Abstract
PURPOSE
Black women are disproportionately affected with triple negative breast cancer and have relatively poor survival. It is not known to what extent differences in clinical presentation of breast cancer in Non-Hispanic White (NHW) women and Black women can be accounted for by the presence of mutations in the BRCA1 and BRCA2 (BRCA) genes. We sought to evaluate the frequency of BRCA pathogenic variants in a population-based sample of young Black women with breast cancer.
PATIENTS AND METHODS
Black women diagnosed with invasive breast cancer at or before age 50 from 2009 to 2012 were recruited to the study through the Florida Cancer Registry. Participants underwent genetic counseling, completed a study questionnaire and consented to release of their medical records. Saliva specimens were collected for BRCA sequencing and large rearrangement testing through MLPA.
RESULTS
A DNA sample was evaluated for 396 women of whom 49 (12.4%) had a mutation in BRCA1 or BRCA2. Eight recurrent mutations accounted for 49% of all pathogenic variants.
CONCLUSIONS
The prevalence of BRCA mutations among our Florida-based sample of young Black women with breast cancer exceeds that previously reported for NHW women. It is appropriate to recommend BRCA testing in all young Black women with invasive breast cancer.
Keywords: Breast cancer, BRCA1, BRCA2, Black women, Disparities
Introduction
One in 53 Black women in the United States will develop breast cancer by the age of 50, compared to one in 48 NHW women, but one in 278 Black women will die of breast cancer by age 50 (compared to one in 476 NHW non-Jewish women).1 Furthermore, for patients under age 50, the 10-year survival rate is 64% for Black women, compared to 81% for NHW women.1 To some extent the disparities in survival can be accounted for by stage at diagnosis2 and by the high proportion of triple-negative cancers in Black women,3, 4 but other biologic and social factors may contribute to the observed disparity. Given that most BRCA1- associated breast cancers occur before age 50 and that cancers in BRCA1 carriers are predominantly triple-negative,5 it is reasonable to ask to what extent mutations in BRCA1 (and BRCA2) contribute to the racial disparity in breast cancer incidence among young women. The frequency of mutations in both BRCA genes among breast cancer patients of all ages in the United States has been estimated to be 5%, but this estimate is primarily based on studies of NHW women.6 The frequency is estimated to be 6% in women with breast cancer at or below age 407 and 11.2% based on a study of 1824 unselected women with triple-negative breast cancer.8
The prevalence of BRCA mutations in unselected Black women is not yet known with precision, in part because of the limited testing performed among Blacks which may be the result of lower access and awareness.9–11 Furthermore, most studies have focused on clinic-based patients as opposed to patients unselected for family history.12 Of the three prior population-based studies which included BRCA testing in US-based Black women,6, 13, 14 two only tested for BRCA1 mutations,13, 14 and none included large rearrangement testing which accounts for up to 10% of mutations.15, 16 In one study, the prevalence was particularly high among the 30 women in the sample diagnosed below age 35, of whom 5 had a mutation (16.7%).13 In the sole prior population-based effort including both BRCA1 and BRCA2 testing, the overall mutation frequency was 4% among the 463 Black women with breast cancer diagnosed between ages 35 to 64; however age-stratified mutation frequencies were not provided.6
In a population-based sample of young Black women with breast cancer from Florida, we sought to estimate the frequency of BRCA mutations and to describe the clinical characteristics of those with and without a BRCA mutation.
Materials and Methods
Eligible study subjects were Black women diagnosed with invasive breast cancer, ≤ age 50, between 2009–2012, living in Florida at the time of diagnosis. Upon approval of the institutional review boards (IRB) of the University of South Florida and the Florida Department of Health (DOH), registry-based recruitment was initiated. The Florida State Cancer Registry released patient information, clinical information (i.e., age at diagnosis, stage of diagnosis, histologic subtype) and demographic information (i.e., county of residence, marital status, primary payer at diagnosis) on all eligible participants with newly diagnosed breast cancer within 6–18 months of diagnosis who self-reported Black race (which included those of African-American, Afro-Carribean, and other/mixed descent).
Participants were recruited using a state-mandated recruitment method.17 Study participants were asked to: 1) provide verbal and written informed consent, including medical records release; 2) complete a study questionnaire, which included socio-demographic, epidemiologic, and lifestyle factors; 3) attend a telephone genetic counseling session to discuss inherited breast cancer with a certified genetic counselor, at which time a three generation pedigree was drawn; and 4) provide a saliva sample through the mail for DNA extraction and BRCA testing.
Genetic testing included full gene sequencing and comprehensive rearrangement testing (MLPA) of the BRCA1 and BRCA2 genes.18 All BRCA alterations were evaluated through available clinical and research data, however a variant was classified as pathogenic if there were several lines of evidence confirming its pathogenicity through the multi-factorial model.19 All variants were searched in the literature and through the publicly available Breast Cancer Information Core (BIC) database.20
Demographic and clinical characteristics of the 396 participants with BRCA testing were compared to non-participants using the Chi-squared test. The mutation prevalence and 95% confidence interval (CI) were calculated for the entire sample as well as for subgroups defined by age of onset, family history and triple-negative status. A patient was considered to have a positive family history if there was a first or second degree relative with breast cancer ≤50 or ovarian cancer at any age. Additionally, BRCA carriers and non-carriers were compared using Pearson Chi-square tests to evaluate differences in triple-negative status, family history family breast and/or ovarian cancer in first and/or second degree relative and age at diagnosis (≤45 versus >45). The pedigrees of all study participants were reviewed to determine if the proband met the criteria for BRCA testing according to National Comprehensive Cancer Network (NCCN) practice guidelines.21 All statistical tests were 2-sided and considered significant at a level of p ≤ 0.05.
