Abstract
There is increasing scientific and clinical interest in elucidating the biology of type I Interferons, which began approximately 60 years ago with the concept of “viral interference”, a property that reduces the ability of a virus to infect cells. Although our understanding of the multiple cellular and molecular functions of interferons has advanced significantly, much remains to be learned and type I Interferons remain an active and fascinating area of inquiry. In this review, we cover some general aspects of type I interferon genes, with emphasis on interferon-alpha, and various aspects of molecular mechanisms triggered by type I interferons and toll-like receptor signaling by the Janus activated kinase/signal transducer activation of transcription (JAK-STAT) pathway and interferon regulatory factor pathway. We will also describe the role of type I interferons in autoimmune and inflammatory diseases, and its potential use as therapeutic agent.
Keywords: Interferon alpha, Interferon beta, Interferon signature, autoimmune diseases, systemic lupus erythematosus, rheumatoid arthritis, multiple sclerosis, idiopathic inflammatory myopathies
Introduction
The interferons (IFN)s are a family of cytokines with antiviral, antiproliferative, and antitumor activities, as well as immunomodulatory effects on the innate and adaptive immune responses (Lengyel, 1982; Pestka et al., 1987). The awareness that more than one type of IFN existed developed gradually as a result of the molecular cloning of the different IFN genes. Since the first published description of IFN (Isaacs and Lindenmann, 1957,Isaacs et al., 1957), there has been an explosive growth in our understanding of genes encoding the IFNs and their receptors, their complex signaling cascade and regulation, and their biological activities (Pestka et al., 2004). Historically, IFNs have been classified into two major types, type I and type II, based on their interactions with the IFN receptor subunits, peptide mapping, and sequencing homology (Pestka et al., 1987, Pestka et al., 2004). Recently, a novel class of cytokines with IFN-like activities has been described and designated as type III IFNs (IFN-λ1-3) (Osterlund et al., 2007). In humans, the type I IFN system consists of a family of IFN proteins encoded by at least 13 IFN alpha (IFNA) subtype genes (IFN-α1, -α2, -α4, -α5, -α6, -α7, -α8, -α10, -α13, -α14, -α16, -α17 and -α21), and one IFN beta gene (IFNB), one IFN-Epsilon gene, one IFN-Kappa gene, and IFN-Omega gene, all of which bind to the type I interferon receptor composed of the IFNAR1 and IFNAR2 chains (Uze et al., 2007). Sequence data indicate the human IFNA gene family shares 70-80% sequence homology within the IFNA subtypes, and about 35% identity with IFNB (Diaz et al., 1994).
This article reviews current understanding of the type I IFN gene family, molecular functions of type I IFNs, and the role of type I IFN in autoimmune disease such as systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), multiple sclerosis (MS), and idiopathic inflammatory myopathies (IIM). As type I IFNs have been implicated in these human disease processes, therapies are currently being developed targeting this pathway. We have used the nomenclature approved by the Human Genome Mapping Workshop for IFN genes. For example, IFNA designates a gene or locus, whereas IFN-α refers to the protein (Diaz et al., 1996).
Human interferon-alpha gene family
In humans, the genes encoding IFNA are found as a family of 13 intronless genes clustered together within a region spanning ~ 400 kb on the short arm of chromosome 9 (cytogenetic bands 9p22-9p21) (Shows et al., 1982). There are 12 functional human IFNA gene products. All of these IFN-α proteins exhibit high homology in their primary, secondary, and tertiary structures (Karpusas et al., 1997, Mitsui and Senda, 1997, Thomas et al., 2011). They also bind to the same receptor (IFNAR1/IFNAR2) and signal through similar mechanisms eliciting similar biological activity (Pestka et al., 2004; Viscomi, 1997). Currently, there is a small body of experimental data demonstrating differences in the biological activities of human IFN-α subtypes, although some studies suggest that even minor variations in the primary sequences of individual subtypes of human IFNA genes may lead to distinct antiviral and immunoregulatory functions in T cells, B cells, and dendritic cells (DCs) (Pestka et al., 2004, Yanai et al., 2001, Foster et al., 1996, Dipaola et al., 1994, Hilkens et al., 2003, Hibbert and Foster, 1999). Data from the murine IFNA gene family suggests that diverse IFN-α proteins may vary in their affinity for the IFN receptor subunits, resulting in differences in IFN signaling. Interestingly, mouse fibroblasts transfected with different type I IFNA/B transgenes (i.e., IFNA1, IFNA4, IFNA5, IFNA6, Ifna9 and IFNB) showed different degrees of protection against herpes simplex virus type 1 (HSV-1) and HSV-2; suggesting differences in the downstream activation of genes responsible for the antiviral activities of IFN-α subtypes (Harle et al., 2002). Some studies also suggest differences in cell- and ligand-specific expression and different kinetics between IFN-α subtypes, suggesting other mechanisms for diversity in downstream response beyond conformational changes at the type I IFN receptor (Hillyer et al., 2012). The high degree of amino acid sequence similarity within the IFN-α proteins suggests a common ancestral gene. Gene clusters such as the IFNA cluster are genomic regions that comprise multiple similar copies in close proximity, and are thought to be generated by local duplication of a common ancestral segment. (Chen et al., 2007, Song et al., 2011). A study published by Woelk and colleagues (Woelk et al., 2007) using gene conversion analysis of 156 IFNA genes from mammalian species (chimpanzee, dog, mouse, rat, and rhesus macaque) in which gene-specific clustering is also evident, identified specific sequences and fragments involved in gene conversion and gene duplication events. This study suggested that both of these evolutionary mechanisms contributed to the evolution of IFNA gene clusters. Other studies have been unable to clarify whether gene conversion or recent duplication play a role for gene-specific clustering of IFNA genes (Hughes, 1995). An evolutionary analysis of human and mouse IFNA genes failed to find evidence of gene conversion in humans but some interlocus recombination was identified among mouse IFNA genes (Hughes, 1995).
Interplay between type I IFN signaling and Toll-like receptor response
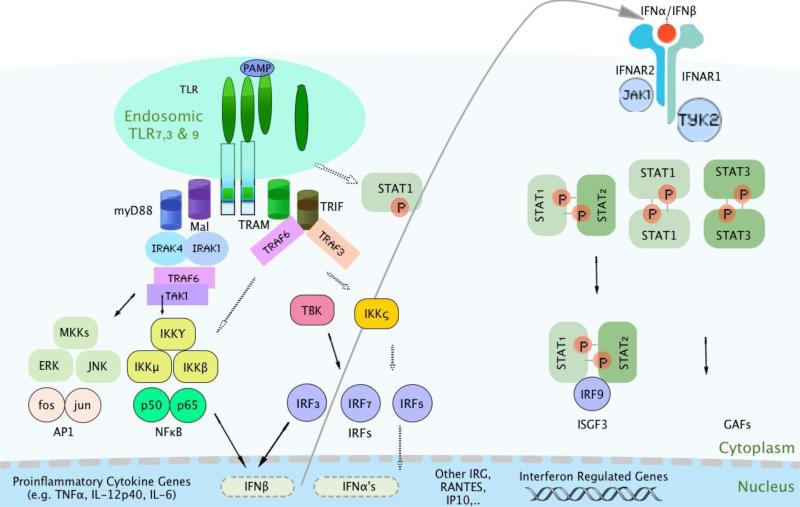
Type I IFNs elicit antiviral, antiproliferative and immunomodulatory responses by binding to the type I interferon receptor. The receptor consists of the IFNAR1 and IFNAR2 transmembrane proteins, and two associated cytoplasmic tyrosine kinases, the Janus kinase 1 (JAK1) and tyrosine kinase 2 (TYK2). The IFNAR2 subunit is considered as the primary binding chain as it binds type I IFNs with relatively high affinity, whereas the IFNAR1 subunit does not bind type I IFNs with detectable affinity but is absolutely required for signal transduction from the heterodimeric IFNAR complex and for type I IFN biological activity (Cohen et al., 1995, Arduini et al., 1999, Uze et al., 2007). Thus, both the IFNAR1 and IFNAR2 subunits are required to mediate the biological effects of all type I IFNs. As shown in Figure 1, the biological effects of IFNs are mediated through the Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway. STAT1 and STAT2 mediate the antiviral and inflammatory effects of IFN-α/IFN-β (Aaronson and Horvath, 2002}. Upon IFNAR engagement, IFNs induce tyrosine phosphorylation of STAT1 and STAT2 proteins and, together with IFN-regulatory factor 9 (IRF9) , form the IFN-stimulated gene factor 3 (ISGF3) transcription factor complex (Mowen and David, 1998), which then translocates to the nucleus and binds to IFN-stimulated response elements (ISREs) in the promoters of IFN-regulated genes (IRGs). In addition, canonical type I IFN signalling may activate STAT1 homodimers that bind to interferon-gamma-activating factor (GAF), which translocates to the nucleus and activates transcription of IFN-stimulated genes (David, 2002). In contrast, IFN-α-activated STAT3 is thought to inhibit STAT1-dependent gene activation, thereby down-regulating IFN-α-mediated induction of inflammatory mediators, attenuating the inflammatory properties of type I IFNs (Ho and Ivashkiv, 2006).
Figure 1.
