Abstract
Microbes play an important role in human health and disease. In the setting of heart failure (HF), substantial hemodynamic changes such as hypoperfusion and congestion in the intestines can alter gut morphology, permeability, function, and possibly the growth and composition of gut microbiota. These changes can disrupt the barrier function of the intestines, and exacerbate systemic inflammation through microbial or endotoxin translocation into systemic circulation. Furthermore, cardio-renal alterations via metabolites derived from gut microbiota can potentially mediate or modulate HF pathophysiology. Recently, trimethylamine N-oxide (TMAO) has emerged as a key mediator which provides mechanistic link between gut microbiota and multiple cardiovascular diseases, including HF. Potential intervention strategies which may target this microbiota-driven pathology include dietary modification, prebiotics or probiotics, and selective binders of microbial enzymes or molecules – yet further investigations into their safety and efficacy are warranted.
Keywords: microbiome, TMAO, heart failure
Introduction
There is a growing literature to support a role of the gut in the pathogenesis of heart failure (HF) in what is often referred to as the “gut hypothesis of heart failure.” The gut hypothesis implies that decreased cardiac output and redistribution of systemic circulation can lead to a decrease in intestinal perfusion, muscoal ischemia, and ultimately a disrupted intestinal mucosa. This disruption in intestinal barrier function in turn can lead to increased gut permeability, increased bacterial translocation and increased circulating endotoxins that can contribute to the underlying inflammation seen in patients with HF. With new insights into the role of gut microbiota in health and disease, the contribution of gut microbiota and its metabolites have broadened the scope of the gut hypothesis. Herein, we will summarize the evidence regarding the intricate interaction between intestine and heart in the setting of HF, as well as the association of gut microbiota with HF. We will further discuss some key molecules derived from gut microbiota and potential pathogenic mechanisms that may be relevant to HF. Although approaches to modulating gut microbiota to treat HF are not yet established, possible intervention strategies aimed at these novel potential therapeutic targets are currently under active investigation.
Gut Microbiota in Health and Disease
There are approximately 1014 bacterial organisms belonging to more than 2,000 species within our bodies, the vast majority being in the gut [1]. These are commensal microorganisms that colonize in the human gut and play a crucial role in protection from environmental exposure, digestion and absorption of nutrients [2–4]. The phylogenetic composition of the bacterial communities evolves towards an adult-like configuration during the first few years of life. The shaping of gut microbiota is largely influenced by lifestyle factors and/or environmental exposure rather than by inherited genetic factors [5].
There is increasing evidence that the nature of the microbiome plays an important role in human health and disease. Recent development of high-throughput assays such as next-generation sequencing analysis of bacterial 16S ribosomal DNA has made it possible to explore the composition of huge numbers of diverse microbiota in the gut in animals and humans [5–8]. Numerous studies have shown alterations of gut microbiota in a variety of conditions or diseases such as obesity [7, 9], fatty liver, insulin resistance [10], diabetes mellitus [8], and hypertension [11]. Experiments in fecal transplantation from conventionally-raised animals to germ-free or microbiota-ablated ones have shown that these lifestyle alterations contribute, at least in part, to the pathogenesis of these diseases [7–11].
Gut Microbiota and Heart Failure
Alteration of Gut and Gut Microbiota in Heart Failure
The gut is a blood-demanding organ, and villi (and microvilli) are prone to functional ischemia due to reduced blood flow [12]. The arteries form dense capillary networks close to the top of the villi. This anatomical arrangement allows countercurrent exchange of oxygen from the arteries to the veins along their course within the villus. This results in a descending gradient of tissue oxygen concentration from the base to the tip of the villus. This gradient is inversely related to blood flow [12], hence it is directly influenced by alterations in perfusion in the context of HF. In patients with HF, intestinal ischemia can be demonstrated by a decrease in intestinal mucosal pH [13] or diminished passive carrier-mediated transport of D-xylose [14]. Possibly as a result of intestinal ischemia [12, 15] and congestion [16], the morphology, permeability, and function of the intestines are substantially altered in HF, especially in advanced stages with cardiac cachexia [14, 17, 18]. In fact, the increase in bowel wall thickness due to edema can be directly visualized in patients with HF [17].
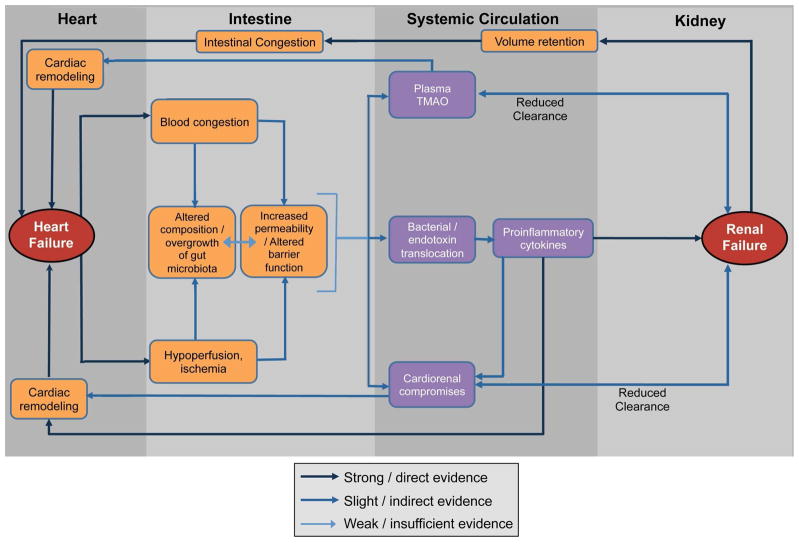
The mechanistic links between gut microbiota and HF are becoming better and established. While evidence is still accruing, higher concentrations of adherent bacteria have been identified in the intestinal mucosal biofilm of patients with HF compared with control subjects [17]. As gut luminal hypoxia, hypercapnia, changes in local pH, redox state, and norepinephrine are all known to be potent activators of bacterial virulence in microbiota [19], the composition of intestinal microbiota may shift rapidly during intestinal ischemia and reperfusion [20] or following an increase in portal vein pressure [21]. Hypoperfusion and congestion in intestine due to reduced cardiac output can further disrupt the barrier function of the intestine and can promote systemic inflammation through bacterial translocation, potentially leading to further HF exacerbations (Figure). However, major changes in the gut microbial composition have not been observed in animal models of HF, such as one induced by coronary artery ligation in the rat [22]. In this regard, there is a paucity of data regarding altered gut microbial composition that is unique to human HF.