Results
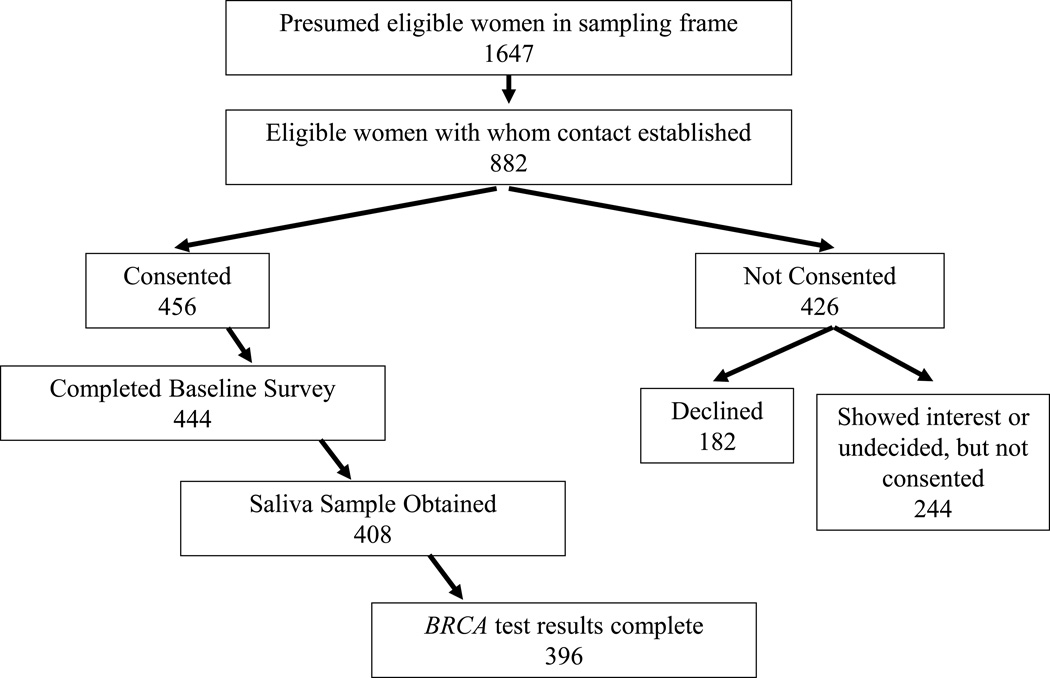
There were 1647 Black women with breast cancer identified in Florida who qualified for the study. Contact was established with 882 of the eligible women. Among these, 456 women provided written informed consent for the study and BRCA testing was completed for 396 women (Figure 1).
Figure 1.
Recruitment Schema
Of the 426 women who chose not to participate, 182 declined testing outright and 244 women expressed interest in participating but did not follow through with testing by the time study recruitment was completed. The 396 tested participants are compared with remaining 1251 women in the sampling frame in Table 1. The median age at diagnosis for the participants was 43 years (range 21 to 50) and 25% had triple-negative (TN) disease. Overall, 87 (22.0%) had a family history of breast cancer and 48 (12.1%) had a family history of ovarian cancer. Race was based on participant self report and those reporting race/ethnicity data in addition to Black/African American, were as follows: 64 Carribeans, 14 Black/NHW and 2 Black/Hispanic.
Table 1.
Clinical and Demographic Comparisons
| Participants with BRCA testing N=396 |
All others in the sampling frame N=1251 |
P- value |
||||
|---|---|---|---|---|---|---|
| N | % | N | % | |||
| Age at diagnosis (years) | ||||||
| Mean (SD) | 42.1 (6.1) | 42.6 (6.4) | ||||
| <35 | 65 | 16.4% | 181 | 14.5% | 0.53 | |
| 36–40 | 74 | 18.7% | 216 | 17.3% | ||
| 41–45 | 107 | 27.0% | 332 | 26.5% | ||
| 46–50 | 150 | 37.9% | 522 | 41.7% | ||
| Stage at Diagnosis (n (%)) | ||||||
| Localized | 216 | 54.5% | 594 | 47.5% | 0.04 | |
| Regional/Distant | 174 | 44.0% | 626 | 50.0% | ||
| Unstaged | 6 | 1.5% | 31 | 2.5% | ||
| Histologic Subtype (n (%)) | ||||||
| Ductal | 308 | 77.8% | 1015 | 81.1% | 0.16 | |
| Lobular | 25 | 6.3% | 48 | 3.8% | ||
| Mixed | 26 | 6.6% | 87 | 7.0% | ||
| Other/Unknown | 37 | 9.3% | 101 | 8.1% | ||
| Receptor Status (n (%)) | ||||||
| Triple-negative** | 66 | 16.7% | 200 | 16.0% | 0.89 | |
| Not triple-negative | 194 | 49.0% | 607 | 48.5% | ||
| unknown | 136 | 34.3% | 444 | 35.5% | ||
| Married or cohabiting (n (%)) | ||||||
| no | 232 | 58.6% | 687 | 54.9% | 0.44 | |
| yes | 157 | 39.6% | 540 | 43.2% | ||
| unknown | 7 | 1.8% | 24 | 1.9% | ||
| Insurance at Diagnosis (n (%)) | ||||||
| Not Insured | 42 | 10.6% | 119 | 9.5% | 0.64 | |
| Private Insurance | 219 | 55.3% | 735 | 58.8% | ||
| Medicaid | 64 | 16.2% | 201 | 16.1% | ||
| Medicare | 18 | 4.5% | 48 | 3.8% | ||
| Other Insurance | 49 | 12.4% | 128 | 10.2% | ||
| Unknown | 4 | 1.0% | 20 | 1.6% | ||
| Employment at Diagnosis (n (%)) | ||||||
| Unemployed | 44 | 11.1% | 140 | 11.2% | 0.82 | |
| Employed | 247 | 62.4% | 760 | 60.8% | ||
| Unknown | 105 | 26.4% | 351 | 28.1% | ||
| Metropolitan (n (%)) | 380 | 96.0% | 1201 | 96.0% | 1.00 | |
<0.05
this number is solely based on data available through the state cancer registry, in order to provide a fair comparison with all others within the sampling frame. Note that among study participants, triple negative status was obtained on a number of additional participants in whom this information was missing, through collection of medical records.
