Abstract
Sutherlandia frutescens is a botanical widely used in southern Africa for treatment of inflammatory and other conditions. Previously, an ethanolic extract of S. frutescens (SFE) has been shown to inhibit the production of reactive oxygen species (ROS) and nitric oxide (NO) by murine neurons and a microglia cell line (BV-2 cells). In this study we sought to confirm the anti-inflammatory activities of SFE on a widely used murine macrophage cell line (i.e., RAW 264.7 cells) and primary mouse macrophages. Furthermore, experiments were conducted to investigate the anti-inflammatory activity of the flavonol and cycloartanol glycosides found in high quantities in S. frutescens. While the SFE exhibited anti-inflammatory activities upon murine macrophages similar to that reported with the microglia cell line, this effect does not appear to be mediated by sutherlandiosides or sutherlandins. In contrast, chlorophyll in our extracts appeared to be partly responsible for some of the activity observed in our macrophage-dependent screening assay.
Keywords: Sutherlandia frutescens, inflammation, macrophage, chlorophyll
1. Introduction
Sutherlandia frutescens (L.) R. Br, commonly referred to as “cancer bush”, is a medicinal plant traditionally used in southern Africa for a variety of health conditions [1]. S. frutescens has been used to treat patients suffering from various chronic health conditions, such as rheumatoid arthritis, osteoarthritis, and gout where inflammation plays a significant role in the disease etiology or symptoms [2]. Fernandes and coworkers [3] suggested that the antioxidant properties of S. frutescens contribute to its anti-inflammatory actions. Using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) free-radical scavenging assay of acetone, aqueous, ethanol, methanol, and chloroform extracts of S. frutescens [4-6], antioxidant properties were demonstrated without direct evidence of anti-inflammatory activity. In fact, the evidence of anti-inflammatory activity in S. frutescens is fairly limited and mostly indirect. Kundu et al. [7] demonstrated that a methanolic extract of S. frutescens could decrease 12-O-tetra-decanoylphorbol-13-acetate-induced cyclooxygenase-2 (COX-2) expression in mouse skin. The proposed mechanism was a reduction in the activity of activator protein-1 (AP-1), a component of the ERK signaling pathway. Recently, we reported the ability for ethanol extract of S. frutescens to inhibit neuronal excitotoxicity and mitigate lipopolysaccharide (LPS)-induced ROS and NO production in BV-2 microglial cells [8].
Several biologically active compounds have been identified in extracts of S. frutescens, including: L-canavanine, pinitol, and gamma-aminobutyric acid (GABA) [9, 10]. Fu and colleagues [11] characterized two groups of compounds unique to S. frutescens: Sutherlandiosides (a group of cycloartane glycosides with structural variants denoted by designations A, B, C, D) and sutherlandins (a group of flavonol glycosides derivatives of 3-hydroxy-3-methylglutarate with structural designations of A-D, also). Investigation into the biological activities associated with these cycloartane- and flavonol-glycosides from S. frutescens has been limited [11-13]. No reports exist regarding the biological functions/activities of either sutherlandiosides or sutherlandins on innate immune cells.
Macrophages are innate immune cells that play critical roles in the inflammatory responses [14]. These cells are activated by a complex of immune-mediators including pathogen associated molecules (such as LPS from gram-negative bacteria) and host factors (e.g., TNF-α, IFNγ) [15, 16]. Co-stimulation with LPS and IFNγ has been widely used as a means of stimulating/activating macrophages. Following stimulation, the subunits of nicotinamide adenine dinucleotide phosphate (NADPH) oxidases are assembled resulting in the production of large quantities of ROS [17]. ROS is known to play critical roles in anti-microbial activity and serves as secondary signaling molecules to modulate the inflammatory responses [18]. Additionally, LPS and IFNγ co-stimulation of murine macrophages promotes the release of nitric oxide (NO) through induction of nitric oxide synthase (iNOS), which depends on several signaling pathways, including: NF-κB, MAP kinase (e.g. ERK1/2), and JAK-STAT1 [19-21]. However, over-production of ROS or NO can result in oxidative and nitrosative stress that can lead to health-related problems (e.g., cancer, inflammatory disease) [22-24]. The objective of this study was to investigate the anti-inflammatory activity of S. frutescens in macrophages, and to better define the putative bioactive compounds responsible for this activity.
2. Materials and Methods
2.1. Reagents
Lipopolysaccharide (LPS, from E. coli 0111:B4) was purchased from Sigma Chemical Co. (St. Louis, MO, USA). Murine interferon-γ (IFNγ) was purchased from R&D Systems (Minneapolis, MN, USA). Fetal bovine serum (FBS) was from Thermo Scientific (Logan, Utah, USA). Antibodies used for Western blotting include: goat anti-rabbit IgG-horseradish peroxidase, goat anti-mouse IgG-horseradish peroxidase, and iNOS polyclonal from Santa Cruz Biotechnology (Santa Cruz, CA USA); phospho- and total-ERK1/2 antibodies from Cell Signaling Technology (Danvers, MA, USA); phospho-STAT1α antibody from Thermo Fisher Scientific (Rockford, IL, USA); and total-STAT antibody from Millipore (Billerica, MA, USA). The 5-(and-6)-chloromethyl-2’,7’-dichlorodihydrofluorescein diacetate, acetyl ester (CM-H2DCFDA) for ROS detection was purchased from Invitrogen, Inc. (Carlsbad, CA, USA). For the cytokines and chemokines analysis, a Multi-Plex kit (Cat. MCYTOMAG-70K-PMX) was obtained from EMD Millipore (Billerica, MA, USA). Protease inhibitor cocktail (P8340) was purchased from Sigma (St. Louis, MO, USA).
2.2. Preparation of ethanolic extract of S. frutescens (SFE)
S. frutescens (L.) R. Br. desiccated powder was purchased from Big Tree Nutraceutical (Fish Hoek, South Africa) and verified as described previously [25]. For preparation of SFE, 10 g of S. frutescens was stirred into 250 ml 95% ethanol. The mixture were left to stand overnight at room temperature, after which it was centrifuged, filter sterilized (0.2 μm, Fisher Scientific, Pittsburgh, PA, USA), and then stored at −80°C until used. Fifty milliliters of extract was dried at 55 °C. The yield of dry matter from the SFE was 4.16 mg/mL. Prior to use in cell culture experiments, the ethanolic extract was dried at low temperature under a vacuum (Centrivap Concentrator, LABCONCO, Kansas City, MO, USA). The dried extract was then re-suspended in a volume of dimethyl sulfoxide (DMSO) that was one-tenth of the original volume of the ethanolic extract. The addition of SFE to cells was carried out such that the final concentration of DMSO was always kept constant at 0.1% (vol/vol), including those cells designated “negative” and “positive” controls. Preliminary experiments demonstrated that this concentration of DMSO was without effect on cell viability as well as the other biological readouts measured (data not shown).