Schematic diagram shows major signaling pathways stimulated by IFN-α/β (mediated by the type I IFN receptor) and viral pathogen-associated molecular patterns (PAMPs) and/or ICs-containing nucleic acids (mediated by endosomal TLRs). PAMPs or their mimics are detected by TLRs and RNA helicases. The signaling pathways finally lead to the induction of IFN-α/β as well as several IFN-inducible genes. IFN-α/β is then secreted and signals through the type I IFN receptor and the JAK/STAT pathway to regulate the expression of IFN-inducible genes, thereby generating antiviral, anti-proliferative, and immunomodulatory effects.
The canonical IFN-JAK/STAT signal transduction pathway is not isolated, but communicates extensively with other signal transduction pathways such as the innate pattern recognition receptors (PRRs), which include Toll-like receptors (TLRs), RIG-I-like receptors (RLGs), NOD-like receptors, and C-type lectin receptors (Takeuchi and Akira, 2010). The virus-induced expression of IFNA/IFNB genes is primarily controlled at the gene transcription level by the IRFs and IFN-stimulated genes (Honda and Taniguchi, 2006a). Immune complexes (ICs) containing nucleic acids can access intracellular TLRs (TLR3, TLR7/8 and TLR9) after binding to Fc receptors and induce IFN-α production by IRF3, IRF7, and IRF5 (Honda and Taniguchi, 2006a, Honda and Taniguchi, 2006b). Signaling through TLRs can broadly be categorized into two pathways; the MyD88 and the TRIF-dependent pathway. All TLRs, except TLR3, activate through the MyD88-dependent pathway. Only TLR3 and TLR4 activate through the TRIF-dependent pathway (Honda and Taniguchi, 2006b). The MyD88-dependent pathway recruits several effector molecules such as IRAK1/4 and tumor necrosis factor receptor-associated factor 6 (TRAF6) (Kawai and Akira, 2006). These molecules are linked to at least three major downstream pathways: the NF-κB pathway, the pathway involving mitogen-activated protein kinases (MAPKs), and IRF pathways. Depending on the stimulus and the responding cell types, activation of these pathways results in transcription of various cytokines including IFN-α/β (Honda and Taniguchi, 2006b). In human plasmacytoid DCs (pDCs), IRFs such as IRF3, 5, and 7 are activated by TLR7 and TLR9 signaling pathways, enabling type I IFN production (Baccala et al., 2007). In a model of virus-mediated IFNA/IFNB gene induction in fibroblasts, IRF3 and IRF7 were both required for efficient induction of IFNA and IFNB genes, as they cooperate with each other as DNA-binding transcription factors at the promoter (Sato et al., 2000). Studies have suggested that IRF3 is mainly responsible for the initial induction of IFNB, whereas IRF7 is involved in the late phase of both IFNA and IFNB gene induction. Honda et al (Honda et al., 2005) showed a robust induction of IFNA/IFNB mRNA expression upon CpGstimulation (a TLR9 agonist) of splenic-derived pDCs, which was abolished in splenic-derived pDCs from Irf7−/−mice, despite the normal expression of TLR9 mRNA. In contrast, induction of IFNA/IFNB mRNA expression occurred normally in Irf3−/−pDCs. Similar results were obtained upon stimulation with synthetic single-stranded RNA (TLR7 agonist). Their results suggest that IRF7 is essential and IRF3 is dispensable for MyD88-dependent induction of IFNA/IFNB genes via the TLR9 and TLR7 in pDCs in these animal models.
Role of interferon-α in cellular autoimmunity
The induction of IFNA gene expression may represent a finely tuned mechanism by which different cell types within the innate and adaptive immunity systems produce specific IFN-α subtypes in response to different stimuli, and in different physiological and pathological conditions. Although IFN-α and IFN-β are produced by a wide range of cells such as macrophages, fibroblasts, and endothelial cells, plasmacytoid dendritic cells (pDCs) are thought to be the major cell type responsible for producing high levels of IFN-α in response to RNA or DNA viruses. PDCs are thought to produce type I IFN in response to nucleic acid-containing immune complexes through activation of TLRs 7 and 9 (Ronnblom et al., 2003), which is relevant in autoimmune conditions such as SLE in which these types of immune complexes are prevalent. IFN-α has been reported to modulate the number and function of several key immune effector cells such as B cells, T effector cells, and regulatory T cells in autoimmune disease. For instance, type I IFN induced by pDCs significantly stimulated full differentiation of autoreactive B cells into Ig-secreting plasma cells and promote B cell survival in B cells purified from anti-snRNP Ig Tg mice upon TLR7/9 stimulation (Ding et al., 2009). These responses were partially abrogated by neutralization of IFN-α/β and IL-6 (Ding et al., 2009). IFN-α also plays a major role in T cells by inducing immunogenic T cell responses. Ag-specific naïve CD8+ T cells primed in the presence of IFN-α undergo marked proliferation and acquisition of effector functions (Le Bon et al., 2006). In addition, T cells isolated from the skin of psoriasis patients show an increased and prolonged IFN-α signaling pathway activation when compared with infiltrating T cells from skin of non-psoriatic donors (Eriksen et al., 2005). With regard to Foxp3+ Treg cells, it has been shown that IFN-α mediates the inactivation of human Treg cells by downregulating intracellular cAMP levels and negative regulation of T-cell receptor signaling, and might be responsible for autoimmune dysfunctions associated with IFN-α treatment of hematologic malignancies (Bacher et al., 2013). Similarly, the blockade of Treg cell development by IFN-α-producing antigen-presenting cells has been suggested as a pathogenic factor in untreated active SLE patients (Yan et al., 2008).
Type I Interferon in systemic lupus erythematosus
Increased serum IFN-α and IFN-α-induced gene expression are frequently observed in systemic lupus erythematosus (SLE) patients, suggesting that type I IFNs are important in the molecular pathogenesis of the disease (Ytterberg and Schnitzer, 1982, Crow et al., 2003, Baechler et al., 2003). High circulating levels of type I IFN are frequent in SLE patients, and correlate with SLE disease severity, as well as with the presence of SLE-associated autoantibodies (Baechler et al., 2003; Ronnblom et al., 2003; Feng et al., 2006; Niewold, 2008; Weckerle et al., 2012). In addition, healthy first degree relatives of SLE patients frequently have higher serum IFN-α levels compared to healthy unrelated individuals (Mavragani et al., 2007; Niewold et al., 2008a), suggesting that high serum IFN-α is a heritable risk factor for SLE. It is likely that the high concentrations of circulating IFN-α is produced by excessive pDC activation. Aberrant activation of endosomal TLR within pDCs via ICs is one of the mechanisms behind the activation of pDCs in SLE (Alexopoulou et al., 2001). In support of this model, in vitro studies have shown that DNA-containing ICs purified from serum of active SLE patients, but not protein-containing ICs from other autoimmune rheumatic diseases, induces pDCs to produce IFN-α, and other pro-inflammatory cytokines and chemokines, known to contribute directly to the pathogenesis of SLE (Means et al., 2005; Ronnblom et al., 2003; Lovgren et al., 2004).
Recent studies have shown that both anti-double-stranded DNA (ds-DNA) and anti-RNA binding protein autoantibodies can trigger IFN-α production in in vitro systems (Ronnblom et al., 2003; Lovgren et al., 2006; Rekvig and Nossent, 2003). These ICs are associated with high IFN in SLE patients, but are not sufficient to cause high levels of circulating IFN in humans (Niewold et al., 2008c). These data would suggest that underlying genetic susceptibility, possibly resulting in a hyperactive TLR system, may also be required for the ICs to result in systemic increases in IFN-α. This hypothesis has been supported by emerging data demonstrating genetic associations between variants in the IRF pathways with both SLE susceptibility and increased circulating IFN-α in SLE patients. For instance, single nucleotide polymorphisms (SNPs) in the IFN-regulatory factor 5 (IRF5)and tyrosine kinase 2 (TYK2) genes have been associated with SLE susceptibility in several populations (Sigurdsson et al., 2005, Graham et al., 2006, Kawasaki et al., 2008, Feng et al., 2010). In addition to the association of SNPs in IRF5 with SLE susceptibility, we have shown an association of the IRF5 risk haplotype with higher serum IFN-α activity in SLE patients (Niewold et al., 2008b). Also, we have observed associations between genetic variations in IRF genes and SLE-associated autoantibody profiles. For example, the same genetic variants in IRF5 which are associated with increased IFN-α are also associated with autoantibody formation, and these effects are independent (Niewold et al., 2012, Cherian et al., 2012). This suggests a feed-forward mechanism, in which the same genetic variant predisposes the patient to autoantibody formation (possibly via TLR pathway stimulation in B cells), and then the autoantibodies result in increased IFN-α production by pDCs in the setting of the same genetic variants downstream of TLRs (Jensen and Niewold, 2015). IRF-7 is considered a master regulator of type I IFN induction and IFN-stimulated gene expression (Honda et al., 2005). Similar to IRF5, SLE-associated genetic variants in IRF7 are associated with both increased circulating IFN-α as well as autoantibody formation (Salloum et al., 2010). This parallel pattern observed with both IRF5 and IRF7 variations supports the idea that genetic variants downstream of TLRs follow a similar feed-forward mechanism with respect to IFN-α (Salloum and Niewold, 2011). It seems that the autoantibody ICs are important for high circulating IFN-α in SLE patients, suggesting an induced model in which the upstream stimulus acts upon overactive variants in the downstream signaling pathway. A recent gene expression microarray study showed that this was particularly important in African-American SLE patients, as activation of IFN-related pathways was dependent on the presence of RNA-binding proteins (anti-RBP) antibodies in this ethnic background, while evidence for IFN pathway activation was observed in some European-American SLE patients who lacked these antibodies (Ko et al., 2013). STAT4 is a transcription factor belonging to the STAT protein family, and a genetic variant of STAT4 has been associated with the risk of SLE (Remmers et al., 2007). This risk allele is also correlated with presence of SLE-associated anti-ds-DNA autoantibodies (Sigurdsson et al., 2008). STAT4 is required for optimal IFN transcription downstream of IFNAR activation, as well as for the transcription of IFN-α induced genes (Tyler et al., 2007). We find that the SLE-associated STAT4 allele is associated with increased sensitivity to IFN-α in SLE patients, being associated with an increased amount of IFN-induced gene expression for a given amount of IFN-α in circulation (Kariuki et al., 2009). A similar phenomenon was observed with the SLE-risk allele of the interferon induced with helicase C domain 1 (IFIH1) gene (Robinson et al., 2011), supporting the idea that genetic variants can tune the IFN pathway by both modulating IFN-α production and sensitivity to IFN-α.