Systemic Inflammation Caused by Bacterial Translocation
Even though the levels of circulating pro-inflammatory cytokines such as tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6), and C-reactive protein are elevated in HF [23–28], trials that targeted these cytokines in patients with HF failed to show benefit on cardiac function or prognosis [29]. Alternatively, it has been proposed that endotoxins may be an important stimulus for cytokine production in patients with HF through their action on mononuclear cells [24]. Bacteria or bacterial endotoxins such as lipopolysaccharide (LPS) can enter the mesenteric lymph nodes or the systemic circulation through the intestine if its barrier function is impaired, as it is the case in various diseases such as multiple organ failure, sepsis, liver cirrhosis, ischemia reperfusion, or burn injury [30–33]. The mechanism which leads to bacterial translocation from the gut to systemic circulation in the setting of HF can be explained by impaired host defense [34] as well as alterations in gut microbiota and intestinal barrier function that can be induced by hemodynamic changes in the intestines [15, 20, 21, 35].
There is evidence that bacteria or endotoxins are translocated into systemic circulation in humans in the absence of systemic or local infections. Indeed, bacterial DNA can be commonly detected in systemic circulation in the general population [36, 37], although the origins of these bacterial DNA remain unknown. Higher concentrations of bacterial DNA have been quantified in patients with heart disease when compared to healthy subjects, and even the compositions of the bacteria were different between these groups [38]. It has therefore been proposed that the concentration of bacterial DNA and its composition had a considerable impact on the onset of cardiovascular events [37]. In fact, levels of endotoxin and inflammatory markers (IL-6 and TNF-α, and sTNF-R1) increased during acute cardiac decompensation while endotoxin levels decreased after stabilization [14, 39, 40]. Furthermore, endotoxin levels were higher in the hepatic veins compared to those measured in the left ventricle (LV) or pulmonary artery, suggesting possible endotoxin translocation from the gut into the circulation [40]. Moreover, selective eradication of intestinal aerobic gram-negative bacilli with enteral non-absorbable polymyxin B has resulted in a decrease in fecal endotoxin concentrations, monocyte production of some pro-inflammatory cytokines in patients with HF, and improved flow-mediated dilation, a marker of endothelial function [41]. These findings provide proof of concept that the gut microbiota can affect the systemic inflammatory response in patients with HF, even though this may not translate into substantial changes in circulating endotoxin or pro-inflammatory cytokine levels [41].
Cardiorenal Compromise Derived from Gut Microbiota
Besides endotoxins, metabolites derived from metabolism of dietary nutrients by gut microbiota have been proposed to exert endocrine effects and promote host disease processes. A broad mass-spectrometry based metabolomics study demonstrated a surprisingly large effect of the gut microbiome on blood metabolites, including indole derivatives such as indoxyl sulfate and phenyl derivatives such as p-cresol sulfate by comparing plasma extracts from germ-free and conventional mice [42]. These molecules, often referred to as “uremic toxins”, can originate from microbial metabolism and are retained in the bodies of patients with chronic kidney disease (CKD) [43]. Among them, indoxyl sulfate is a metabolite derived from dietary tryptophan [42] and is associated with the progression of renal dysfunction as well as vascular pathology by oxidative stress-induced modification of pro-inflammatory mediators [44]. Elevated systemic levels of indoxyl sulfate have been associated with greater risk of cardiovascular events and mortality in patients with CKD [45]. In vitro studies have also demonstrated that indoxyl sulfate has a direct effect on cardiomyocytes (inducing hypertrophy) [46–48] and cardiac fibroblasts (inducing collagen synthesis) [46]. In mice, administration of indoxyl sulfate can induce the development of LV hypertrophy without affecting blood pressure or renal function [47], corresponding to the association between higher levels of indoxyl sulfate and LV mass index in patients with CKD [47].
