Abstract
Background
The mechanism(s) of persistent and long-standing persistent (LSP) atrial fibrillation (AF) is/are poorly understood. We performed high density, simultaneous, bi-atrial, epicardial mapping of persistent and LSP AF in patients undergoing open heart surgery (OHS) 1) to test the hypothesis that persistent and LSP AF are due to one or more drivers, either focal or reentrant, and 2) to characterize associated atrial activation.
Methods and Results
Twelve patients with persistent and LSP AF (1 month - 9 years duration) were studied at OHS. During AF, electrograms (AEGs) were recorded from both atria simultaneously for 1-5 minutes from 510-512 epicardial electrodes with ECG lead II. Thirty-two consecutive seconds of activation sequence maps were produced per patient. During AF, multiple foci (QS unipolar AEGs) of different cycle lengths (mean 175±18 ms) were present in both atria in 11/12 patients. Foci (2-4 per patient, duration 5-32 secs) were either sustained or intermittent, were predominantly found in the lateral left atrial free wall, and likely acted as drivers. Random and nonrandom breakthrough activation sites (initial r or R in unipolar AEGs) were also found. In 1/12 patients, only breakthrough sites were found. All wave fronts emanated from foci and/or breakthrough sites, and largely either collided or merged with each other at variable sites. Repetitive focal QS activation occasionally generated repetitive wannabe reentrant activation in 5/12 patients. No actual reentry was found.
Conclusions
During persistent and LSP AF in 12 patients, wave fronts emanating from foci and/or breakthrough sites maintained AF. No reentry was demonstrated.
Keywords: atrial fibrillation, arrhythmia (mechanisms), electrophysiology mapping, cardiac electrophysiology, reentry
Introduction
Over the years, there have been many mapping studies of persistent and long-standing persistent (LSP) atrial fibrillation (AF) in patients. They involved recording of atrial electrograms (AEGs) from variable numbers and types of electrodes; sequential or simultaneous multisite site mapping; and endocardial and/or epicardial mapping, the latter including body surface potential mapping.1-14 Nevertheless, the mechanism(s) that maintain persistent and LSP AF is/are poorly understood.15 Focal and reentrant drivers, i.e., a source or sources responsible for maintaining AF, have been reported, but not well characterized, and there remain many questions about recording techniques and analysis algorithms used.16 We performed high density, simultaneous, multisite (510 - 512 electrodes), bi-atrial, epicardial mapping of persistent and LSP AF in patients undergoing open heart surgery 1) to test the hypothesis that persistent and LSP AF is due to a driver which is either focal or reentrant, and 2) to further characterize its associated atrial activation.
Methods
The research protocol was approved by the committee on the conduct of human research at University Hospitals Case Medical Center. Twelve patients with persistent and LSP AF (1 month - 9 years duration) were studied at open heart surgery. All patients gave written informed consent before their surgery. All patients had multiple comorbidities including valvular heart disease (12/12), hypertension (11/12), heart failure (10/12), coronary heart disease (2/12), and diabetes (1/12). (Table 1)
Table 1.
Patient Characteristics.
| Patient No. | Age | Gender | AF Duration | CAD | Valvular Disease | Hypertension | Heart Failure | Diabetes |
|---|---|---|---|---|---|---|---|---|
| 1 | 80 | F | 3 months | – | MR, TR | + | + | – |
| 2 | 60 | M | > 1 year | – | MR | + | + | – |
| 3 | 57 | M | > 1 year | – | MS | + | + | – |
| 4 | 68 | F | 1 month | – | MR | + | – | – |
| 5 | 67 | M | 1 month | – | MR | + | + | – |
| 6 | 62 | M | 1 month | – | MR | – | + | – |
| 7 | 79 | F | > 1 year | – | TR | + | + | – |
| 8 | 78 | M | 9 years | + | AS | + | – | – |
| 9 | 70 | M | 9 years | – | AS,TR | + | + | – |
| 10 | 70 | F | 8.5 years | – | AS | + | + | + |
| 11 | 80 | F | 2.5 years | – | TR | + | + | – |
| 12 | 63 | M | > 1 year | + | MR,TR | + | + | – |
AS, aortic stenosis; CAD, coronary artery disease; MR, mitral regurgitation; TR, tricuspid regurgitation; +, present; and –absent
Simultaneous, Bi-Atrial, High Density, Epicardial Mapping of Persistent and Long-Standing Persistent AF During Open Heart Surgery
(a) Data Acquisition
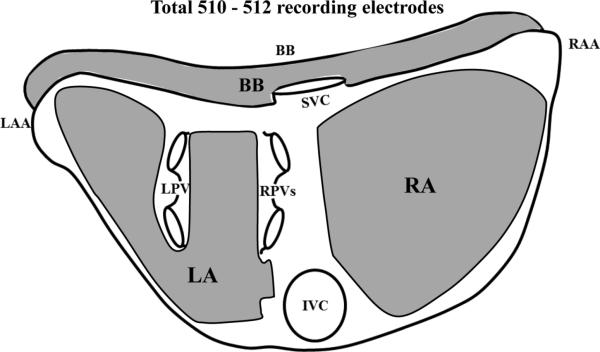
Atrial epicardial mapping studies were performed during open heart surgery in patients with persistent and LSP AF after the heart was exposed using standard surgical procedures. Studies were performed on a beating heart at normothermia either prior to or during cardiopulmonary bypass. All patients underwent transesophageal echocardiography study prior to mapping AF to assess the presence of a left atrial thrombus. If the latter was found, the patient did not undergo mapping. Three electrode arrays with a total of 510 - 512 electrodes covering a total area of 92.85 cm2 were placed on the atrial epicardial surface for simultaneous recording. The mapping arrays had 2 versions for electrode distribution. Version 1, used in the first four patients studied, contained a total of 510 unipolar electrodes arranged in pairs: left atrium (LA) - 232 electrodes, right atrium (RA) - 166 electrodes, Bachmann's bundle (BB) - 112 electrodes. The interelectrode distance between each bipolar electrode pair in the array was 1.2 mm, and the distance between the center of each bipolar pair and its neighbors varied from 5.6 -7.0 mm. Version 2, used in the next 8 patients, had a total of 512 unipolar electrodes arranged in pairs: LA - 256 electrodes, RA - 160 electrodes, BB - 96 electrodes. The interelectrode distance between each bipolar electrode pair was 1.5 mm, and the distance between the center of each bipolar pair and its neighbors varied from 5.2 -7.0 mm. Both versions were the same shape, and covered the same total area (Figure 1). AEGs from 510 - 512 electrodes (255 - 256 bipolar pairs) along with ECG lead II were simultaneously recorded for 1-5 minutes during persistent and LSP AF. Data were digitally recorded, and processed with an Active Two system (BioSemi, Amsterdam, The Netherlands). All AEGs were sampled at 1,024 Hz, and digitized at 24 bits. Data were transferred in real time, and stored on a personal computer for further analysis (CEPAS, Cuoretech Pty Ltd, Sydney, Australia).