Currently, a number of anti-IFN-α monoclonal antibodies are in clinical trials for SLE, and thus far the proof of concept data is encouraging. Preliminary data from a phase I clinical trial of the anti-IFN-α monoclonal antibody MEDI-545 in SLE patients suggested possible disease activity improvement among SLE patients (Wallace et al., 2007). Furthermore, in another phase I clinical study, the authors reported a dose-dependent inhibition of IFN-α/β-inducible genes in both peripheral blood and skin biopsies in SLE patients treated with a single dose of an anti-IFN monoclonal antibody, as well as a possible reduction in clinical disease activity (Yao et al., 2009). Further data from phase II and phase III trials will be needed to assess the clinical efficacy of anti-IFN-α agents in SLE.
Type I interferon in autoimmune myositis
Similar to SLE, studies in the last several years have documented marked over-expression of type I IFN-inducible genes and IFN-regulated proteins in the peripheral blood and inflamed muscle tissues of patients with autoimmune myositis, with pDCs infiltrating inflamed tissues (Tezak et al., 2002, Zhou et al., 2004, Greenberg et al., 2005, Baechler et al., 2007, Lopez de Padilla et al., 2007, Niewold et al., 2009, Shrestha et al., 2010, Higgs et al., 2011). Many of these IFN-induced genes are similar to those which are upregulated in SLE. Also, studies have suggested a genetic or heritable component to the high type I IFN levels observed in this disease, similar to seen in SLE (Niewold et al., 2010, Niewold et al., 2011). The expression of the type I IFN-inducible genes in dermatomyositis (DM) also correlates positively with disease activity and with titers of anti-Jo1 and anti-Ro autoantibodies, which can be observed in this disease (Baechler et al., 2007, Niewold et al., 2009). As a result of these observations, great interest has been directed toward the role of type I IFNs in the pathogenesis of DM. Recent studies suggest that the engagement of endosomal TLRs by ICs containing anti-Jo-1 or anti-Ro 60 autoantibodies and self-antigens may activate endogenous IFN-α production in myositis patients (Eloranta et al., 2007, Balboni et al., 2013). In line with these findings, a study published by Cappelletti and colleagues showed that TLR3 mRNA transcript and protein were upregulated in muscle tissue from DM patients compared to controls, and were uniquely found to be associated with muscle type I IFN-dependent transcripts (Cappelletti et al., 2011). More recently, the endoplasmic reticulum stress response pathway (unfolded protein response) has been suggested to contribute to skeletal muscle damage and dysfunction in autoimmune myositis. Proposed mechanisms by which the unfolded protein response contributes to muscle pathology include induction of MHC class I expression in immature myoblast precursors, and augmented type I IFN production by muscle cells as well as by the infiltrating pDCs in the inflamed muscle tissue (Nagaraju et al., 2005, Tournadre et al., 2010, Vitadello et al., 2010, Tournadre et al., 2012).
Type I interferon in Multiple sclerosis
Multiple sclerosis (MS) is a disorder of the central nervous system characterized by inflammation, demyelination, and neurodegeneration with presumed autoimmune origin. The pathological lesion of MS consists of multiple focal demyelinated plaques within the central nervous system with variable degree of inflammation and gliosis (Frohman et al., 2006). The inflammatory infiltrates in acute and relapsing-remitting MS lesions are composed mainly of activated macrophages and CD8+ cytotoxic T lymphocytes, which are predominantly clustered in the perivascular white matter (Noseworthy et al., 2000; Frohman et al., 2006, Popescu et al., 2013). In contrast to other autoimmune diseases, MS patients have lower levels of circulating type I IFN than controls (Hertzog et al., 1991, Reder and Feng, 2013, Feng et al., 2012). Additionally, the mRNA expression levels of IFNα/βregulated antiviral proteins 2′,5′-OAS and MxA are significantly lower in peripheral mononuclear cells from untreated MS patients with exacerbations or rapid disease progression compared to MS patients with stable disease (Feng et al., 2002). And while type I IFNs are thought to induce some autoimmune conditions such as SLE as noted above, MS is effectively treated by administering recombinant human IFN-β. In MS, treatment with IFN-β abrogates the development of new inflammatory lesions as detected using MRI, reduces relapses in many patients with relapsing-remitting MS, and slows disease progression (Jacobs et al., 1996). In contrast, IFN gamma (type II IFN) appears to exacerbate the disease (Panitch et al., 1987).
The mechanistic basis for low IFN expression in MS is not well-understood, but some evidence suggests that IFN signal transduction is defective in MS patients. In the above-mentioned study in untreated relapsing-remitting MS patients (Feng et al., 2002), blood mononuclear cells also showed downregulation of IRF1 and IRF2 genes, two key transcription factors that regulate many type I IFN-regulated genes and IFN-α-induced expression of the antiviral proteins 2′,5′-OAS and MxA. Nevertheless, the IRF1 and IRF2 expression levels only increased slightly above the baseline levels in response to IFN-β-1b therapy (Feng et al., 2002), suggesting that MS patients may have a defect in IFN signaling which is only partially reversed by therapy. Although most MS patients display low levels of type I IFN-regulated genes, a subset of MS patients has a high IFN signature as well as more clinical and MRI disease activity before therapy, and these patients often do not respond to IFN-β treatment (Comabella et al., 2009, Hundeshagen et al., 2012, Matas et al., 2014), suggesting that MS immunopathogenesis may differ between patients. The heterogeneity among MS patients with respect to IFN induction and expression of IFN-induced genes, may, in part, explain the varied clinical response to IFN-β treatment observed in a MS population. Additionally, the MS-like illness neuromyelitis optica is associated with higher circulating IFN levels, and similarly does not respond well to IFN-β treatment (Feng et al., 2012).
Type I IFN in rheumatoid arthritis
Several observations suggest that type I IFN is involved in the pathogenesis of rheumatoid arthritis (RA). First, IFN immunotherapy has been reported to induce RA (Passos de Souza et al., 2001; Ionescu et al., 2008; Cacopardo et al., 2013). Second, type I IFN has been reported in the circulation and synovial tissues of a subset of RA patients (Olsen et al., 2004, Mavragani et al., 2010). A recent study reported by van der Pouw Kraan and colleagues showed that many IFN-inducible transcripts up-regulated in RA patients were very similar to those up-regulated in SLE patients, including G1P2/ISG15 (interferon-induced protein 15), MX1 (Myxovirus resistance 1), IFIT1 (interferon-induced with tetratricopeptide repeats 1), IFIT2, IRF7, and IFRG28 (28 kDa interferon responsive protein) (van der Pouw Kraan et al., 2007). In this study, RA patients with higher IFN-response gene expression in peripheral blood had increased activity of complement, coagulation cascades, and fatty acid metabolism pathways compared to patients with low IFN-induced gene expression in peripheral blood cells. In addition, phenotypic analysis has shown prominent expression of IFN-β protein by macrophages, DCs, and fibroblast-like synoviocytes in rheumatoid synovium compared with controls (van Holten et al., 2005). Fibroblast-like synoviocytes play a major role in the initiation and perpetuation of synovial inflammation, suggesting that type I IFN might be involved in chronic inflammation and joint destruction in RA.
While the relevance of the IFN signature to RA disease activity and progression remains unclear, some studies suggest that type I IFN in circulation may predict response to immunotherapy in RA (Mavragani et al., 2010, Thurlings et al., 2010). Thurlings and colleagues recently showed that RA patients with a low IFN signature had a better clinical response to rituximab compared with patients with a high IFN high signature (Thurlings et al., 2010). Similarly, Sekiguchi and colleagues also showed that in RA patients treated with infliximab (anti-TNF-alpha antibody) and that had a high IFN signature at baseline, disease activity was higher as measured using the disease activity of 28 joints (DAS28) score (Sekiguchi et al., 2008). Furthermore, they showed that changes in IFN-regulated gene expression during infliximab treatment correlated with changes in DAS28 score and individual clinical parameters, more prominently in responders. Interestingly, an increase in the IFN-regulated gene levels was associated with disease activity flare after 2 weeks in the non-responders. Mavragani et al examined pre-treatment serum from RA patients who were to receive anti-TNF-α, and found that the ratio of IFN-β/IFN-α in circulation provided some ability to predict those patients who would respond to anti-TNF-α treatment (Mavragani et al., 2010). Thus, type I IFN might be a feasible biomarker for predicting or monitoring response to biologic agents in RA.