In total, 35 different mutations in the two BRCA genes were detected in 49 different and unrelated women (prevalence 12.4%; 95% CI: 9.5%–16.0%), including 32 in BRCA1 only, 15 in BRCA2 only, and two in both BRCA1 and BRCA2 (Table 2). Of these 35 mutations, seven have not been reported in the BIC database.20 Eight mutations were observed in two or more unrelated participants. Overall, 24 of 49 mutation carriers had a mutation that was seen more than once and these eight recurrent mutations accounted for 49% of all the mutations detected. Three different large rearrangements were detected through MLPA (BRCA1 del Exon 8; BRCA1 dup Exon13 and BRCA2 del Exon 4).
Table 2.
Summary of BRCA mutations among study participants
| Unique Participants |
Gene | Exon | BIC Nucleotide Designation |
Number of mutations |
HGVS Nucleotide Designation |
Mutation Typea | #BIC | #BIC African/Carribeanb |
|---|---|---|---|---|---|---|---|---|
| 1 | BRCA1 | 2 | 122G>T | 1 | c.3G>T | M | 10 | 0 |
| 2 | BRCA1 | 5 | 301G>A | 1 | c.182G>A | M | 6 | 1 |
| 3 | BRCA1 | 6 | IVS5-11T>G | 1 | c.213-11T>G | IVS | 108 | 0 |
| 4 | BRCA1 | 8 | deletion of exon 8 | 1 | delExon8 | LR | * | * |
| 5 | BRCA1 | 11 | 943ins10 | 2 | c.824_825insAGCCATGTGG | F | 34 | 21 |
| 6 | BRCA1 | 11 | c.824_825insAGCCATGTGG | F | ||||
| 7 | BRCA1 | 11 | 2190delA | 1 | c.2071delA | F | 25 | 0 |
| 8 | BRCA1 | 11 | 3135delC | 1 | c.3016delC | F | 0 | 0 |
| 9 | BRCA1 | 11 | 3477delGT | 1 | c.3358_3359delGT | F | 4 | 0 |
| 10 | BRCA1 | 11 | 3600del11 | 1 | c.3481_3491delGAAGATACTAG | F | 64 | 0 |
| 11 | BRCA1 | 13 | IVS13+1G>A | 5 | c.4357+1G>A | IVS | 23 | 13 |
| 12 | BRCA1 | 13 | c.4357+1G>A | IVS | ||||
| 13 | BRCA1 | 13 | c.4357+1G>A | IVS | ||||
| 14 | BRCA1 | 13 | c.4357+1G>A | IVS | ||||
| 15 | BRCA1 | 13 | c.5467+1G>A | IVS | ||||
| BRCA2 | 10 | 1779delAT | 1 | c.1570_1571delAT | F | 0 | 0 | |
| 16 | BRCA1 | 13 | duplication of exon 13 | 1 | dupExon13 | LR | * | * |
| 17 | BRCA1 | 14 | 4603G>T | 1 | c.4484G>T | M | 26 | 3 |
| 18 | BRCA1 | 16 | IVS16+6T>C | 4 | c.4986+6T>C | IVS | 10 | 8 |
| 19 | BRCA1 | 16 | c.4986+6T>C | IVS | ||||
| 20 | BRCA1 | 16 | c.4986+6T>C | IVS | ||||
| 21 | BRCA1 | 16 | c.4986+6T>C | IVS | ||||
| 22 | BRCA1 | 18 | IVS18+1G>C | 1 | c.5152+1G>A | IVS | 1 | 0 |
| 23 | BRCA1 | 19 | 5296del4 | 3 | c.5177_5180delGAAA | F | 39 | 8 |
| 24 | BRCA1 | 19 | c.5177_5180delGAAA | F | ||||
| 25 | BRCA1 | 19 | c.5177_5180delGAAA | F | ||||
| 26 | BRCA1 | 20 | 5370C>T | 3 | c.5251C>T | N | 44 | 3 |
| 27 | BRCA1 | 20 | c.5251C>T | N | ||||
| 28 | BRCA1 | 20 | c.5251C>T | N | ||||
| BRCA2 | 4 | deletion of exon 4 | 1 | delExon4 | LR | * | * | |
| 29 | BRCA1 | 21 | 5443T>G | 3 | c.5324T>G | M | 31 | 16 |
