Abstract
Background
The long-term efficacy of combination of chemotherapy with dasatinib in patients with Philadelphia-chromosome positive (Ph+) acute lymphoblastic leukemia (ALL) is not well-established.
Methods
Patients received dasatinib with 8 cycles of alternating hyperCVAD and high dose cytarabine and methotrexate. Patients in complete remission (CR) continued maintenance dasatinib, vincristine and prednisone for 2 years followed by dasatinib indefinitely. Patients eligible for allogeneic stem cell transplant (SCT) received it in first CR.
Results
72 patients with a median age of 55 years (range 21 – 80) were treated; 69 (96%) achieved CR. Among them, 57 (83%) achieved cytogenetic (CG) CR after 1 cycle and 64 (93%) achieved a major molecular response (MMR) at a median of 4 weeks (range, 2 – 38 weeks). Minimal residual disease by flow cytometry was negative in 65 (94 %) patients at a median of 3 weeks (range, 2–37). Dasatinib-related grade 3 and 4 adverse events included bleeding, pleural/pericardial effusions, and elevated transaminases. With a median follow-up of 67 months (range, 33–97), 33 patients (46%) are alive and 30 (43%) are in CR; 12 underwent an allogeneic SCT. Thirty nine patients have died (3 at induction, 19 after relapse, 7 post SCT performed in CR1, and 10 in CR). The median disease free and overall survival are 31 months (range, 0.3 to 97) and 47 months (range, 0.2 to 97). Seven relapsed patients had ABL mutations including 4 T315I.
Conclusion
Combination of chemotherapy with dasatinib is effective in achieving long-term remissions in patients with newly diagnosed Ph+ ALL.
Keywords: Philadelphia chromosome, acute lymphoblastic leukemia, dasatinib, chemotherapy, combination
INTRODUCTION
The introduction of tyrosine kinase inhibitors (TKIs) has revolutionized the management of a number of hematological malignancies and has changed the natural history of patients with chronic myeloid leukemia (CML).1 The Philadelphia chromosome (Ph) is also detected in about a third of adult patients with acute lymphoblastic leukemia with an even higher incidence among the older patients.2 Early trials demonstrated the high efficacy of TKIs in patients with relapsed Ph+ ALL even as monotherapy.3,4 However, it was clear, that despite their potency, these agents were likely associated with more durable responses when administered together with combination chemotherapy regimens designed for patients with ALL.5–7 Dasatinib, has been used both for salvage as well as for the initial therapy of patients with Ph+ ALL.8
Preclinical studies have demonstrated the improved efficacy of dasatinib against BCR-ABL bearing leukemic cells when combined with traditional ALL-specific chemotherapy agents.9 We have therefore combined dasatinib with the hyperCVAD regimen for the treatment of patients with Ph+ ALL and have previously reported the high efficacy and manageable toxicity of this combination in this setting.10 Important questions that remain are the durability of these responses and whether this regimen can omit the need for an allogeneic SCT in patients without an available donor or those who are unable to undergo transplant due to advanced age or comorbid medical conditions. The primary objective of the study was the clinical efficacy including response, and the duration of disease-free survival (DFS), and overall survival (OS). Secondary objectives were toxicity, immunophenotypic response rate, and molecular response rate. Previously, we have reported on the response rate, minimal residual disease evalutaion and early follow-up of patients in this study.10,11 Herein, we report the long term outcomes of patients treated with this regimen and discuss the potential ways that this strategy can be improved further.
PATIENTS AND METHODS
Eligibility Criteria
Newly diagnosed patients older than 18 with a proven diagnosis of Ph+ ALL based on the detection of either the cytogenetic abnormality and/or the fusion transcript product of the translocation (BCR-ABL) were eligible for participation in the study. Patients who had received 1 or 2 prior courses of chemotherapy (typically prior to the detection of the Ph abnormality) were also eligible for enrollment but were only evaluable for toxicity and outcome assessment. Patients had to have an Eastern Cooperative Oncology Group performance status of 2 or less, as well as adequate renal, hepatic and cardiac function with serum creatinine ≤ 3.0 mg/dl, serum bilirubin ≤ 3.0 mg/dl (unless considered due to the leukemia) and no clinical evidence of congestive heart failure. Patients were excluded if they had active infections that were not controlled with antibiotics, or if they were pregnant, had significant history of bleeding disorders, or had a clinically significant pleural or pericardial effusion (unless it was directly related to the leukemia). All patients had to sign an informed consent approved by the University of Texas – MD Anderson Cancer Center Institutional Review Board, and the study was conducted in accordance to the declaration of Helsinki.