Figure 1.
Locations of the epicardial recording arrays covering a total area of 92.85 cm2. BB, Bachmann's bundle; RA, right atrium; RAA, right atrial appendage; LA, left atrium; LAA, left atrial appendage; SVC, superior vena cava; IVC, inferior vena cava; LPVs, left pulmonary veins; RPVs, right pulmonary veins. See text for discussion.
(b) Activation Sequence Analysis
Sequential activation maps of persistent and LSP AF were constructed for a period of 32 consecutive seconds per patient. Data were analyzed automatically for activation times using a custom designed algorithm. Data in both their raw unipolar format, and computer-processed bipolar format were used to assist in the manual selection of activation times when necessary. The following was the method of selection of bipolar activation times used in our study.17 The moment of activation at each site was taken as 1) the peak of the rapid deflection in a predominantly monophasic AEG, 2) the time when the first rapid deflection crossed the baseline in a predominantly biphasic AEG, or 3) for sites in which polyphasic electrograms (AEGs with two or more deflections) were recorded, activation was assigned to the major deflection (highest amplitude or fastest downstroke). If there were two discrete deflections during one atrial complex in the AEGs (i.e., a double potential), the activation was assigned to the highest amplitude peak. Activation from neighboring sites was also used to aid in determining activation of complex electrograms recorded during high density mapping. Propagation of activation was depicted on activation sequence maps using 10 millisecond isochronal lines. All isochronal lines were determined by activation times from recorded AEGs. All bipolar AEGs were subjected to cycle length (CL) variation and dominant frequency (DF) analyses to detect mean CL, standard deviation (SD), and DF.18 Data were presented as the mean CL ± SD since 1,000/DF was equivalent to the mean CL.
(c) Analysis of Unipolar Atrial Electrogram Morphology
Once the activation sequence maps were constructed, the earliest sites of activation compared to their neighbors were identified, and the morphology of the unipolar electrogram was characterized at these sites.
Definitions
A focus was defined as a site a) of the earliest activation compared to its neighboring sites, b) manifesting a QS morphology in the unipolar AEG, and c) from which wave fronts emanated. A sustained focus was defined as a focus from which wave fronts emanated continuously during the 32 seconds of analysis. An intermittent focus was defined as a focus that was not continuous during 32 seconds of analysis. A breakthrough activation site was defined as a site a) of earliest activation compared to its neighbors, b) manifesting a unipolar AEG morphology with an initial r or R wave, and c) from which wave fronts emanated. Nonrandom breakthrough activation was defined as a breakthrough activation site that a) recurred, b) had at least one episode in which there were three or more consecutive breakthrough activations, and c) from which wave fronts emanated. All other breakthrough activation sites were considered random. Reentry was defined as circus movement with head–tail interaction. “Wannabe” reentry was defined as a wave front that circulated around a functional line of block that wanted-to-become (“wannabe”) a reentrant circuit, but could not complete the revolution because it either collided with a wave front from a focal or breakthrough site, or encountered a line of block.17
Results
Overall Findings
Table 2 shows summary data for all patients. During persistent and LSP AF, multiple foci (mean 2.7) of different CLs were present in both atria in 11/12 patients. Foci were either sustained or intermittent during the 32 seconds of analysis. In 6/12 patients, sustained foci (mean 1.3, range 1 – 2) were identified, and in 11/12 patients, intermittent foci (mean 2, range 1 – 3) were identified. A total of eight sustained foci were identified (mean CL 170 ± 19 ms, range 142 – 200 ms; duration 32 secs). A total of 21 intermittent foci were present (mean CL 176 ± 18 ms, range 143 – 211 ms). The longest duration of consecutive QS activation of an intermittent focus ranged from 1.4 – 19.7 secs. The temporal behavior of intermittent foci, i.e. the duration of individual episodes of QS activation, was variable: bursts of QS activation lasted 0.2 – 19.7 secs (2 – 113 beats), and the total duration of QS activation per intermittent focus (sum of all bursts) ranged from 5 – 30.5 secs (see supplementary data, Figure 1). Periods during which the intermittent focal QS activation was inactive were associated with activation of that site by a wave front from other focal or breakthrough sites. No reentry was demonstrated in any patients studied. However, wannabe reentry was present in all 12 patients. In 5/12 patients, repetitive focal QS activation occasionally generated repetitive wannabe reentry, the longest duration ranged from 0.4 – 9.2 secs. Nonrandom breakthrough activations were present in 7/12 patients, including the one patient without any demonstrated focal QS activation. Their mean CLs ranged from 136 – 195 ms, and their longest duration ranged from 0.3 – 2.7 secs. Random breakthrough activations were present in all patients. In 1/12 patients, during the 32 secs of analysis, no foci were identified, but several breakthrough sites, both random and nonrandom, were present.
Table 2.
Summary of analysis data during persistent and long-standing persistent AF.
