Abstract
Current theoretical conceptualizations of regulatory development suggest that attention processes and emotion regulation processes share common neurophysiological underpinnings and behavioral antecedents such that emotion regulation abilities may build upon early attentional skills. To further elucidate this proposed relationship, we tested whether early neurophysiological processes measured during an attention task in infancy predicted in-task attention behavior, and whether infant's attention behavior was subsequently associated with their ability to regulate emotion in early childhood (N=388). Results indicated that, greater EEG power change (from baseline to task) at medial frontal locations (F3 and F4) during an attention task at 10 months were associated with concurrent observed behavioral attention. Specifically, greater change in EEG power at the right frontal location (F4) was associated with more attention, and greater EEG power at the left frontal location (F3) was associated with less attention, indicating a potential right hemisphere specialization for attention processes already present in the first year of life. In addition, after controlling for 5-month attention behavior, increased behavioral attention at 10-months was negatively associated with children's observed frustration to emotional challenge at age 3. Finally, the indirect effects from 10-month EEG power change at F3 and F4 to 3-year emotion regulation via infants' 10-month behavioral attention were significant, suggesting that infant's attention behavior is one mechanism through which early neurophysiological activity is related to emotion regulation abilities in childhood.
Keywords: emotion regulation, attention, EEG power, infancy, early childhood
Deficits in early emotion regulation abilities are considered to be central to childhood psychological maladjustment and thought to partially constrain subsequent development in a variety of domains (Keenan, 2000; Calkins & Fox, 2002; Calkins, 2008). Current conceptualizations of emotion regulation acknowledge that the regulation of emotion is not purely an emotional process. Instead, emotion regulation draws on fundamental neurophysiological, cognitive, and behavioral processes, all of which become elaborated and integrated over the course of development (Calkins, 1994, 2008; Fox & Calkins, 2003). Thus, a clearer understanding of how these different domains develop and integrate across infancy and early childhood has been suggested as critical for advancing our knowledge of how emotion regulation abilities contribute to overall behavioral adjustment (Posner & Rothbart, 2000).
The role of children's attentional capacities in the modulation of arousal has been of particular interest to developmental scientists. The first year of life is characterized by significant maturation of neural processes thought to underlie attention, as well as an increase in more sophisticated attention behaviors (Colombo, Harlan, & Mitchell, 1999; Posner & Fan, 2008). This is also the time when infants begin to develop and utilize rudimentary attention-based strategies to regulate emotional arousal (Grolnick, McMenamy, & Kurowski, 2006). Thus, understanding the development of neural mechanisms underlying infants' attention behavior may provide important insight into how early attention and emotional self-regulatory processes are linked, and how they are related to later adaptive functioning. In the current paper we utilize a biobehavioral developmental perspective, which advocates investigating both biological and behavioral levels of a given factor in the context of one another, rather than in isolation (Beauchaine, 2001). We examine the influence of attentional processes measured at both the neural and behavioral level during early infancy on children's ability to regulate emotion during early childhood. We posit that the development of attention-related neural activity during infancy may impact later emotion regulation behavior through its influence on children's developing attentional skills.
Emotion Regulation and Attentional Abilities
Emotion regulation processes are often defined as behaviors, skills, and strategies, whether automatic or effortful, which serve to modulate emotional experiences and expressions (Calkins & Hill, 2007). Although the development of emotion regulation relies on reciprocal and transactional associations across multiple developmental domains, previous theoretical and empirical work makes clear that attentional skills in particular are closely tied to the ability to regulate distress during infancy (Bell & Calkins, 2012; Calkins, 2004; Kopp, 1989; Thompson, Lewis, & Calkins, 2008). In fact, emotion regulation in the first year has been largely described and defined in terms of attentional and motoric control mechanisms that operate primarily to reduce, inhibit, amplify, or balance infant's affective responses (Calkins, 2004; Posner & Rothbart, 2000). Attention orienting, specifically, has been identified as playing a critical role in the early regulation of emotion (Rothbart, Sheese, Rueda, & Posner, 2011). This is because orienting attention toward a stimulus, or away from it, has the effect of amplifying or reducing the affective valence with which it is associated, therefore changing the affective experience and potential salience for the infant (Rothbart, Posner, & Rosicky, 1994).
Rothbart and colleagues (Rothbart, Posner, & Boylan, 1990) were among the first to observe that attentional orienting skills were related to decreases in negative affect in infants. Others have also found that distraction strategies, in which children shift their attention away from the source of arousal and orient toward a more positive or neutral stimulus, may assist the child in managing early frustration and fear responses (Calkins, Smith, Gill & Johnson, 1998; Diaz & Bell, 2011; Stifter & Braungart, 1995). Indeed, much research, including contingency studies, has demonstrated that infants who avert their gaze or distract away from a distressing stimulus (i.e., frustrating or fearful) show reduced negative affect in the moment, and less anxious behavior over time (Crockenberg & Leerkes, 2004, 2006; Stifter & Spinrad, 2002).
Given this concurrent association, qualitative shifts in attentional abilities across the first twelve months of life may be fundamental to qualitative shifts in early emotional self-regulation. From three to six months of age, infants develop the ability to coordinate their use of attention engagement and disengagement in response to emotion inducing situations in their environment. Thus, younger infants rely on simple attentional means such as gaze avoidance to regulate negative affect (Mangelsdorf, Sharpiro & Marzolf, 1995), but by six months of age an increase in attentional control allows infants to reliably distract or avert attention from the source of distress (Johnson, Posner, & Rothbart, 1999; Posner & Rothbart, 1998; Rothbart et al., 1990). By the end of the first year, fundamental changes in the development of executive attention occur, which result in increasingly more sophisticated volitional attention control (Ruff & Rothbart, 1996). The emergence of executive attention within this time period is coincident with a shift in emotion regulation strategies around 10 months of age during which infants are able to employ more organized and sophisticated sequences of behavior in a purposeful and flexible manner when responding to environmental cues (Calkins 2004; Calkins, Dedmon, Gill, Lomax, & Johnson, 2002; Kopp, 1982; Mangelsdorf et al., 1995).
The development of early more rudimentary self-regulatory processes is theorized to set the stage for, and may even constrain, the development of more complex and effortful emotion regulation that occurs over the course of early childhood (Calkins, 2010; Calkins, 2011). Specifically, infants who develop and are able to flexibly utilize attentional abilities to control arousal may have a larger repertoire of sophisticated self-regulatory strategies to effortfully draw from when encountering emotional situations independently. In addition, the practice of these emerging skills early on may lead to greater automaticity, so that by the time the child is ready to enter an environment with increasingly complex social and cognitive demands they are able to easily modulate their own arousal and behavior in a way that is socially appropriate without having to tax valuable cognitive resources (Calkins & Marcovitch, 2010).
The previously described work suggests that a detailed understanding of the development of attention during infancy has significant implications for understanding the origins of emotion regulation (Posner, Rothbart, Sheese, & Voelker, 2014). Although a large body of literature has examined attentional behavior as it relates to emotion regulation skills and strategies in infancy, models examining multiple levels of child attention (i.e., biological and behavioral) in combination with one another are needed to better elucidate developmental pathways to emotional adjustment (Cicchetti & Dawson, 2002; Lewis & Todd, 2007).
Neurophysiological Correlates of Attention Behavior
The emergence of attention behavior in development is theorized to be associated with underlying connectivity of neural circuitry that develops and strengthens across childhood (Colombo & Cheatham, 2006; Posner & Fan, 2008). Because of its role in the volitional control of attention, the executive attention network has been implicated as most critical for supporting developing regulatory abilities and has therefore been the focus of much theoretical and empirical work. Data from a number of studies have shown that situations requiring the volitional control that is associated with executive attention activate a neural network comprised of the Anterior Cingulate Cortex (ACC) in the medial frontal lobe and parts of the prefrontal cortex (Posner & Fan, 2008). These brain regions are thought to play an important role in the executive attention network because they serve to coordinate, regulate, and process information from other neural networks and are involved in the control of both cognition and emotion (Posner, 2012; Posner, Rothbart, Sheese, & Tang, 2007). Thus, the involvement of attention in emotional regulation is thought to be facilitated by this overlap in the neural circuitry within the ACC and prefrontal areas (Beauregard, Levesque, & Paquette, 2004; Posner & Fan, 2008; Rubia et al., 2008; Smith, Taylor, Brammer, Toone, & Rubia, 2006).