Type I IFNs and Tumor Necrosis Factor Alpha Cross-Regulation
TNF-α is a pleiotropic cytokine with potent proinflammatory effects, and is elevated in inflammatory diseases such as RA and inflammatory bowel disease (Feldmann and Maini, 2001). TNF-α is a recognized and relevant therapeutic target to attenuate the chronic inflammation in those diseases. As TNF-α inhibitors have gained wider clinical use for inflammatory diseases such as RA, psoriatic arthritis, juvenile idiopathic arthritis, and inflammatory bowel disease, they have been associated with the new onset of cutaneous and systemic autoimmune disorders such as the paradoxical development of psoriasis and psoriasiform eruptions, leukocytoclastic vasculitis, and anti-TNF-induced lupus in patients receiving anti-TNF-α therapy for Crohn's disease or RA (Sfikakis et al., 2005, Flendrie et al., 2005, Williams et al., 2009, Sokumbi et al., 2012, Denadai et al., 2013). These manifestations can occur with any of the available TNF inhibitors and withdrawal of anti-TNF-α therapy usually leads to resolution of symptoms. Moreover, a subset of SLE patients treated with anti-TNF-α therapy has shown increased titers of anti-dsDNA antibodies (Aringer et al., 2004). The immune mechanisms underlying the development of new onset features of other immune disorders distinct from the primary rheumatologic disease remain unclear. Although it has been reported that the monocyte-gene expression profile in SLE is predominantly IFN-driven, while the RA cytokine profile is mainly TNFα-driven, both types of responses are present in SLE and RA (Smiljanovic et al., 2012, Weckerle et al., 2012), and these cytokines are hypothesized to counteract each other. Accumulating evidence suggests that the inhibition of TNF-α might cause disequilibrium in the balance of serum cytokine profiles, potentially skewing the immune system towards activated IFN-α signaling pathway. The immunostimulatory effects of IFN-α can then lead to pathological activation of T cells and DCs and a subsequent inflammatory response (Palucka et al., 2005, Fiorentino, 2007, Aringer and Crow, 2008, Asarch et al., 2009). A study in Sjogren's syndrome patients supports this idea, as TNF-α inhibition resulted in increased type I IFN-induced gene expression in peripheral blood immune cells (Mavragani et al., 2007). Similarly, TNF-α blockade in inflammatory myositis patients resulted in an increase of pre-existing high IFN-induced gene expression, and TNF-α blockade was associated with clinical worsening (Dastmalchi et al., 2008).
Conclusions
Type I IFNs exert many effects on the immune system including antiviral, control of proliferation, apoptosis, antitumor activity, and immune modulation. We could not review all of these effects, but have summarized current data regarding type I IFNs in a range of autoimmune diseases. The fact that type I IFNs can be both beneficial and detrimental in autoimmune diseases illustrates both the central nature of type I IFN in human immunity and tolerance, as well as the complexity inherent in the system. The cellular and molecular mechanisms underlying the pathogenic role of IFN-α and IFN-β in autoimmunity are not well understood, and this represents an active area of investigation in immunology and translational research. Such studies may lead to a better understanding of the involvement of these pleiotropic cytokines in chronic inflammation and immunity, and targeting IFN transduction pathways could potentially enable new therapeutic avenues.
Highlights.
The interferon-alpha (IFNA) gene cluster consists of at 13 IFNA homologous genes
The type I IFN genes exhibit diverse biological functions in cellular immunity
IFN-α plays a role in several autoimmune diseases, including lupus and myositis
Clinical trials with anti-IFN-α antibodies are underway in autoimmune disease
In contrast, IFN-β is an effective treatment for multiple sclerosis
Acknowledgments
This review and the corresponding Gene Wiki article are written as part of the Gene Wiki Review series--a series resulting from a collaboration between the journal GENE and the Gene Wiki Initiative. The Gene Wiki Initiative is supported by National Institutes of Health (GM089820). Additional support for Gene Wiki Reviews is provided by Elsevier, the publisher of GENE.
Supported by grants from: the NIH (AR060861, AR057781, AI071651), Rheumatology Research Foundation, CureJM Foundation, the Mayo Clinic Foundation, and the Lupus Foundation of Minnesota.
Abbreviations
- IFN
interferon
- IFNA
interferon alpha gene
- IFN-α
interferon α protein
- IFNB
interferon beta gene
- IFN-β
interferon β protein
- SLE
systemic lupus erythematosus
- IIM
idiopathic inflammatory myopathies
- DC
dendritic cells
- JAK
Janus kinase
- STAT
signal transducer and activator of transcription
- TYK2
tyrosine kinase 2
- IRF
IFN-regulatory factor
- PDCs
plasmacytoid dendritic cells
- ICs
immune complexes
- SNP
single nucleotide polymorphism
- IFNAR
Type I interferon receptor
- TLR
toll-like receptor
- ICs
Immune complexes
- MS
multiple sclerosis
- RA
rheumatoid arthritis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors declare no conflict of interest.
References
- Aaronson DS, Horvath CM. A road map for those who don't know JAK-STAT. Science. 2002;296:1653–5. doi: 10.1126/science.1071545. [DOI] [PubMed] [Google Scholar]
- Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–8. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- Arduini RM, Strauch KL, Runkel LA, Carlson MM, Hronowski X, Foley SF, Young CN, Cheng W, Hochman PS, Baker DP. Characterization of a soluble ternary complex formed between human interferon-beta-1a and its receptor chains. Protein science : a publication of the Protein Society. 1999;8:1867–77. doi: 10.1110/ps.8.9.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aringer M, Crow MK. A bridge between interferon-alpha and tumor necrosis factor in lupus. The Journal of rheumatology. 2008;35:1473–6. [PubMed] [Google Scholar]
- Aringer M, Graninger WB, Steiner G, Smolen JS. Safety and efficacy of tumor necrosis factor alpha blockade in systemic lupus erythematosus: an open-label study. Arthritis and rheumatism. 2004;50:3161–9. doi: 10.1002/art.20576. [DOI] [PubMed] [Google Scholar]
- Asarch A, Gottlieb AB, Lee J, Masterpol KS, Scheinman PL, Stadecker MJ, Massarotti EM, Bush ML. Lichen planus-like eruptions: an emerging side effect of tumor necrosis factor-alpha antagonists. Journal of the American Academy of Dermatology. 2009;61:104–11. doi: 10.1016/j.jaad.2008.09.032. [DOI] [PubMed] [Google Scholar]
- Baccala R, Hoebe K, Kono DH, Beutler B, Theofilopoulos AN. TLR-dependent and TLR-independent pathways of type I interferon induction in systemic autoimmunity. Nature medicine. 2007;13:543–51. doi: 10.1038/nm1590. [DOI] [PubMed] [Google Scholar]
- Bacher N, Raker V, Hofmann C, Graulich E, Schwenk M, Baumgrass R, Bopp T, Zechner U, Merten L, Becker C, Steinbrink K. Interferon-alpha suppresses cAMP to disarm human regulatory T cells. Cancer research. 2013;73:5647–56. doi: 10.1158/0008-5472.CAN-12-3788. [DOI] [PubMed] [Google Scholar]
- Baechler EC, Batliwalla FM, Karypis G, Gaffney PM, Ortmann WA, Espe KJ, Shark KB, Grande WJ, Hughes KM, Kapur V, Gregersen PK, Behrens TW. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:2610–5. doi: 10.1073/pnas.0337679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baechler EC, Bauer JW, Slattery CA, Ortmann WA, Espe KJ, Novitzke J, Ytterberg SR, Gregersen PK, Behrens TW, Reed AM. An interferon signature in the peripheral blood of dermatomyositis patients is associated with disease activity. Molecular medicine. 2007;13:59–68. doi: 10.2119/2006-00085.Baechler. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balboni I, Niewold TB, Morgan G, Limb C, Eloranta ML, Ronnblom L, Utz PJ, Pachman LM. Interferon-alpha induction and detection of anti-ro, anti-la, anti-sm, and anti-rnp autoantibodies by autoantigen microarray analysis in juvenile dermatomyositis. Arthritis and rheumatism. 2013;65:2424–9. doi: 10.1002/art.38038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacopardo B, Benanti F, Pinzone MR, Nunnari G. Rheumatoid arthritis following PEG-interferon-alfa-2a plus ribavirin treatment for chronic hepatitis C: a case report and review of the literature. BMC research notes. 2013;6:437. doi: 10.1186/1756-0500-6-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappelletti C, Baggi F, Zolezzi F, Biancolini D, Beretta O, Severa M, Coccia EM, Confalonieri P, Morandi L, Mora M, Mantegazza R, Bernasconi P. Type I interferon and Toll-like receptor expression characterizes inflammatory myopathies. Neurology. 2011;76:2079–88. doi: 10.1212/WNL.0b013e31821f440a. [DOI] [PubMed] [Google Scholar]