| Patient No. | Total # foci | Sustained foci |
Intermittent foci |
Nonrandom breakthrough |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| # | Location | Mean CL ± SD(ms) | # | Location | Longest duration (s) | Mean CL ± SD(ms) | # | Location | Longest duration (s) | Mean CL ± SD(ms) | ||
| 1 | 4 | 1 | LA | 179 ± 8 | 3 | right BB btwn PV left BB |
9.8 3.9 3.3 |
178 ± 11 170 ± 12 179 ± 12 |
– | |||
| 2 | 3 | – | 3 | left BB right BB LA |
5.6 8.7 1.4 |
184 ± 16 196 ± 17 172 ± 4 |
1 | LA | 0.7 | 195 ± 11 | ||
| 3 | – | – | – | 2 | LA LA |
2.7 2.5 |
174 ± 15 175 ± 16 |
|||||
| 4 | 3 | 2 | LA btwn PV |
182 ± 11 200 ± 13 |
1 | right BB | 3.7 | 171 ± 12 | 1 | RA | 1.2 | |
| 5 | 2 | 1 | left BB | 147 ± 10 | 1 | RA | 4 | 164 ± 17 | 2 | right BB right BB |
2.3 1.4 |
173 ± 15 139 ± 21 |
| 6 | 3 | 2 | left BB RA |
168 ± 10 174 ± 11 |
1 | right BB | 7.7 | 177 ± 14 | – | |||
| 7 | 2 | – | 2 | LA btwn PV |
13.5 3.4 |
188 ± 9 203 ± 13 |
– | |||||
| 8 | 2 | – | 2 | btwn PV LA |
2.9 2.7 |
211 ± 33 169 ± 9 |
1 | LA | 1.5 | 187 ± 21 | ||
| 9 | 3 | – | 3 | left BB btwn PV LA |
19.7 7.3 3.7 |
174 ± 8 182 ± 4 145 ± 12 |
1 | LA | 1.5 | 153 ± 24 | ||
| 10 | 2 | – | 2 | LA right BB |
9.2 5.7 |
179 ± 9 201 ± 12 |
– | |||||
| 11 | 2 | 1 | LA | 171 ± 9 | 1 | btwn PV | 9.9 | 185 ± 14 | – | |||
| 12 | 4 | 1 | LA | 142 ± 6 | 3 | RA RA RA |
4.4 4.3 2 |
143 ± 9 159 ± 12 149 ± 8 |
2 | LA btwn PV |
1.1 0.3 |
177 ± 13 136 ± 1 |
| mean | 2.7 ± 0.8 | 1.3 ± 0.5 | 170 ± 19 | 2 ± 0.9 | 62 ± 4 | 176 ± 18 | 1.4 ± 0.5 | 1.5 ± 1 | 166 ± 20 | |||
BB, Bachmann's Bundle; btwn, between; CL, cycle length; LA, left atrium; PV, pulmonary veins; RA, right atrium; SD, standard deviation; – , None
The locations of all focal and nonrandom breakthrough sites are summarized in Table 2. The foci were found in both atria. However, sustained foci were predominantly located in the LA. All wave fronts emanating from sustained and intermittent foci, as well as breakthrough activation sites (random and nonrandom) contributed to the colliding and merging of wave fronts at different sites, and occasionally generated fibrillatory conduction manifested by a functional line of block. Foci likely acted as drivers because of their QS morphology, the fact that they repetitively manifested the earliest activation compared to their neighboring sites, and wave fronts always emanated from them. The same was true for breakthrough sites, except they all manifested initial r or R waves in their recorded unipolar AEGs. We were unable to identify the likely site of origin for the epicardial breakthrough activation.
Spatial Distribution of Foci and Breakthrough Sites and Temporal Behavior of Foci
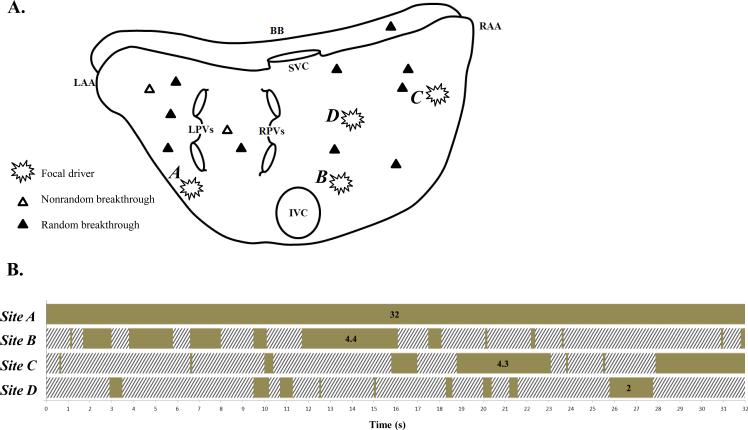
Figure 2 is a representative example of AF due to activation from multiple foci and breakthrough sites of different CLs (Table 2, patient #12). The top panel (A) is a summary of the location of foci and breakthrough activation sites identified in the activation sequence maps during 32 seconds. Four foci identified by burst symbols ( ) were present, one sustained (site A) in the LA, and 3 intermittent (sties B, C, and D) in the RA. Also, several breakthrough sites were present in both atria. A nonrandom breakthrough site was identified by an open delta symbol (Δ), and a random breakthrough site by a closed delta symbol (▲). Site A had a mean CL of 142 ± 6, range 130 – 154 ms, and a duration of 32 secs. The three intermittent foci (B, C, and D) had mean CLs of 143 ± 9, 159 ± 12, and 149 ± 8 ms, respectively. In these latter three sites, the longest continuous periods of focal QS activation were 4.4 secs, 4.3 secs, and 2 secs, respectively. These data are summarized in Table 2. The bottom panel (B) of Figure 2 shows the temporal behavior of focal QS activation during the analyzed 32 seconds. The time bar illustrates the time course of active (solid) and inactive (hatched) focal QS activation from selected focal sites A – D (see following).
) were present, one sustained (site A) in the LA, and 3 intermittent (sties B, C, and D) in the RA. Also, several breakthrough sites were present in both atria. A nonrandom breakthrough site was identified by an open delta symbol (Δ), and a random breakthrough site by a closed delta symbol (▲). Site A had a mean CL of 142 ± 6, range 130 – 154 ms, and a duration of 32 secs. The three intermittent foci (B, C, and D) had mean CLs of 143 ± 9, 159 ± 12, and 149 ± 8 ms, respectively. In these latter three sites, the longest continuous periods of focal QS activation were 4.4 secs, 4.3 secs, and 2 secs, respectively. These data are summarized in Table 2. The bottom panel (B) of Figure 2 shows the temporal behavior of focal QS activation during the analyzed 32 seconds. The time bar illustrates the time course of active (solid) and inactive (hatched) focal QS activation from selected focal sites A – D (see following).
Figure 2.
Data from a representative example of AF due to activation from multiple foci and breakthrough sites of different CLs (from patient #12). Top Panel (A): Summary of the location of foci and breakthrough sites seen in activation sequence maps during 32 seconds of analysis. Foci are denoted by burst symbols ( ). Breakthrough activation is denoted by open or closed delta symbols (Δ identifies a nonrandom breakthrough site; ▲ identifies a random breakthrough site). See text for discussion. Bottom Panel (B): Temporal behavior of focal QS activation with a time bar indicating focal QS activation from selected sites A - D during the analyzed 32 seconds (patient #12). The time bar illustrates the time course of active (solid) and inactive (hatched) focal QS activation from selected focal sites (see text for discussion). BB, Bachmann's bundle; RA, right atrium; RAA, right atrial appendage; LA, left atrium; LAA, left atrial appendage; SVC, superior vena cava; IVC, inferior vena cava; LPVs, left pulmonary veins; RPVs, right pulmonary veins.