There is accumulating evidence that there is at least rudimentary overlap in the functional connectivity of brain areas comprising neural networks that subserve attention processes in very early infancy, although clearly activation between brain regions becomes increasingly coordinated during the latter half of the 1st year with significant development continuing into early childhood (Berger, Tzur, & Posner, 2006; Fair et al., 2012; Posner, Rothbart, Sheese, & Voelker, 2012, 2014). Work using fMRI functional connectivity (i.e., measurement of synchronization in activity from distinct brain areas) during a resting state has revealed that in neonates, parietal areas associated with an attention orienting network exhibit strong connectivity to lateral and medial frontal areas associated with the executive attention network (Posner et al., 2012). Despite the fact that these rudimentary connections (centered at a seed region) are present at birth, a series of recent studies has shown that extensive development of neural networks controlling attentional and cognitive control processes continues to occur rapidly, with adult-like connectivity present by the end of the first year of life (Gao, Zhu, Giovanello, Smith, Shen, et al., 2009; Gao, Gilmore, Shen, Smith, Zhu, & Lin, 2013).
Developmental imaging work in older children has shown that amount of ACC activation is related to both a child's observed performance on cognition and attention based laboratory tasks, as well as parental reports of emotional control abilities (Posner & Rothbart, 1998). Although this work has led to promising theoretical conceptualizations of the development of the executive attention network and the interconnectedness of attention and emotion processes in the infant brain, empirical work providing data in infancy is lacking. A better understanding of these associations in infancy is particularly important because the early emergence of more sophisticated regulatory strategies is thought to be heavily reliant on the development of the neural systems underlying attention during the latter half of the first year of life (Fox & Calkins, 2003; Grolnick, Bridges, & Connell, 1996; Kopp, 2002; Silk, Shaw, Skuban, Oland, & Kovacs, 2006). Delays in this development may be one source of individual difference in the development of effective emotion regulation and may result in elevated risk for developing early psychopathology such as internalizing and externalizing behavior problems (e.g. Calkins, 1994; Calkins, 2010; Calkins & Keane, 2004).
One reason empirical work in this area is lacking is the limited availability of neurophysiological methods that can be utilized with infant samples. Neural activity measured at the scalp level via electroencephalogram (EEG) methodology, particularly within areas of the frontal cortex, is one method that can provide a direct measure of attention processes in the infant brain. Attentional control exerts its influence in the brain by modulating the activity of neural systems involved in information processing such that information processing in the attended channel is facilitated, while processing in irrelevant channels is inhibited (Rueda, Posner, & Rothbart, 2005; Orekahova, Stroganova, & Posikera, 2001). The EEG signal is a measure of brain electrical activity that is recorded via electrodes on the scalp and results from summated postsynaptic neuronal potentials firing in synchrony (Davidson, Jackson & Larson, 2000). This synchronization of activity leads to a dominant frequency of oscillation that is measureable at electrode sites placed at specific scalp locations (e.g. Kagan, Snidman, Kahn, & Towsley, 2007). From this, measures of EEG power (i.e., root-mean-square average amplitude of the EEG signal within a frequency band of interest) can be derived and provide information about the extent of cortical activity at rest (i.e. baseline) and in response to specific situations or stimuli (i.e., task related changes).
Task related changes in EEG power (in comparison to baseline) are hypothesized to represent neural activity in brain areas underlying specific scalp electrodes, and have been used as indicators of cognitive processing during laboratory tasks with infants and adults (see Klimesch, 1999 for a review). Although work with adults and infants has revealed potential associations between attention performance and multiple EEG frequency bands at multiple scalp locations (see Orekahova, Stroganova, & Posikera, 1999 and a review by Saby & Marshall, 2012), the infant alpha band (6-9 Hz) has been identified by infant EEG researchers as the dominant frequency to investigate both cognitive and emotional processing (Bell, 2001, 2002, 2012; Orekhova et al., 2001; Marshall, Bar Haim & Fox, 2002), and is thought to approximate the alpha band in adults. Further, a relationship between EEG alpha activity and attentional modulation of cortical networks has a long and prevalent history and is commonly accepted in the field (Ray & Cole, 1985; Orekhova et al., 2001). For example, very early work attempting to link EEG and attention in infancy found a change in posterior rhythm similar to the adult alpha rhythm to be associated with visual stimulation (Lindsley, 1939). More systematic work with 7-12 month old infants replicated and extended this finding and specified that infant alpha power over fronto-central scalp locations increased during a visual stimulation condition compared to a condition of complete darkness (Stroganova, Orekhova, & Posikera, 1999).
This work was further extended by examining the association between infant alpha power and actual performance on attentional tasks. Specifically, better attention performance was associated with higher amplitude EEG power values at fronto-central and parietal locations in 7-12 month old infants during internally (anticipatory) and externally (visual display) controlled attention tasks, although this result was strongest for EEG measured during the externally controlled attention condition (Orekhova et al., 2001). Additional work with infants and the 6-9 Hz frequency band has shown that higher baseline EEG power values at all scalp locations, and larger baseline to task increases in EEG power at frontal scalp locations specifically, were associated with longer looking on a visual habituation task (Diaz & Bell, 2011) and better performance on working memory tasks that rely on attention shifting (e.g., Bell, 2002; Bell & Wolfe, 2007; Cuevas & Bell, 2011). These finding may be indicative of different levels of brain maturation resulting in individual differences in attention behavior in the first year (e.g., Cuevas & Bell, 2014; Marshall et al., 2002). Thus, utilizing EEG methodology to examine attention task-related changes in 6-9 Hz EEG power at medial frontal scalp locations (electrode sites F3 and F4) may allow for a clearer understanding regarding the association between early neural activity potentially linked with the executive attention network and observed attention behavior, and its relation to the development of later emotion regulation.
The Current Study
Early attentional processes are theorized to be one mechanism through which infants begin to develop more sophisticated attention-based strategies for regulating their emotional arousal, which has critical implications for overall behavioral adjustment (e.g., Bell & Calkins, 2012; Fox & Calkins 2003; Posner & Rothbart, 1998; Rothbart et al, 1994; Rothbart, Sheese, & Posner, 2007; Sheese, Rothbart, Posner, White, & Fraundorf, 2008). However, very little work examines the impact of attentional processes at multiple levels of child functioning on children's emotional control. To address this gap in the literature we utilize a bio-behavioral developmental perspective and examine the relation between infants' attention behavior and underlying neurophysiological activity, and their ability to emotionally regulate during childhood. We focused our investigation on the second half of the first year of life because it is during this time that attention-related neural networks become even more integrated, resulting in greater sustained attentional abilities that can be utilized to regulate emotional arousal. We hypothesized that 1) greater neurophysiological activity at 10 months, as indexed by increases in EEG power from baseline to task at frontal electrode sites, would be associated with greater observed attentional skills at 10 months, 2) greater attentional abilities at 10 months would be associated with greater emotional control at age 3, and 3) neurophysiological activity at 10 months would be associated with children's ability to emotionally regulate at age 3 through its influence on developing attentional behaviors.
Method
Participants
As part of a longitudinal study examining individual differences in the development of cognition and emotion across early development, 410 infants were recruited by two research locations (XXXX, and XXXX), with each location recruiting half of the total sample. Infants were recruited via commercial mailing lists, newspaper birth announcements, and word of mouth. Of the 410 infants, 22 were reported to be low birth weight or developmentally delayed at age 3 and were excluded from the final sample. Thus, the current study utilized data from 388 infants who were born within 15 days of their calculated due dates and were healthy at the time of testing. For mothers who reported educational information (N=378), 97% graduated from high school, 6% had a technical degree, 42% had a bachelor's degree, and 22% had a graduate degree. Mothers were, on average, 29 years old (SD = 6) when the infants were born.
At the 5-month visit, 388 infants participated. Infants were on average 162 (SD=7.8) days old, 51% female, 13% African American, 78% European American, and 9% multiracial or other. At the 10-month visit, 352 infants participated. Infants were on average 314 (SD=11.4) days old, 51% female, 13% African American, 78% European American, and 9% multiracial or other. At the 3-year visit, 305 children participated. Children were on average 1129 (SD=31.6) days old, 50% female, 13% African American, 79% European American, and 8% multiracial or other. Families lost to attrition included those who could not be located, moved out of the area, declined participation, or did not respond to phone and letter requests to participate. There were no significant differences between families who did or did not participant at each time point in terms of child sex or race.
Procedures
Data were collected in both research locations using identical protocols. Research assistants from each location were trained together by the project's Principal Investigator on protocol administration, as well as on behavioral and psychophysiological coding. To ensure that identical protocol administration was maintained between the labs, the XXXX site periodically viewed DVD recordings and psychophysiology files collected by the XXXX lab. To ensure that identical coding criteria were maintained between labs, the XXXX lab provided reliability coding for behavioral data and verification of artifact screening for psychophysiology data collected and coded by the XXXX lab.