- Chen JM, Cooper DN, Chuzhanova N, Ferec C, Patrinos GP. Gene conversion: mechanisms, evolution and human disease. Nature reviews. Genetics. 2007;8:762–75. doi: 10.1038/nrg2193. [DOI] [PubMed] [Google Scholar]
- Cherian TS, Kariuki SN, Franek BS, Buyon JP, Clancy RM, Niewold TB. Brief Report: IRF5 systemic lupus erythematosus risk haplotype is associated with asymptomatic serologic autoimmunity and progression to clinical autoimmunity in mothers of children with neonatal lupus. Arthritis and rheumatism. 2012;64:3383–7. doi: 10.1002/art.34571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen B, Novick D, Barak S, Rubinstein M. Ligand-induced association of the type I interferon receptor components. Molecular and cellular biology. 1995;15:4208–14. doi: 10.1128/mcb.15.8.4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comabella M, Lunemann JD, Rio J, Sanchez A, Lopez C, Julia E, Fernandez M, Nonell L, Camina-Tato M, Deisenhammer F, Caballero E, Tortola MT, Prinz M, Montalban X, Martin R. A type I interferon signature in monocytes is associated with poor response to interferon-beta in multiple sclerosis. Brain : a journal of neurology. 2009;132:3353–65. doi: 10.1093/brain/awp228. [DOI] [PubMed] [Google Scholar]
- Crow MK, Kirou KA, Wohlgemuth J. Microarray analysis of interferon-regulated genes in SLE. Autoimmunity. 2003;36:481–90. doi: 10.1080/08916930310001625952. [DOI] [PubMed] [Google Scholar]
- Dastmalchi M, Grundtman C, Alexanderson H, Mavragani CP, Einarsdottir H, Helmers SB, Elvin K, Crow MK, Nennesmo I, Lundberg IE. A high incidence of disease flares in an open pilot study of infliximab in patients with refractory inflammatory myopathies. Annals of the rheumatic diseases. 2008;67:1670–7. doi: 10.1136/ard.2007.077974. [DOI] [PubMed] [Google Scholar]
- David M. Signal transduction by type I interferons. BioTechniques Suppl. 2002:58–65. [PubMed] [Google Scholar]
- Denadai R, Teixeira FV, Steinwurz F, Romiti R, Saad-Hossne R. Induction or exacerbation of psoriatic lesions during anti-TNF-alpha therapy for inflammatory bowel disease: a systematic literature review based on 222 cases. Journal of Crohn's & colitis. 2013;7:517–24. doi: 10.1016/j.crohns.2012.08.007. [DOI] [PubMed] [Google Scholar]
- Diaz MO, Bohlander S, Allen G. Nomenclature of the human interferon genes. Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research. 1996;16:179–80. doi: 10.1089/jir.1996.16.179. [DOI] [PubMed] [Google Scholar]
- Diaz MO, Pomykala HM, Bohlander SK, Maltepe E, Malik K, Brownstein B, Olopade OI. Structure of the human type-I interferon gene cluster determined from a YAC clone contig. Genomics. 1994;22:540–52. doi: 10.1006/geno.1994.1427. [DOI] [PubMed] [Google Scholar]
- Ding C, Cai Y, Marroquin J, Ildstad ST, Yan J. Plasmacytoid dendritic cells regulate autoreactive B cell activation via soluble factors and in a cell-to-cell contact manner. Journal of immunology. 2009;183:7140–9. doi: 10.4049/jimmunol.0901175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dipaola M, Smith T, Ferencz-Biro K, Liao MJ, Testa D. Interferon-alpha 2 produced by normal human leukocytes is predominantly interferon-alpha 2b. Journal of interferon research. 1994;14:325–32. doi: 10.1089/jir.1994.14.325. [DOI] [PubMed] [Google Scholar]
- Eloranta ML, Barbasso Helmers S, Ulfgren AK, Ronnblom L, Alm GV, Lundberg IE. A possible mechanism for endogenous activation of the type I interferon system in myositis patients with anti-Jo-1 or anti-Ro 52/anti-Ro 60 autoantibodies. Arthritis and rheumatism. 2007;56:3112–24. doi: 10.1002/art.22860. [DOI] [PubMed] [Google Scholar]
- Eriksen KW, Lovato P, Skov L, Krejsgaard T, Kaltoft K, Geisler C, Odum N. Increased sensitivity to interferon-alpha in psoriatic T cells. The Journal of investigative dermatology. 2005;125:936–44. doi: 10.1111/j.0022-202X.2005.23864.x. [DOI] [PubMed] [Google Scholar]
- Feldmann M, Maini RN. Anti-TNF alpha therapy of rheumatoid arthritis: what have we learned? Annual review of immunology. 2001;19:163–96. doi: 10.1146/annurev.immunol.19.1.163. [DOI] [PubMed] [Google Scholar]
- Feng D, Stone RC, Eloranta ML, Sangster-Guity N, Nordmark G, Sigurdsson S, Wang C, Alm G, Syvanen AC, Ronnblom L, Barnes BJ. Genetic variants and disease-associated factors contribute to enhanced interferon regulatory factor 5 expression in blood cells of patients with systemic lupus erythematosus. Arthritis and rheumatism. 2010;62:562–73. doi: 10.1002/art.27223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X, Petraglia AL, Chen M, Byskosh PV, Boos MD, Reder AT. Low expression of interferon-stimulated genes in active multiple sclerosis is linked to subnormal phosphorylation of STAT1. Journal of neuroimmunology. 2002;129:205–15. doi: 10.1016/s0165-5728(02)00182-0. [DOI] [PubMed] [Google Scholar]
- Feng X, Reder NP, Yanamandala M, Hill A, Franek BS, Niewold TB, Reder AT, Javed A. Type I interferon signature is high in lupus and neuromyelitis optica but low in multiple sclerosis. Journal of the neurological sciences. 2012;313:48–53. doi: 10.1016/j.jns.2011.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X, Wu H, Grossman JM, Hanvivadhanakul P, FitzGerald JD, Park GS, Dong X, Chen W, Kim MH, Weng HH, Furst DE, Gorn A, McMahon M, Taylor M, Brahn E, Hahn BH, Tsao BP. Association of increased interferon-inducible gene expression with disease activity and lupus nephritis in patients with systemic lupus erythematosus. Arthritis and rheumatism. 2006;54:2951–62. doi: 10.1002/art.22044. [DOI] [PubMed] [Google Scholar]
- Fiorentino DF. The Yin and Yang of TNF-{alpha} inhibition. Archives of dermatology. 2007;143:233–6. doi: 10.1001/archderm.143.2.233. [DOI] [PubMed] [Google Scholar]
- Flendrie M, Vissers WH, Creemers MC, de Jong EM, van de Kerkhof PC, van Riel PL. Dermatological conditions during TNF-alpha-blocking therapy in patients with rheumatoid arthritis: a prospective study. Arthritis research & therapy. 2005;7:R666–76. doi: 10.1186/ar1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster GR, Rodrigues O, Ghouze F, Schulte-Frohlinde E, Testa D, Liao MJ, Stark GR, Leadbeater L, Thomas HC. Different relative activities of human cell-derived interferon-alpha subtypes: IFN-alpha 8 has very high antiviral potency. Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research. 1996;16:1027–33. doi: 10.1089/jir.1996.16.1027. [DOI] [PubMed] [Google Scholar]
- Frohman EM, Racke MK, Raine CS. Multiple sclerosis--the plaque and its pathogenesis. The New England journal of medicine. 2006;354:942–55. doi: 10.1056/NEJMra052130. [DOI] [PubMed] [Google Scholar]
- Graham RR, Kozyrev SV, Baechler EC, Reddy MV, Plenge RM, Bauer JW, Ortmann WA, Koeuth T, Gonzalez Escribano MF, Pons-Estel B, Petri M, Daly M, Gregersen PK, Martin J, Altshuler D, Behrens TW, Alarcon-Riquelme ME. A common haplotype of interferon regulatory factor 5 (IRF5) regulates splicing and expression and is associated with increased risk of systemic lupus erythematosus. Nature genetics. 2006;38:550–5. doi: 10.1038/ng1782. [DOI] [PubMed] [Google Scholar]
- Greenberg SA, Pinkus JL, Pinkus GS, Burleson T, Sanoudou D, Tawil R, Barohn RJ, Saperstein DS, Briemberg HR, Ericsson M, Park P, Amato AA. Interferon-alpha/beta-mediated innate immune mechanisms in dermatomyositis. Annals of neurology. 2005;57:664–78. doi: 10.1002/ana.20464. [DOI] [PubMed] [Google Scholar]
- Harle P, Cull V, Guo L, Papin J, Lawson C, Carr DJ. Transient transfection of mouse fibroblasts with type I interferon transgenes provides various degrees of protection against herpes simplex virus infection. Antiviral research. 2002;56:39–49. doi: 10.1016/s0166-3542(02)00093-1. [DOI] [PubMed] [Google Scholar]
- Hertzog PJ, Wright A, Harris G, Linnane AW, Mackay IR. Intermittent interferonemia and interferon responses in multiple sclerosis. Clinical immunology and immunopathology. 1991;58:18–32. doi: 10.1016/0090-1229(91)90145-z. [DOI] [PubMed] [Google Scholar]
- Hibbert L, Foster GR. Human type I interferons differ greatly in their effects on the proliferation of primary B cells. Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research. 1999;19:309–18. doi: 10.1089/107999099314009. [DOI] [PubMed] [Google Scholar]