). Breakthrough activation is denoted by open or closed delta symbols (Δ identifies a nonrandom breakthrough site; ▲ identifies a random breakthrough site). See text for discussion. Bottom Panel (B): Temporal behavior of focal QS activation with a time bar indicating focal QS activation from selected sites A - D during the analyzed 32 seconds (patient #12). The time bar illustrates the time course of active (solid) and inactive (hatched) focal QS activation from selected focal sites (see text for discussion). BB, Bachmann's bundle; RA, right atrium; RAA, right atrial appendage; LA, left atrium; LAA, left atrial appendage; SVC, superior vena cava; IVC, inferior vena cava; LPVs, left pulmonary veins; RPVs, right pulmonary veins.
Unipolar QS Electrogram Morphology at Focal Sites
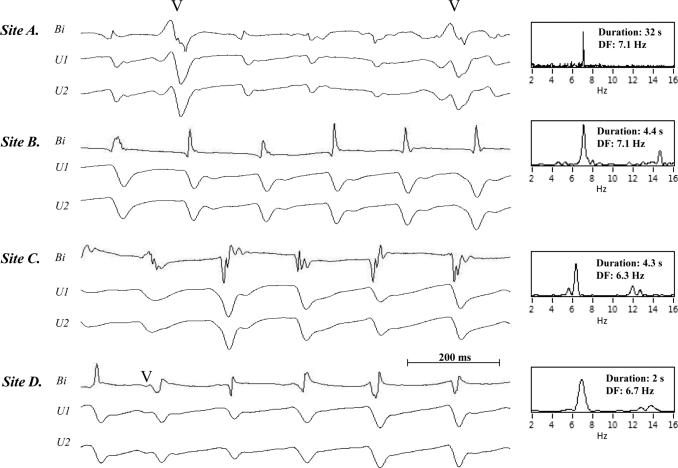
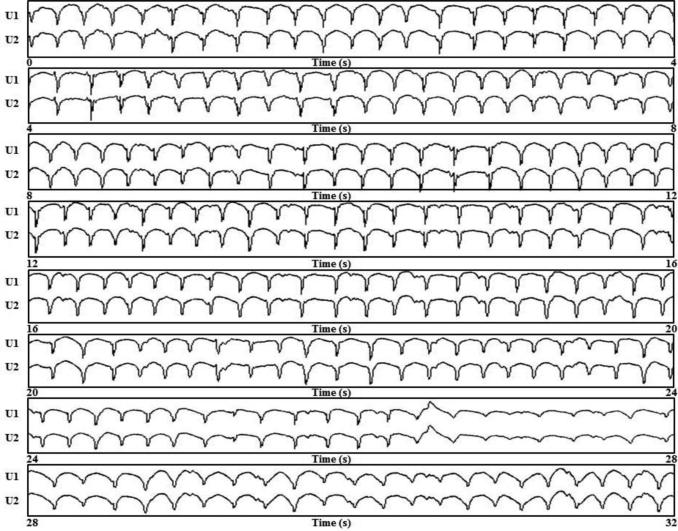
Figure 3 shows a representative example of selected bipolar AEGs along with each unipolar component of the bipolar AEGs from sustained focal site A (recording time 0.6 – 1.5 secs), and from intermittent focal sites B (recording time 4.2 – 5.1 secs), C (recording time 20 – 20.9 secs) and D (recording time 27 – 27.9 secs). All sites and recording times were referenced from Figure 2 (Table 2, LSP AF patient #12). In the right panel of Figure 3, DFs of the bipolar AEGs are shown for the longest episode of continuous QS activation which occurred in the analyzed 32 seconds. The unipolar AEGs from the four foci, A–D, demonstrated a QS morphology, indicating that each site was likely the origin of a focal impulse. However, occasionally a small r wave appeared at the beginning of some of the unipolar complexes, suggesting that the precise site of origin of the impulse moved slightly. Figure 4 shows continuous 32 seconds from a sustained focus with unipolar AEGs having a QS morphology from a patient with persistent AF (Table 2, patient # 6). These unipolar morphologies were typical of all sustained and intermittent foci present in all persistent and LSP AF patients.
Figure 3.
A representative example (patient #12) of focal QS morphology. Selected bipolar AEGs along with each unipolar component of the bipolar AEG from the one sustained focus and three intermittent foci during QS activation previously shown in the time bar activation summary in Figure 2 (A is shown during 0.6 – 1.5 secs recording period, B is shown during 4.2 – 5.1 secs recording period, C is shown during 20 – 20.9 secs recording period, D is shown during 27 – 27.9 secs recording period). The power spectrum is shown to the right of the traces in each panel with the dominant frequency for the DF analysis during the longest continuous activation duration (A: 32 secs recording period, B: 4.4 secs recording period, C: 4.3 secs recording period, and D: 2 secs recording period in Figure 2).
Figure 4.
A representative example of both unipolar AEGs showing a QS morphology during 32 continuous seconds of sustained focal activation from a site in patient # 6 (Table 2).