Contributions of Gut Microbiota-derived Metabolites: Trimethylamine N-oxide (TMAO)
The concept that gut microbiota contribute to the pathogenesis and disease progression of cardiovascular diseases has been supported by the recent discovery of dietary-induced, gut microbiota-derived metabolites identified in high fasting plasma levels from patients experiencing incident major adverse cardiac events compared to those without – namely choline, betaine, and trimethylamine N-oxide (TMAO) [49]. Choline is a necessary nutrient to synthesize phospholipids in the cell membrane, methyl group metabolism and synthesis of the neurotransmitter acetylcholine. What has not been appreciated until now is that gut microbiota can convert the choline moiety of dietary phosphatidylcholine into trimethylamine as substrate for metabolism, which is subsequently absorbed into the human host and converted into TMAO by hepatic flavin-containing monooxygenases (FMO) [52]. Indeed, changes in bacterial composition have been shown to serve as a primary driver of TMAO levels [53]. Circulating TMAO levels are greatly influenced by dietary intake of foods that are high in substrates of TMAO production, such as phosphatidylcholine or L-carnitine. In animal models, dietary supplementation with choline (as substrate) or TMAO promoted atherosclerosis in ApoE-null mice, a process that was ameliorated when gut microbiota were acutely suppressed by broad-spectrum antibiotics (i.e. no gut microbiota-associated TMAO production) in animals [49], as well as in humans [54]. Furthermore, plasma TMAO levels were positively related to the number of coronary vessels with significant atherosclerotic stenosis [49]. Also, increased plasma TMAO levels were associated with an increased risk of major adverse cardiovascular events [54, 55]
TMAO also serves as a key driver of CKD progression. The Framingham Heart Study, which included 1,434 participants without CKD at baseline, revealed that choline and TMAO levels were independent predictors of future CKD [56]. Mechanistically, choline or TMAO fed mice showed increased fibrosis in the kidneys and elevation of renal injury markers such as kidney injury molecule-1 and cystatin-C [55]. In humans, TMAO levels were elevated in plasma from patients with CKD [55, 57] and end stage renal failure with hemodialysis [58]. Interestingly, in the HF population the TMAO level was also positively related to the severity of renal impairment [59, 60].
Recently, TMAO levels were found to be higher in subjects with HF compared to non-HF counterparts [59]. This has been replicated in other cohorts, whereby higher TMAO levels portend increased risk of HF hospitalization [61]. Choline, betaine and TMAO levels were correlated with B-type natriuretic peptide [59], echocardiographic indices of diastolic function (such as E/Ea and left atrial volume index) [60] but not systolic function [59, 60]. An increase in TMAO levels was associated with increased mortality risk after adjustment for traditional cardiac risk factors and cardiorenal indices [59]. Notably, whereas all 3 measures (choline, betaine, and TMAO) were predictive of the composite endpoint of all-cause mortality plus cardiac transplantation, only the prognostic value of TMAO remained statistically significant after adjustments for traditional cardiac risk factors and cardiorenal indices [60]. Elevated TMAO levels have also shown equivalent adverse prognostic value both in patients with ischemic and non-ischemic etiologies, notwithstanding that TMAO has an atherogenic effect [59]. Thus, it can be speculated that TMAO might have a direct deleterious effect on the heart independent of its atherogenic effect, although the detailed mechanism has not been elucidated.
The mechanisms contributing to the rise in TMAO in the setting of HF are likely multifactorial. Clearly, renal impairment itself can lead to accumulation of TMAO via decrease in clearance (Figure), even though patients with preserved renal function may exhibit elevated TMAO levels. In animal studies involving choline-fed mice, there was a direct impact of elevated TMAO levels on myocardial fibrosis and activation of associated profibrotic pathways as a consequence of gut microbiota metabolism [55]. It is also conceivable that TMAO promotes microvascular disease in heart tissue in patients with HF even without clinically evident coronary disease. Alternatively, direct infusion of TMAO appears to augment angiotensin-II mediated hypertensive effects beyond the infusion period, suggesting a synergistic adverse effect on neurohormonal derangements [62]. Bacterial overgrowth or changes in the composition of gut microbiota in patients with HF due to changes in the microenvironment [19] or possibly intestinal ischemia [20] may explain this phenomenon, since TMAO levels can be altered with changes in bacterial composition [53]. Increased production (from increased dietary sources or microbial/host enzymes) or reduced clearance (because of renal insufficiency) may also contribute to these effects. Since stronger correlations were observed between all 3 metabolites and LV diastolic functional indices rather than systolic function [59, 60], these metabolites may influence the steepness of the pressure-volume fitting curves.
In summary, TMAO, a gut-microbiota derived metabolite, is associated with HF disease severity and may play a crucial role in its pathogenesis through multifaceted effects on cardiovascular pathology and renal injury. Furthermore, TMAO may provide a previously under-recognized mechanistic link between the intestines and cardio-renal pathophysiology.
Potential Intervention Strategies
Therapeutic tools available to modulate the microbiota-driven pathogenesis of HF remain limited. However, based on evidence that mechanistically links gut microbiota and HF, potential intervention strategies include targeting the composition of the microbiota or the biochemical pathways (Table). The composition of the microbiota can be modulated by diet [63], antibiotics, prebiotics/probiotics, or fecal transplantation. The biochemical pathways involved in microbiota-driven pathology can be modulated through the binder of the culprit molecule or pharmacologic intervention targeting host/bacterial metabolism (e.g., inhibition of FMO to prevent TMAO production) [52]. A mucosal barrier protector which prevents gut microflora translocation restoring the functionality and physiological permeability of the intestinal barrier [64, 65] may also be a therapeutic option.
Table.
