FAAH leads to TRPV1-mediated analgesic effects in neuropathic rats and possible lipoxygenase-mediated remodeling of anandamide metabolism. PLoS ONE [Electronic Resource] 2013;8:e60040. doi: 10.1371/journal.pone.0060040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stewart GB, Altman DG, Askie LM, Duley L, Simmonds MC, Stewart LA. Statistical analysis of individual participant data meta-analyses: a comparison of methods and recommendations for practice. PLoS One. 2012;7:e46042. doi: 10.1371/journal.pone.0046042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sznitman SR, Zolotov Y. Cannabis for Therapeutic Purposes and public health and safety: A systematic and critical review. Int J Drug Policy. 2014 doi: 10.1016/j.drugpo.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 84.Volkow ND, Baler RD, Compton WM, Weiss SR. Adverse health effects of marijuana use. N Engl J Med. 2014;370:2219–2227. doi: 10.1056/NEJMra1402309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wagenmakers E-J, Lee M, Lodewyckx T, Iverson G. Bayesian Versus Frequentist Inference. In: Hoijtink H, Klugkist I, Boelen P, editors. Bayesian Evaluation of Informative Hypotheses. Statistics for Social and Behavioral Sciences. Springer; New York: 2008. pp. 181–207. [Google Scholar]
- 86.Wallace JM. Update on pharmacotherapy guidelines for treatment of neuropathic pain. Current Pain and Headache Reports. 2007;11(13):208–214. doi: 10.1007/s11916-007-0192-6. 2007. [DOI] [PubMed] [Google Scholar]
- 87.Ware MA, Rueda S, Singer J, Kilby D. Cannabis use by persons living with HIV/AIDS: Patterns and prevalence of use. Journal of Cannabis Therapeutics. 2003;3(2):3–15. 2003. [Google Scholar]
- 88.Ware MA, Wang T, Shapiro S, Robinson A, Ducruet T, Huynh T, Gamsa A, Bennett GJ, Collet JP. Smoked cannabis for chronic neuropathic pain: a randomized controlled trial. CMAJ. 2010;182:E694–701. doi: 10.1503/cmaj.091414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ware MA, Wang T, Shapiro S, Robinson A, Ducruet T, Huynh T, Gamsa A, Bennett GJ, Collet JP. Smoked cannabis for chronic neuropathic pain: A randomized controlled trial. Cmaj. 2010;182(114):E694–E701. doi: 10.1503/cmaj.091414. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wiffen PJ, Derry S, Moore RA, Aldington D, Cole P, Rice AS, Lunn MP, Hamunen K, Haanpaa M, Kalso EA. Antiepileptic drugs for neuropathic pain and fibromyalgia - an overview of Cochrane reviews. Cochrane Database Syst Rev. 2013;11:CD010567. doi: 10.1002/14651858.CD010567.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wilkinson ST, D'Souza DC. Problems with the medicalization of marijuana. JAMA. 2014;311:2377–2378. doi: 10.1001/jama.2014.6175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wilsey B, Marcotte T, Deutsch R, Gouaux B, Sakai S, Donaghe H. Low-dose vaporized cannabis significantly improves neuropathic pain. J Pain. 2013;14:136–148. doi: 10.1016/j.jpain.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wilsey B, Marcotte T, Deutsch R, Gouaux B, Sakai S, Donaghe H. Low-dose vaporized cannabis significantly improves neuropathic pain. J Pain. 2013;14:136–148. doi: 10.1016/j.jpain.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wilsey B, Marcotte T, Tsodikov A, Millman J, Bentley H, Gouaux B, Fishman S. A randomized, placebo-controlled, crossover trial of cannabis cigarettes in neuropathic pain. J Pain. 2008;9:506–521. doi: 10.1016/j.jpain.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wilsey B, Marcotte T, Tsodikov A, Millman J, Bentley H, Gouaux B, Fishman S. A Randomized, Placebo-Controlled, Crossover Trial of Cannabis Cigarettes in Neuropathic Pain. J Pain. 2008;9(6):506–521. doi: 10.1016/j.jpain.2007.12.010. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Woolf CJ. Dissecting out mechanisms responsible for peripheral neuropathic pain: implications for diagnosis and therapy. Life Sci. 2004;74:2605–2610. doi: 10.1016/j.lfs.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 97.Woolf CJ, Mannion RJ. Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet. 1999;353:1959–1964. doi: 10.1016/S0140-6736(99)01307-0. [DOI] [PubMed] [Google Scholar]
- 98.Woolridge E, Barton S, Samuel J, Osorio J, Dougherty A, Holdcroft A. Cannabis use in HIV for pain and other medical symptoms. J Pain Symptom Manage. 2005;29:358–367. doi: 10.1016/j.jpainsymman.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 99.Wright S. Cannabinoid-based medicines for neurological disorders - Clinical evidence. Molecular Neurobiology. 2007;36(31):129–136. doi: 10.1007/s12035-007-0003-4. 2007. [DOI] [PubMed] [Google Scholar]
- 100.Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics. 1988;44:1049–1060. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.