- Higgs BW, Liu Z, White B, Zhu W, White WI, Morehouse C, Brohawn P, Kiener PA, Richman L, Fiorentino D, Greenberg SA, Jallal B, Yao Y. Patients with systemic lupus erythematosus, myositis, rheumatoid arthritis and scleroderma share activation of a common type I interferon pathway. Annals of the rheumatic diseases. 2011;70:2029–36. doi: 10.1136/ard.2011.150326. [DOI] [PubMed] [Google Scholar]
- Hilkens CM, Schlaak JF, Kerr IM. Differential responses to IFN-alpha subtypes in human T cells and dendritic cells. Journal of immunology. 2003;171:5255–63. doi: 10.4049/jimmunol.171.10.5255. [DOI] [PubMed] [Google Scholar]
- Hillyer P, Mane VP, Schramm LM, Puig M, Verthelyi D, Chen A, Zhao Z, Navarro MB, Kirschman KD, Bykadi S, Jubin RG, Rabin RL. Expression profiles of human interferon-alpha and interferon-lambda subtypes are ligand- and cell-dependent. Immunology and cell biology. 2012;90:774–83. doi: 10.1038/icb.2011.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho HH, Ivashkiv LB. Role of STAT3 in type I interferon responses. Negative regulation of STAT1-dependent inflammatory gene activation. The Journal of biological chemistry. 2006;281:14111–8. doi: 10.1074/jbc.M511797200. [DOI] [PubMed] [Google Scholar]
- Honda K, Taniguchi T. IRFs: master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors. Nature reviews. Immunology. 2006a;6:644–58. doi: 10.1038/nri1900. [DOI] [PubMed] [Google Scholar]
- Honda K, Taniguchi T. Toll-like receptor signaling and IRF transcription factors. IUBMB life. 2006b;58:290–5. doi: 10.1080/15216540600702206. [DOI] [PubMed] [Google Scholar]
- Honda K, Yanai H, Negishi H, Asagiri M, Sato M, Mizutani T, Shimada N, Ohba Y, Takaoka A, Yoshida N, Taniguchi T. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature. 2005;434:772–7. doi: 10.1038/nature03464. [DOI] [PubMed] [Google Scholar]
- Hughes AL. The evolution of the type I interferon gene family in mammals. Journal of molecular evolution. 1995;41:539–48. doi: 10.1007/BF00175811. [DOI] [PubMed] [Google Scholar]
- Hundeshagen A, Hecker M, Paap BK, Angerstein C, Kandulski O, Fatum C, Hartmann C, Koczan D, Thiesen HJ, Zettl UK. Elevated type I interferon-like activity in a subset of multiple sclerosis patients: molecular basis and clinical relevance. Journal of neuroinflammation. 2012;9:140. doi: 10.1186/1742-2094-9-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ionescu C, Micu L, Constantinescu I, Hortopan M, Ursaciuc C, Voiculescu M. Prolonged treatment with interferon alpha and peginterferon induces rheumatoid arthritis syndrome and erythema nodosum. Journal of gastrointestinal and liver diseases : JGLD. 2008;17:211–2. doi: 10.1007/s11749-008-0114-x. [DOI] [PubMed] [Google Scholar]
- Isaacs A, Lindenmann J. Virus interference. I. The interferon. Proceedings of the Royal Society of London. Series B, Containing papers of a Biological character. Royal Society. 1957;147:258–67. doi: 10.1098/rspb.1957.0048. [DOI] [PubMed] [Google Scholar]
- Isaacs A, Lindenmann J, Valentine RC. Virus interference. II. Some properties of interferon. Proceedings of the Royal Society of London. Series B, Containing papers of a Biological character. Royal Society. 1957;147:268–73. doi: 10.1098/rspb.1957.0049. [DOI] [PubMed] [Google Scholar]
- Jacobs LD, Cookfair DL, Rudick RA, Herndon RM, Richert JR, Salazar AM, Fischer JS, Goodkin DE, Granger CV, Simon JH, Alam JJ, Bartoszak DM, Bourdette DN, Braiman J, Brownscheidle CM, Coats ME, Cohan SL, Dougherty DS, Kinkel RP, Mass MK, Munschauer FE, 3rd, Priore RL, Pullicino PM, Scherokman BJ, Whitham RH, et al. Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group (MSCRG). Annals of neurology. 1996;39:285–94. doi: 10.1002/ana.410390304. [DOI] [PubMed] [Google Scholar]
- Jensen MA, Niewold TB. Interferon regulatory factors: critical mediators of human lupus. Translational research : the journal of laboratory and clinical medicine. 2015;165:283–295. doi: 10.1016/j.trsl.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariuki SN, Kirou KA, MacDermott EJ, Barillas-Arias L, Crow MK, Niewold TB. Cutting edge: autoimmune disease risk variant of STAT4 confers increased sensitivity to IFN-alpha in lupus patients in vivo. Journal of immunology. 2009;182:34–8. doi: 10.4049/jimmunol.182.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpusas M, Nolte M, Benton CB, Meier W, Lipscomb WN, Goelz S. The crystal structure of human interferon beta at 2.2-A resolution. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:11813–8. doi: 10.1073/pnas.94.22.11813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T, Akira S. TLR signaling. Cell death and differentiation. 2006;13:816–25. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- Kawasaki A, Kyogoku C, Ohashi J, Miyashita R, Hikami K, Kusaoi M, Tokunaga K, Takasaki Y, Hashimoto H, Behrens TW, Tsuchiya N. Association of IRF5 polymorphisms with systemic lupus erythematosus in a Japanese population: support for a crucial role of intron 1 polymorphisms. Arthritis and rheumatism. 2008;58:826–34. doi: 10.1002/art.23216. [DOI] [PubMed] [Google Scholar]
- Ko K, Koldobskaya Y, Rosenzweig E, Niewold TB. Activation of the Interferon Pathway is Dependent Upon Autoantibodies in African-American SLE Patients, but Not in European-American SLE Patients. Frontiers in immunology. 2013;4:309. doi: 10.3389/fimmu.2013.00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bon A, Durand V, Kamphuis E, Thompson C, Bulfone-Paus S, Rossmann C, Kalinke U, Tough DF. Direct stimulation of T cells by type I IFN enhances the CD8+ T cell response during cross-priming. Journal of immunology. 2006;176:4682–9. doi: 10.4049/jimmunol.176.8.4682. [DOI] [PubMed] [Google Scholar]
- Lengyel P. Biochemistry of interferons and their actions. Annual review of biochemistry. 1982;51:251–82. doi: 10.1146/annurev.bi.51.070182.001343. [DOI] [PubMed] [Google Scholar]
- Lopez de Padilla CM, Vallejo AN, McNallan KT, Vehe R, Smith SA, Dietz AB, Vuk-Pavlovic S, Reed AM. Plasmacytoid dendritic cells in inflamed muscle of patients with juvenile dermatomyositis. Arthritis and rheumatism. 2007;56:1658–68. doi: 10.1002/art.22558. [DOI] [PubMed] [Google Scholar]
- Lovgren T, Eloranta ML, Bave U, Alm GV, Ronnblom L. Induction of interferon-alpha production in plasmacytoid dendritic cells by immune complexes containing nucleic acid released by necrotic or late apoptotic cells and lupus IgG. Arthritis and rheumatism. 2004;50:1861–72. doi: 10.1002/art.20254. [DOI] [PubMed] [Google Scholar]
- Lovgren T, Eloranta ML, Kastner B, Wahren-Herlenius M, Alm GV, Ronnblom L. Induction of interferon-alpha by immune complexes or liposomes containing systemic lupus erythematosus autoantigen- and Sjogren's syndrome autoantigen-associated RNA. Arthritis and rheumatism. 2006;54:1917–27. doi: 10.1002/art.21893. [DOI] [PubMed] [Google Scholar]
- Matas E, Bau L, Martinez-Iniesta M, Romero-Pinel L, Mane MA, Cobo-Calvo A, Martinez-Yelamos S. Baseline MxA mRNA expression predicts interferon beta response in multiple sclerosis patients. PloS one. 2014;9:e112758. doi: 10.1371/journal.pone.0112758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavragani CP, La DT, Stohl W, Crow MK. Association of the response to tumor necrosis factor antagonists with plasma type I interferon activity and interferon-beta/alpha ratios in rheumatoid arthritis patients: a post hoc analysis of a predominantly Hispanic cohort. Arthritis and rheumatism. 2010;62:392–401. doi: 10.1002/art.27226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavragani CP, Niewold TB, Moutsopoulos NM, Pillemer SR, Wahl SM, Crow MK. Augmented interferon-alpha pathway activation in patients with Sjogren's syndrome treated with etanercept. Arthritis and rheumatism. 2007;56:3995–4004. doi: 10.1002/art.23062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Means TK, Latz E, Hayashi F, Murali MR, Golenbock DT, Luster AD. Human lupus autoantibody-DNA complexes activate DCs through cooperation of CD32 and TLR9. The Journal of clinical investigation. 2005;115:407–17. doi: 10.1172/JCI23025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsui Y, Senda T. Elucidation of the basic three-dimensional structure of type I interferons and its functional and evolutionary implications. Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research. 1997;17:319–26. doi: 10.1089/jir.1997.17.319. [DOI] [PubMed] [Google Scholar]