2.3. Determination and isolation of sutherlandiosides, sutherlandins and chlorophyll from S. frutescens
Sutherlandioside B, a mixture of sutherlandiosides A, C, D, a mixture of sutherlandins A and B, and a mixture of sutherlandins C and D were isolated from S. frutescens as described by Brownstein et al. [26], a modified isolation procedure originally described by Fu et al. [11, 27]. The sutherlandiosides and sutherlandins in the crude extracts and the quality of semi-purified fractions were determined using liquid chromatography (LC)-evaporative light scattering detection or LC-UV as described by Avula et al. [28]. The purity of sutherlandioside B was > 95% based on the analysis result, while the purity of fractions with sutherlandiosides A, C, D, sutherlandins A and B, and sutherlandins C and D was better than 90%. Each bioactive purified from these extracts as well as the original crude extract were screened for the presence of bacterial endotoxin using a highly-sensitive NF-κB reporter cell line described below and were found to be free of such contamination.
The concentration of chlorophyll in the crude ethanolic extract of S. frutescens was estimated by spectrophotometry (Beckman, Brea, CA, USA) using the method described by Lichtenthaler [29]. Isolation of the native chlorophylls present in S. frutescens were conducted by following the method described by Bidigare and colleagues [30]. The purity of chlorophylls (a mixture of chlorophyll a and chlorophyll b) isolated from S. frutescens by using this procedure was > 95%. Furthermore, neither sutherlandiosides nor sutherlandins were detected in the isolated chlorophyll fraction.
2.4. Cell culture
The murine macrophage cell line, RAW 264.7, was purchased from the American Type Culture Collection (Manassas, VA, USA). Cells were cultured in Dulbecco’s modified Eagle medium (DMEM, Gibco, Grand Island, NY, USA) supplemented with 5% fetal bovine serum (FBS), and maintained at 37 °C in an atmosphere of 5% CO2. A colleague, Dr. Val Mossine, provided us with RAW 264.7 cells stably-transfected with a nuclear factor-κB (NF-κB) reporter that were generated as described previously [31]. The NF-κB reporter construct contained five copies of the element from the promoter of NF-κB, a copy of green fluorescent protein (GFP), and puromycin resistance genes. These cells were maintained under the same condition as wild-type RAW 264.7 cells.
2.5. Isolation of primary murine macrophages
Primary murine macrophages were obtained from healthy five to ten-week old male Balb/c mice (Jackson Laboratory, Bar Harbor, ME, USA) as described in a previous study [32]. Cells were then diluted to a final concentration of 2 × 106 cell/mL, and were seeded in 96-well plates (Corning® Costar®, Corning, NY, USA) at a density of 2 × 105 cells/well. After incubation for 2 h, non-adherent cells (e.g., lymphocytes, erythrocytes) were removed by gently washing twice with DMEM leaving a monolayer of murine macrophages ready for subsequent experiments.
2.6. Cell viability
Cell viability was measured using the resazurin assay and green fluorescents protein (GFP) assay as described previously [25]. The RAW 264.7 cells were exposed to different concentrations of SFE or fractions from S. frutescens for 1 h prior to co-stimulation with murine IFNγ and LPS at the concentration used for ROS and NO stimulation (i.e., 1 ng/mL and 100 ng/mL or 0.1 ng/mL and 10 ng/mL) for 20 h (resazurin assay) and 3 h (GFP assay). Cells without any treatment (neither stimuli nor S. frutescens) were included as a negative control, while cells co-stimulated with LPS and IFNγ but not treated with S. frutescens were considered as a positive control.
2.7. Analysis of reactive oxygen species (ROS) production
RAW 264.7 cells were cultured overnight in 96-well plates (1 × 105 cells/well) in DMEM contained 5% FBS to reach greater than 95% confluence. The cells were then cultured in DMEM with 1% FBS for 2 h, then pretreated with various dilutions of SFE for 60 min. Cells were then co-stimulated with LPS (100 ng/mL) and IFNγ (1 ng/mL) for 18 h. Intracellular ROS was measured using the oxidant-sensitive fluorescent probe CM-H2DCFDA as described previously [25].
2.8. Nitric oxide (NO) measurement
The concentration of NO in the cell culture medium was measured using Griess reagents [33]. Similar to ROS assay, RAW 264.7 cells were cultured overnight in 96-well plates (1 × 105 cells/well) in DMEM/5% FBS. The cells were pretreated with various dilutions of SFE for 1 prior to co-stimulation with LPS (10 ng/mL) and IFNγ (0.1 ng/mL) for 20 h. The cell culture medium was harvested for NO production and inflammatory cytokines/chemokines analysis. For the NO assay, 50 μL of cell culture supernatants from each well were mixed with 50 μL of fresh DMEM medium in a 96-well ELISA plate. Fifty microliters of each Griess reagent (7.5 mM sulfanilamide solution, 0.75 M HCL, 7.5 mM napthyl ethylenediamine solution) were added sequentially to the wells. The absorbance at 548 nm was determined with a filter-based multi-mode microplate reader (Biotek, Winooski, VT, USA), and nitrite concentrations were calculated from a sodium nitrite standard curve that had a linear response in the range of 5 to 100 μM.
2.9. Cytokine analysis
RAW 264.7 cells were treated with SFE and co-stimulated with inflammatory mediators as described above. Concentrations of cytokines/chemokines in culture media were measured using a MILLIPLEX magnetic bead panel kit (murine cytokines/chemokines, Cat. MCYTOMAG-70K-PMX, EMD Millipore, Billerica, MA, USA) according to the instructions provided by manufacturer. The plate was read using the MAGPIX® plate reader with xPONENT software (Luminex, Austin, TX, USA). Data were analyzed using the MILLIPLEX™ Analyst software.
2.10. Western blot analysis
RAW 264.7 cells were plated in 6-well plates, and cultured overnight to > 95% confluence. Cells were pretreated with various concentrations of ethanolic extract of S. frutescens (SFE) for 1 h, and then co-stimulated with LPS (10 ng/mL) and IFNγ (0.1 ng/mL) for 30 min or 60 min. Samples harvested at 30 min were used for analysis of ERK1/2, and those harvested at 60 min were used for STAT1α. The cells were washed with ice-cold PBS, and lysed in radio-immunoprecipitation assay buffer (50 mM Tris-HCl, 150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, and 0.1% sodium dodecyl sulfate and a protease inhibitor cocktail). The protein concentrations of each cell lysate were then measured with the BCA protein assay kit (Pierce Biotechnology, Rockford, IL). Next, 2.4 μg of protein from each sample was loaded on a lane in the gel and separated by 8% SDS-PAGE. The proteins were transferred onto 0.45 μm nitrocellulose membranes (Bio-Rad Laboratories, Hercules, CA, USA) at 300 mA for 3 h. The membranes were blocked for 1 h with 5% non-fat dried milk in Tris-buffered saline. Subsequently, the blots were probed with the primary antibodies in TBS containing 5% non-fat dried milk at 4°C overnight. After washing, antibody-antigen complexes were then detected using goat anti-rabbit or goat anti-mouse IgG-horseradish peroxidase (1:5000) conjugated antibodies and an enhanced chemiluminescence detection kit (Amersham Pharmacia Biotech, Piscataway, NJ, USA). Monoclonal anti-β-actin peroxidase was used as the loading control. The blots were scanned, and the intensity of protein bands was quantified using the QuantityOne program (BioRad, Hercules, CA, USA).