Potential intervention strategies which can modulate gut microbiota–driven pathogenesis in heart failure
| Strategies | Target | Supporting evidence | ||
|---|---|---|---|---|
| animals | humans | Comments | ||
| Diet modulation | The abundance/composition of gut microbiota | (−) | (−) | No evidence at present |
| Antibiotics | The abundance/composition of gut microbiota | (+) | (±) | Oral administration of vancomycin presented a reduction of myocardial infarct size in rat ischemia reperfusion model [69]. Oral administration of polymyxin B decreased monocyte production of some pro-inflammatory cytokines in patients with HF and improved flow-mediated dilation [41]. |
| Prebiotics/probiotics | The abundance/composition of gut microbiota | (+) | (±) |
Lactobacillus plantarum led to the attenuation of ischemia-reperfusion injury in rats [69]. Lactobacillus rhamnosus (GR-1) attenuated LV hypertrophy and improved LVEF in a rat myocardial infarction model [22]. In a randomized controlled pilot study Saccharomyces boulardii improved LVEF and decreased serum creatinine and C-reactive protein in HF patients [71]. |
| Binders of key mediators | Uremic toxins derived from gut microbiota (AST-120) | (+) | (−) | AST-120 prevented progression of LV hypertrophy and cardiac fibrosis in rats with CKD [72] and CKD+HF [73] |
| TMAO | (−) | (−) | No evidence at present | |
Antibiotic Therapy
Although an association between infectious organisms and atherosclerosis has previously been postulated, randomized controlled studies have failed to show a benefit of antibiotic therapy for secondary prevention of cardiovascular events [66, 67]. However, antibiotics can influence the microbiota-driven pathophysiology by changing the abundance or composition of the microbiota community in the gut. In patients with alcohol-related cirrhosis, treatment with rifaximin, a non-absorbable oral antibiotic, resulted in increases in glomerular filtration rate and natriuresis, while reducing plasma endotoxin, IL-6 and TNF-α levels [68]. Rats treated with vancomycin presented a reduction of myocardial infarct size in an ischemia reperfusion model [69]. Interestingly, direct administration of vancomycin into the coronary circulation had no effect on severity of myocardial infarction. Also, the oral antibiotic polymyxin B decreased monocyte production of some pro-inflammatory cytokines in patients with HF and improved flow-mediated dilation, a marker of endothelial function [41]. These findings provide a proof of concept that modulation of gut microbiota can remotely affect the pathophysiology of various diseases including HF. However, we also need to recognize the potential unfavorable effects of antibiotics, such as microbial substitution and generation of antibiotic-resistant bacteria, as commonly seen in clinical practice. Therefore, this strategy is still challenging, and careful consideration to its application will be required to minimize the side effects of the antibiotics agents. Further investigations are needed to determine if judicious administration of antibiotics in specific circumstances can favorably affect cardiac function or clinical outcomes in the HF population.
Prebiotic and Probiotic Therapy
Prebiotics are a fermented ingredient that induces the growth or activity of microorganisms, and probiotics are generally live microbial feed supplements that benefit the host animal by improving its intestinal microbial balance [70]. It has been shown that many kinds of metabolite production, including TMAO, can be altered by probiotic modulation in germ-free mice colonized with human flora [53]. An accumulating body of evidence has demonstrated that intervention to microbiota by a probiotic product can favorably affect cardiac morphology and function in an animal model. Treatment with probiotics that contain the leptin-suppressing bacteria Lactobacillus plantarum led to the attenuation of ischemia-reperfusion injury in rats [69]. In addition, in a rat myocardial infarction model, probiotic administration (Lactobacillus rhamnosus GR-1) attenuated LV hypertrophy and improved LV ejection fraction (LVEF) [22]. Interestingly, there were no major changes in gut microbial composition between the GR-1 and control treatment groups, suggesting that the GR-1 strain did not colonize and that this is not a prerequisite for its beneficial effect. Recently, a randomized controlled pilot study showed probiotic therapy with Saccharomyces boulardii was beneficial for patients with HF, as evidenced by improvement of cardiac systolic function (LVEF) and decreases in serum creatinine and C-reactive protein during short term follow-up [71]. Whether probiotic intervention strategies can yield long-term benefits remains to be determined.
Binders of Key Mediators
Agents that target the key mediators promoting the pathogenesis of disease are intriguing. The removal of such gut-microbiota derived metabolites (e.g., TMAO) or their precursors (e.g., TMA) from the gut by oral administration of specific oral non-absorbant binders could be a promising intervention targeting gut microbiota-driven pathogenesis. Oral charcoal adsorbent (AST-120) has been clinically used to remove uremic toxins such as indoxyl sulfate for patients with advanced renal failure. It has been shown that AST-120 also prevents progression of LV hypertrophy and cardiac fibrosis in rats with CKD [72] and in a combination model of CKD+HF [73] without affecting blood pressure. However, the efficacy of these intervention strategies has not yet been demonstrated in human HF.
Conclusion
Millions of years of co–evolution between humans and microorganisms have led to a mutualistic relationship, in which diverse ecosystems of gut microbiota contribute to the maintenance of our metabolic homeostasis. The interaction of heart and gut, or heart-intestine axis, has emerged as a novel concept to provide new insights into the intricate mechanisms of HF. However, at present the role of gut microbiota-targeted interventions remains uncertain in the absence of solid, well-conducted clinical studies. Indeed, reliable and reproducible biomarkers may help to identify those who might benefit from such interventions. Further advances in this area have a huge potential for yielding significant breakthroughs in the development of novel therapeutic tools for metabolic modulation of HF.
Figure 1. Interactions between Heart Failure, Gut Microbiota, and Renal Failure.