Treatment Regimen
Initially patients received dasatinib 50 mg orally twice daily for the first 14 days of each of 8 cycles of alternating hyperCVAD (hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone) and high dose cytarabine and methotrexate (induction/consolidation cycles). After the demonstration of their equivalence, the study was amended to replace the twice daily dosing regimen with dasatinib 100 mg daily.12 After the enrollment of the first 42 patients, the protocol was further amended to give dasatinib 100 mg daily in the first 14 days of the first cycle followed by 70 mg daily continuously from the second cycle. Patients achieving complete remission (CR) continued to receive maintenance dasatinib (initially 50 mg orally twice daily and after amendment 100 mg daily) as well as vincristine and prednisone monthly for 2 years followed by dasatinib indefinitely. Patients could receive intensification courses of hyperCVAD on months 6 and 13 of maintenance depending on their clinical status and presence of MRD. All patients received prophylactic intrathecal (IT) chemotherapy to prevent central nervous system (CNS) relapse. Initially, the number of doses of IT chemotherapy was determined using a previously reported risk score including presentation LDH value and the bone marrow proliferative index (6 for patients with low risk of CNS relapse and 8 for those with high risk).13 Later all patients received 8 IT chemotherapy treatments administered twice per course. Patients presenting with CNS disease received twice weekly IT chemotherapy until the cerebrospinal fluid (CSF) became clear of blasts; they then received once weekly CNS therapy for 4 more doses. Patients with cranial nerve palsies received radiation therapy to the base of the skull. Full details of the regimen and the dose of individual agents have been previously published.5,14 Patients eligible for allogeneic SCT proceeded to it in first CR. All patients received standard supportive measures based on the prevailing institutional guidelines including transfusion of blood products, prophylactic and therapeutic use of antibiotics, and measures to prevent and treat tumor lysis syndrome, as previously reported.10
Response Criteria
Standard, published criteria for determining response were used to assess the efficacy of the regimen.15 Achievement of complete response (CR) required a bone marrow examination showing trilineage hematopoiesis with < 5% blasts and with the absence of circulating blasts and extramedullary disease. Furthermore, an absolute neutrophil counts (ANC) >1.0 × 109/L and platelet counts >100 × 109/L were required. Remission of CNS disease was defined as the presence of no lymphoblasts in the CSF regardless of white blood cell (WBC) count. Relapse was defined as the reappearance of lymphoblasts in the peripheral blood or bone marrow (> 5%) or in any extramedullary site. Cytogenetic and molecular responses were defined as previously reported.11
Follow-up Assessments
Baseline evaluations included a full history and physical examination, as well as complete blood count (CBC), full chemistry panel, bone marrow aspirate examination for morphology, flow cytometry and detection of the Ph abnormality using standard cytogenetics, fluorescent in situ hybridization (FISH) and reverse transcription quantitative polymerase chain reaction (RT-qPCR) for the BCR-ABL fusion transcripts. CBC and chemistry panel was repeated at least weekly during the intensive chemotherapy cycles and at least monthly during the maintenance phase. Bone marrow exam including cytogenetics (CG), and RT-qPCR test for BCR-ABL was repeated on days 14 and 21 of the first cycle, every 2 to 3 cycles during the intensive phase, and approximately every 3 months during the maintenance phase. Baseline CSF evaluation was conducted on day 2 of the induction course and any other time when IT chemotherapy was administered. Minimal residual disease (MRD) assessment was performed on all bone marrow specimens as previously reported.10,11
Statistical Analysis and Survival Definitions
Overall survival was measured from the time of study enrollment to the time of death or last follow-up. Event-free survival was from the study entry to the time of relapse, progression or death. Disease free survival was calculated from the time of achievement of CR until relapse or death from any cause. Survival outcomes were evaluated using the Kaplan-Meier method and were compared with the log-rank test. Differences between cohorts were evaluated using the χ2 test for nominal variables and the Mann-Whitney U test and Fisher exact test for continuous values.