2.11. NF-κB activation assay
The impact of S. frutescens extract on NF-κB activation was investigated using RAW 264.7 cells transfected with the NF-κB reporter [31] as described by Lei et al [25]. Transfected cells were cultured overnight to reach confluence. The cells were exposed to various concentrations of S. frutescens extract or enriched fractions for 1 h prior to co-stimulation with IFNγ (0.1 ng/mL) and LPS (10 ng/mL) for 3 h to activate NF-κB signaling. After removing the medium, 70 μL lysis buffer was added to each well to lyse cells. Ten microliters of cell lysate was transferred into a white 96-well plate. Twenty microliters of BSA and 30 μL luciferase substrates were added and the plate was read immediately to detect light output using a microplate reader (Biotek, Winooski, VT, USA).
2.12. Statistical analysis
All data shown represent the means ± standard error of means from at least three independent experiments with each treatment condition conducted in triplicate. The post-comparisons were performed using the Tukey’s multiple comparison tests. A p < 0.05 was considered to indicate statistical significance.
3. Results
3.1. ROS production
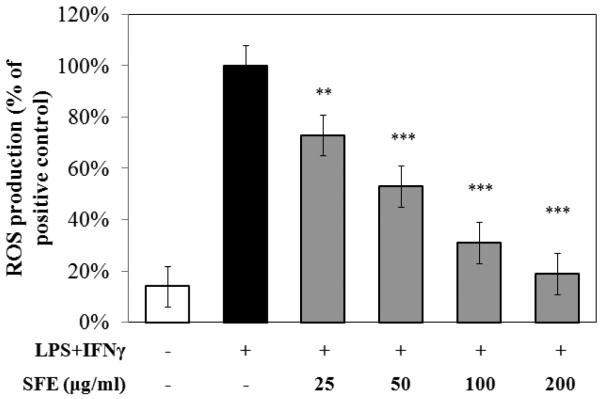
To evaluate intracellular ROS production, RAW 267.4 cells were treated with SFE for 1 h prior to co-stimulation with LPS and IFNγ for 18h. After this time, ROS production was measured every 10 min for 3 h. Cells co-stimulated with LPS and IFNγ, but without SFE, were included as a positive control. The SFE extract did not affect cell viability as assessed by the resazurin assay and green fluorescent proteins (GFP) assay (data not shown). SFE significantly inhibited ROS production by RAW 264.7 cells co-stimulated with LPS and IFNγ in a dose-dependent manner (Fig. 1).
Fig. 1. Ethanolic extract of S. frutescens (SFE) reduced reactive oxygen species (ROS) production by murine macrophage cell line (i.e. RAW 264.7 cells) in a dose-dependent manner.
ROS production is expressed as percentage of that relative to cells co-stimulated with LPS (100 ng/mL) and IFNγ (1 ng/mL) after exposure to various concentrations of SFE for 18 h. The Y-axis is showing percentage of ROS production compared to the positive control, and X-axis shows different treatments. The data were from three independent experiments each conducted in triplicate. * p < 0.05, ** p < 0.01, *** p < 0.0001.
3.2. NO production and expression of iNOS
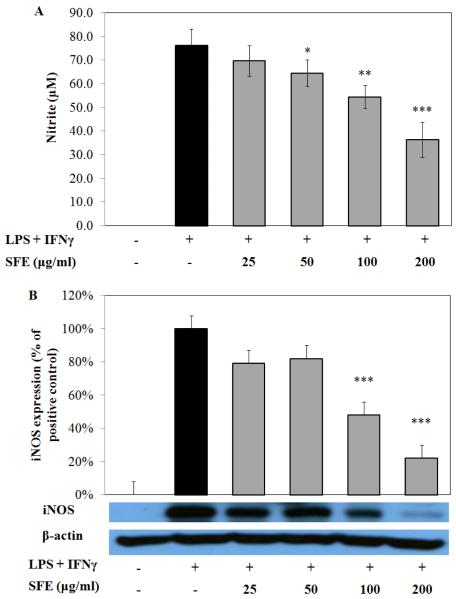
RAW 264.7 cells produced 76.3 ± 6.8 μM NO after co-stimulating with LPS (10 ng/mL) and IFNγ (0.1 ng/mL) for 20 h. When cells were pretreated with SFE, LPS-induced NO production was reduced in a dose related pattern (Fig. 2A).
Fig. 2. Ethanolic extract of S. frutescens (SFE) dose-dependently reduced nitric oxide (NO) production and inducible nitric oxide synthase (iNOS) expression murine macrophage cell line (i.e. RAW 264.7 cells) in a dose-dependent manner.
Cells were pretreated with SFE, then costimulated with LPS (10 ng/mL) and IFNγ (0.1 ng/mL) for 20 h. NO production was significantly inhibited by exposure of SFE induced by inflammatory stimuli (A). Data were from three independent experiments each conducted in triplicate. The iNOS expression induced by the stimuli was significantly reduced by SFE(B). The Western blot figure is one reprehensive experiment, and the bar graph is data from three independent experiments. * p < 0.05, ** p < 0.01, *** p < 0.0001.
Exposure to SFE (200 μg/mL) reduced NO concentration to 36.4 ± 7.4 μM, which is about 47% less than the positive control. In an effort to identify whether the reduction of NO was due to inhibition of the enzyme, iNOS expression was evaluated by Western blot. As shown in Fig. 2B, iNOS expression was reduced by 60% in RAW 264.7 cells that were pretreated with 200 μg/mL SFE and co-stimulated with LPS and IFNγ. The effects of ethanolic extract of S. frutescens on iNOS expression were consistent with the results of NO production and thus indicated that S. frutescens extract reduced LPS-induced NO production by diminishing the expression of iNOS.