| 30 | BRCA1 | 21 | c.5324T>G | M | ||||
| 31 | BRCA1 | 21 | c.5324T>G | M | ||||
| 32 | BRCA1 | 22 | 5506C>A | 2 | c.5387C>A | N | 1 | 0 |
| 33 | BRCA1 | 22 | c.5387C>A | N | ||||
| 34 | BRCA1 | 23 | IVS23+1G>A | 1 | c.5467+1G>A | IVS | 5 | 3 |
| 35 | BRCA2 | 3 | 343delG | 1 | c.115delG | F | 0 | 0 |
| 36 | BRCA2 | 10 | 1331C>G | 1 | c.1103C>G | N | 0 | 0 |
| 37 | BRCA2 | 10 | 1933delCA | 1 | c.1705_1706delCA | F | 0 | 0 |
| 38 | BRCA2 | 10 | 2115del7 | 1 | c.1887_1893delTACATTT | F | 0 | 0 |
| 39 | BRCA2 | 11 | 3827delGT | 1 | c.3599_3600delGT | F | 6 | 0 |
| 40 | BRCA2 | 11 | 3908delTG | 1 | c.3680_3681delTG | F | 8 | 0 |
| 41 | BRCA2 | 11 | 4699del4 | 2 | c.4471_4474delCTGA | F | 1 | 0 |
| 42 | BRCA2 | 11 | c.4471_4474delCTGA | F | ||||
| 43 | BRCA2 | 11 | 5844del5 | 1 | c.5616_5620delAGTAA | F | 7 | 7 |
| 44 | BRCA2 | 11 | 6207insA | 1 | c.5979_5980insA | F | 0 | 0 |
| 45 | BRCA2 | 11 | 6365C>A | 1 | c.6137C>A | N | 0 | 0 |
| 46 | BRCA2 | 22 | 9005T>A | 1 | c.8777T>A | N | 0 | 0 |
| 47 | BRCA2 | 23 | 9197G>A | 1 | c.8969G>A | N | 2 | 1 |
| 48 | BRCA2 | 24 | 9481insA | 1 | c.9253_9254insA | F | 19 | 0 |
| 49 | BRCA2 | 25 | 9610C>T | 1 | c.9382C>T | N | 50 | 1 |
The prevalence of mutations was 22% for women diagnosed at age 35 and below, 13% for women diagnosed from age 36 to 40, 11% for women diagnosed from age 41 to 45 and 8% for women diagnosed from age 46 to 50 (Table 3). A trend in decreasing mutation prevalence with increasing age of onset was present only for BRCA1 carriers; among BRCA2 carriers, the overall prevalence of mutations was 4.3% and this was similar for women in all age categories.
Table 3.
Age-stratified and cumulative mutation prevalence
| Age Group |
Total N |
BRCA Mutation Prevalencea |
BRCA1 Mutation Prevalence |
BRCA2 Mutation Prevalence |
|---|---|---|---|---|
| Age-stratified: | ||||
| ≤35 | 72 | 22.2% | 19.4% | 4.2% |
| 36–40 | 79 | 12.7% | 10.1% | 2.5% |
| 41–45 | 105 | 11.4% | 5.7% | 5.7% |
| 46–50 | 140 | 7.9% | 4.3% | 4.3% |
| Cumulative: | ||||
| ≤35 | 72 | 22.2% | 19.4% | 4.2% |
| ≤40 | 151 | 17.2% | 14.6% | 3.3% |
| ≤45 | 256 | 14.8% | 10.9% | 4.3% |
| ≤50 | 396 | 12.4% | 8.6% | 4.3% |
2 participants with both a BRCA1 and BRCA2 mutation are only counted once in the overall BRCA mutation frequency
A total of 73 different variants of uncertain significance (VUS) were detected among 86 non-carriers (21.7%) and 6 BRCA carriers of a pathogenic variant of which 57 were suspected to be benign (Supplemental Table 1). These included 4 VUS results that were potentially pathogenic, but lacked sufficient data to be classified based on the multi-factorial model criteria used to quantify likelihood of pathogenicity,19 and an additional 12 straight VUS (Table 4).
Table 4.