The hemodynamic changes due to heart failure range in microcirculation in intestinal villi. This results in the alterations in intestinal permeability and gut microbiota. Microbial and endotoxin translocation, trimethylamine N-oxide (TMAO), and cardiorenal compromises can mediate the pathology which leads to further exacerbation of heart failure and renal damage. Impaired clearance of these metabolites due to renal dysfunction further promotes this pathology and constitutes a vicious cycle.
Highlights.
In the setting of heart failure, substantial hemodynamic changes such as hypoperfusion and congestion in the intestines can alter gut morphology, permeability, function, and possibly the growth and composition of gut microbiota.
Cardio-renal alterations via metabolites derived from gut microbiota can potentially mediate or modulate HF pathophysiology.
Trimethylamine N-oxide (TMAO) has emerged as a key mediator which provides mechanistic link between gut microbiota and multiple cardiovascular diseases, including HF.
Potential intervention strategies may target this microbiota-driven pathology, including dietary modification, prebiotics or probiotics, and selective binders of microbial enzymes or molecules.
Acknowledgments
Funding Source
This work is funded by the National Institutes of Health and the Office of Dietary Supplements (R01HL103931, P20HL113452, 1R01DK106000). Dr. Nagatomo is the recipient of the Postdoctoral Fellowship award from the Myocarditis Foundation.
Footnotes
Disclosure
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Neish AS. Microbes in gastrointestinal health and disease. Gastroenterology. 2009;136:65–80. doi: 10.1053/j.gastro.2008.10.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–20. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 3.Nicholson JK, Holmes E, Wilson ID. Gut microorganisms, mammalian metabolism and personalized health care. Nat Rev Microbiol. 2005;3:431–8. doi: 10.1038/nrmicro1152. [DOI] [PubMed] [Google Scholar]
- 4.Li M, Wang B, Zhang M, Rantalainen M, Wang S, Zhou H, et al. Symbiotic gut microbes modulate human metabolic phenotypes. Proc Natl Acad Sci U S A. 2008;105:2117–22. doi: 10.1073/pnas.0712038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–7. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gill SR, Pop M, Deboy RT, Eckburg PB, Turnbaugh PJ, Samuel BS, et al. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312:1355–9. doi: 10.1126/science.1124234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–31. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 8.Wen L, Ley RE, Volchkov PY, Stranges PB, Avanesyan L, Stonebraker AC, et al. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature. 2008;455:1109–13. doi: 10.1038/nature07336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Backhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. 2004;101:15718–23. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dumas ME, Barton RH, Toye A, Cloarec O, Blancher C, Rothwell A, et al. Metabolic profiling reveals a contribution of gut microbiota to fatty liver phenotype in insulin-resistant mice. Proc Natl Acad Sci U S A. 2006;103:12511–6. doi: 10.1073/pnas.0601056103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mell B, Jala VR, Mathew AV, Byun J, Waghulde H, Zhang Y, et al. Evidence for a link between Gut Microbiota and Hypertension in the Dahl rat model. Physiol Genomics. 2015 doi: 10.1152/physiolgenomics.00136.2014. physiolgenomics 00136 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takala J. Determinants of splanchnic blood flow. Br J Anaesth. 1996;77:50–8. doi: 10.1093/bja/77.1.50. [DOI] [PubMed] [Google Scholar]
- 13.Krack A, Richartz BM, Gastmann A, Greim K, Lotze U, Anker SD, et al. Studies on intragastric PCO2 at rest and during exercise as a marker of intestinal perfusion in patients with chronic heart failure. Eur J Heart Fail. 2004;6:403–7. doi: 10.1016/j.ejheart.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Sandek A, Bjarnason I, Volk HD, Crane R, Meddings JB, Niebauer J, et al. Studies on bacterial endotoxin and intestinal absorption function in patients with chronic heart failure. Int J Cardiol. 2012;157:80–5. doi: 10.1016/j.ijcard.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 15.Hsiao JK, Huang CY, Lu YZ, Yang CY, Yu LC. Magnetic resonance imaging detects intestinal barrier dysfunction in a rat model of acute mesenteric ischemia/reperfusion injury. Invest Radiol. 2009;44:329–35. doi: 10.1097/RLI.0b013e3181a16762. [DOI] [PubMed] [Google Scholar]
- 16.Hashimoto N, Ohyanagi H. Effect of acute portal hypertension on gut mucosa. Hepatogastroenterology. 2002;49:1567–70. [PubMed] [Google Scholar]