3.3. Cytokines and chemokines production
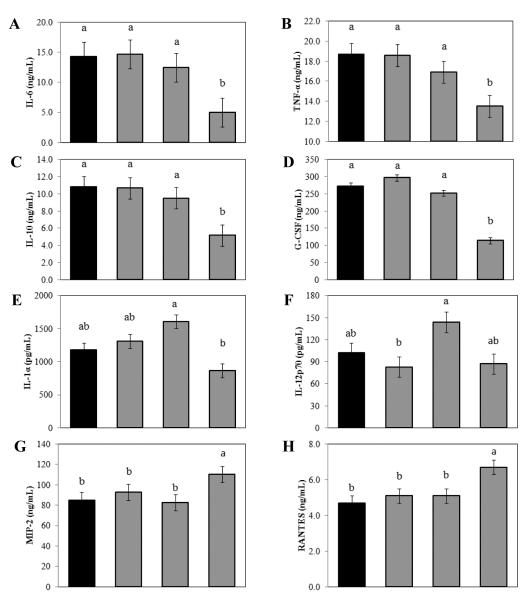
Treating RAW 264.7 cells with LPS/IFNγ resulted in the production of a number of cytokines and chemokines including IL-1α, TNF-α, IL-6, IL-10, IL-12p70, G-CSF, MIP-2, and RANTES (Fig. 3). Exposure to 200 μg/mL SFE significantly decreased the production of interleukin (IL)-6 and TNF-α by 65% and 28%, respectively. On the other hand, treatment with SFE at 200 μg/mL increased secretion of MIP-2 and RANTES. The SFE also modulated other inflammatory cytokines and chemokines, such as IL-1α, IL-10, and G-CSF but the changes were not dose-dependent. Treatment of stimulated primary murine macrophages with SFE had similar effects on the production of most cytokines as in LPS/IFNγ stimulated primary murine macrophages (Table 1).
Fig. 3. Ethanolic extract of S. frutescens (SFE) modulated the production of inflammatory cytokines and chemokines by murine macrophage cell line (i.e. RAW 264.7 cells).
Exposure to SFE (200μg/ml) significantly reduced the production of G-CSF, IL-6, TNF-α, and IL-10 (A to D). SFE increased the production of IL-1α, IL-12p70 at 25 or 50 μg/mL, however, the highest concentration of SFE reduced them to similar or less than that of the group without SFE (E and F). SFE significantly increased the production of MIP-2 and RANTES (G and H). Data are from three independent experiments, and are expressed as mean ± SME, and letters (a and b) are showing the difference among treatments based on the statistical analysis results.
Table1.
The impact of ethanolic extract of S. frutescens (SFE) on the production of cytokines and chemokines by LPS + IFNγ-stimulated primary murine macrophages (ng/ml)
| LPS+IFNγ | LPS+IFNγ+SFE25 | LPS+IFNγ+SFE50 | LPS+IFNγ+SFE200 | |
|---|---|---|---|---|
| IL-6 | 153 ± 12 | 185 ± 8 | 185 ± 31 | 106 ± 67 |
| TNF-α | 13.1 ± 1.0 | 15.6 ± 0.7 | 14.5 ± 2.1 | 10.7 ± 4.1 |
| IL-10 | 0.27 ± 0.09 | 0.26 ± 0.11 | 0.27 ± 0.08 | 0.29 ± 0.03 |
| G-CSF | 4.78 ± 0.86 | 8.09 ± 0.28 | 8.61 ± 1.95 | 5.73 ± 3.88 |
| IL-1α | 2.46 ± 0.28 | 1.85 ± 0.74 | 2.27 ± 0.34 | 1.46 ± 0.49* |
| IL-12p40 | 0.98 ± 0.16 | 0.58 ± 0.12** | 0.51 ± 0.14** | 0.27 ± 0.10*** |
| IL-12p70 | 0.91 ± 0.16 | 0.81 ± 0.30 | 0.69 ± 0.29 | 0.37 ± 0.11* |
| IL-9 | 2.62 ± 0.73 | 1.79 ± 0.52 | 2.23 ± 0.65 | 2.40 ± 1.29 |
| IL-13 | 128 ± 13 | 141 ± 29 | 123 ± 14 | 116 ± 25 |
| GM-CSF | 1.74 ± 0.49 | 1.86 ± 0.59 | 1.58 ± 0.88 | 1.21 ± 0.28 |
| KC | 61.7 ± 4.0 | 85.7 ± 13.7 | 96.1 ± 4.5 | 105.8 ± 40.7 |
| IP-10 | 1.58 ± 0.38 | 1.37 ± 0.31 | 1.75 ± 0.56 | 1.66 ± 1.44 |
| MCP-1 | 33.8 ± 11.0 | 34.5 ± 10.4 | 39.4 ± 11.3 | 30.1 ± 22.5 |
| MIP-1α | 25.8 ± 7.9 | 24.4 ± 18.5 | 36.0 ± 7.6 | 31.8 ± 16.1 |
| MIP-1b | 25.2 ± 4.4 | 25.8 ± 4.3 | 28.5 ± 4.7 | 28.1 ± 14.4 |
| MIP-2 | 144 ± 17 | 199 ± 29 | 223 ± 21 | 210 ± 106 |
| RANTES | 8.35 ± 1.39 | 7.56 ± 1.09 | 8.00 ± 1.13 | 6.16 ± 3.49 |
All the data were from three independent experiments.
p < 0.05,
p < 0.01,
p < 0.001.
GM-CSF, granulocyte-macrophage colony stimulating factor; IL, interleukin; IP, interferon gamma-induced protein; KC, chemokine (C-X-C motif) ligand 1, MCP, monocyte chemotactic protein, MIP, macrophage inflammatory protein, RANTES, regulated on activation, normal T cell expressed and secreted.
3.4. NF-κB activation
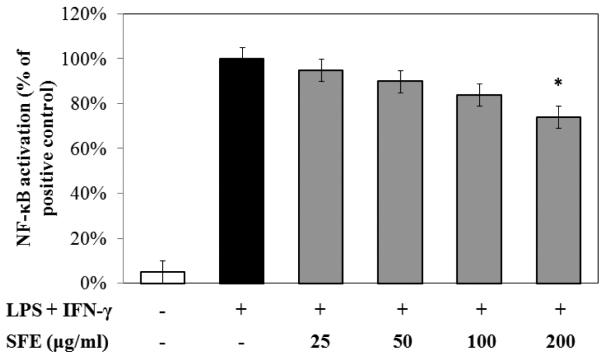
NF-κB has been regarded a major signaling pathway for mediating inflammatory responses. To evaluate the impact of SFE on NF-κB activation, RAW 267.4 cells containing a luciferase reporter for NF-κB were treated with SFE for 1 h, followed by 3 h stimulation with LPS and IFNγ, and cell lysates were analyzed using the luciferase assay protocol. Co-stimulation with LPS and IFNγ increased NF-κB activation by ~50-fold and exposure to SFE diminished the activation (Fig. 4). Nevertheless, with this protocol, significant reduction of 26% was only observed with SFE at 200 μg/mL.