Variants of Uncertain Significance (VUS) Classified as Suspected Deleterious or Straight VUS
| Gene | Exon | Variant | Typea | #Observed in Study | #Observed in BIC | Splicing priors | Align GVGD | Novel | Final Interpretationb | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | BRCA2 | 18 | 8237C>T | M | 1 | 9 | N/A | C15 | SD | |
| 2 | BRCA1 | 3 | 229C>G | M | 1 | 1 | N/A | C65 | SD | |
| 3 | BRCA1 | 11 | 1950C>G | M | 1 | 0 | 0.64 | C0 | x | SD |
| 4 | BRCA1 | 11 | 1694delTCA | F | 1 | 0 | N/A | N/A | x | SD |
| 5 | BRCA1 | 18 | 5242C>T | M | 1 | 0 | N/A | C65 | SV | |
| 6 | BRCA2 | 27 | 10315A>G | M | 1 | 0 | N/A | C0 | x | SV |
| 7 | BRCA1 | 11 | 1965delTCT | ID | 1 | 0 | N/A | N/A | x | SV |
| 8 | BRCA1 | 3 | IVS2-13C>G | I | 1 | 10 | 0.34 | N/A | SV | |
| 9 | BRCA1 | 17 | IVS16-20A>G | I | 2 | 26 | 0.3 | N/A | SV | |
| 10 | BRCA1 | 3 | IVS2-13C>A | I | 1 | 1 | 0.34 | N/A | SV | |
| 11 | BRCA1 | 3 | IVS2-13C>G | I | 1 | 10 | 0.34 | N/A | SV | |
| 12 | BRCA1 | 12 | 4300C>T | M | 1 | 0 | N/A | C55 | x | SV |
| 13 | BRCA1 | 22 | 5467T>C | M | 1 | 0 | N/A | C45 | x | SV |
| 14 | BRCA2 | 11 | 2074del3 | ID | 1 | 0 | N/A | N/A | x | SV |
| 15 | BRCA2 | 12 | 7084A>C | M | 1 | 0 | N/A | C45 | SV | |
| 16 | BRCA2 | 2 | 214A>C | 5'U | 1 | 11 | N/A | N/A | SV |
Abbreviations: Frameshift (F); Missense (M); Intronic (I); Inframe Deletion (ID); 5'Untranslated Region (5'U)
Abbreviations: Suspected Deleterious (SD); Straight VUS (SV)
A mutation was present in 30% of women with triple-negative disease and in 7.1% of women with ER-positive disease. The prevalence of mutations was 23.8% among women with a family history of early-onset breast and/or ovarian cancer and was 7.3% among women with no family history (or a minimal family history). With respect to family history, a mutation was found in 31.0% (27/87) of women with early onset breast cancer in the family and in 12.5% (6/48) of women with ovarian cancer in the family. BRCA carriers compared to non-carriers had a significantly higher frequency of: 1) triple-negative disease; 2) family history of breast and/or ovarian cancer; and 3) breast cancer age of onset ≤45 (Figure 2). Furthermore, of those with a BRCA mutation, 41% (20/49) had no first and/or second-degree relative with breast and/or ovarian cancer.
Figure 2.
BRCA carriers versus non-carriers
Given that national practice guidelines recommend referral for genetic counseling for any woman diagnosed with breast cancer at or under age 50,21 all participants met criteria for referral for genetic counseling. 359 women (90%) met the national guideline for genetic testing (breast cancer at ≤45 or breast cancer age 45–50 with a family history of cancer or triple-negative disease). The criteria did not discriminate well between those who did and who did not have a mutation; the mutation frequency was 12.8% (46 of 359) among those women who qualified for testing under national guidelines and was 8% (3 of 37) among those who did not qualify. In total, there were 118 participants who were diagnosed above age 45 with non-triple-negative breast cancer who did not meet BRCA testing criteria solely based on personal cancer history. Of these, nine (7.6%) had a BRCA mutation, which encompasses 18% of all carriers in our sample.
Discussion
In this population-based study from Florida, we estimate the mutation prevalence among young Black women with breast cancer to be approximately double that reported previously for NHW women in a similar age category.7 There have been three population-based studies in the US in which participants were recruited through a cancer registry and in which BRCA testing was conducted on both NHW and Black women.8,11,12 John et al13 reported a BRCA1 mutation frequency of 16.7% among Black women with breast cancer under 35 years from California, similar to our study where 19.4% of patients in this age group had a mutation. Malone et al6 reported that a BRCA1 mutation was present in 4.0% of Black cases (all ages) and in 4.5% for NHW women. Interestingly among Black women, BRCA2 mutations were more numerous (65% of the total) whereas BRCA1 mutations were more common in NHWs (53% of the total). In our study 17 of the 51 mutations (among the 49 BRCA carriers identified) were in BRCA2.
The prevalence of BRCA1 mutations declined with increasing age of diagnosis, but in BRCA2 was similar across all age groups recognizing that our sampling frame was limited to those diagnosed ≤50. The age distribution was similar among patients in Nigeria. Among 434 Nigerian women with breast cancer,22 the BRCA1 mutation frequency was 9.4% in women diagnosed before age 50 and was 3.6% in women diagnosed above age 50. In contrast, BRCA2 mutations were similar for women diagnosed below (3.4%) versus at or above 50 (4.7%).
Approximately 15% of breast cancers are triple-negative, but the proportion of triple-negatives is higher in Black women than in NHW women.5 In our study, 25% of the patients had a triple negative breast cancer. Among these, 30% had a BRCA1 mutation similar to the 26.5% frequency reported by Sharma et al23 among (primarily NHW) women with triple-negative breast cancer diagnosed before age 50. Among a clinic-based sample of 469 women with triple-negative breast cancer, the BRCA mutation prevalence was significantly lower among Blacks (20.4%) than NHW (33.3%) but the number of mutation carriers in this study was small. Taken together, these findings suggest that the higher rate of triple-negative disease does not fully account for the high BRCA mutation frequency observed in our study.
We identified eight recurrent mutations. Five of these (BRCA1 mutations 943ins10, M1775R (5443T>G), 5296del4, IVS13+G>A, and IVS16+6T>C) were also identified in the study based on data from an American commercial genetic testing company,24 where they comprised 31.5% of all mutations detected in African Americans. In our study, these five mutations constituted 34.7% of all mutations found. Similarly, 11 recurrent mutations accounted for over 60% of BRCA mutations in a large Nigerian study of breast cancer patients,22, 25 of which 1 was also seen in our study. We identified three recurrent mutations not previously reported (i.e., BRCA1 5370C>T, BRCA1 5506C>A, and BRCA2 4699del4) which accounted for 14.3% of the 49 mutations observed in our study. If one used a panel test based on only recurrent mutations only, the sensitivity might be 50%. However, given the decline in the cost of genetic testing it is probably unwise and inefficient to restrict mutation testing to a limited number of founder mutations in Black women.