- 17.Sandek A, Bauditz J, Swidsinski A, Buhner S, Weber-Eibel J, von Haehling S, et al. Altered intestinal function in patients with chronic heart failure. J Am Coll Cardiol. 2007;50:1561–9. doi: 10.1016/j.jacc.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 18.Arutyunov GP, Kostyukevich OI, Serov RA, Rylova NV, Bylova NA. Collagen accumulation and dysfunctional mucosal barrier of the small intestine in patients with chronic heart failure. Int J Cardiol. 2008;125:240–5. doi: 10.1016/j.ijcard.2007.11.103. [DOI] [PubMed] [Google Scholar]
- 19.Alverdy J, Zaborina O, Wu L. The impact of stress and nutrition on bacterial-host interactions at the intestinal epithelial surface. Curr Opin Clin Nutr Metab Care. 2005;8:205–9. doi: 10.1097/00075197-200503000-00016. [DOI] [PubMed] [Google Scholar]
- 20.Wang F, Li Q, He Q, Geng Y, Tang C, Wang C, et al. Temporal variations of the ileal microbiota in intestinal ischemia and reperfusion. Shock. 2013;39:96–103. doi: 10.1097/SHK.0b013e318279265f. [DOI] [PubMed] [Google Scholar]
- 21.Llamas MA, Aller MA, Marquina D, Nava MP, Arias J. Bacterial translocation to mesenteric lymph nodes increases in chronic portal hypertensive rats. Dig Dis Sci. 2010;55:2244–54. doi: 10.1007/s10620-009-1001-3. [DOI] [PubMed] [Google Scholar]
- 22.Gan XT, Ettinger G, Huang CX, Burton JP, Haist JV, Rajapurohitam V, et al. Probiotic administration attenuates myocardial hypertrophy and heart failure after myocardial infarction in the rat. Circ Heart Fail. 2014;7:491–9. doi: 10.1161/CIRCHEARTFAILURE.113.000978. [DOI] [PubMed] [Google Scholar]
- 23.Levine B, Kalman J, Mayer L, Fillit HM, Packer M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med. 1990;323:236–41. doi: 10.1056/NEJM199007263230405. [DOI] [PubMed] [Google Scholar]
- 24.Anker SD, Egerer KR, Volk HD, Kox WJ, Poole-Wilson PA, Coats AJ. Elevated soluble CD14 receptors and altered cytokines in chronic heart failure. Am J Cardiol. 1997;79:1426–30. doi: 10.1016/s0002-9149(97)00159-8. [DOI] [PubMed] [Google Scholar]
- 25.Roig E, Orus J, Pare C, Azqueta M, Filella X, Perez-Villa F, et al. Serum interleukin-6 in congestive heart failure secondary to idiopathic dilated cardiomyopathy. Am J Cardiol. 1998;82:688–90. A8. doi: 10.1016/s0002-9149(98)00388-9. [DOI] [PubMed] [Google Scholar]
- 26.Sato Y, Takatsu Y, Kataoka K, Yamada T, Taniguchi R, Sasayama S, et al. Serial circulating concentrations of C-reactive protein, interleukin (IL)-4, and IL-6 in patients with acute left heart decompensation. Clin Cardiol. 1999;22:811–3. doi: 10.1002/clc.4960221211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maeda K, Tsutamoto T, Wada A, Mabuchi N, Hayashi M, Tsutsui T, et al. High levels of plasma brain natriuretic peptide and interleukin-6 after optimized treatment for heart failure are independent risk factors for morbidity and mortality in patients with congestive heart failure. J Am Coll Cardiol. 2000;36:1587–93. doi: 10.1016/s0735-1097(00)00912-8. [DOI] [PubMed] [Google Scholar]
- 28.Nagatomo Y, Yoshikawa T, Kohno T, Yoshizawa A, Anzai T, Meguro T, et al. Effects of beta-blocker therapy on high sensitivity c-reactive protein, oxidative stress, and cardiac function in patients with congestive heart failure. J Card Fail. 2007;13:365–71. doi: 10.1016/j.cardfail.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 29.Mann DL, McMurray JJ, Packer M, Swedberg K, Borer JS, Colucci WS, et al. Targeted anticytokine therapy in patients with chronic heart failure: results of the Randomized Etanercept Worldwide Evaluation (RENEWAL) Circulation. 2004;109:1594–602. doi: 10.1161/01.CIR.0000124490.27666.B2. [DOI] [PubMed] [Google Scholar]
- 30.Doig CJ, Sutherland LR, Sandham JD, Fick GH, Verhoef M, Meddings JB. Increased intestinal permeability is associated with the development of multiple organ dysfunction syndrome in critically ill ICU patients. Am J Respir Crit Care Med. 1998;158:444–51. doi: 10.1164/ajrccm.158.2.9710092. [DOI] [PubMed] [Google Scholar]
- 31.Cirera I, Bauer TM, Navasa M, Vila J, Grande L, Taura P, et al. Bacterial translocation of enteric organisms in patients with cirrhosis. J Hepatol. 2001;34:32–7. doi: 10.1016/s0168-8278(00)00013-1. [DOI] [PubMed] [Google Scholar]
- 32.Deitch EA. Bacterial translocation or lymphatic drainage of toxic products from the gut: what is important in human beings? Surgery. 2002;131:241–4. doi: 10.1067/msy.2002.116408. [DOI] [PubMed] [Google Scholar]
- 33.Riddington DW, Venkatesh B, Boivin CM, Bonser RS, Elliott TS, Marshall T, et al. Intestinal permeability, gastric intramucosal pH, and systemic endotoxemia in patients undergoing cardiopulmonary bypass. Jama. 1996;275:1007–12. [PubMed] [Google Scholar]
- 34.Krack A, Sharma R, Figulla HR, Anker SD. The importance of the gastrointestinal system in the pathogenesis of heart failure. Eur Heart J. 2005;26:2368–74. doi: 10.1093/eurheartj/ehi389. [DOI] [PubMed] [Google Scholar]