Upon arrival at the research laboratory, participants were greeted by a research assistant who explained the study procedures and obtained signed consent from the mother. After a brief warm-up period, children were fitted with the EEG cap and participated in a variety of behavioral laboratory tasks assessing cognitive and emotional development. The session was digitally recorded for later behavioral coding. Parents were paid $50 for each laboratory visit.
Measures
Neural Activity
EEG was recorded during baseline and during a visual attention task at 5 and 10 months. To obtain baseline EEG at both the 5 and 10 month visit, infants sat on their mothers' laps and watched a research assistant manipulate a toy containing brightly colored balls on a testing table 1.1 meters in front of them. This baseline procedure quiets the infant, yields minimal eye and gross motor movements, and allows the infant to tolerate the EEG cap (e.g. Bell, 2001, 2012; Diaz & Bell, 2011; Fox, Henderson, Rubin, Calkins & Schmidt, 2001).
Recordings were made from 16 left and right scalp sites: frontal pole (Fp1, Fp2), medial frontal (F3, F4), lateral frontal (F7, F8), central (C3, C4), temporal (T7, T8), lateral parietal (P7, P8), medial parietal (P3, P4), and occipital (O1, O2). All electrode sites were referenced to Cz during recording. EEG was recorded using a stretch cap (Electro-Cap, Inc.; Eaton, OH; E1-series cap) with tin electrodes in the 10/20 system pattern. After the cap was placed on the head, a small amount of abrasive gel was placed into each recording site and the scalp gently rubbed. Conductive gel was then added to the recoding sites. Electrode impedances were measured and accepted if they were below 20 kΩ.
The electrical activity from each lead was amplified using separate James Long Company Bioamps (James Long Company; Caroga Lake, NY). During data collection, the high pass filter was a single pole RC filter with a 0.1 Hz cut-off (3 dB or half-power point) and 6 dB per octave roll-off. The low pass filter was a two-pole Butterworth type with a 100 Hz cut-off (3 dB or half-power point) and 12 dB octave roll-off. Activity for each lead was displayed on the monitor of the acquisition computer. The EEG was digitized on-line at 512 samples per second for each channel to eliminate the effects of aliasing. The acquisition software was Snapshot-Snapstream (HEM Data Corp.; Southfield, MI) and the raw data were stored for later analyses. Prior to the recording of each subject a 10 Hz, 50 uV peak-to-peak sine wave was input through each amplifier. This calibration signal was digitized for 30 s and stored for subsequent analysis.
Spectral analysis of the calibration signal and computation of power at the 9 to 11 Hz frequency band was accomplished. The power figures were used to calibrate the power derived from the subsequent spectral analysis of the EEG. Next, EEG data were examined and analyzed using EEG Analysis software developed by James Long Company. Data were re-referenced via software to an average reference configuration. The average reference EEG data were artifact scored for eye movements using a peak-to-peak criterion of 100 uV or greater. Artifact associated with gross motor movements over 200 uV peak-to-peak was also scored. These artifact-scored epochs were eliminated from all subsequent analyses. No artifact correction procedures were used. The data then were analyzed with a discrete Fourier transform (DFT) using a Hanning window of 1-s width and 50% overlap.
Power was computed for the 6- to 9-Hz frequency band. The 6-9 Hz frequency band is the dominant frequency for infants this age (Bell & Fox, 1992; Marshall et al., 2002), is thought to approximate the alpha band in adults, and has been used by infant EEG researchers to investigate both cognitive and emotional processing (Bell, 2001, 2002, 2012; Diaz & Bell, 2011; Fox et al., 2001; Orekhova, et al., 2001). Power was expressed as mean square microvolts and data were transformed using the natural log (ln) to normalize the distribution.
Because task-related changes in EEG power (in comparison to baseline) are hypothesized to represent activation of brain areas underlying specific scalp electrodes (Cuevas & Bell, 2011), we derived a measure of neural activity at frontal scalp locations by subtracting baseline EEG power from EEG power during the attention task at the medial frontal electrode sites (F3 and F4). We chose to only examine these medial frontal sites because of the well-established role of the frontal cortex for attention processes (Posner & Fan, 2008).
Attention Behavior
At the 5- and 10-month laboratory visit infants sat on their mothers' laps 1.1m from the edge of the testing table (90 cm [L] × 60 cm [W] × 75 cm [H]) and were presented with a glove puppet adorned with facial features on the palms of each glove and small bells attached to each fingertip. Glove puppets were presented four separate times, each ending when the infant looked away for at least 3 seconds. Although this procedure ensured that each infant was given multiple opportunities to direct their attention to the stimuli, it resulted in different task times for each participant (Cuevas & Bell, 2014). Thus, a proportion score of time looking at the glove puppet in relation to the total task time was utilized as the measure of infant attention. In the current study, a greater proportion of time spent attending to the puppet was indicative of greater behavioral attention ability.
Looking data were coded offline to determine proportion of time looking. A video camera was placed behind and above the experimenter's head and focused to maintain a close-up view of the glove and the infant's face. A research assistant coded each infant's look duration from a videotape/DVD of the laboratory session using the Video Coding System software developed by James Long Company (Caroga Lake, NY). An additional independent observer coded at least 20% of the videos to confirm reliability of coding. Intraclass correlations exceeded .91 for proportion of looking at each age at each study site.
Emotion Regulation
At the 36 month laboratory visit children participated in a frustrating puzzle task. During the task children were given a developmentally difficult alphabet puzzle with all of the pieces scattered around it and asked to work independently. The letters were heavily bubbled making their shape somewhat abstract, and there was no corresponding picture of the letter on the puzzle to use as a guide, so it was not easy to decipher exactly where the piece should fit. No child was able to complete the puzzle without assistance. A camera was focused on the child and child behavior was videotaped for off-line coding by trained research assistants. Coders assessed children's intensity and latency of anger, as well as their language valance and content, and rated children's global frustration on a 4-point scale (i.e., 1 = low incidence of frustration; 4 = high incidence). Because adaptive emotion regulation includes the ability to express and modulate emotions in a way that is socially appropriate, higher observed frustration scores are indicative of less regulatory ability. Global frustration was coded in 30-s epochs, which were then averaged to create a mean score for frustration across the entire task. Higher scores were indicative of greater frustration. An additional independent observer coded at least 20% of the videos to confirm reliability of coding. Intraclass correlations exceeded .94 for observed child frustration at each study site.
Results
Descriptive statistics and correlations for all study variables are presented in Table 1. A path analysis was conducted to examine the associations between brain electrical activity at frontal scalp locations during attention in infancy (i.e., EEG power change scores), observed attention behavior in infancy, and children's behavioral regulation of emotional frustration. Mplus (Version 7; Muthén & Muthén, 2012) was used to conduct the analyses and Full Information Maximum Likelihood (FIML) was used to handle missing data. Because baseline power values are included in the calculation of the change scores and may therefore impact the magnitude of change in power from baseline to task, 5-month and 10-month baseline power values were controlled for in the model. Moreover, given our interest in isolating the developmental time period associated with the second half of the first year when examining whether neural activity was associated with developmental changes in attention behavior and subsequent emotion regulation, neural activity and attention behavior at 5 months were controlled for in the model. In doing this, we were able to account for attentional skills in very early infancy and focus on the way in which developmental changes in neural and behavioral attentional processes occurring from 5 to 10 months of age influenced later emotion regulation.