Our findings suggest that, based on mutation prevalence, it is appropriate to recommend BRCA testing in all Black women with invasive breast cancer diagnosed before age 50, regardless of family history. This criterion benefits from simplicity and ease of application because it does not require knowledge of a patient’s family history or receptor status. Prior studies suggest that family histories are not always taken in oncology practices,26 and therefore, if family history is used to determine eligibility for testing, many women who are candidates might not be offered testing. In our study, 118 participants did not meet national practice criteria for testing solely based on personal history; of these 7.6% had a BRCA mutation. These women would qualify for testing based on our less stringent criteria.
BRCA status is becoming increasingly important at point of cancer diagnosis, to inform optimal treatment. Specifically, among women with triple-negative breast cancer, increasing evidence shows that neoadjuvant cis-platinum and oophorectomy are both effective treatments when targeted to women with a BRCA1 mutation.27 With regards to cancer prevention, testing itself does not ensure benefit; the ultimate benefit from genetic testing comes from uptake of cancer risk management options, including breast cancer screening, as well as preventive mastectomy or oophorectomy. In the absence of achieving such a benefit, there is little purpose to genetic testing. We do not have good estimates for the uptake of preventive mastectomy or oophorectomy in Black populations and our estimates of surgical preferences to date have been based on studies of NHW women.
In our study, a number of Black women expressed interest in being tested but did not follow through (244/882), the reason for which is unclear. Prior publications have also shown low uptake of BRCA testing among minority populations,24, 28 compared to NHW. Contributing factors are thought to include lower awareness and access to testing9–11 but other factors including patient preferences and attitudes to genetic testing should be considered. In our study, testing was free of charge and access to testing should not have influenced the decision. A recent study reported that among breast cancer patients who were interested in genetic testing, healthcare providers were less likely to discuss genetic testing with minority women compared to NHW women.27 Furthermore it is not clear to what extent Black women in Florida this study will communicate a positive test result to healthy relatives. In the Bahamas, Trottier et al reported that when the proband was the route of communication fewer than 20% of at risk relatives came for testing.29 Consequently, it is important that future studies which evaluate genetic testing among underserved populations also address uptake of cancer risk management options and sharing of test results with family members in order for the full potential of genetic testing for BRCA1 and BRCA2 to be realized.
Our study had several strengths. We recruited a representative sample of young Black breast cancer patients based on clinical and demographic variables available through the State Cancer Registry. Furthermore, our population-based study comprises the largest US-based sample of Black women ≤50 in whom comprehensive BRCA testing (including sequencing and large rearrangement testing of both BRCA1 and BRCA2) has been conducted. Limitations include the possibility for survival bias among participants given the lag between diagnosis and recruitment. However prior studies have not suggested mortality differences based on BRCA carrier status thus reducing the likelihood of impact to mutation prevalence estimates. Additionally, given that race was based on self-report rather than conduct of ancestry analyses, our findings are of particular relevance to the Florida-based population and may not be generalizable to all states. Moreover, although there were no differences in demographic and clinical variables between our participants compared to the entire sampling frame (Table 1), it is not possible to evaluate ascertainment bias based on family history because family history information is not available through our state cancer registry dataset. However, it is noteworthy that our initial recruitment materials framed the study goals broadly as evaluating the etiology and outcomes of breast cancer, rather than a study of genetic testing. Thus, it is unlikely those with stronger family histories are represented within our sample. Finally, given the Florida Black population encompasses women of Caribbean descent and the mutation rate is high in Bahamian women,31 we considered whether this may have contributed to our high mutation prevalence. However the mutation prevalence was slightly lower among women of Caribbean descent (7/64; 10.9%) than among the non-Caribbeans (42/332; 12.7%), which demonstrates that this did not inflate our observed mutation rates.
Our study represents amongst the largest efforts to recruit a population-based sample of young Black women for BRCA testing to evaluate mutation prevalence. The 12.4% BRCA mutation prevalence observed in our study is much higher than that previously reported in Caucasians, and suggests that BRCA mutations may account for the higher incidence of breast cancer observed among young Black women. Furthermore, 41% of women with BRCA mutations had no first and/or second-degree relative with breast and/or ovarian cancer, suggesting that family history may have limited specificity in identifying mutation carriers. Our findings suggest that it may be appropriate to recommend BRCA testing in all young Black women with invasive breast cancer.
Supplementary Material
Acknowledgments
We thank the following members of our Community Advisory Panel for their valuable input: Joyce Austin, Sue Friedman, Benita Hayes, Evora Pimento, Peggie Sherry, Cheryl Clinton, Gwendolyn Dawson, Gloria Wood, Linda Paige, Deneen Wyman, Khaliah Fleming, and Valerie Poindexter.
Research support:
This work was supported by Florida Biomedical (IBG10-34199), the American Cancer Society (RSG-11-268-01-CPPB), and the Florida Breast Cancer Foundation. Support for Deborah Cragun’s time was provided by a NCI R25T training grant (5R25CA147832-04). This work has been supported in part by the Biostatistics and Survey Core at the Moffitt Cancer Center (P30-CA076292). Data provided by the Florida Department of Health, Florida Cancer Data System (FCDS) are made available to aid public health surveillance and research to advance cancer control and prevention activities to better serve the population at risk for developing cancer and improve treatment for cancer patients. The contents of this study are solely the responsibility of the authors and do not necessarily reflect the official view of the Florida Department of Health, Florida Cancer Data System.