- 35.Ozban M, Aydin C, Cevahir N, Yenisey C, Birsen O, Gumrukcu G, et al. The effect of melatonin on bacterial translocation following ischemia/reperfusion injury in a rat model of superior mesenteric artery occlusion. BMC Surg. 2015;15:18. doi: 10.1186/s12893-015-0003-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Amar J, Serino M, Lange C, Chabo C, Iacovoni J, Mondot S, et al. Involvement of tissue bacteria in the onset of diabetes in humans: evidence for a concept. Diabetologia. 2011;54:3055–61. doi: 10.1007/s00125-011-2329-8. [DOI] [PubMed] [Google Scholar]
- 37.Amar J, Lange C, Payros G, Garret C, Chabo C, Lantieri O, et al. Blood microbiota dysbiosis is associated with the onset of cardiovascular events in a large general population: the D.E.S.I.R. study. PLoS One. 2013;8:e54461. doi: 10.1371/journal.pone.0054461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dinakaran V, Rathinavel A, Pushpanathan M, Sivakumar R, Gunasekaran P, Rajendhran J. Elevated levels of circulating DNA in cardiovascular disease patients: metagenomic profiling of microbiome in the circulation. PLoS One. 2014;9:e105221. doi: 10.1371/journal.pone.0105221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Niebauer J, Volk HD, Kemp M, Dominguez M, Schumann RR, Rauchhaus M, et al. Endotoxin and immune activation in chronic heart failure: a prospective cohort study. Lancet. 1999;353:1838–42. doi: 10.1016/S0140-6736(98)09286-1. [DOI] [PubMed] [Google Scholar]
- 40.Peschel T, Schonauer M, Thiele H, Anker SD, Schuler G, Niebauer J. Invasive assessment of bacterial endotoxin and inflammatory cytokines in patients with acute heart failure. Eur J Heart Fail. 2003;5:609–14. doi: 10.1016/s1388-9842(03)00104-1. [DOI] [PubMed] [Google Scholar]
- 41.Conraads VM, Jorens PG, De Clerck LS, Van Saene HK, Ieven MM, Bosmans JM, et al. Selective intestinal decontamination in advanced chronic heart failure: a pilot trial. Eur J Heart Fail. 2004;6:483–91. doi: 10.1016/j.ejheart.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 42.Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC, et al. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci U S A. 2009;106:3698–703. doi: 10.1073/pnas.0812874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Evenepoel P, Meijers BK, Bammens BR, Verbeke K. Uremic toxins originating from colonic microbial metabolism. Kidney Int Suppl. 2009:S12–9. doi: 10.1038/ki.2009.402. [DOI] [PubMed] [Google Scholar]
- 44.Yamamoto S, Zuo Y, Ma J, Yancey PG, Hunley TE, Motojima M, et al. Oral activated charcoal adsorbent (AST-120) ameliorates extent and instability of atherosclerosis accelerated by kidney disease in apolipoprotein E-deficient mice. Nephrol Dial Transplant. 2011;26:2491–7. doi: 10.1093/ndt/gfq759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barreto FC, Barreto DV, Liabeuf S, Meert N, Glorieux G, Temmar M, et al. Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin J Am Soc Nephrol. 2009;4:1551–8. doi: 10.2215/CJN.03980609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lekawanvijit S, Adrahtas A, Kelly DJ, Kompa AR, Wang BH, Krum H. Does indoxyl sulfate, a uraemic toxin, have direct effects on cardiac fibroblasts and myocytes? Eur Heart J. 2010;31:1771–9. doi: 10.1093/eurheartj/ehp574. [DOI] [PubMed] [Google Scholar]
- 47.Yang K, Wang C, Nie L, Zhao X, Gu J, Guan X, et al. Klotho Protects Against Indoxyl Sulphate-Induced Myocardial Hypertrophy. J Am Soc Nephrol. 2015 doi: 10.1681/ASN.2014060543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang K, Xu X, Nie L, Xiao T, Guan X, He T, et al. Indoxyl sulfate induces oxidative stress and hypertrophy in cardiomyocytes by inhibiting the AMPK/UCP2 signaling pathway. Toxicol Lett. 2015;234:110–9. doi: 10.1016/j.toxlet.2015.01.021. [DOI] [PubMed] [Google Scholar]
- 49.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lombardi B, Pani P, Schlunk FF. Choline-deficiency fatty liver: impaired release of hepatic triglycerides. J Lipid Res. 1968;9:437–46. [PubMed] [Google Scholar]
- 51.Zeisel SH, Da Costa KA, Franklin PD, Alexander EA, Lamont JT, Sheard NF, et al. Choline, an essential nutrient for humans. Faseb J. 1991;5:2093–8. [PubMed] [Google Scholar]
- 52.Bennett BJ, de Aguiar Vallim TQ, Wang Z, Shih DM, Meng Y, Gregory J, et al. Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab. 2013;17:49–60. doi: 10.1016/j.cmet.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martin FP, Wang Y, Sprenger N, Yap IK, Lundstedt T, Lek P, et al. Probiotic modulation of symbiotic gut microbial-host metabolic interactions in a humanized microbiome mouse model. Mol Syst Biol. 2008;4:157. doi: 10.1038/msb4100190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368:1575–84. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tang WH, Wang Z, Kennedy DJ, Wu Y, Buffa JA, Agatisa-Boyle B, et al. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ Res. 2015;116:448–55. doi: 10.1161/CIRCRESAHA.116.305360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rhee EP, Clish CB, Ghorbani A, Larson MG, Elmariah S, McCabe E, et al. A combined epidemiologic and metabolomic approach improves CKD prediction. J Am Soc Nephrol. 2013;24:1330–8. doi: 10.1681/ASN.2012101006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bell JD, Lee JA, Lee HA, Sadler PJ, Wilkie DR, Woodham RH. Nuclear magnetic resonance studies of blood plasma and urine from subjects with chronic renal failure: identification of trimethylamine-N-oxide. Biochim Biophys Acta. 1991;1096:101–7. doi: 10.1016/0925-4439(91)90046-c. [DOI] [PubMed] [Google Scholar]