Table 1. Descriptive Statistics and Correlations among Model Variables.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
|---|---|---|---|---|---|---|---|---|---|
| 1. Δ Power F3 5 months | -- | ||||||||
| 2. Δ Power F4 5 months | .43** | -- | |||||||
| 3. Δ Power F3 10 months | -.01 | .05 | -- | ||||||
| 4. Δ Power F4 10 months | .08 | .09 | .52** | -- | |||||
| 5. Baseline Power F3 10 months | -.16** | -.20** | -.40** | -.25** | -- | ||||
| 6. Baseline Power F4 10 months | -.22** | -.21** | -.28** | -.40** | .80** | -- | |||
| 7. Observed Attention 5 months | .02 | .10 | -.03 | -.03 | -.05 | -.03 | -- | ||
| 8. Observed Attention 10 months | .03 | .12* | -.12* | .03 | .12* | -.07 | .35** | -- | |
| 9. Observed Frustration 36 months | -.05 | -.05 | .07 | -.02 | -.11 | -.08 | -.07 | -.22** | -- |
|
| |||||||||
| Mean | .03 | .05 | .07 | .09 | 2.62 | 2.63 | 58.94 | 60.65 | 1.40 |
| Standard Deviation | .31 | .32 | .31 | .35 | .53 | .52 | 17.07 | 13.21 | .52 |
| Minimum | -.95 | -1.10 | -.91 | -.94 | .94 | 1.22 | 20.80 | 27.66 | 1.00 |
| Maximum | 1.47 | 1.41 | .94 | 2.23 | 4.30 | 4.11 | 94.84 | 94.73 | 4.00 |
| Skew (SE) | .12(.13) | .09(13) | -.05(.13) | .58(.13) | -.10(.13) | .09(.13) | .01(.13) | .07(.13) | 1.60(.15) |
| N | 355 | 355 | 331 | 331 | 334 | 334 | 370 | 343 | 273 |
Note:
p < .05,
p < .01
Evaluation of model fit was assessed by examining the comparative fit index (CFI) (Marsh & Hau, 2007) and the root mean square error of approximation (RMSEA) (Cole & Maxwell, 2003). Values close to or greater than .90 are desirable for the CFI, while RMSEA values should be less than or equal to .06 for good model fit (Hu & Bentler, 1999). Based on these criteria, the hypothesized model fit well, χ2 (29, N = 388) = 54.17, p = .01, CFI = .95, RMSEA = .05 [CI = .03, .07] (standardized coefficients are presented in Figure 1). The first aim of the study was to assess whether neural activity within the frontal cortex during an attention based task was associated with concurrent observed attention behavior. To measure neural activity we calculated the change in EEG power from a baseline task to an attention task at left frontal (F3) and right frontal (F4) electrode sites and modeled each as a predictor of observed attention. Results indicated that at 10-months, change in EEG power at the left frontal location was associated negatively with proportion of time spent attending, while change in EEG power at the right frontal location was associated positively with looking behaviors (see Figure 1). These results suggest that greater increases in EEG power within the left frontal cortex were associated with less attention to the stimulus, while greater increases in EEG power in the right frontal cortex were associated with greater attention.
Figure 1.
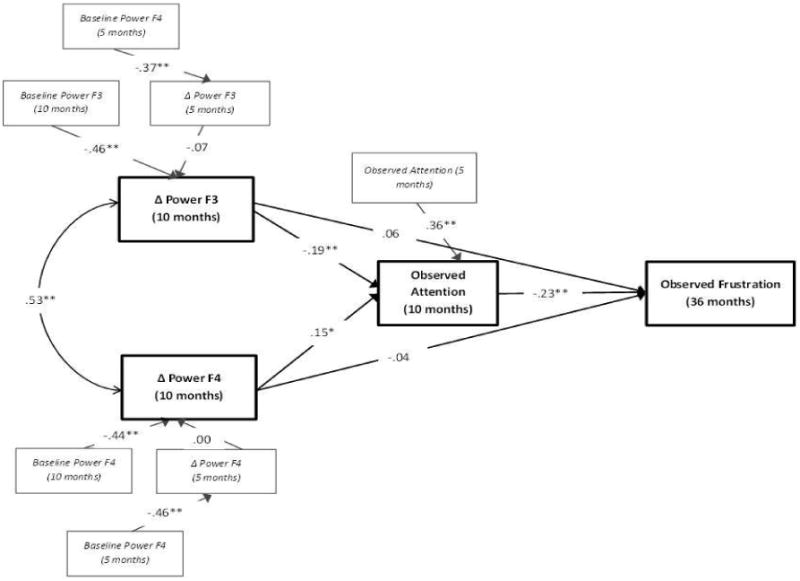
Standardized estimates for the model predicting 10-month observed infant attention and 36-month observed frustration. Italicized wording delineates variables included for the purposes of controlling for previous levels. *p < .05, **p < .01
We also examined whether attention in infancy was associated with children's ability to regulate their emotions during early childhood. Results revealed that proportion of time spent attending to the glove puppet at 10-months was associated negatively with children's observed frustration during the challenging puzzle task at age 3 (see Figure 1). These findings suggest that development in attentional abilities during the second half of the first year may have lasting impacts on emotion regulation in early childhood; the ability to sustain attentional engagement at 10 months, in particular, may be an important predictor for later emotion regulation abilities.
Finally, because infant attention is theorized to lay the foundation for later emotion regulation and behavioral control across childhood, and attentional control is built from rapidly developing neural networks, we investigated whether development of attention was one mechanism through which early neural activity within the frontal cortex is associated with later behavioral regulation of emotion. Thus, using a bias-corrected bootstrapping procedure (10,000 draws), we tested the indirect effect of EEG power change scores at F3 and F4 on observed frustration at 36 months through 10-month observed attention behavior. This approach has been shown to generate the most accurate confidence intervals for indirect effects, reducing Type 1 error rates and increasing power over other similar tests (MacKinnon, Lockwood, & Williams, 2004). The indirect effect from EEG power change at F3 to observed frustration at 36 months was significant (unstandardized estimate = .07, 95% BC Bootstrap CI [.02, .15]), indicating that greater power increase within the left frontal cortex during an attention task at 10 months was associated with greater 36-month frustration through its influence on infants' observed attention skills. The indirect effect from EEG power change at F4 to observed frustration at 36 months was also significant (unstandardized estimate = -.05, 95% BC Bootstrap CI [-.12, -.01]). However, in contrast to power change within the left frontal cortex, greater power increase within the right frontal cortex during the attention task at 10 months was associated with less 36-month frustration through its influence on infants' 10-month observed attention.
Discussion
Current conceptualizations of development acknowledge that complex interactions across levels of child functioning (Gottlieb, 1997; Sameroff, 2010, Shonkoff, 2010) impact developmental pathways, and developmental outcomes in any domain are at least partially dependent on fundamental neurophysiological and behavioral processes that become elaborated and integrated over time (Calkins, 1994, 2008; Thompson et al., 2008). Although a bio-behavioral perspective of development underscores the need to assess the contribution of underlying biological processes to produce patterns of behavior that are influential to the development of fundamental skills, very little work has examined the role of attentional processes at both the behavioral and neurophysiological level on children's developing emotional control.
Previous developmental work utilizing samples of older children and adults demonstrates that activity within the ACC is associated with observed attentional behavior (Posner & Rothbart, 1998; Posner & Rothbart, 2000, 2009). Empirical work providing data to support these ideas in infancy, however, is severely lacking. Therefore, the first goal of the current study was to test whether neural activity within the frontal cortex during an attention based task was associated with concurrent observed attention behavior during the second half of the first year of life. As hypothesized, we found that greater neurophysiological activity at medial frontal scalp locations during an attention task at 10 months was associated with greater time spent attending during the task. However, our findings indicate that this relationship was specific to the right frontal scalp location (F4). In contrast, an increase in power from baseline to task at the analogous left frontal scalp location (F3) was associated with less time spent attending at 10 months. In combination, these results suggest that neural activity within the frontal cortex may play a particularly important role in the development of infants' observed attention.
Our finding that right frontal activity, in particular, was related to greater observed attention fits with previous work from both clinical and developmental research showing a right hemisphere bias for attention based performance. Developmental neuroimaging work in older children (5 to 16 years), for example, has shown a significant correlation between the volume of area of the right anterior cingulate of the ACC, which is known to be related to brain development and functional maturation of neural networks, and the ability to perform tasks requiring focal attention (Casey et al., 1997b; Durston et al., 2001). Similarly, developmental neuroimaging work with older children with attention-deficit/hyperactivity disorder (ADHD) and age-matched controls has demonstrated that performance on tasks requiring the use of attention and response inhibition correlated with differences in volume of structures of the frontal cortex which were found to be abnormal in children with ADHD; these correlations were present predominately in the right hemisphere, thereby supporting a role of right frontal circuitry in attention and response inhibition abilities that are compromised in ADHD (Casey et al., 1997a).
Brain development is a dynamic and constantly evolving process and the relationship between neural activity and behavior also changes across developmental time periods. This may be particularly true with regard to neural systems involved in attention, cognitive control, and regulatory abilities (e.g. Posner, 2012). For example, a recent large-scale multi-center examination of structural maturation of the brain using MRI technology in children aged 4 to 21 years found that surface area of the ACC accounted for a significant proportion of the variance in cognitive control abilities measured by the flanker task of the NIH toolbox, which relies heavily on attentional control (Fjell, Walhovd, Brown, Kuperman, Chung, et al., 2012). This relationship was most prominent in the right anterior cingulate area of the ACC and strongest for the youngest children in the sample; this correlation decreased to non-significance with age. These findings support the hemispheric difference we observed in early infancy. Because there appears to be an advantage for right hemisphere dominance for attentional processing in older children and adults, our findings suggest that by 10-months of age this advantage is already evident and is associated with performance on an attention task. Taken together, these results may suggest that during infancy, when the most change in neural development is occurring, the adult like pattern of a right hemisphere advantage may have long-term consequences for subsequent abilities (e.g. self-regulation and emotion regulation) later in development.