Footnotes
Conflict of Interest: The authors declare that no conflict of interest exists.
REFERENCES
- 1.Surveillance, epidemiology, and end results program (seer) 2015 [Google Scholar]
- 2.Iqbal J, Ginsburg O, Rochon PA, Sun P, Narod SA. Differences in breast cancer stage at diagnosis and cancer-specific survival by race and ethnicity in the united states. Jama. 2015;313:165–173. doi: 10.1001/jama.2014.17322. [DOI] [PubMed] [Google Scholar]
- 3.Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, Karaca G, Troester MA, Tse CK, Edmiston S, Deming SL, Geradts J, Cheang MC, Nielsen TO, Moorman PG, Earp HS, Millikan RC. Race, breast cancer subtypes, and survival in the carolina breast cancer study. Jama. 2006;295:2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 4.Lund MJ, Butler EN, Bumpers HL, Okoli J, Rizzo M, Hatchett N, Green VL, Brawley OW, Oprea-Ilies GM, Gabram SG. High prevalence of triple-negative tumors in an urban cancer center. Cancer. 2008;113:608–615. doi: 10.1002/cncr.23569. [DOI] [PubMed] [Google Scholar]
- 5.Young SR, Pilarski RT, Donenberg T, Shapiro C, Hammond LS, Miller J, Brooks KA, Cohen S, Tenenholz B, Desai D, Zandvakili I, Royer R, Li S, Narod SA. The prevalence of brca1 mutations among young women with triple-negative breast cancer. BMC cancer. 2009;9:86. doi: 10.1186/1471-2407-9-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malone KE, Daling JR, Doody DR, Hsu L, Bernstein L, Coates RJ, Marchbanks PA, Simon MS, McDonald JA, Norman SA, Strom BL, Burkman RT, Ursin G, Deapen D, Weiss LK, Folger S, Madeoy JJ, Friedrichsen DM, Suter NM, Humphrey MC, Spirtas R, Ostrander EA. Prevalence and predictors of brca1 and brca2 mutations in a population-based study of breast cancer in white and black american women ages 35 to 64 years. Cancer research. 2006;66:8297–8308. doi: 10.1158/0008-5472.CAN-06-0503. [DOI] [PubMed] [Google Scholar]
- 7.Nelson HD, Fu R, Goddard K, Mitchell JP, Okinaka-Hu L, Pappas M, Zakher B. Risk assessment, genetic counseling, and genetic testing for brca-related cancer: Systematic review to update the u.S. Preventive services task force recommendation. Rockville MD: 2013. [PubMed] [Google Scholar]
- 8.Couch FJ, Hart SN, Sharma P, Toland AE, Wang X, Miron P, Olson JE, Godwin AK, Pankratz VS, Olswold C, Slettedahl S, Hallberg E, Guidugli L, Davila JI, Beckmann MW, Janni W, Rack B, Ekici AB, Slamon DJ, Konstantopoulou I, Fostira F, Vratimos A, Fountzilas G, Pelttari LM, Tapper WJ, Durcan L, Cross SS, Pilarski R, Shapiro CL, Klemp J, Yao S, Garber J, Cox A, Brauch H, Ambrosone C, Nevanlinna H, Yannoukakos D, Slager SL, Vachon CM, Eccles DM, Fasching PA. Inherited mutations in 17 breast cancer susceptibility genes among a large triple-negative breast cancer cohort unselected for family history of breast cancer. J Clin Oncol. 2015;33:304–311. doi: 10.1200/JCO.2014.57.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Armstrong K, Micco E, Carney A, Stopfer J, Putt M. Racial differences in the use of brca1/2 testing among women with a family history of breast or ovarian cancer. Jama. 2005;293:1729–1736. doi: 10.1001/jama.293.14.1729. [DOI] [PubMed] [Google Scholar]
- 10.Levy DE, Byfield SD, Comstock CB, Garber JE, Syngal S, Crown WH, Shields AE. Underutilization of brca1/2 testing to guide breast cancer treatment: Black and hispanic women particularly at risk. Genet Med. 2011;13:349–355. doi: 10.1097/GIM.0b013e3182091ba4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pagan JA, Su D, Li L, Armstrong K, Asch DA. Racial and ethnic disparities in awareness of genetic testing for cancer risk. Am J Prev Med. 2009;37:524–530. doi: 10.1016/j.amepre.2009.07.021. [DOI] [PubMed] [Google Scholar]
- 12.Newman LA. Breast cancer in african-american women. Oncologist. 2005;10:1–14. doi: 10.1634/theoncologist.10-1-1. [DOI] [PubMed] [Google Scholar]
- 13.John EM, Miron A, Gong G, Phipps AI, Felberg A, Li FP, West DW, Whittemore AS. Prevalence of pathogenic brca1 mutation carriers in 5 us racial/ethnic groups. Jama. 2007;298:2869–2876. doi: 10.1001/jama.298.24.2869. [DOI] [PubMed] [Google Scholar]
- 14.Newman B, Mu H, Butler LM, Millikan RC, Moorman PG, King MC. Frequency of breast cancer attributable to brca1 in a population-based series of american women. Jama. 1998;279:915–921. doi: 10.1001/jama.279.12.915. [DOI] [PubMed] [Google Scholar]