Fig. 4. Ethanolic extract of S. frutescens (SFE) inhibited NF-κB activation in RAW 264.7 cells.
The NF-κB activity was measured using the luciferase reporter assay. SFE had modest reduction effect on NF-κB activation. The bars indicated the percentage of activated-cells treated with different concentration of SFE compared to that of cells stimulated with LPS/IFNγ without any Sutherlandia treatment (100%) to calculate the percentage. * p < 0.05.
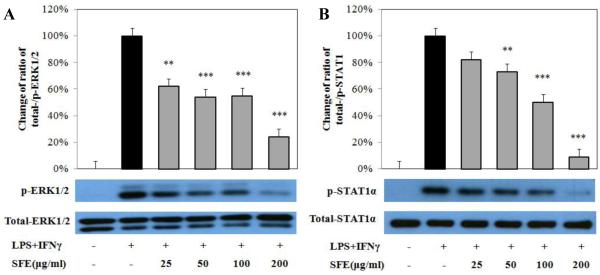
3.5. Phosphorylation of ERK1/2 and STAT1α
In addition to the NF-κB signaling pathway, we also investigated the effect of SFE on the MAP kinase (i.e. ERK1/2) and JAK-STAT1 signaling pathways. Exposure to SFE significantly reduced the phosphorylation of ERK1/2 activated by LPS and IFNγ up to 70% without changing the expression of total ERK1/2, and this reduction was observed in all doses used (25 to 200 μg/mL) (Fig. 5A). Similar to the effect on ERK1/2, SFE had no effect on expression of total STAT1α, however, SFE also significantly and dose-dependently inhibited the activation of STAT1α induced by LPS and IFNγ (Fig. 5B).
Fig. 5. Ethanolic extract of S. frutescens (SFE) inhibited the phosphorylation of ERK1/2 (A) and STAT1α (B) activated by LPS and IFNγ.
RAW 264.7 cells were pretreated with SFE for 1 h, followed by co-stimulation of LPS and IFNγ for 30 min (ERK1/2) or 60 min (STAT1α). The phosphorylated- and total-ERK1/2 and STAT1α was analyzed using Western blot. The Western blot band graphs showing a representative experiment of SFE pretreatment on the phosphorylation of ERK1/2 or STAT1α induced by LPS/IFNγ in RAW 264.7 cells. The bar graphs are showing the ratios of p-ERK1/2 or p-STAT1α to total ERK1/2 or STAT1α from four independent experiments using samples from co-stimulation of LPS and IFNγ as control (100%). Data are expressed as mean ± SD. ** P<0.01, *** P<0.001.
3.6. Effects of sutherlandiosides and sutherlandins on responses induced by co-stimulation with LPS/IFNγ
To identify the bioactive compound(s) in S. frutescens, various fractions prepared from S. frutescens were tested on their ability to inhibit LPS/IFNγ-induced production of NO and ROS as well as activation of NF-κB using the murine macrophage (i.e. RAW 264.7 cells). The LC-ELSD analysis determined that the ethanolic extract of S. frutescens contained about 1442 ± 95 μg/mL sutherlandioside B, which was the most abundant cycloartanol glycoside in this medicinal plant. The concentrations of sutherlandioside C and sutherlandioside D were about 400 μg/mL. There was no detectable sutherlandioside A in the ethanolic extract.
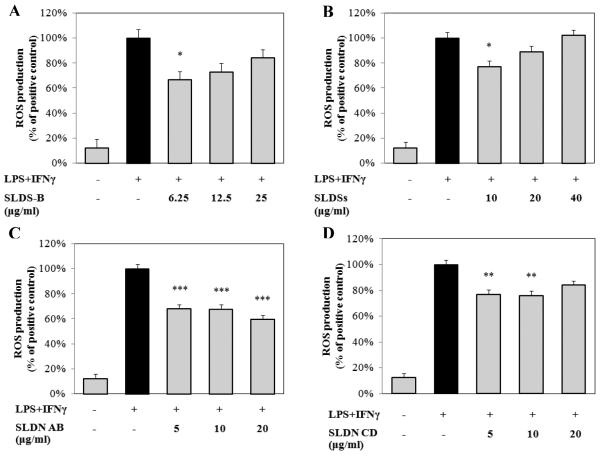
Since the crude SFE was most potent at reducing ROS generation we evaluated each of the fractions that contained one or more of the sutherlandiosides or sutherlandins for its impact on LPS/IFNγ-induced ROS production by RAW 264.7 cells. As shown in Fig. 6A and 6B, sutherlandiosides possessed modest amounts of ROS reducing activity (i.e., 25-30%). However, when either sutherlandioside-enriched fraction was used at higher concentrations, the bioactivity fell off and was no longer statistically different from the untreated cells. The concentrations of sutherlandiosides tested reflect those present in the same dilutions of the crude SFE that we evaluated in earlier experiments (see Fig. 1). Treatment of RAW 264.7 cells with sutherlandins A and B significantly reduced the ROS production by 30-40% (Fig. 6C). On the other hand, treatment with sutherlandins C and D had much less activity on ROS production compared to sutherlandins A and B (Fig. 6D).
Fig. 6. Compounds isolated from S. frutescens reduced the production of reactive oxygen species (ROS) by murine macrophage cell line (i.e. RAW 264.7).
Sutherlandioside B (SLDS-B)(A), combination of sutherlandiosides A + C + D (SLDSs)(B) , combination of sutherlandins A + B (SLDN AB)(C), and sutherlandins C + D (SLDN CD) (D)significantly decreased the production of ROS induced by LPS/IFNγ. Data were expressed as mean ± SME (n = 3). * p < 0.05, ** p < 0.01, *** p < 0.001.
While sutherlandioside B possessed some antioxidant activity (i.e., reduced ROS generation), these compounds failed to significantly affect LPS/IFNγ-induced NO production or NF-κB signaling in RAW 264.7 cells (data not shown). Similar results (i.e., no activity) were found when RAW 264.7 cells were treated with fractions enriched with sutherlandiosides A + C + D, sutherlandins A + B, or sutherlandins C + D (data not shown).