Activation Sequence Maps Generated from Comprehensive Analysis
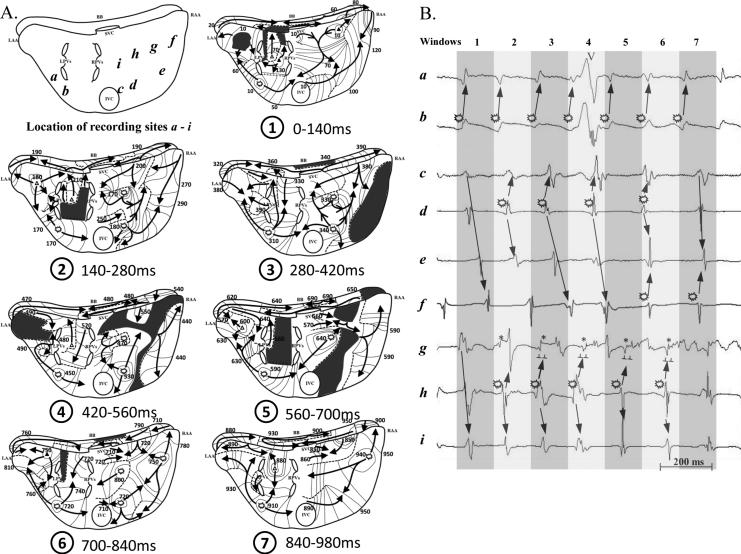
Figure 5 is a representative example of AF associated with multiple foci of different CLs in patient #12 (Table 2). Figure 5A shows activation sequence maps during seven consecutive 140 ms windows (from 9.4 to 10.2 secs in the Figure 2B time bar), and a schematic identifying locations of recording sites a - i. Bipolar AEGs from selected sites a through i (locations identified on the schematic) are shown in Figure 5B. For each 140 ms window, both atria were activated by wave fronts emanating from a variable number of focal and breakthrough sites. One sustained focus was present in the posterior LA free wall ( site b), and three intermittent foci were present: one in the low RA (
site b), and three intermittent foci were present: one in the low RA ( site d); one in the sulcus terminalis region (
site d); one in the sulcus terminalis region ( site h); and one in the high RA (
site h); and one in the high RA ( site f). Also, five distinct breakthrough sites were present: two between the region of the pulmonary veins (PVs); one in the high RA region; and two in the LA free wall. As demonstrated in all activation windows, wave fronts propagated away from their respective focal and breakthrough sites, and then largely either merged with and/or collided with other wave fronts. Note that activation from focal sites did not always result in radial activation due to collision or merging with another wave front or encountering a functional line of block.
site f). Also, five distinct breakthrough sites were present: two between the region of the pulmonary veins (PVs); one in the high RA region; and two in the LA free wall. As demonstrated in all activation windows, wave fronts propagated away from their respective focal and breakthrough sites, and then largely either merged with and/or collided with other wave fronts. Note that activation from focal sites did not always result in radial activation due to collision or merging with another wave front or encountering a functional line of block.
Figure 5.
A representative example of AF associated with multiple foci of different CLs. A: Activation sequence maps during 7 consecutive 140 ms windows, and a schematic (upper left panel) identifying the locations of recording sites a - i. Foci are denoted by burst symbols ( ). Breakthrough activation is denoted by open or closed delta symbols (Δ identifies a nonrandom breakthrough site; ▲ identifies a random breakthrough site). The black arrows indicate activation wave fronts. Black areas identify areas not activated during each 140 ms window. Dashed lines indicate a functional line of block. See text for discussion. B: Bipolar AEGs from selected sites a through i (locations identified on the schematic in panel A) during each time frame, i.e., each 140ms windows. The alternating dark and light gray columns represent each activation window in panel A. See text for discussion.
). Breakthrough activation is denoted by open or closed delta symbols (Δ identifies a nonrandom breakthrough site; ▲ identifies a random breakthrough site). The black arrows indicate activation wave fronts. Black areas identify areas not activated during each 140 ms window. Dashed lines indicate a functional line of block. See text for discussion. B: Bipolar AEGs from selected sites a through i (locations identified on the schematic in panel A) during each time frame, i.e., each 140ms windows. The alternating dark and light gray columns represent each activation window in panel A. See text for discussion.
Wave fronts from the one sustained focus (site b) produced a pattern of collision seen over several consecutive activation windows. The posterior LA region's wave fronts from sustained focus b spread to the LA free wall, the PV area, and the border of the inferior vena cava (IVC). The wave front from this focus (b) collided with wave fronts from intermittent foci d (window 3) and h (window 5), and collided with a wave front from a breakthrough site in the high RA (window 1). Selected AEGs are illustrated in Figure 5B, with burst symbols, and propagation arrows illustrating the above description.
Wave fronts from each of the three intermittent foci (sites d, f, and h) produced a pattern of collision and merging with other wave fronts. The low RA free wall region's wave front from intermittent focus d spread radially to the RA free wall and intercaval region areas. The wave front from this focus (d) collided with wave fronts from sustained focus b (window 3), intermittent focus h (window 4), and intermittent focus f (window 6). It also merged with a wave front from intermittent focus h in the RA free wall (windows 2 and 3). In addition, focal QS activation from intermittent focus d was occasionally suppressed or concealed by an activation wave front from the border of the IVC (windows 1 and 7). The high RA free wall region's wave front from intermittent focus f spread radially to the RA free wall. The wave front from this focus (f) collided with the wave fronts from intermittent focus d in the high RA (window 6) and the border of the IVC (window 7). Also, focal QS activation from intermittent focus f was occasionally suppressed or concealed by an activation wave front from intermittent focus d (windows 4 and 5) or the border of the IVC (window 1). The sulcus terminalis region's wave fronts from intermittent focus h were blocked at a functional line of block in the superior direction and spread inferiorly to the RA free wall and intercaval regions. The wave front from this focus (h) collided with the wave fronts from sustained focus b (window 5) and an intermittent focus d in the high RA (window 4). In addition, there was merging of wave fronts in the RA free wall (windows 2 and 3). These wave fronts were from intermittent focus d. Focal QS activation from intermittent focus h was occasionally suppressed or concealed by an activation wave front from intermittent focus f (window 7) or a breakthrough site (window 1). Selected AEGs are shown in Figure 5B with burst symbols and propagation arrows illustrating the above description.
Wave fronts from all breakthrough sites merged with and/or collided either with other wave fronts or a functional line of block. For example, in window 1, a breakthrough wave front located in the high RA propagated both in the direction of the mid RA free wall and to the right portion of BB. The mid RA wave front merged with a wave front from the area of the superior vena cava (SVC). This merged wave front collided with both a wave front from LA focal site b and a wave front from the border of the IVC. The wave front propagating to the right portion of BB merged with a wave front propagating from left-to-right on BB at 80ms. This merged wave front then continued to activate the right portion of BB, propagating around to the mid lateral RA free wall before it collided at 120ms with a wave front from the border of IVC.