- 15.Palma MD, Domchek SM, Stopfer J, Erlichman J, Siegfried JD, Tigges-Cardwell J, Mason BA, Rebbeck TR, Nathanson KL. The relative contribution of point mutations and genomic rearrangements in brca1 and brca2 in high-risk breast cancer families. Cancer research. 2008;68:7006–7014. doi: 10.1158/0008-5472.CAN-08-0599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roa B, Rosenthal E, Arnell C, Burbidge LA, Geary W, Schoenberger J, Trost J, Wenstrup R, Judkins T. Brca1 and brca2 large genomic rearrangement testing in a large cohort of hereditary breast/ovarian cancer patients: Prevalence and mutation profiles in risk-stratified patient groups of different ethnicities. ASHG Abstract. 2011 [Google Scholar]
- 17.Pal T, Rocchio E, Garcia A, Rivers D, Vadaparampil S. Recruitment of black women for a study of inherited breast cancer using a cancer registry-based approach. Genet Test Mol Biomarkers. 15:69–77. doi: 10.1089/gtmb.2010.0098. [DOI] [PubMed] [Google Scholar]
- 18.Zhang S, Royer R, Li S, McLaughlin JR, Rosen B, Risch HA, Fan I, Bradley L, Shaw PA, Narod SA. Frequencies of brca1 and brca2 mutations among 1,342 unselected patients with invasive ovarian cancer. Gynecologic oncology. 2011;121:353–357. doi: 10.1016/j.ygyno.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 19.Plon SE, Eccles DM, Easton D, Foulkes WD, Genuardi M, Greenblatt MS, Hogervorst FB, Hoogerbrugge N, Spurdle AB, Tavtigian SV. Sequence variant classification and reporting: Recommendations for improving the interpretation of cancer susceptibility genetic test results. Hum Mutat. 2008;29:1282–1291. doi: 10.1002/humu.20880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.BIC. Breast cancer information core. 2015;2015 Web-site: Http://www.Nhgri.Nih.Gov/intramural_research/lab_transfer/bic/ [Google Scholar]
- 21.Genetic/familial high-risk assessment: Breast and ovarian. NCCN Practice Guidelies. 2015;2015 [Google Scholar]
- 22.Fackenthal JD, Zhang J, Zhang B, Zheng Y, Hagos F, Burrill DR, Niu Q, Huo D, Sveen WE, Ogundiran T, Adebamowo C, Odetunde A, Falusi AG, Olopade OI. High prevalence of brca1 and brca2 mutations in unselected nigerian breast cancer patients. International journal of cancer. Journal international du cancer. 2012;131:1114–1123. doi: 10.1002/ijc.27326. [DOI] [PubMed] [Google Scholar]
- 23.Sharma P, Klemp JR, Kimler BF, Mahnken JD, Geier LJ, Khan QJ, Elia M, Connor CS, McGinness MK, Mammen JM, Wagner JL, Ward C, Ranallo L, Knight CJ, Stecklein SR, Jensen RA, Fabian CJ, Godwin AK. Germline brca mutation evaluation in a prospective triple-negative breast cancer registry: Implications for hereditary breast and/or ovarian cancer syndrome testing. Breast Cancer Res Treat. 2014;145:707–714. doi: 10.1007/s10549-014-2980-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hall MJ, Reid JE, Burbidge LA, Pruss D, Deffenbaugh AM, Frye C, Wenstrup RJ, Ward BE, Scholl TA, Noll WW. Brca1 and brca2 mutations in women of different ethnicities undergoing testing for hereditary breast-ovarian cancer. Cancer. 2009;115:2222–2233. doi: 10.1002/cncr.24200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang J, Fackenthal JD, Zheng Y, Huo D, Hou N, Niu Q, Zvosec C, Ogundiran TO, Hennis AJ, Leske MC, Nemesure B, Wu SY, Olopade OI. Recurrent brca1 and brca2 mutations in breast cancer patients of african ancestry. Breast Cancer Res Treat. 2012;134:889–894. doi: 10.1007/s10549-012-2136-z. [DOI] [PubMed] [Google Scholar]
- 26.Wood ME, Kadlubek P, Pham TH, Wollins DS, Lu KH, Weitzel JN, Neuss MN, Hughes KS. Quality of cancer family history and referral for genetic counseling and testing among oncology practices: A pilot test of quality measures as part of the american society of clinical oncology quality oncology practice initiative. J Clin Oncol. 2014;32:824–829. doi: 10.1200/JCO.2013.51.4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Isakoff SJ, Mayer EL, He L, Traina TA, Carey LA, Krag KJ, Rugo HS, Liu MC, Stearns V, Come SE, Timms KM, Hartman AR, Borger DR, Finkelstein DM, Garber JE, Ryan PD, Winer EP, Goss PE, Ellisen LW. Tbcrc009: A multicenter phase ii clinical trial of platinum monotherapy with biomarker assessment in metastatic triple-negative breast cancer. J Clin Oncol. 2015 doi: 10.1200/JCO.2014.57.6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hall MJ, Olopade OI. Disparities in genetic testing: Thinking outside the brca box. J Clin Oncol. 2006;24:2197–2203. doi: 10.1200/JCO.2006.05.5889. [DOI] [PubMed] [Google Scholar]
- 29.Trottier M, Lunn J, Butler R, Curling D, Turnquest T, Royer R, Akbari MR, Donenberg T, Hurley J, Narod SA. Strategies for recruitment of relatives of brca mutation carriers to a genetic testing program in the bahamas. Clin Genet. 2014 doi: 10.1111/cge.12468. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.