Our findings are the first of our knowledge to demonstrate an advantage for right frontal alpha power during attention in infancy and extend previous findings of a right hemisphere specialization for attention processes to the first year of life. In addition, our finding that more left frontal activation during the attention task was related to less observed attention behavior suggests a potential disadvantage for left frontal activation during attention in infancy. It should be noted, however, that our findings regarding right hemisphere activation being associated with better attentional engagement in infancy are preliminary and were part of a larger study designed to look at multiple aspects of cognitive and emotional development. These ideas and questions should be further explored in longitudinal studies designed to look specifically at the neural bases of attention development and emotion regulation in infancy and early childhood.
We focused our investigation exclusively on the 6-9 Hz frequency band of the EEG, which is the most prevalent frequency band used in infant EEG research. However, previous work with adults and older children has also found a relationship between the theta frequency band (4-8 Hz) and performance on tasks that require cognitive control processes that depend heavily on attention (e.g. Cavanagh, Cohen, & Allen, 2009; Cavanagh, Frank, Klein, & Allen, 2010; Liu, Woltering, & Lewis, 2014). Importantly, using high density EEG methodology, these studies have also demonstrated that theta activity may originate from the ACC during tasks which require cognitive control (e.g. Cavanagh et al., 2009, 2010; Womelsdorf, Johnson, Vinck, & Everling, 2011). Previous infant work has examined theta band activity (3.6 – 6 Hz in infant samples) in relation to attention behavior in development and found that although there was an association between sustained attention and theta band activity, the direction of this relationship reversed between 8 and 10 months of age; increased theta power was associated with greater sustained attention at 8 months, but less at 9 and 10 months (Orekhova et al., 1999). Thus, the examination of EEG power in additional frequency bands in relation to the development of attention may be important in future work with infant and toddler samples.
A number of theoretical and empirical works have supported the role of attention behavior in children's emotion regulation abilities (e.g., Bell & Calkins, 2012; Crockenberg & Leerkes, 2006; Posner & Rothbart, 2000; Stifter & Spinrad, 2002). Very little research, however, has addressed whether the early development of attention behaviors impacts emotion regulation abilities in childhood. Thus, an additional aim of the current study was to assess whether the development of observed attentional abilities in infancy, as indexed by a greater proportion of time spent attending to a task, was associated with greater emotional control in early childhood.
As hypothesized, infants who demonstrated more attention to task stimuli at 10 months of age showed less evidence of frustration during a challenge task at 3 years of age.
Empirical work has documented normative declines in looking duration from 3 to 5 months of age, a number of studies have linked shorter looking durations (SL) during this time period to faster information processing better inhibition or disengagement of attention (see Colombo, 2002 for a review), and better EF and cognitive outcomes (e.g. Cuevas & Bell, 2014). By 7 months of age, however, looking behavior plateaus or increases and the relation between shorter looking duration and greater information processing is less clear (Colombo, Harlan, & Mitchell, 1999). The increase in infants' time spent attending during the second half of the first year is attributed to the emergence of endogenous or sustained attention processes. Sustained attention processes are more volitional and controlled and have been theoretically and empirically linked to the emergence of the executive attention system, which is thought to underlie downstream development of emotion regulation (Berger et al., 2006).
One way in which early attentional skills may related to children's later ability to emotionally regulate is through increased practice regulating emotional arousal. A greater ability to direct, orient, and control attention is likely to afford children more opportunities to directly engage with their environment. Engagement in qualitatively different social and emotional contexts that vary in arousal may subsequently provide increased opportunities for children to hone their regulatory skills (Sroufe, 1996). These opportunities to practice and employ varying regulatory strategies across infancy and toddlerhood may subsequently impact the sophistication of emotion regulation processes during early childhood. In addition, greater attentional abilities may influence the amount of information children receive and retain during social contexts and subsequently their social functioning (e.g., Eisenberg et al., 1993; Eisenberg, Spinrad, & Morris, 2002). For example, children who are better able to attend to relevant stimuli may have an easier time picking up on social partners' responses to emotional behavior, which convey information regarding appropriate emotional expression. An early history of attentional engagement in social interactions, during which children can pick up these social cues, is likely to be an important factor for later adaptive emotion regulation.
Finally, given the theorized role of neural activity in the development of increasing behavioral attentional abilities, and the relation between attentional skills and the regulation of emotion, we tested whether neurophysiological activity during infancy is associated with children's ability to emotionally regulate during early childhood through its influence on developing attentional behavior. As hypothesized, we found that neurophysiological activity at 10 months was associated with children's ability to emotionally regulate at age 3 through its influence on infants ability to attend to stimuli. However, the specific pattern of findings was again differentiated by hemisphere. Greater neural activity in the right hemisphere during an attention task during infancy was associated with a greater ability to emotionally regulate in early childhood through its impact on attention behavior. Thus, greater right hemisphere activity during an attention task leads to more attentional engagement in the moment; greater attentional engagement in infancy is then associated with a better ability to regulate one's own emotions independently in childhood. In contrast, greater left hemisphere activity was associated with less emotion regulation due to its negative impact on attentional engagement.
These findings suggest that right frontal activation during attention may be an optimal pattern of neurophysiological response from very early in development, and that deviations in this pattern of neural response may have downstream consequences for not only attention behaviors but also other areas of functioning that rely on attention development including emotion regulation. Moreover, our findings support the argument that both early neurophysiological and behavioral attentional processes play a role in children's later emotion regulation, and that child functioning across biological and behavioral levels should be considered in combination with one another to assess questions of developmental process.
The current study has multiple implications for developmental theory and research. First, findings highlight the importance of examining developmental processes from a bio-behavioral perspective and underscore the need for researchers to examine associations across multiple developmental domains and levels of child functioning. In addition, because a significant indirect effect emerged linking early neurophysiological attentional capacities to later emotion regulation through attention behavior, findings suggest that neural activity during attentional tasks during infancy may be one factor that can help identify children at risk for later deficits in emotional control. Additional research, however, is still needed to better understand the longitudinal relations between attention and emotion regulation processes. Analyses presented here are in line with prior longitudinal work highlighting the role of early attentional orienting on later emotional functioning (e.g., Rothbart et al.,2011; Sheese, Rothbart, Posner, White, & Fraundorf, 2008), but they do not address potential reciprocal relations among the development of both constructs over time. Recent research has shown infants early emotional expression during the first year of life to be associated with later attentional control by age 7 (Posner et al., 2014). Thus, future longitudinal developmental models examining reciprocal and bi-directional pathways from infancy throughout childhood may be particularly useful in identifying when and how emotion and attention processes integrate and support one another.
Although the current study had several strengths, there are some noteworthy limitations. First, we measured emotion regulation during a frustration task. Neurophysiological and behavioral attentional abilities may not be as salient of a predictor in the regulation of positive emotions due to the fact that attention aversion from a negative stimuli is not necessary. However, children may still reduce positive arousal or excitement by distracting themselves toward neutral stimuli in a very similar way. Although very little empirical work has addressed this, Stifter and Moyer (1991) were able to demonstrate that 5 month old infants avert their gaze as a means of regulating high levels of positive arousal during a peek-a-boo task. Thus, more research is needed to disentangle possible differentiating effects of attention on the regulation of both positive and negative emotion.
Second, because the focus of the current paper was on the association between early attention and later emotion regulation, we did not examine how attention is related to later cognitive regulation. There is a large body of literature, however, that links early attention to cognition in childhood (e.g., Sigman, Cohen, & Beckwith, 1997), including work conducted in our own lab (XXXX, 2014). Thus future bio-behavioral research is needed to examine the way in which early neurophysiological and behavioral attentional processes may influence one another, and subsequently, later abilities such as working memory, inhibitory control, and problem solving. Finally, although the current sample was relatively diverse, the majority of infants were from families in which at least one parent had a college education or higher (61%). Infants from higher income families with higher educated parents are often exposed to increased parental sensitivity and cognitive stimulation, both of which have been found to be associated with children's attention and emotion regulation (e.g., Berry, Deater-Deckard, McCartney, Wang, & Petrill, 2013; Chazan-Cohen et al., 2009; Feldman et al., 2009; Wijnroks, 1998). Therefore, an important goal for future work will be to assess these associations in more at-risk populations.
In conclusion, this work extends current literature and demonstrates that neural activity within the frontal cortex may play a particularly important role in the development of early attention behaviors that are associated with the emergence of more sophisticated emotion regulation behavior and, importantly, that this dynamic process begins in the first year of life. To our knowledge, this is the first study to provide empirical data demonstrating that neural activity during an attention task in infancy is related to both concurrent attention behavior, as well as future emotion regulation behavior through its influence on attentional abilities in infancy. In addition, findings underscore the importance of assessing the longitudinal relations across developmental domains and levels, and identifying potential mediating mechanisms, when attempting to understand developmental processes involved in the regulation of emotion.