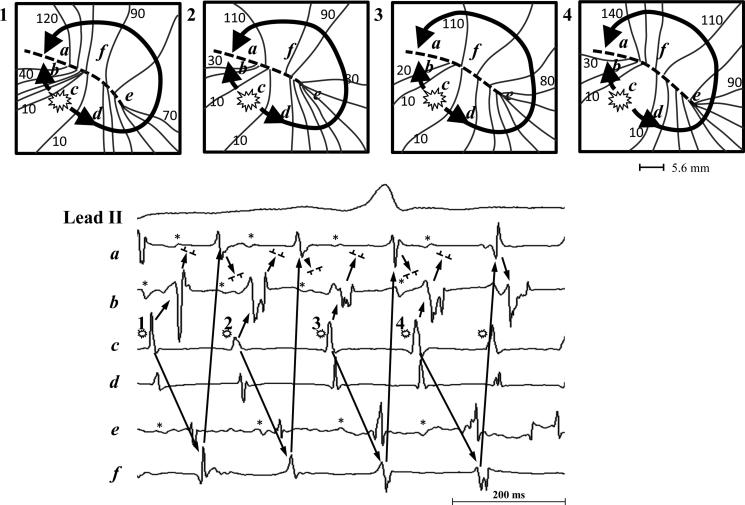
Focal QS Activation Mimicking Reentry
Occasionally focal QS activation generated a pivoting wave front around a functional line of block that initiated wannabe reentry. Figure 6 is a representative example of a repetitive wannabe reentry pattern initiated by an intermittent focus in the RA which occurred during recording time 12 - 12.6 secs (Site B in Figure 2, patient #12 in Table 2). The top panel of Figure 6 shows a zoomed in area of the activation maps of four consecutive beats during AF. The bottom panel of Figure 6 shows selected AEGs recorded simultaneously during AF from a focus (site c) and five nearby sites (a, b, d - f) around a functional line of block (dashed line). The wave front generated from the repetitive QS activation of the intermittent focal site c produced a repetitive wannabe reentrant activation. This occurred when propagation from the focus traveling along one side of a functional line of block pivoted around one end, but failed to complete the rotation when it collided with a wave front coming from the opposite direction which was also generated from the same focus. This activation sequence was repeated over a period of 2.5 seconds. Repetitive focal QS activation generating repetitive wannabe reentry was identified in 5/12 patients (see supplementary data, video 1 and Figure 2).
Figure 6.
A representative example of a repetitive focal QS activation generating repetitive wannabe reentry. Top panel: A zoomed in area of the activation maps of four consecutive beats during AF. Bottom panel: Selected AEGs recorded simultaneously during AF from a focus (site c) and five nearby sites (a, b, d - f) around a functional line of block (dashed line). See text for discussion.
Discussion
Major Findings
In our high density simultaneous bi-atrial epicardial mapping study in 12 patients with persistent and LSP AF, we generated 32 seconds of classical activation sequence maps per patient from activation times that were determined by a comprehensive analysis of unipolar AEGs, bipolar AEGs, and activation of neighboring sites; and also characterized unipolar electrograms at the earliest activation sites. Our results demonstrated that 1) persistent and LSP AF were maintained by activation wave fronts emanating from both foci and/or breakthrough sites, and they likely acted as drivers; 2) foci were either sustained or intermittent, and had a characteristic QS morphology in the unipolar atrial electrogram; 3) intermittent foci were more common than sustained foci; 4) breakthrough sites were all intermittent (random or nonrandom), were characterized by rS or RS morphology of the unipolar atrial electrograms, were present in all patients, and, in one patient, were the only source of wave fronts observed; 5) nonrandom breakthrough behaved like intermittent foci; 6) the atrial activation patterns during persistent and LSP AF generated from foci and breakthrough sites were largely of collision and merging of wave fronts at continuously varying sites, with occasional fibrillatory conduction manifested by a functional line of block; 7) all wave fronts propagating on the atrial epicardium extinguished by collision or merging with other wave fronts, and sometimes by colliding with a functional line of block; 8) focal QS activation occasionally generated repetitive wannabe reentrant activation patterns; 9) no reentrant circuits were demonstrated.
In Support of Focal Drivers
We recognize that because we did not perform ablation of the focal sites, we cannot state definitively that the focal sites were drivers. However, data from our study do support the likelihood that focal sites act as drivers: 1) the focal site was the earliest site activated compared to its neighbors; 2) the unipolar AEG at the earliest site manifested a QS morphology; 3) wave fronts always emanated from the focal site; and 4) the focal site manifested repetitive QS activation. The same data were true for breakthrough sites, except they did not manifest a QS unipolar AEG morphology.
Previous Mapping Studies During Persistent and Long-Standing Persistent AF in Patients
Contact mapping
Using low resolution endocardial basket catheter mapping in paroxysmal and persistent AF, Narayan et al.11, 14 reported finding putative focal drivers and “rotors” with spatial stability. Our findings also demonstrated likely focal drivers, but, using classical activation sequence epicardial mapping, did not find any evidence of reentry of any type.
Over a period of two decades, several epicardial mapping studies were performed in patients with persistent and LSP AF.1-8, 10, 13 Those epicardial mapping studies analyzed by classical activation sequence mapping not only showed rapid and regular focal activation in the LA and/or RA,2, 3, 5, 6 but also repetitive activation patterns in the LA1, 4 suggestive of a driver. However, they did not further characterize the putative driver. Other studies analyzed by DF analysis showed rapid and regular activation patterns that were also considered to be evidence of a driver.7, 8 However, high density sequential area epicardial mapping by de Groot et al.10 in patients with LSP AF demonstrated neither focal drivers nor reentry. They primarily found epicardial breakthrough sites, but also some intermittent focal activity (0.8%). They introduced a third mechanism based on breakthrough activation which is independent of both focal and ordered reentrant activity, and called it the “double layer hypothesis.” In another recent study, sequential, epicardial mapping of portions of the LA and RA by Kalman's group13 in patients with persistent and LSP AF, intermittent foci (≤ 2 beats) and intermittent reentry (≥ 2 rotations) were identified, but neither sustained focal nor sustained reentrant activation were found. Notably, these latter two sequential (RA and LA) epicardial mapping studies covered less than 20% (total covered area in the two studies: 5.65 cm2 or 6.75 cm2) of the atria per recording. In our study, we simultaneously recorded from an area of 92.85 cm2 with bi-atrial, high density, epicardial mapping during persistent and LSP AF in patients, and all our data were recorded and analyzed over a duration of 32 continuous secs.
In sum, when comparing our study with other epicardial mapping studies, our finding of intermittent focal and breakthrough activation during AF concurs, in whole or in part, with the findings of others.2, 3, 5, 6, 10, 13 However, importantly, our data further characterize both the sites from which the wave fronts emanate, and the nature of the atrial activation patterns (largely collision and merging of wave fronts) during persistent and LSP AF. Finally, we did not find any reentrant circuits.