RESULTS
Patients
Between September 2006 and March 2012, 72 patients including 63 patients with untreated Ph+ ALL and 9 patients with 1 or 2 prior cycles of chemotherapy (before Ph+/BCR-ABL+ status was known) were enrolled and treated in the study. The median age of the patients is 55 years (range 21 – 80 years) with 46 patients being older than 50. The median WBC at diagnosis was 12 × 109/L (range, 0.4 – 658.1 × 109/L). Ten patients had CNS involvement at presentation. Other pre-treatment characteristics of the patients are summarized in Table 1.
Table 1.
Patient characteristics
| Demographics | Median [range], or No (%) |
|---|---|
| Patients (Pts) | 72 |
| Median Age at dx, Years [Range] | 55 [21–80] |
| Age > 50 years | 46 (64) |
| Male/Female | 40/32 |
| Laboratory | |
| WBC (× 109/L) | 12 [0.4 – 658.1] |
| WBC > 30 × 109/L | 26 (36) |
| Cytogenetic | |
| Ph+ alone | 13 (18) |
| Ph+ with other | 45 (63) |
| Diploid, FISH+ or PCR+ | 14 (19) |
| BCR-ABL Transcript | |
| e1a2 | 52 |
| b2a2 | 13 |
| b2a2+b3a2 | 2 |
| b3a2/e1a3 | 2/2 |
| Not done | 1 |
Response and Survival