3.7. Anti-inflammatory activity of chlorophyll extracted from S. frutescens
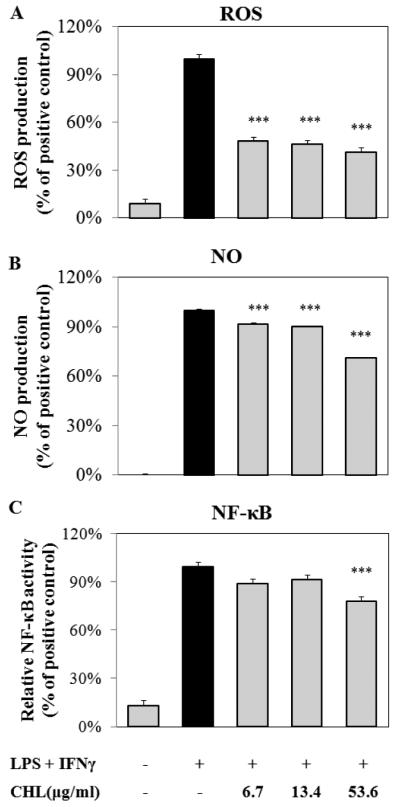
The ethanolic extract of S. frutescens displayed a dark and vibrant green color suggesting the presence of chlorophyll in this extract. In order to evaluate the role of chlorophyll on the bioactivity we observed with crude S. frutescens extracts, we isolated chlorophyll from S. frutescens by preparative HPLC. Our purified chlorophyll preparation was a mixture of chlorophyll a and chlorophyll b isoforms at a ratio of ~2:1. The concentrations of purified chlorophyll used in this experiment matched those present in the dilutions of the crude ethanolic extract of S. frutescens used elsewhere. Our results show that the chlorophyll in SFE potently reduced LPS/IFNγ-induced ROS production (Fig. 7A), however, this effect was not dose-dependent. Similar to SFE, the chlorophyll-enriched fraction isolated from S. frutescens had only a modest impact on NO biosynthesis (Fig. 7B) and NF-κB activation (Fig. 7C).
Fig. 7. Chlorophyll (CHL) isolated from S. frutescens exhibited anti-oxidant activity in murine macrophage cell line (i.e. RAW 264.7).
The highest concentration of chlorophyll was about 60 μM (based on the molecular weight of chlorophyll b). The LPS/IFNγ-induced ROS production (A) was significantly reduced by treatment of chlorophyll. Chlorophyll had modestly, but significantly, attenuated the production of NO (B) and activation of NF-κB (C). Data were from three independent experiments and were expressed as mean ± SME (n = 3). *** p < 0.001.
4. Discussion
In this study, we report that an ethanolic extract of S. frutescens (SFE) dose-dependently diminished the production of two key inflammatory mediators (i.e., ROS and NO), yet only had a modest impact on inflammatory cytokine (e.g., TNF-α and IL-6) production by murine macrophages. For much of this research we used a common murine macrophage cell line (i.e. RAW 264.7), while conducting a more limited set of experiments with macrophages isolated from healthy mice. Furthermore, we relied on a well-characterized method for inducing inflammation in macrophages (i.e., treatment with LPS and IFNγ). Overall, these experiments confirm and extend our previous findings with a murine microglia cell line (i.e., BV-2 cells) that SFE possesses both anti-oxidant and anti-inflammatory activities [8].
Another major objective of this research was to investigate the bioactivity of some of the novel compounds found in S. frutescens to better understand the immune-modulatory potential of this medicinal plant. Sutherlandiosides and sutherlandins have previously been identified as unique compounds found in S. frutescens, yet knowledge of their bioactivity is rudimentary [11, 27, 28]. In this study, we found that a sutherlandioside B-enriched fraction had no discernible impact on the production of NO or activation of NF-κB, yet it significantly reduced the production of ROS in RAW 264.7 cells co-stimulated with LPS and IFNγ. Similarly, a SFE fraction containing sutherlandiosides A, C, and D was without activity in our assay system. The sutherlandins had no impact on production of NO or activation of NF-κB, however, they were able to modestly reduce the production of ROS.
Not surprisingly the ethanolic extract of S. frutescens contained plant pigments, most notably chlorophylls. The in vivo and in vitro biological activities associated with chlorophyll has been the subject of considerable research [34]. In fact, others have reported that chlorophyllin, a synthetic, water-soluble form of chlorophyll, reduces the production of NO and the activation of NF-κB in LPS-stimulated RAW 264.7 cells [35]. Natural chlorophylls, however, exists as a group of structurally-related, fat-soluble compounds that possess a magnesium atom in the center of a tetrapyrrole structure with a phytol-side chain of variable length. During the production of chlorophyllin the magnesium atom in the porphyrin ring is replaced with a copper atom and the phytol tail is removed. While these changes make “chlorophyll” more stable and water-soluble, they also have a significant impact on its bioactivity. Since native chlorophylls are lipid-soluble, their absorption and distribution within cells is likely to be restricted to cell membranes. The uptake of natural chlorophylls into cells in culture is relatively inefficient relative to the water-soluble derivative, chlorophyllin [34]. Since much of what is known about the bioactivity of chlorophylls is based on studies that use chlorophyllin [36, 37], rather than native chlorophylls, we thought it was important for us to directly assess the contribution of these plant pigments in our extract. We found that native chlorophylls extracted directly from S. frutescens only modestly reduced the production of NO and NF-κB signaling in RAW 264.7 cells co-stimulated with LPS and IFNγ. In contrast, native chlorophyll diminished ROS production by ~50% independent of the dosage used to treat the cells. Whether this reduction of cellular ROS production was by direct quenching or modulation of the activity of NADPH oxidase was not determined in these experiments. Regardless, our results with native chlorophylls suggest that only some, but not all, anti-inflammatory activity in an ethanolic extract of this medicinal plant was mediated by this common plant pigment.
In this study we observed that the ethanolic extract of S. frutescens and some of the bioactive compounds isolated from this plant showed greater impact on the production of ROS than on NO or cytokine production. We believe that our research into some of the various signaling pathways provided some insight into the possible mechanism(s) for the anti-inflammatory activity of SFE. Upon LPS and IFNγ co-stimulation of macrophages, several protein kinases, such as protein kinase C, ERK, and p38, are activated. These activated kinases phosphorylate subunits of NADPH oxidase, such as p47phox [38]. The activated subunits are recruited to the membrane resulting in the activation of NADPH oxidase to produce ROS for host defense [18]. We demonstrated that the phosphorylation of ERK was strongly reduced by treatment with SFE, and that this reduction seemed to correlate with the impact of SFE on ROS production. Stimulation with LPS and/or IFNγ also activates the NF-κB pathway, which induces the expression of iNOS to produce NO [23]. The modest effect of SFE (and chlorophyll) on the activation of NF-κB was consistent with the limited impact they had on macrophage NO production. We believe that additional experiments with alternative bioactives isolated from S. frutescens would improve our understanding of how this medicinal plant impacts human health.