Non-contact mapping
Recently, a noninvasive mapping study by Rudy's group9 showed that in paroxysmal and persistent AF patients, the most common activation pattern consisted of multiple (2 – 5) concurrent wavelets (92%), with simultaneous focal activation from areas near the pulmonary veins (69%) and non-pulmonary veins (62%). Reentry was seen rarely, and was rarely sustained > 1 rotation. Also, in a noninvasive mapping study in patients with persistent and LSP AF, Haissaguerre et al.12 identified driver mechanisms, of which 80.5% were reentries, and 19.5% were focal breakthroughs. Their maps showed incessantly changing beat-to-beat wave fronts, and varying spatiotemporal behavior of driver activities. Reentries were not sustained (median, 2.6 rotations lasting 449 ± 89 ms), meandered substantially, but recurred repetitively in the same region.
In comparison with our study, we found both sustained and intermittent types of foci, as well as nonrandom and random breakthrough sites. No reentry was demonstrated, but occasionally, repetitive focal QS activation generated repetitive wannabe reentry. Also, it is interesting that the focus-initiated wannabe reentry we described has some similarity to their noninvasive mapping study in which focal activation initiated reentry. Of course, wannabe reentry is not actual reentry.
Implications
Our data describe a new paradigm for the mechanism that maintains persistent and LSP AF. Understanding mechanism presents opportunities for new potential approaches to the treatment of persistent and LSP AF, especially for ablation. The results to date of ablation in persistent and LSP AF have been far from optimal, and the current approach to ablation remains largely empiric because we have not understood well enough the mechanism(s) maintaining persistent and LSP AF. Study of the mechanism(s) maintaining AF has been limited by the difficulty in mapping this complex arrhythmia. The data from our study should provide opportunities for a targeted, rather than an empiric approach to ablation.
Study Limitation
Because our mapping studies were only performed in patients with persistent and LSP AF, the findings cannot be assumed to apply to patients with paroxysmal AF. Additionally, the fact that our study patients all had valvular heart disease might make this patient population unique when considering AF mechanism(s), despite the fact that they also had other comorbidities often found in AF patients.
AEGs from the endocardium, including from the atrial septum, were not obtained, precluding identification and characterization of the source of the epicardial breakthroughs (and perhaps also further characterizing foci). We did not record from the PVs, but our LA recording array was in close proximity to them.
A unipolar QS electrogram might not identify the exact location of a focus because the impulse may originate from an area close by from which conduction does not generate enough of a signal to be appreciated in the unipolar atrial electrogram. The same may also apply to activation from a sub-epicardial reentrant circuit. Although we characterized some foci as continuous over the 32 secs of analysis, we do not know if ultimately, they became intermittent. Additional studies beyond the capability of mapping studies will be required to find the exact mechanism of the foci and breakthrough sites.
Although understanding the mechanism of both random and nonrandom breakthrough activation is still uncertain, they clearly play a role in the collision and merging of wave fronts that largely characterized atrial activation in our study. We cannot rule out the possibility that breakthrough activation was the result of reentry, including sub-epicardial reentry and/or foci originating at a distance from the breakthrough site.
Finally, mapping studies alone are not the gold standard for definitely proving that drivers maintain AF. Recalling the admonition of Mines,19 in order to say definitively that AF is due to multiple drivers, the gold standard is first to identify the putative drivers, and then prove that with their disappearance, the AF stops. However, because all analyses were performed offline, this was not possible.
Conclusions
In our high density, simultaneous, bi-atrial, epicardial mapping study in patients with persistent and LSP AF, activation from foci and/or breakthrough sites, from which wave fronts emanated, was an important mechanism in the maintenance of AF. These wave fronts collided and merged continuously at multiple and variable sites. Although occasional wannabe reentry was seen, no reentry was demonstrated. These data identify a new paradigm for persistent and LSP AF.
Supplementary Material
Acknowledgments
Funding Sources: This work was supported in part by grants from R01 HL074189 from the National Institutes of Health, National Heart, Lung, and Blood Institute, Bethesda, MD; BRTT/WCI TECH 05-066 from the Ohio Wright Center of Innovations, a Third Frontier program from the State of Ohio, Columbus, OH; and from the Jennie Zoline, Blue Dot, Glenstone, Frank & Gerry Pearl, and MCJ Amelior Foundations.
Footnotes
Disclosures: None
References
- 1.Sueda T, Nagata H, Shikata H, Orihashi K, Morita S, Sueshiro M, Okada K, Matsuura Y. Simple left atrial procedure for chronic atrial fibrillation associated with mitral valve disease. Ann Thorac Surg. 1996;62:1796–1800. doi: 10.1016/s0003-4975(96)00613-3. [DOI] [PubMed] [Google Scholar]
- 2.Holm M, Johansson R, Brandt J, Luhrs C, Olsson SB. Epicardial right atrial free wall mapping in chronic atrial fibrillation. Documentation of repetitive activation with a focal spread--a hitherto unrecognised phenomenon in man. Eur Heart J. 1997;18:290–310. doi: 10.1093/oxfordjournals.eurheartj.a015233. [DOI] [PubMed] [Google Scholar]
- 3.Harada A, Konishi T, Fukata M, Higuchi K, Sugimoto T, Sasaki K. Intraoperative map guided operation for atrial fibrillation due to mitral valve disease. Ann Thorac Surg. 2000;69:446–450. doi: 10.1016/s0003-4975(99)01091-7. discussion 450-441. [DOI] [PubMed] [Google Scholar]
- 4.Wu T-J, Doshi RN, Huang H-LA, Blanche C, Kass RM, Trento A, Cheng WEN, Karagueuzian HS, Peter CT, Chen P-S. Simultaneous biatrial computerized mapping during permanent atrial fibrillation in patients with organic heart disease. J Card Electrophysiol. 2002;13:571–577. doi: 10.1046/j.1540-8167.2002.00571.x. [DOI] [PubMed] [Google Scholar]