- Mowen K, David M. Role of the STAT1-SH2 domain and STAT2 in the activation and nuclear translocation of STAT1. The Journal of biological chemistry. 1998;273:30073–6. doi: 10.1074/jbc.273.46.30073. [DOI] [PubMed] [Google Scholar]
- Nagaraju K, Casciola-Rosen L, Lundberg I, Rawat R, Cutting S, Thapliyal R, Chang J, Dwivedi S, Mitsak M, Chen YW, Plotz P, Rosen A, Hoffman E, Raben N. Activation of the endoplasmic reticulum stress response in autoimmune myositis: potential role in muscle fiber damage and dysfunction. Arthritis and rheumatism. 2005;52:1824–35. doi: 10.1002/art.21103. [DOI] [PubMed] [Google Scholar]
- Niewold TB. Interferon alpha-induced lupus: proof of principle. Journal of clinical rheumatology : practical reports on rheumatic & musculoskeletal diseases. 2008;14:131–2. doi: 10.1097/RHU.0b013e318177627d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niewold TB, Adler JE, Glenn SB, Lehman TJ, Harley JB, Crow MK. Age- and sex-related patterns of serum interferon-alpha activity in lupus families. Arthritis and rheumatism. 2008a;58:2113–9. doi: 10.1002/art.23619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niewold TB, Kariuki SN, Morgan GA, Shrestha S, Pachman LM. Elevated serum interferon-alpha activity in juvenile dermatomyositis: associations with disease activity at diagnosis and after thirty-six months of therapy. Arthritis and rheumatism. 2009;60:1815–24. doi: 10.1002/art.24555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niewold TB, Kariuki SN, Morgan GA, Shrestha S, Pachman LM. Gene-gene-sex interaction in cytokine gene polymorphisms revealed by serum interferon alpha phenotype in juvenile dermatomyositis. The Journal of pediatrics. 2010;157:653–7. doi: 10.1016/j.jpeds.2010.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niewold TB, Kelly JA, Flesch MH, Espinoza LR, Harley JB, Crow MK. Association of the IRF5 risk haplotype with high serum interferon-alpha activity in systemic lupus erythematosus patients. Arthritis and rheumatism. 2008b;58:2481–7. doi: 10.1002/art.23613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niewold TB, Kelly JA, Kariuki SN, Franek BS, Kumar AA, Kaufman KM, Thomas K, Walker D, Kamp S, Frost JM, Wong AK, Merrill JT, Alarcon-Riquelme ME, Tikly M, Ramsey-Goldman R, Reveille JD, Petri MA, Edberg JC, Kimberly RP, Alarcon GS, Kamen DL, Gilkeson GS, Vyse TJ, James JA, Gaffney PM, Moser KL, Crow MK, Harley JB. IRF5 haplotypes demonstrate diverse serological associations which predict serum interferon alpha activity and explain the majority of the genetic association with systemic lupus erythematosus. Annals of the rheumatic diseases. 2012;71:463–8. doi: 10.1136/annrheumdis-2011-200463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niewold TB, Rivera TL, Buyon JP, Crow MK. Serum type I interferon activity is dependent on maternal diagnosis in anti-SSA/Ro-positive mothers of children with neonatal lupus. Arthritis and rheumatism. 2008c;58:541–6. doi: 10.1002/art.23191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niewold TB, Wu SC, Smith M, Morgan GA, Pachman LM. Familial aggregation of autoimmune disease in juvenile dermatomyositis. Pediatrics. 2011;127:e1239–46. doi: 10.1542/peds.2010-3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. The New England journal of medicine. 2000;343:938–52. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- Olsen N, Sokka T, Seehorn CL, Kraft B, Maas K, Moore J, Aune TM. A gene expression signature for recent onset rheumatoid arthritis in peripheral blood mononuclear cells. Annals of the rheumatic diseases. 2004;63:1387–92. doi: 10.1136/ard.2003.017194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterlund PI, Pietila TE, Veckman V, Kotenko SV, Julkunen I. IFN regulatory factor family members differentially regulate the expression of type III IFN (IFN-lambda) genes. Journal of immunology. 2007;179:3434–42. doi: 10.4049/jimmunol.179.6.3434. [DOI] [PubMed] [Google Scholar]
- Palucka AK, Blanck JP, Bennett L, Pascual V, Banchereau J. Cross-regulation of TNF and IFN-alpha in autoimmune diseases. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:3372–7. doi: 10.1073/pnas.0408506102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panitch HS, Hirsch RL, Haley AS, Johnson KP. Exacerbations of multiple sclerosis in patients treated with gamma interferon. Lancet. 1987;1:893–5. doi: 10.1016/s0140-6736(87)92863-7. [DOI] [PubMed] [Google Scholar]
- Passos de Souza E, Evangelista Segundo PT, Jose FF, Lemaire D, Santiago M. Rheumatoid arthritis induced by alpha-interferon therapy. Clinical rheumatology. 2001;20:297–9. doi: 10.1007/pl00011206. [DOI] [PubMed] [Google Scholar]
- Pestka S, Krause CD, Walter MR. Interferons, interferon-like cytokines, and their receptors. Immunological reviews. 2004;202:8–32. doi: 10.1111/j.0105-2896.2004.00204.x. [DOI] [PubMed] [Google Scholar]
- Pestka S, Langer JA, Zoon KC, Samuel CE. Interferons and their actions. Annual review of biochemistry. 1987;56:727–77. doi: 10.1146/annurev.bi.56.070187.003455. [DOI] [PubMed] [Google Scholar]
- Popescu BF, Pirko I, Lucchinetti CF. Pathology of multiple sclerosis: where do we stand? Continuum. 2013;19:901–21. doi: 10.1212/01.CON.0000433291.23091.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reder AT, Feng X. Aberrant Type I Interferon Regulation in Autoimmunity: Opposite Directions in MS and SLE, Shaped by Evolution and Body Ecology. Frontiers in immunology. 2013;4:281. doi: 10.3389/fimmu.2013.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekvig OP, Nossent JC. Anti-double-stranded DNA antibodies, nucleosomes, and systemic lupus erythematosus: a time for new paradigms? Arthritis and rheumatism. 2003;48:300–12. doi: 10.1002/art.10739. [DOI] [PubMed] [Google Scholar]
- Remmers EF, Plenge RM, Lee AT, Graham RR, Hom G, Behrens TW, de Bakker PI, Le JM, Lee HS, Batliwalla F, Li W, Masters SL, Booty MG, Carulli JP, Padyukov L, Alfredsson L, Klareskog L, Chen WV, Amos CI, Criswell LA, Seldin MF, Kastner DL, Gregersen PK. STAT4 and the risk of rheumatoid arthritis and systemic lupus erythematosus. The New England journal of medicine. 2007;357:977–86. doi: 10.1056/NEJMoa073003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson T, Kariuki SN, Franek BS, Kumabe M, Kumar AA, Badaracco M, Mikolaitis RA, Guerrero G, Utset TO, Drevlow BE, Zaacks LS, Grober JS, Cohen LM, Kirou KA, Crow MK, Jolly M, Niewold TB. Autoimmune disease risk variant of IFIH1 is associated with increased sensitivity to IFN-alpha and serologic autoimmunity in lupus patients. Journal of immunology. 2011;187:1298–303. doi: 10.4049/jimmunol.1100857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronnblom L, Eloranta ML, Alm GV. Role of natural interferon-alpha producing cells (plasmacytoid dendritic cells) in autoimmunity. Autoimmunity. 2003;36:463–72. doi: 10.1080/08916930310001602128. [DOI] [PubMed] [Google Scholar]
- Salloum R, Franek BS, Kariuki SN, Rhee L, Mikolaitis RA, Jolly M, Utset TO, Niewold TB. Genetic variation at the IRF7/PHRF1 locus is associated with autoantibody profile and serum interferon-alpha activity in lupus patients. Arthritis and rheumatism. 2010;62:553–61. doi: 10.1002/art.27182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salloum R, Niewold TB. Interferon regulatory factors in human lupus pathogenesis. Translational research : the journal of laboratory and clinical medicine. 2011;157:326–31. doi: 10.1016/j.trsl.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Suemori H, Hata N, Asagiri M, Ogasawara K, Nakao K, Nakaya T, Katsuki M, Noguchi S, Tanaka N, Taniguchi T. Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-alpha/beta gene induction. Immunity. 2000;13:539–48. doi: 10.1016/s1074-7613(00)00053-4. [DOI] [PubMed] [Google Scholar]
- Sekiguchi N, Kawauchi S, Furuya T, Inaba N, Matsuda K, Ando S, Ogasawara M, Aburatani H, Kameda H, Amano K, Abe T, Ito S, Takeuchi T. Messenger ribonucleic acid expression profile in peripheral blood cells from RA patients following treatment with an anti-TNF-alpha monoclonal antibody, infliximab. Rheumatology. 2008;47:780–8. doi: 10.1093/rheumatology/ken083. [DOI] [PubMed] [Google Scholar]
- Sfikakis PP, Iliopoulos A, Elezoglou A, Kittas C, Stratigos A. Psoriasis induced by anti-tumor necrosis factor therapy: a paradoxical adverse reaction. Arthritis and rheumatism. 2005;52:2513–8. doi: 10.1002/art.21233. [DOI] [PubMed] [Google Scholar]