There are a few limitations in the present study that are worth noting. First, in this study we only investigated the bioactivity of a limited number of constituents found in S. frutescens. Our rationale for this was based on the fact that both the sutherlandiosides and sutherlandins are thought to be unique to this medicinal plant and also that these compounds were found in relatively high quantities such that they are being consider useful biomarkers for S. frutescens. In fact, to our knowledge this is the first research report describing biological activity for these unique botanical compounds on innate immune cells. However, this investigation was limited to macrophage-mediated production of ROS, NO, and cytokines. It is entirely possible that these compounds exhibit important biological activities other than those examined here and in cells other than macrophages. For example, collaborators have recently reported dose- and time-dependent growth inhibition in human prostate cancer cells from a methanolic extract from this botanical [39]. While others have recently reported that a S. frutescens extract down-regulated PI-3 kinase and Akt phosphorylation in human colon cancer cells [40]. Finally, some might find our investigation into the role that chlorophyll played in the in vitro antioxidant and anti-inflammatory activities of this plant extract to be limited in scope. This was purposeful, because our sole interest in chlorophyll was to account for its contribution to the immune modulating activities we were investigating in our crude botanical extract. We believe we did just that and that we can conclude that there exists some biological activity present in the SFE independent of the native chlorophylls present.
5. Conclusions
In conclusion, an ethanolic extract of S. frutescens (SFE) had a significant and dose-dependent inhibitory effect on select inflammatory responses by a murine macrophage cell line and in primary murine macrophages. We demonstrated that this anti-inflammatory activity was associated with diminished activation of ERK, STAT1α and NF-κB dependent signaling pathways. Furthermore, we found that neither sutherlandiosides nor sutherlandins accounted for this anti-inflammatory activity. Additional research is needed to identify and characterize the bioactive compound(s), other than chlorophyll, that are responsible for anti-inflammatory activity observed in SFE.
Highlights.
Extracts of S. frutescens (SFE) reduce macrophage production of ROS and NO
SFE alters NF-κB, ERK1/2, and JAK-STAT1 signaling pathways in macrophages
Sutherlandiosides and sutherlandins were not responsible for these effects
Native chlorophyll were partly responsible for the bioactivity in this extract
Acknowledgements
This publication and project was made possible by Grant Number P50AT006273 from the National Center for Complementary and Integrative Health (NCCIH), the Office of Dietary Supplements (ODS), and the National Cancer Institute (NCI) and the support of the University of Missouri’s College of Agriculture, Food, and Natural Resources, and the Food-for-the-21st Century Program. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCCIH, ODS, or NCI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].van Wyk BE, Albrecht C. A review of the taxonomy, ethnobotany, chemistry and pharmacology of Sutherlandia frutescens (Fabaceae) J Ethnopharmacol. 2008;119:620–9. doi: 10.1016/j.jep.2008.08.003. [DOI] [PubMed] [Google Scholar]
- [2].Ojewole JA. Anticonvulsant property of Sutherlandia frutescens R. BR. (variety Incana E. MEY.) [Fabaceae] shoot aqueous extract. Brain Res Bull. 2008;75:126–32. doi: 10.1016/j.brainresbull.2007.08.002. [DOI] [PubMed] [Google Scholar]
- [3].Fernandes AC, Cromarty AD, Albrecht C, van Rensburg CE. The antioxidant potential of Sutherlandia frutescens. J Ethnopharmacol. 2004;95:1–5. doi: 10.1016/j.jep.2004.05.024. [DOI] [PubMed] [Google Scholar]
- [4].Katerere DR, Eloff JN. Antibacterial and antioxidant activity of Sutherlandia frutescens (Fabaceae), a reputed anti-HIV/AIDS phytomedicine. Phytother Res: PTR. 2005;19:779–81. doi: 10.1002/ptr.1719. [DOI] [PubMed] [Google Scholar]
- [5].Chen YC. Bioactivities of selected Sutherlandia frutescens (L.) R. BR. leaf extracts. University of Missouri-Columbia; 2007. Master's Degree Thesis. [Google Scholar]
- [6].Tobwala S, Fan W, Hines CJ, Folk WR, Ercal N. Antioxidant potential of Sutherlandia frutescens and its protective effects against oxidative stress in various cell cultures. BMC Complement Altern Med. 2014;14:271. doi: 10.1186/1472-6882-14-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kundu JK, Mossanda KS, Na HK, Surh YJ. Inhibitory effects of the extracts of Sutherlandia frutescens (L.) R. Br. and Harpagophytum procumbens DC. on phorbol ester-induced COX-2 expression in mouse skin: AP-1 and CREB as potential upstream targets. Cancer Lett. 2005;218:21–31. doi: 10.1016/j.canlet.2004.07.029. [DOI] [PubMed] [Google Scholar]
- [8].Jiang J, Chuang DY, Zong Y, Patel J, Brownstein K, Lei W, et al. Sutherlandia frutescens ethanol extracts inhibit oxidative stress and inflammatory responses in neurons and microglial cells. PloS One. 2014;9:e89748. doi: 10.1371/journal.pone.0089748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Tai J, Cheung S, Chan E, Hasman D. In vitro culture studies of Sutherlandia frutescens on human tumor cell lines. J Ethnopharmacol. 2004;93:9–19. doi: 10.1016/j.jep.2004.02.028. [DOI] [PubMed] [Google Scholar]
- [10].Faleschini MT, Myer MS, Harding N, Fouchè G. Chemical profiling with cytokine stimulating investigations of Sutherlandia frutescens L. R. (Br.) (Fabaceae) S Afr J Bot. 2013;85:48–55. [Google Scholar]
- [11].Fu X, Li XC, Smillie TJ, Carvalho P, Mabusela W, Syce J, Johnson Q, Folk W, Avery MA, Khan IA. Cycloartane glycosides from Sutherlandia frutescens. J Nat Prod. 2008;71:1749–53. doi: 10.1021/np800328r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Muller AC, Patnala S, Kis O, Bendayan R, Kanfer I. Interactions between phytochemical components of Sutherlandia frutescens and the antiretroviral, atazanavir in vitro: implications for absorption and metabolism. J pharm Pharm Sci. 2012;15:221–33. doi: 10.18433/j3ns3x. [DOI] [PubMed] [Google Scholar]
- [13].Prevoo D, Swart P, Swart AC. The influence of Sutherlandia frutescens on adrenal steroidogenic cytochrome P450 enzymes. J Ethnopharm. 2008;118:118–26. doi: 10.1016/j.jep.2008.03.019. [DOI] [PubMed] [Google Scholar]