- 5.Yamauchi S, Ogasawara H, Saji Y, Bessho R, Miyagi Y, Fujii M. Efficacy of intraoperative mapping to optimize the surgical ablation of atrial fibrillation in cardiac surgery. Ann Thorac Surg. 2002;74:450–457. doi: 10.1016/s0003-4975(02)03696-2. [DOI] [PubMed] [Google Scholar]
- 6.Nitta T, Ishii Y, Miyagi Y, Ohmori H, Sakamoto S-i, Tanaka S. Concurrent multiple left atrial focal activations with fibrillatory conduction and right atrial focal or reentrant activation as the mechanism in atrial fibrillation. J Thorac Cardiovasc Surg. 2004;127:770–778. doi: 10.1016/j.jtcvs.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 7.Sahadevan J, Ryu K, Peltz L, Khrestian CM, Stewart RW, Markowitz AH, Waldo AL. Epicardial mapping of chronic atrial fibrillation in patients: Preliminary observations. Circulation. 2004;110:3293–3299. doi: 10.1161/01.CIR.0000147781.02738.13. [DOI] [PubMed] [Google Scholar]
- 8.Sanders P, Berenfeld O, Hocini M, Jais P, Vaidyanathan R, Hsu L-F, Garrigue S, Takahashi Y, Rotter M, Sacher F, Scavee C, Ploutz-Snyder R, Jalife J, Haissaguerre M. Spectral analysis identifies sites of high-frequency activity maintaining atrial fibrillation in humans. Circulation. 2005;112:789–797. doi: 10.1161/CIRCULATIONAHA.104.517011. [DOI] [PubMed] [Google Scholar]
- 9.Cuculich PS, Wang Y, Lindsay BD, Faddis MN, Schuessler RB, Damiano RJ, Jr., Li L, Rudy Y. Noninvasive characterization of epicardial activation in humans with diverse atrial fibrillation patterns. Circulation. 2010;122:1364–1372. doi: 10.1161/CIRCULATIONAHA.110.945709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Groot NM, Houben RP, Smeets JL, Boersma E, Schotten U, Schalij MJ, Crijns H, Allessie MA. Electropathological substrate of longstanding persistent atrial fibrillation in patients with structural heart disease: Epicardial breakthrough. Circulation. 2010;122:1674–1682. doi: 10.1161/CIRCULATIONAHA.109.910901. [DOI] [PubMed] [Google Scholar]
- 11.Narayan SM, Krummen DE, Clopton P, Shivkumar K, Miller JM. Direct or coincidental elimination of stable rotors or focal sources may explain successful atrial fibrillation ablationon-treatment analysis of the confirm trial (conventional ablation for af with or without focal impulse and rotor modulation). J Am Coll Cardiol. 2013;62:138–147. doi: 10.1016/j.jacc.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haissaguerre M, Hocini M, Denis A, Shah AJ, Komatsu Y, Yamashita S, Daly M, Amraoui S, Zellerhoff S, Picat M-Q, Quotb A, Jesel L, Lim H, Ploux S, Bordachar P, Attuel G, Meillet V, Ritter P, Derval N, Sacher F, Bernus O, Cochet H, Jais P, Dubois R. Driver domains in persistent atrial fibrillation. Circulation. 2014;130:530–538. doi: 10.1161/CIRCULATIONAHA.113.005421. [DOI] [PubMed] [Google Scholar]
- 13.Lee G, Kumar S, Teh A, Madry A, Spence S, Larobina M, Goldblatt J, Brown R, Atkinson V, Moten S, Morton JB, Sanders P, Kistler PM, Kalman JM. Epicardial wave mapping in human long-lasting persistent atrial fibrillation: Transient rotational circuits, complex wavefronts, and disorganized activity. Eur Heart J. 2014;35:86–97. doi: 10.1093/eurheartj/eht267. [DOI] [PubMed] [Google Scholar]
- 14.Swarup V, Baykaner T, Rostamian A, Daubert JP, Hummel J, Krummen DE, Trikha R, Miller JM, Tomassoni GF, Narayan SM. Stability of rotors and focal sources for human atrial fibrillation: Focal impulse and rotor mapping (firm) of af sources and fibrillatory conduction. J Cardiovasc Electrophysiol. 2014;25:1284–1292. doi: 10.1111/jce.12559. [DOI] [PubMed] [Google Scholar]
- 15.Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA, Crijns HJ, Damiano RJ, Jr., Davies DW, DiMarco J, Edgerton J, Ellenbogen K, Ezekowitz MD, Haines DE, Haissaguerre M, Hindricks G, Iesaka Y, Jackman W, Jalife J, Jais P, Kalman J, Keane D, Kim YH, Kirchhof P, Klein G, Kottkamp H, Kumagai K, Lindsay BD, Mansour M, Marchlinski FE, McCarthy PM, Mont JL, Morady F, Nademanee K, Nakagawa H, Natale A, Nattel S, Packer DL, Pappone C, Prystowsky E, Raviele A, Reddy V, Ruskin JN, Shemin RJ, Tsao HM, Wilber D. 2012 hrs/ehra/ecas expert consensus statement on catheter and surgical ablation of atrial fibrillation: Recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: A report of the heart rhythm society (hrs) task force on catheter and surgical ablation of atrial fibrillation. Developed in partnership with the european heart rhythm association (ehra), a registered branch of the european society of cardiology (esc) and the european cardiac arrhythmia society (ecas); and in collaboration with the american college of cardiology (acc), american heart association (aha), the asia pacific heart rhythm society (aphrs), and the society of thoracic surgeons (sts). Endorsed by the governing bodies of the american college of cardiology foundation, the american heart association, the european cardiac arrhythmia society, the european heart rhythm association, the society of thoracic surgeons, the asia pacific heart rhythm society, and the heart rhythm society. Heart Rhythm. 2012;9:632–696. e621. doi: 10.1016/j.hrthm.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 16.Benharash P, Buch E, Frank P, Share M, Tung R, Shivkumar K, Mandapati R. Quantitative analysis of localized sources identified by focal impulse and rotor modulation mapping in atrial fibrillation. Circ Arrhythm Electrophysiol. 2015;8:554–561. doi: 10.1161/CIRCEP.115.002721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee S, Sahadevan J, Khrestian CM, Durand DM, Waldo AL. High density mapping of atrial fibrillation during vagal nerve stimulation in the canine heart: Restudying the moe hypothesis. J Card Electrophysiol. 2013;24:328–335. doi: 10.1111/jce.12032. [DOI] [PubMed] [Google Scholar]
- 18.Lee S, Ryu K, Waldo AL, Khrestian CM, Durand DM, Sahadevan J. An algorithm to measure beat-to-beat cycle lengths for assessment of atrial electrogram rate and regularity during atrial fibrillation. J Card Electrophysiol. 2013;24:199–206. doi: 10.1111/jce.12014. [DOI] [PubMed] [Google Scholar]
- 19.Mines GR. On circulating excitations in heart muscles and their possible relation to tachycardia and fibrillation. Trans R Soc Can. 1914;8:43–52. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.