Highlights.
Attention-related neural processes in infancy predicted observed attention behavior
Behavioral attention in infancy predicts emotion regulation at age 3
Infant neural activity predicted emotion regulation at age 3 through attention behavior
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Margaret M. Swingler, Email: mmswingl@uncg.edu.
Susan D. Calkins, Email: sdcalkin@uncg.edu.
Martha Ann Bell, Email: mabell@vt.edu.
References
- Beauchaine TP. Vagal tone, development, and Gray's motivational theory: Toward an integrated model of autonomic nervous system functioning in psychopathology. Development and Psychopathology. 2001;13(2):183–214. doi: 10.1017/s0954579401002012. doi: http://dx.doi.org/10.1017/S0954579401002012. [DOI] [PubMed] [Google Scholar]
- Beauregard M, Levesque J, Paquette V. Neural basis of conscious and voluntary self regulation of emotion. In: Beauregard M, editor. Consciousness, Emotional Self-Regulation and the Brain. Philadelphia: John Benjamins Publishing Company; 2004. pp. 163–194. [Google Scholar]
- Bell MA. Brain electrical activity associated with cognitive processing during a looking version of the A-not-B object permanence task. Infancy. 2001;2:311–330. doi: 10.1207/S15327078IN0203_2. [DOI] [PubMed] [Google Scholar]
- Bell MA. Power changes in infant EEG frequency bands during a spatial working memory task. Psychophysiology. 2002;39:450–458. doi: 10.1017/S0048577201393174. [DOI] [PubMed] [Google Scholar]
- Bell MA. A psychobiological perspective on working memory performance at 8 months of age. Child Development. 2012;83:251–265. doi: 10.1111/j.1467-8624.2011.01684.x. [DOI] [PubMed] [Google Scholar]
- Bell MA, Calkins SD. Attentional control and emotion regulation in early development. In: Posner MI, editor. Cognitive neuroscience of attention. 2nd. New York, NY, US: Guilford Press; 2012. pp. 322–330. [Google Scholar]
- Bell MA, Fox NA. The relations between frontal brain electrical activity and cognitive development during infancy. Child Development. 1992;63:1142–1163. doi: 10.1111/j.1467-8624.1992.tb01685.x. [DOI] [PubMed] [Google Scholar]
- Bell MA, Wolfe CD. Changes in brain functioning from infancy to early childhood: Evidence from EEG power and coherence during working memory tasks. Developmental Neuropsychology. 2007;31(1):21–38. doi: 10.1080/87565640709336885. [DOI] [PubMed] [Google Scholar]
- Berger A, Tzur G, Posner MI. Infant brains detect arithmetic errors. Proceedings of the National Academy of Sciences. 2006;103:12649–12653. doi: 10.1073/pnas.0605350103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry D, Deater-Deckard K, McCartney K, Wang Z, Petrill SA. Gene-environment interaction between dopamine receptor D4 7-repeat polymorphism and early maternal sensitivity predicts inattention trajectories across middle childhood. Development and Psychopathology. 2013;25(2):291–306. doi: 10.1017/S095457941200106X. doi: http://dx.doi.org/10.1017/S095457941200106X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins SD. Individual differences in the biological aspects of temperament. In: Bates JE, Wachs TD, editors. Temperament: Individual Differences at the Interface of Biology and Behavior. Washington, DC US: American Psychological Association; 1994. pp. 199–217. doi: http://dx.doi.org/10.1037/10149-007. [Google Scholar]
- Calkins SD. Early attachment process and the development of emotional self-regulation. In: Baumeister RF, Vohs KD, editors. The handbook of self-regulation. Hillsdale, N.J.: Lawrence Erlbaum; 2004. [Google Scholar]
- Calkins SD. The emergence of self-regulation: Biological and behavioral control mechanisms supporting toddler competencies. In: Brownell C, Kopp C, editors. Transitions in early socioemotional development: The toddler years. NY: Guilford Press; 2008. [Google Scholar]
- Calkins SD. Psychobiological models of adolescent risk: Implications for intervention and prevention. Introduction to the Special Issue. Developmental Psychobiology. 2010;52:213–215. doi: 10.1002/dev.20435. [DOI] [PubMed] [Google Scholar]
- Calkins SD. Biopsychosocial models and the study of family processes and child adjustment. Journal of Marriage and Family. 2011;73:817–821. doi: 10.1111/j.1741-3737.2011.00847.x. Doi: http://dx.doi.org/10.1111/j.1741-3737.2011.00847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins SD, Dedmon SE, Gill KL, Lomax LE, Johnson LM. Frustration in infancy: Implications for emotion regulation, physiological processes, and temperament. Infancy. 2002;3(2):175–197. doi: 10.1207/S15327078IN0302_4. [DOI] [PubMed] [Google Scholar]
- Calkins SD, Fox NA. Self-regulatory processes in early personality development: A multilevel approach to the study of childhood social withdrawal and aggression. Development and Psychopathology. 2002;14:477–498. doi: 10.1017/s095457940200305x. doi: http://dx.doi.org/10.1017/S095457940200305X. [DOI] [PubMed] [Google Scholar]
- Calkins SD, Hill A. Caregiver influences on emerging emotion regulation: Biological and environmental transactions in early development. In: Gross JJ, editor. Handbook of emotion regulation. New York, NY, US: Guilford Press; 2007. pp. 229–248. [Google Scholar]
- Calkins SD, Keane SP. Cardiac vagal regulation across the preschool period: Stability, continuity, and implications for childhood adjustment. Developmental Psychobiology. 2004;45:101–112. doi: 10.1002/dev.20020. [DOI] [PubMed] [Google Scholar]
- Calkins SD, Marcovitch S. Emotion regulation and executive functioning in early development: Integrated mechanisms of control supporting adaptive functioning. In: Calkins SD, Bell MA, editors. Child Development at the Intersection of Emotion and Cognition. Washington, DC, US: American Psychological Association; 2010. pp. 37–57. doi: http://dx.doi.org/10.1037/12059-003. [Google Scholar]
- Calkins SD, Smith CL, Gill KL, Johnson MC. Maternal interactive style across contexts: Relations to emotional, behavioral, and physiological regulation during toddlerhood. Social Development. 1998;7:350–369. doi: http://dx.doi.org/10.1111/1467-9507.00072. [Google Scholar]
- Casey BJ, Castellanos FX, Giedd JN, Marsh WL, Hamburger SD, Schubert AB, et al. Rapoport JL. Implication of right frontostriatal circuitry in response inhibition and attention-deficit/hyperactivity disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 1997a;36(3):374–383. doi: 10.1097/00004583-199703000-00016. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Trainor R, Giedd J, Vauss Y, Vaituzis CK, Hamburger S, et al. Rapoport JL. The role of the anterior cingulate in automatic and controlled processes: A developmental neuroanatomical study. Developmental Psychobiology. 1997b;30(1):61–69. doi: 10.1002/(SICI)1098-2302(199701)30:1<61∷AID-DEV6>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Cavanagh JF, Cohen MX, Allen JJB. Prelude to and resolution of an error: EEG phase synchrony reveals cognitive control dynamics during action monitoring. Journal of Neuroscience. 2009;29:98–105. doi: 10.1523/JNEUROSCI.4137-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Frank MJ, Klein TJ, Allen JJB. Frontal theta links prediction errors to behavioral adaptation in reinforcement learning. Neuroimage. 2010;49:3198–3209. doi: 10.1016/j.neuroimage.2009.11.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chazan-Cohen R, Raikes H, Brooks-Gunn J, Ayoub C, Pan BA, Kisker EE, et al. Fuligni AS. Low-income children's school readiness: Parent contributions over the first five years. Early Education and Development. 2009;20(6):958–977. doi: 10.1080/10409280903362402. [DOI] [Google Scholar]
- Cicchetti D, Dawson G. Editorial: Multiple levels of analysis. Development and Psychopathology. 2002;14:417–420. doi: 10.1017/s0954579402003012. doi: http://dx.doi.org/10.1017/S0954579402003012. [DOI] [PubMed] [Google Scholar]
- Cole DA, Maxwell SE. Testing mediational models with longitudinal data: Questions and tips in the use of structural equation modeling. Journal of Abnormal Psychology. 2003;112(4):558–577. doi: 10.1037/0021-843X.112.4.558. doi: http://dx.doi.org/10.1037/0021-843X.112.4.558. [DOI] [PubMed] [Google Scholar]
- Colombo J. Infant attention grows up: The emergence of a developmental cognitive neuroscience perspective. Current Directions in Psychological Science. 2002;11:196–200. doi: 10.1111/1467-8721.00199. [DOI] [Google Scholar]