- [14].Gordon S. Alternative activation of macrophages. Nat Rev Immuno. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- [15].Schroder K, Sweet MJ, Hume DA. Signal integration between IFN gamma and TLR signalling pathways in macrophages. Immunobiology. 2006;211:511–24. doi: 10.1016/j.imbio.2006.05.007. [DOI] [PubMed] [Google Scholar]
- [16].An HJ, Kim IT, Park HJ, Kim HM, Choi JH, Lee KT. Tormentic acid, a triterpenoid saponin, isolated from Rosa rugosa, inhibited LPS-induced iNOS, COX-2, and TNF-alpha expression through inactivation of the nuclear factorkappab pathway in RAW 264.7 macrophages. Int Immunopharmaco. 2011;11:504–10. doi: 10.1016/j.intimp.2011.01.002. [DOI] [PubMed] [Google Scholar]
- [17].Nauseef WM. Assembly of the phagocyte NADPH oxidase. Histochem Cell Biol. 2004;122:277–91. doi: 10.1007/s00418-004-0679-8. [DOI] [PubMed] [Google Scholar]
- [18].Lam GY, Huang J, Brumell JH. The many roles of NOX2 NADPH oxidase-derived ROS in immunity. Semin Immunopathol. 2010;32:415–30. doi: 10.1007/s00281-010-0221-0. [DOI] [PubMed] [Google Scholar]
- [19].Chan ED, Riches DW. IFN-gamma + LPS induction of iNOS is modulated by ERK, JNK/SAPK, and p38(mapk) in a mouse macrophage cell line. Am J Physiol-Cell Ph. 2001;280:C441–50. doi: 10.1152/ajpcell.2001.280.3.C441. [DOI] [PubMed] [Google Scholar]
- [20].Hallman M, Ramet M, Ezekowitz RA. Toll-like receptors as sensors of pathogens. Pediatr Res. 2001;50:315–21. doi: 10.1203/00006450-200109000-00004. [DOI] [PubMed] [Google Scholar]
- [21].Honda K, Taniguchi T. IRFs: master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors. Nat Rev Immunol. 2006;6:644–58. doi: 10.1038/nri1900. [DOI] [PubMed] [Google Scholar]
- [22].Xiang M, Namani A, Wu S, Wang X. Nrf2: bane or blessing in cancer? Journal of Cancer Research and Clinical Oncology. 2014;140:1251–9. doi: 10.1007/s00432-014-1627-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Aktan F. iNOS-mediated nitric oxide production and its regulation. Life sciences. 2004;75:639–53. doi: 10.1016/j.lfs.2003.10.042. [DOI] [PubMed] [Google Scholar]
- [24].Coleman JW. Nitric oxide in immunity and inflammation. Int Immunopharmacol. 2001;1:1397–406. doi: 10.1016/s1567-5769(01)00086-8. [DOI] [PubMed] [Google Scholar]
- [25].Lei W, Browning J, Jr., Eichen PA, Lu CH, Mossine VV, Rottinghaus GE, et al. Immuno-stimulatory activity of a polysaccharide-enriched fraction of Sutherlandia frutescens occurs by the toll-like receptor-4 signaling pathway. J Ethnopharmacol. 2015;172:247–53. doi: 10.1016/j.jep.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Brownstein KJ, Rottinghaus GE, Knight M, Ito Y, Folk W. Isolation of Sutherlandioside B from by Spiral Countercurrent Chromatography. J Liq Chromatogr Related Technol. 2015;38:423–9. doi: 10.1080/10826076.2014.913518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Fu X, Li XC, Wang YH, Avula B, Smillie TJ, Mabusela W, et al. Flavonol glycosides from the south African medicinal plant Sutherlandia frutescens. Planta Med. 2010;76:178–81. doi: 10.1055/s-0029-1186030. [DOI] [PubMed] [Google Scholar]
- [28].Avula B, Wang YH, Smillie TJ, Fu X, Li XC, Mabusela W, et al. Quantitative determination of flavonoids and cycloartanol glycosides from aerial parts of Sutherlandia frutescens (L.) R. BR. by using LC-UV/ELSD methods and confirmation by using LC-MS method. J Pharmaceut Biomed. 2010;52:173–80. doi: 10.1016/j.jpba.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lichtenthaler HK, Kuhn G, Prenzel U, Meier D. Chlorophyll-protein levels and degree of thylakoid stacking in radish chloroplasts from high-light, low-light and bentazon-treated plants. Physiol Plant. 1982;56:183–8. [Google Scholar]
- [30].Bidigare RR, Kennicutt MCI, Keeney-Kennicutt WL. Isolation and purification of chlorophylls a and b for the determination of stable carbon and nitrogen isotope compositions. Anal Chem. 1991;63:130–3. [Google Scholar]
- [31].Mossine VV, Waters JK, Hannink M, Mawhinney TP. piggyBac transposon plus insulators overcome epigenetic silencing to provide for stable signaling pathway reporter cell lines. PloS one. 2013;8:e85494. doi: 10.1371/journal.pone.0085494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Feng C, Keisler DH, Fritsche KL. Dietary omega-3 polyunsaturated fatty acids reduce IFN-gamma receptor expression in mice. J Interferon Cytokine Res. 1999;19:41–8. doi: 10.1089/107999099314405. [DOI] [PubMed] [Google Scholar]
- [33].Wang J, Mazza G. Inhibitory effects of aAnthocyanins and other phenolic compounds on nitric oxide production in LPS/IFN-Ɣ-activated RAW 264.7 macrophages. J Agric Food Chem. 2002;50:850–7. doi: 10.1021/jf010976a. [DOI] [PubMed] [Google Scholar]
- [34].Ferruzzi MG, Blakeslee J. Digestion, absorption, and cancer preventative activity of dietary chlorophyll derivatives. Nutr Res. 2007;27:1–12. [Google Scholar]
- [35].Kamat JP, Boloor KK, Devasagayam TP. Chlorophyllin as an effective antioxidant against membrane damage in vitro and ex vivo. Biochim Biophys Acta. 2000;1487:113–27. doi: 10.1016/s1388-1981(00)00088-3. [DOI] [PubMed] [Google Scholar]
- [36].Sharma D, Kumar SS, Sainis KB. Antiapoptotic and immunomodulatory effects of chlorophyllin. Mol Immunol. 2007;44:347–59. doi: 10.1016/j.molimm.2006.02.031. [DOI] [PubMed] [Google Scholar]
- [37].Cho KJ, Han SH, Kim BY, Hwang SG, Park KK, Yang KH, et al. Chlorophyllin suppression of lipopolysaccharide-induced nitric oxide production in RAW 264.7 cells. Toxicol Appl Pharmacol. 2000;166:120–7. doi: 10.1006/taap.2000.8958. [DOI] [PubMed] [Google Scholar]
- [38].Bae YS, Oh H, Rhee SG, Yoo YD. Regulation of reactive oxygen species generation in cell signaling. Mol Cells. 2011;32:491–509. doi: 10.1007/s10059-011-0276-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Lin H, Jackson GA, Lu Y, Drenkhahn SK, Brownstein KJ, Starkey NJ, et al. Inhibition of Gli/hedgehog signaling in prostate cancer cells by "cancer bush" [Sutherlandia frutescens extract. Cell Biol Int. 2015 doi: 10.1002/cbin.10544. doi: 10.1002/cbin.10544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Leisching G, Loos B, Nell T, Engelbrecht A-M. Sutherlandia frutescens treatment induces apoptosis and modulates the PI3-kinase pathway in colon cancer cells. SAMJ. 2015;100:20–6. [Google Scholar]