- Shows TB, Sakaguchi AY, Naylor SL, Goedell DV, Lawn RM. Clustering of leukocyte and fibroblast interferon genes of human chromosome 9. Science. 1982;218:373–4. doi: 10.1126/science.6181564. [DOI] [PubMed] [Google Scholar]
- Shrestha S, Wershil B, Sarwark JF, Niewold TB, Philipp T, Pachman LM. Lesional and nonlesional skin from patients with untreated juvenile dermatomyositis displays increased numbers of mast cells and mature plasmacytoid dendritic cells. Arthritis and rheumatism. 2010;62:2813–22. doi: 10.1002/art.27529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdsson S, Nordmark G, Garnier S, Grundberg E, Kwan T, Nilsson O, Eloranta ML, Gunnarsson I, Svenungsson E, Sturfelt G, Bengtsson AA, Jonsen A, Truedsson L, Rantapaa-Dahlqvist S, Eriksson C, Alm G, Goring HH, Pastinen T, Syvanen AC, Ronnblom L. A risk haplotype of STAT4 for systemic lupus erythematosus is over-expressed, correlates with anti-dsDNA and shows additive effects with two risk alleles of IRF5. Human molecular genetics. 2008;17:2868–76. doi: 10.1093/hmg/ddn184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdsson S, Nordmark G, Goring HH, Lindroos K, Wiman AC, Sturfelt G, Jonsen A, Rantapaa-Dahlqvist S, Moller B, Kere J, Koskenmies S, Widen E, Eloranta ML, Julkunen H, Kristjansdottir H, Steinsson K, Alm G, Ronnblom L, Syvanen AC. Polymorphisms in the tyrosine kinase 2 and interferon regulatory factor 5 genes are associated with systemic lupus erythematosus. American journal of human genetics. 2005;76:528–37. doi: 10.1086/428480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiljanovic B, Grun JR, Biesen R, Schulte-Wrede U, Baumgrass R, Stuhlmuller B, Maslinski W, Hiepe F, Burmester GR, Radbruch A, Haupl T, Grutzkau A. The multifaceted balance of TNF-alpha and type I/II interferon responses in SLE and RA: how monocytes manage the impact of cytokines. Journal of molecular medicine. 2012;90:1295–309. doi: 10.1007/s00109-012-0907-y. [DOI] [PubMed] [Google Scholar]
- Sokumbi O, Wetter DA, Makol A, Warrington KJ. Vasculitis associated with tumor necrosis factor-alpha inhibitors. Mayo Clinic proceedings. 2012;87:739–45. doi: 10.1016/j.mayocp.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song G, Hsu CH, Riemer C, Zhang Y, Kim HL, Hoffmann F, Zhang L, Hardison RC, Green ED, Miller W. Conversion events in gene clusters. BMC evolutionary biology. 2011;11:226. doi: 10.1186/1471-2148-11-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–20. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- Tezak Z, Hoffman EP, Lutz JL, Fedczyna TO, Stephan D, Bremer EG, Krasnoselska-Riz I, Kumar A, Pachman LM. Gene expression profiling in DQA1*0501+ children with untreated dermatomyositis: a novel model of pathogenesis. Journal of immunology. 2002;168:4154–63. doi: 10.4049/jimmunol.168.8.4154. [DOI] [PubMed] [Google Scholar]
- Thomas C, Moraga I, Levin D, Krutzik PO, Podoplelova Y, Trejo A, Lee C, Yarden G, Vleck SE, Glenn JS, Nolan GP, Piehler J, Schreiber G, Garcia KC. Structural linkage between ligand discrimination and receptor activation by type I interferons. Cell. 2011;146:621–32. doi: 10.1016/j.cell.2011.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurlings RM, Boumans M, Tekstra J, van Roon JA, Vos K, van Westing DM, van Baarsen LG, Bos C, Kirou KA, Gerlag DM, Crow MK, Bijlsma JW, Verweij CL, Tak PP. Relationship between the type I interferon signature and the response to rituximab in rheumatoid arthritis patients. Arthritis and rheumatism. 2010;62:3607–14. doi: 10.1002/art.27702. [DOI] [PubMed] [Google Scholar]
- Tournadre A, Lenief V, Eljaafari A, Miossec P. Immature muscle precursors are a source of interferon-beta in myositis: role of Toll-like receptor 3 activation and contribution to HLA class I up-regulation. Arthritis and rheumatism. 2012;64:533–41. doi: 10.1002/art.33350. [DOI] [PubMed] [Google Scholar]
- Tournadre A, Lenief V, Miossec P. Expression of Toll-like receptor 3 and Toll-like receptor 7 in muscle is characteristic of inflammatory myopathy and is differentially regulated by Th1 and Th17 cytokines. Arthritis and rheumatism. 2010;62:2144–51. doi: 10.1002/art.27465. [DOI] [PubMed] [Google Scholar]
- Tyler DR, Persky ME, Matthews LA, Chan S, Farrar JD. Pre-assembly of STAT4 with the human IFN-alpha/beta receptor-2 subunit is mediated by the STAT4 N-domain. Molecular immunology. 2007;44:1864–72. doi: 10.1016/j.molimm.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uze G, Schreiber G, Piehler J, Pellegrini S. The receptor of the type I interferon family. Current topics in microbiology and immunology. 2007;316:71–95. doi: 10.1007/978-3-540-71329-6_5. [DOI] [PubMed] [Google Scholar]
- van der Pouw Kraan TC, Wijbrandts CA, van Baarsen LG, Voskuyl AE, Rustenburg F, Baggen JM, Ibrahim SM, Fero M, Dijkmans BA, Tak PP, Verweij CL. Rheumatoid arthritis subtypes identified by genomic profiling of peripheral blood cells: assignment of a type I interferon signature in a subpopulation of patients. Annals of the rheumatic diseases. 2007;66:1008–14. doi: 10.1136/ard.2006.063412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Holten J, Smeets TJ, Blankert P, Tak PP. Expression of interferon beta in synovial tissue from patients with rheumatoid arthritis: comparison with patients with osteoarthritis and reactive arthritis. Annals of the rheumatic diseases. 2005;64:1780–2. doi: 10.1136/ard.2005.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viscomi GC. Structure-activity of type I interferons. Biotherapy. 1997;10:59–86. doi: 10.1007/BF02678218. [DOI] [PubMed] [Google Scholar]
- Vitadello M, Doria A, Tarricone E, Ghirardello A, Gorza L. Myofiber stress-response in myositis: parallel investigations on patients and experimental animal models of muscle regeneration and systemic inflammation. Arthritis research & therapy. 2010;12:R52. doi: 10.1186/ar2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DJ, Petri M, Olsen N, Kirou KA, Dennis G, Yao Y, Jallal B, Coyle AJ, Zeng L, White B. MEDI-545, an Anti-Interferon Alpha Monoclonal Antibody, Shows Evidence of Clinical Activity in Systemic Lupus Erythematosus. Arthritis and rheumatism. 2007;56:S526–S527. [Google Scholar]
- Weckerle CE, Mangale D, Franek BS, Kelly JA, Kumabe M, James JA, Moser KL, Harley JB, Niewold TB. Large-scale analysis of tumor necrosis factor alpha levels in systemic lupus erythematosus. Arthritis and rheumatism. 2012;64:2947–52. doi: 10.1002/art.34483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams EL, Gadola S, Edwards CJ. Anti-TNF-induced lupus. Rheumatology. 2009;48:716–20. doi: 10.1093/rheumatology/kep080. [DOI] [PubMed] [Google Scholar]
- Woelk CH, Frost SD, Richman DD, Higley PE, Kosakovsky Pond SL. Evolution of the interferon alpha gene family in eutherian mammals. Gene. 2007;397:38–50. doi: 10.1016/j.gene.2007.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan B, Ye S, Chen G, Kuang M, Shen N, Chen S. Dysfunctional CD4+,CD25+ regulatory T cells in untreated active systemic lupus erythematosus secondary to interferon-alpha-producing antigen-presenting cells. Arthritis and rheumatism. 2008;58:801–12. doi: 10.1002/art.23268. [DOI] [PubMed] [Google Scholar]
- Yanai Y, Sanou O, Kayano T, Ariyasu H, Yamamoto K, Yamauchi H, Ikegami H, Kurimoto M. Analysis of the antiviral activities of natural IFN-alpha preparations and their subtype compositions. Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research. 2001;21:835–41. doi: 10.1089/107999001753238088. [DOI] [PubMed] [Google Scholar]
- Yao Y, Richman L, Higgs BW, Morehouse CA, de los Reyes M, Brohawn P, Zhang J, White B, Coyle AJ, Kiener PA, Jallal B. Neutralization of interferon-alpha/beta-inducible genes and downstream effect in a phase I trial of an anti-interferon-alpha monoclonal antibody in systemic lupus erythematosus. Arthritis and rheumatism. 2009;60:1785–96. doi: 10.1002/art.24557. [DOI] [PubMed] [Google Scholar]
- Ytterberg SR, Schnitzer TJ. Serum interferon levels in patients with systemic lupus erythematosus. Arthritis and rheumatism. 1982;25:401–6. doi: 10.1002/art.1780250407. [DOI] [PubMed] [Google Scholar]
- Zhou X, Dimachkie MM, Xiong M, Tan FK, Arnett FC. cDNA microarrays reveal distinct gene expression clusters in idiopathic inflammatory myopathies. Medical science monitor : international medical journal of experimental and clinical research. 2004;10:BR191–7. [PubMed] [Google Scholar]