- Colombo J, Cheatham CL. The emergence and basis of endogenous attention in infancy and early childhood. In: Kail RV, editor. Advances in child development and behavior. Vol. 34. San Diego, CA, US: Elsevier Academic Press; 2006. pp. 283–322. [DOI] [PubMed] [Google Scholar]
- Colombo J, Harlan JE, Mitchell DW. The Development of look duration in infancy: Evidence for a triphasic course. Poster presented at the annual meeting of the Society for Research in Child Development; Albuquerque, NM. 1999. [Google Scholar]
- Crockenberg SC, Leerkes EM. Infant and maternal behaviors regulate infant reactivity to novelty at 6 months. Developmental Psychology. 2004;40:1123–1132. doi: 10.1037/0012-1649.40.6.1123. doi: http://dx.doi.org/10.1037/0012-1649.40.6.1123. [DOI] [PubMed] [Google Scholar]
- Crockenberg SC, Leerkes EM. Infant and maternal behavior moderate reactivity to novelty to predict anxious behavior at 2.5 years. Development and Psychopathology. 2006;18(1):17–34. doi: 10.1017/S0954579406060020. doi: http://dx.doi.org/10.1017/S0954579406060020. [DOI] [PubMed] [Google Scholar]
- Cuevas K, Bell MA. EEG and ECG from 5 to 10 months of age: Developmental changes in baseline activation and cognitive processing during a working memory task. International Journal of Psychophysiology. 2011;80(2):119–128. doi: 10.1016/j.ijpsycho.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas K, Bell M. Infant attention and early childhood executive function. Child Development. 2014;85:397–404. doi: 10.1111/cdev.12126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ, Jackson DC, Larson CL. Human electroencephalography. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of psychophysiology. 2nd. New York, NY, US: Cambridge University Press; 2000. pp. 27–52. [Google Scholar]
- Diaz A, Bell MA. Information processing efficiency and regulation at five months. Infant Behavior & Development. 2011;34:239–247. doi: 10.1016/j.infbeh.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durston S, Hulshoff Pol HE, Casey BJ, Giedd JN, Buitelaar JK, Van Engeland H. Anatomical MRI of the developing human brain: What have we learned? Journal of American Academy Child Adolescent Psychiatry. 2001;40:1012–1020. doi: 10.1097/00004583-200109000-00009. doi: http://dx.doi.org/10.1097/00004583-200109000-00009. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Fabes RA, Bernzweig J, Karbon M, Poulin R, Hanish L. The relations of emotionality and regulation to preschoolers' social skills and sociometric status. Child Development. 1993;64(5):1418–1438. doi: 10.2307/1131543. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Spinrad TL, Morris AS. Regulation, resiliency, and quality of social functioning. Self and Identity. 2002;1(2):121–128. doi: http://dx.doi.org/10.1080/152988602317319294. [Google Scholar]
- Fair DA, Cohen AL, Power JD, Dosenbach NUF, Church JA, Miezin FM, Schlaggar BL, Petersen SE. Functional brain networks develop from a “local to distributed” organization. Public Library of Science Computational Biology. 2009;5:1–13. doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R, Granat A, Pariente C, Kanety H, Kuint J, Gilboa-Schechtman E. Maternal depression and anxiety across the postpartum year and infant social engagement, fear regulation, and stress reactivity. Journal of the American Academy of Child & Adolescent Psychiatry. 2009;48(9):919–927. doi: 10.1097/CHI.0b013e3181b21651. doi: http://dx.doi.org/10.1097/CHI.0b013e3181b21651. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Walhovd K, Brown T, Kuperman J, Chung Y, Hagler D, et al. Multi-modal imaging of the self-regulating brain. Proceedings of the National Academy of Sciences USA. 2012;109:19620–19625. doi: 10.1073/pnas.1208243109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox NA, Calkins SD. The development of self-control of emotion: Intrinsic and extrinsic influences. Motivation and Emotion. 2003;27:7–26. doi: http://dx.doi.org/10.1023/A:1023622324898. [Google Scholar]
- Fox NA, Henderson HA, Rubin KH, Calkins SD, Schmidt LA. Continuity and discontinuity of behavioral inhibition and exuberance: Psychophysiological and behavioral influences across the first four years of life. Child Development. 2001;72:1–21. doi: 10.1111/1467-8624.00262. [DOI] [PubMed] [Google Scholar]
- Gao W, Gilmore JH, Shen D, Smith JK, Zhu H, Lin W. The synchronization within and interaction between the default and dorsal attention networks in early infancy. Cerebral Cortex. 2013;23:594–603. doi: 10.1093/cercor/bhs043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Zhu H, Giovanello KS, Smith JK, Shen D, Gilmore JH, Lin W. Evidence on the emergence of the brain's default network from 2-week-old to 2-year-old healthy pediatric subjects. Proceedings of the National Academy of Sciences USA. 2009;106:6790–6795. doi: 10.1073/pnas.0811221106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb G. A systems view of psychobiological development. In: Magnusson D, editor. The lifespan development of individuals: Behavioral, neurobiological, and psychosocial perspectives: A synthesis. New York, NY, US: Cambridge University Press; 1997. pp. 76–103. [Google Scholar]
- Grolnick WS, Bridges LJ, Connell JP. Emotion regulation in two-year-olds: Strategies and emotional expression in four fejfcontexts. Child Development. 1996;67:928–941. doi: http://dx.doi.org/10.2307/1131871. [PubMed] [Google Scholar]
- Grolnick WS, McMenamy JM, Kurowski CO. Emotional self-regulation in infancy and toddlerhood. In: Balter L, Tamis-LeMonda CS, editors. Child psychology: A handbook of contemporary issues. 2nd. New York, NY, US: Psychology Press; 2006. pp. 3–25. [Google Scholar]
- Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling. 1999;6(1):1–55. doi: 10.1080/10705519909540118. [DOI] [Google Scholar]
- Johnson MH, Posner MI, Rothbart MK. Components of visual orienting in early infancy: Contingency learning, anticipatory looking, and disengaging. Journal of Cognitive Neuroscience. 1991;3:335–344. doi: 10.1162/jocn.1991.3.4.335. [DOI] [PubMed] [Google Scholar]
- Kagan J, Snidman N, Kahn V, Towsley S. The preservation of two infant temperaments into adolescence. Monographs of the Society for Research in Child Development. 2007;72:1–95. doi: 10.1111/j.1540-5834.2007.00436.x. [DOI] [PubMed] [Google Scholar]
- Keenan K. Emotion dysregulation as a risk factor for child psychopathology. Clinical Psychology: Science and Practice. 2000;7:418–434. doi: 10.1093/clipsy.7.4.418. [DOI] [Google Scholar]
- Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: A review and analysis. Brain Research Reviews. 1999;29(2-3):169–195. doi: 10.1016/S0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- Kopp CB. Antecedents of self-regulation: A developmental perspective. Developmental Psychology. 1982;18(2):199–214. doi: http://dx.doi.org/10.1037/0012-1649.18.2.199. [Google Scholar]
- Kopp CB. Regulation of distress and negative emotions: A developmental view. Developmental Psychology. 1989;25:343–354. doi: http://dx.doi.org/10.1037/0012-1649.25.3.343. [Google Scholar]
- Kopp CB. Commentary: The codevelopments of attention and emotion regulation. Infancy. 2002;3:199–208. doi: 10.1207/S15327078IN0302_5. [DOI] [PubMed] [Google Scholar]
- Lewis MD, Todd RM. The self-regulating brain: Cortical-subcortical feedback and the development of intelligent action. Cognitive Development. 2007;22(4):406–430. doi: 10.1016/j.cogdev.2007.08.004. [DOI] [Google Scholar]
- Lindsley DB. A longitudinal study of the occipital alpha rhythm in normal children: Frequency and amplitude standards. Journal of General Psychology. 1939;55:197–213. doi: 10.1080/08856559.1939.10533190. [DOI] [Google Scholar]
- Liu ZX, Woltering S, Lewis MD. Developmental change in EEG theta activity in the medial frontal cortex during response control. Neuroimage. 2014;85:873–887. doi: 10.1016/j.neuroimage.2013.08.054. [DOI] [PubMed] [Google Scholar]
- MacKinnon DP, Lockwood CM, Williams J. Confidence limits for the indirect effect: Distribution of the product and resampling methods. Multivariate Behavioral Research. 2004;39(1):99–128. doi: 10.1207/s15327906mbr3901_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangelsdorf SC, Shapiro JR, Marzolf D. Developmental and temperamental differences in emotional regulation in infancy. Child Development. 1995;66(6):1817–1828. doi: http://dx.doi.org/10.2307/1131912. [PubMed] [Google Scholar]