- 58.Bain MA, Faull R, Fornasini G, Milne RW, Evans AM. Accumulation of trimethylamine and trimethylamine-N-oxide in end-stage renal disease patients undergoing haemodialysis. Nephrol Dial Transplant. 2006;21:1300–4. doi: 10.1093/ndt/gfk056. [DOI] [PubMed] [Google Scholar]
- 59.Tang WH, Wang Z, Fan Y, Levison B, Hazen JE, Donahue LM, et al. Prognostic value of elevated levels of intestinal microbe-generated metabolite trimethylamine-N-oxide in patients with heart failure: refining the gut hypothesis. J Am Coll Cardiol. 2014;64:1908–14. doi: 10.1016/j.jacc.2014.02.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tang WH, Wang Z, Shrestha K, Borowski AG, Wu Y, Troughton RW, et al. Intestinal microbiota-dependent phosphatidylcholine metabolites, diastolic dysfunction, and adverse clinical outcomes in chronic systolic heart failure. J Card Fail. 2015;21:91–6. doi: 10.1016/j.cardfail.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lever M, George PM, Slow S, Bellamy D, Young JM, Ho M, et al. Betaine and Trimethylamine-N-Oxide as Predictors of Cardiovascular Outcomes Show Different Patterns in Diabetes Mellitus: An Observational Study. PLoS One. 2014;9:e114969. doi: 10.1371/journal.pone.0114969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ufnal M, Jazwiec R, Dadlez M, Drapala A, Sikora M, Skrzypecki J. Trimethylamine-N-oxide: a carnitine-derived metabolite that prolongs the hypertensive effect of angiotensin II in rats. Can J Cardiol. 2014;30:1700–5. doi: 10.1016/j.cjca.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 63.Kim MS, Hwang SS, Park EJ, Bae JW. Strict vegetarian diet improves the risk factors associated with metabolic diseases by modulating gut microbiota and reducing intestinal inflammation. Environ Microbiol Rep. 2013;5:765–75. doi: 10.1111/1758-2229.12079. [DOI] [PubMed] [Google Scholar]
- 64.Bischoff SC, Barbara G, Buurman W, Ockhuizen T, Schulzke JD, Serino M, et al. Intestinal permeability--a new target for disease prevention and therapy. BMC Gastroenterol. 2014;14:189. doi: 10.1186/s12876-014-0189-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lopetuso LR, Scaldaferri F, Bruno G, Petito V, Franceschi F, Gasbarrini A. The therapeutic management of gut barrier leaking: the emerging role for mucosal barrier protectors. Eur Rev Med Pharmacol Sci. 2015;19:1068–76. [PubMed] [Google Scholar]
- 66.Grayston JT, Kronmal RA, Jackson LA, Parisi AF, Muhlestein JB, Cohen JD, et al. Azithromycin for the secondary prevention of coronary events. N Engl J Med. 2005;352:1637–45. doi: 10.1056/NEJMoa043526. [DOI] [PubMed] [Google Scholar]
- 67.Cannon CP, Braunwald E, McCabe CH, Grayston JT, Muhlestein B, Giugliano RP, et al. Antibiotic treatment of Chlamydia pneumoniae after acute coronary syndrome. N Engl J Med. 2005;352:1646–54. doi: 10.1056/NEJMoa043528. [DOI] [PubMed] [Google Scholar]
- 68.Kalambokis GN, Mouzaki A, Rodi M, Pappas K, Fotopoulos A, Xourgia X, et al. Rifaximin improves systemic hemodynamics and renal function in patients with alcohol-related cirrhosis and ascites. Clin Gastroenterol Hepatol. 2012;10:815–8. doi: 10.1016/j.cgh.2012.02.025. [DOI] [PubMed] [Google Scholar]
- 69.Lam V, Su J, Koprowski S, Hsu A, Tweddell JS, Rafiee P, et al. Intestinal microbiota determine severity of myocardial infarction in rats. Faseb J. 2012;26:1727–35. doi: 10.1096/fj.11-197921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.de Vrese M, Schrezenmeir J. Probiotics, prebiotics, and synbiotics. Adv Biochem Eng Biotechnol. 2008;111:1–66. doi: 10.1007/10_2008_097. [DOI] [PubMed] [Google Scholar]
- 71.Costanza AC, Moscavitch SD, Faria Neto HC, Mesquita ET. Probiotic therapy with Saccharomyces boulardii for heart failure patients: a randomized, double-blind, placebo-controlled pilot trial. Int J Cardiol. 2015;179:348–50. doi: 10.1016/j.ijcard.2014.11.034. [DOI] [PubMed] [Google Scholar]
- 72.Lekawanvijit S, Kompa AR, Manabe M, Wang BH, Langham RG, Nishijima F, et al. Chronic kidney disease-induced cardiac fibrosis is ameliorated by reducing circulating levels of a non-dialysable uremic toxin, indoxyl sulfate. PLoS One. 2012;7:e41281. doi: 10.1371/journal.pone.0041281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fujii H, Nishijima F, Goto S, Sugano M, Yamato H, Kitazawa R, et al. Oral charcoal adsorbent (AST-120) prevents progression of cardiac damage in chronic kidney disease through suppression of oxidative stress. Nephrol Dial Transplant. 2009;24:2089–95. doi: 10.1093/ndt/gfp007. [DOI] [PubMed] [Google Scholar]