- Marsh HW, Hau K. Applications of latent-variable models in educational psychology: The need for methodological-substantive synergies. Contemporary Educational Psychology. 2007;32(1):151–170. doi: http://dx.doi.org/10.1016/j.cedpsych.2006.10.008. [Google Scholar]
- Marshall PJ, Bar-Haim Y, Fox NA. Development of the EEG from 5 months to 4 years of age. Clinical Neurophysiology. 2002;113:1199–1208. doi: 10.1016/S1388-2457(02)00163-3. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus. The comprehensive modelling program for applied researchers: User's guide. 2012;5 doi: 10.1016/S1388-2457(02)00163-3. [DOI] [Google Scholar]
- Orekhova EV, Stroganova TA, Posikera IN. Theta synchronization during sustained anticipatory attention in infants over the second half of the first year of life. International Journal of Psychophysiology. 1999;32:151–172. doi: 10.1016/S0167-8760(99)00011-2. [DOI] [PubMed] [Google Scholar]
- Orekhova EV, Stroganova TA, Posikera IN. Alpha activity as an index of cortical inhibition during sustained internally controlled attention in infants. Clinical Neurophysiology. 2001;112:740–749. doi: 10.1016/S1388-2457(01)00502-8. [DOI] [PubMed] [Google Scholar]
- Posner MI. Imaging attention networks. Neuroimage. 2012;61(2):450–456. doi: 10.1016/j.neuroimage.2011.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Fan J. Attention as an organ system. In: Pomerantz JR, editor. Topics in integrative neuroscience. New York: Cambridge University Press; 2008. pp. 31–61. [Google Scholar]
- Posner MI, Rothbart MK. Summary and commentary: Developing attentional skills. In: Richards JE, editor. Cognitive neuroscience of attention: A developmental perspective. Mahwah, NJ: Erlbaum; 1998. pp. 317–323. [Google Scholar]
- Posner MI, Rothbart MK. Developing Mechanisms of Self-regulation. Development and Psychopathology. 2000;12:427–441. doi: 10.1017/S0954579400003096. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK. Toward a physical basis of attention and self-regulation. Physics of life reviews. 2009;6:103–120. doi: 10.1016/j.plrev.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK, Sheese BE, Tang Y. The anterior cingulate gyrus and the mechanism of self-regulation. Cognitive, Affective & Behavioral Neuroscience. 2007;7:391–395. doi: 10.3758/CABN.7.4.391. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK, Sheese BE, Voelker P. Control networks and neuromodulators of early development. Developmental Psychology. 2012;48(3):827–835. doi: 10.1037/a0025530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK, Sheese BE, Voelker P. Developing attention: Behavioral and brain mechanisms. Advances in Neuroscience. 2014 doi: 10.1155/2014/405094. Article ID 405094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray WJ, Cole HW. EEG alpha activity reflects attentional demands, and beta activity reflects emotional and cognitive processes. Science. 1985;228:750–752. doi: 10.1126/science.3992243. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Posner MI, Boylan A. Regulatory mechanisms in infant development. In: Enns JT, editor. The development of attention: Research and theory. Oxford, England: North-Holland; 1990. pp. 47–66. [Google Scholar]
- Rothbart MK, Posner MI, Rosicky J. Orienting in normal and pathological development. Development and Psychopathology. 1994;6:635–652. doi: http://dx.doi.org/10.1017/S0954579400004715. [Google Scholar]
- Rothbart MK, Sheese BE, Posner MI. Executive attention and effortful control: Linking temperament, brain networks, and genes. Child Development Perspectives. 2007;1:2–7. doi: 10.1111/j.1750-8606.2007.00002.x. [DOI] [Google Scholar]
- Rothbart MK, Sheese BE, Rueda MR, Posner MI. Developing mechanisms of self-regulation in early life. Emotion Review. 2011;3(2):207–213. doi: 10.1177/1754073910387943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Halari R, Smith A, Mohammed M, Scott S, Giampietro V, Taylor E, Brammer M. Dissociated functional brain abnormalities of inhibition in boys with pure conduct disorder and in boys with pure attention deficit hyperactivity disorder. American Journal of Psychiatry. 2008;165:889–897. doi: 10.1176/appi.ajp.2008.07071084. doi: http://dx.doi.org/10.1176/appi.ajp.2008.07071084. [DOI] [PubMed] [Google Scholar]
- Rueda M, Posner MI, Rothbart MK. The Development of Executive Attention: Contributions to the Emergence of Self-Regulation. Developmental Neuropsychology. 2005;28:573–594. doi: 10.1207/s15326942dn2802_2. [DOI] [PubMed] [Google Scholar]
- Ruff H, Rothbart M. Attention in early development: Themes and variations. New York, NY: Oxford University Press; 1996. [Google Scholar]
- Saby JN, Marshall P. The utility of EEG band power analysis in the study of infancy and early childhood. Developmental Neuropsychology. 2012;37(3):253–273. doi: 10.1080/87565641.2011.614663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sameroff A. A unified theory of development: A dialectic integration of nature and nurture. Child Development. 2010;81:6–22. doi: 10.1111/j.1467-8624.2009.01378.x. [DOI] [PubMed] [Google Scholar]
- Sheese BE, Rothbart MK, Posner MI, White LK, Fraundorf SH. Executive attention and self-regulation in infancy. Infant Behavior & Development. 2008;31(3):501–510. doi: 10.1016/j.infbeh.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Shonkoff JP. Building a new biodevelopmental framework to guide the future of early childhood policy. Child Development. 2010;81:357–367. doi: 10.1111/j.1467-8624.2009.01399.x. [DOI] [PubMed] [Google Scholar]
- Sigman M, Cohen SE, Beckwith L. Why does infant attention predict adolescent intelligence? Infant Behavior & Development. 1997;20(2):133–140. doi: 10.1016/S0163-6383(97)90016-3. [DOI] [Google Scholar]
- Silk JS, Shaw DS, Skuban EM, Oland AA, Kovacs M. Emotion regulation strategies in offspring of childhood-onset depressed mothers. Journal of Child Psychology and Psychiatry. 2006;47:69–78. doi: 10.1111/j.1469-7610.2005.01440.x. [DOI] [PubMed] [Google Scholar]
- Smith A, Taylor E, Brammer M, Toone B, Rubia K. Task-specific hypoactivation in prefrontal and temporoparietal brain regions during motor inhibition and task switching in medication-naive children and adolescents with attention deficit hyperactivity disorder. American Journal of Psychiatry. 2006;163:1044–1051. doi: 10.1176/ajp.2006.163.6.1044. doi: http://dx.doi.org/10.1176/appi.ajp.163.6.1044. [DOI] [PubMed] [Google Scholar]
- Sroufe LA. Emotional development: The organization of emotional life in the early years. New York, NY, US: Cambridge University Press; 1996. [Google Scholar]
- Stifter CA, Braungart JM. The regulation of negative reactivity in infancy: Function and development. Developmental Psychology. 1995;31(3):448–455. doi: http://dx.doi.org/10.1037/0012-1649.31.3.448. [Google Scholar]
- Stifter CA, Moyer D. The regulation of positive affect: Gaze aversion activity during mother-infant interaction. Infant Behavior & Development. 1991;14(1):111–123. doi: 10.1016/0163-6383(91)90058-Z. [DOI] [Google Scholar]
- Stifter CA, Spinrad TL. The effect of excessive crying on the development of emotion regulation. Infancy. 2002;3(2):133–152. doi: 10.1207/S15327078IN0302_2. [DOI] [PubMed] [Google Scholar]
- Stroganova TA, Orekhova EV, Posikera IN. EEG alpha rhythm in infants. Clinical Neurophysiology. 1999;110:997–1012. doi: 10.1016/S1388-2457(98)00009-1. [DOI] [PubMed] [Google Scholar]
- Thompson RA, Lewis MD, Calkins SD. Reassessing emotion regulation. Child Development Perspectives. 2008;2:124–131. doi: 10.1111/j.1750-8606.2008.00054.x. [DOI] [Google Scholar]
- Wijnroks L. Early maternal stimulation and the development of cognitive competence and attention of preterm infants. Early Development & Parenting. 1998;7(1):19–30. doi: 10.1002/(SICI)1099-0917(199803)7:1<19∷AID-EDP160>3.0.CO;2-R. [DOI] [Google Scholar]
- Womelsdorf T, Johnston K, Vinck M, Everling S. Theta-activity in anterior cingulate cortex predicts task rules and their adjustments following errors. Proceedings of the National Academy of Sciences. 2010;107:5248–5253. doi: 10.1073/pnas.0906194107. [DOI] [PMC free article] [PubMed] [Google Scholar]