Patients have received a median of 6 cycles (range 1–8) of induction/consolidation. 53 patients received fewer than the intended 8 cycles of induction/consolidation due to poor tolerance (particularly the older patients)(n=31), early death (n=9) early relapse (n=1) or proceeding to allogeneic SCT (n=12). All patients were evaluable for assessment of response to induction; 69 (96%) achieved CR after the first cycle of protocol therapy or were CR at start (5 of the 9 patients with prior therapy before enrollment). Three patients died from infection-related complications before their response to induction could be assessed. After 1 cycle of therapy, 57 of 69 (83%) evaluable patients achieved cytogenetic (CG) CR; 5 had a major CG response (4 had 5% and one had 15% remaining Ph+ metaphases), 2 had insufficient metaphases for CG assessment, and 5 are unknown (no CG exam on day 21 marrow). To date, 45 patients (65%) have achieved complete molecular remission (CMR) and another 19 (28%) have achieved a major (but not complete) molecular response (MMR) at a median of 4 weeks from initiation of treatment (range, 2 – 38 weeks). Minimal residual disease assessment by flow cytometry is negative in 65 (94 %) patients at a median of 3 weeks (range, 2–37 weeks).
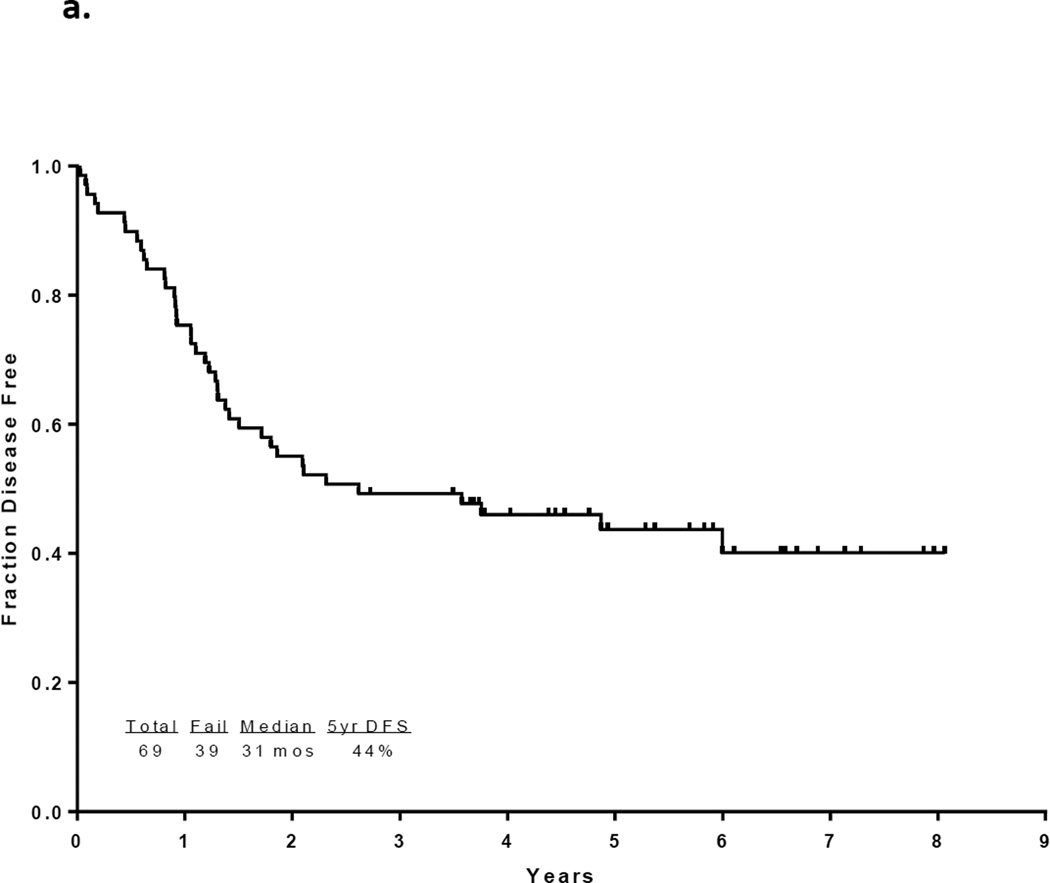
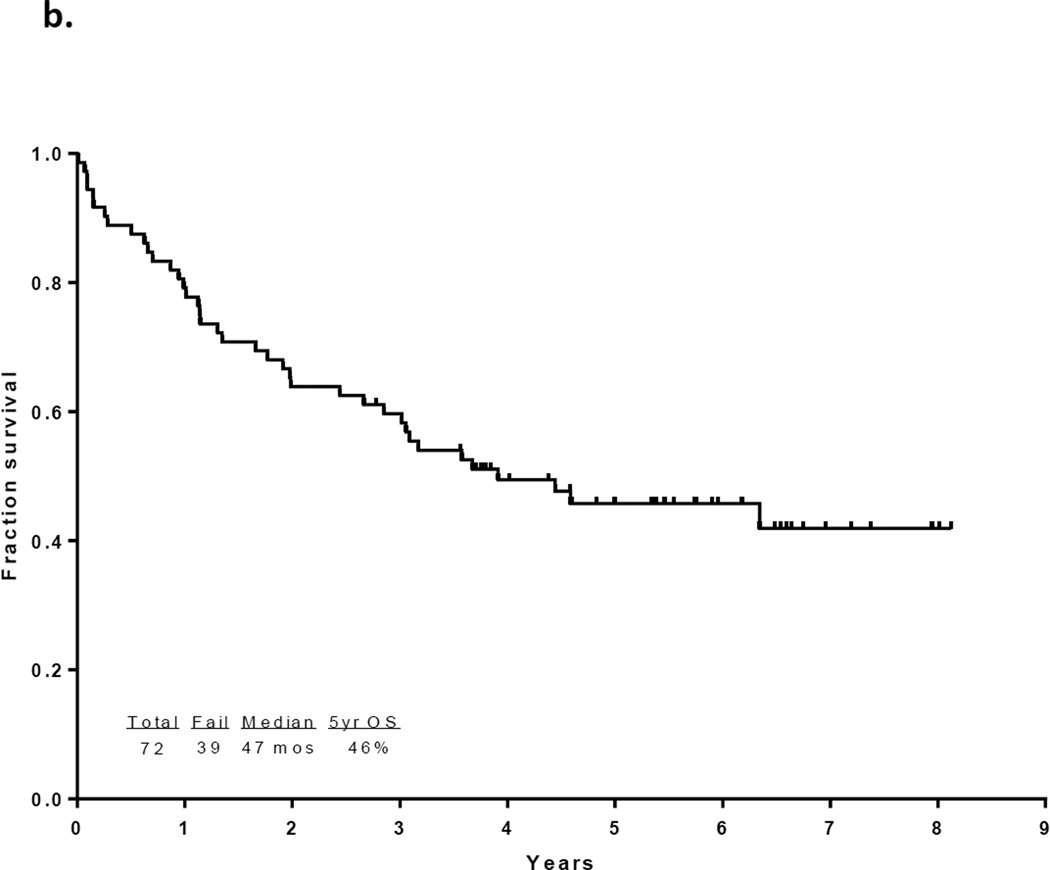
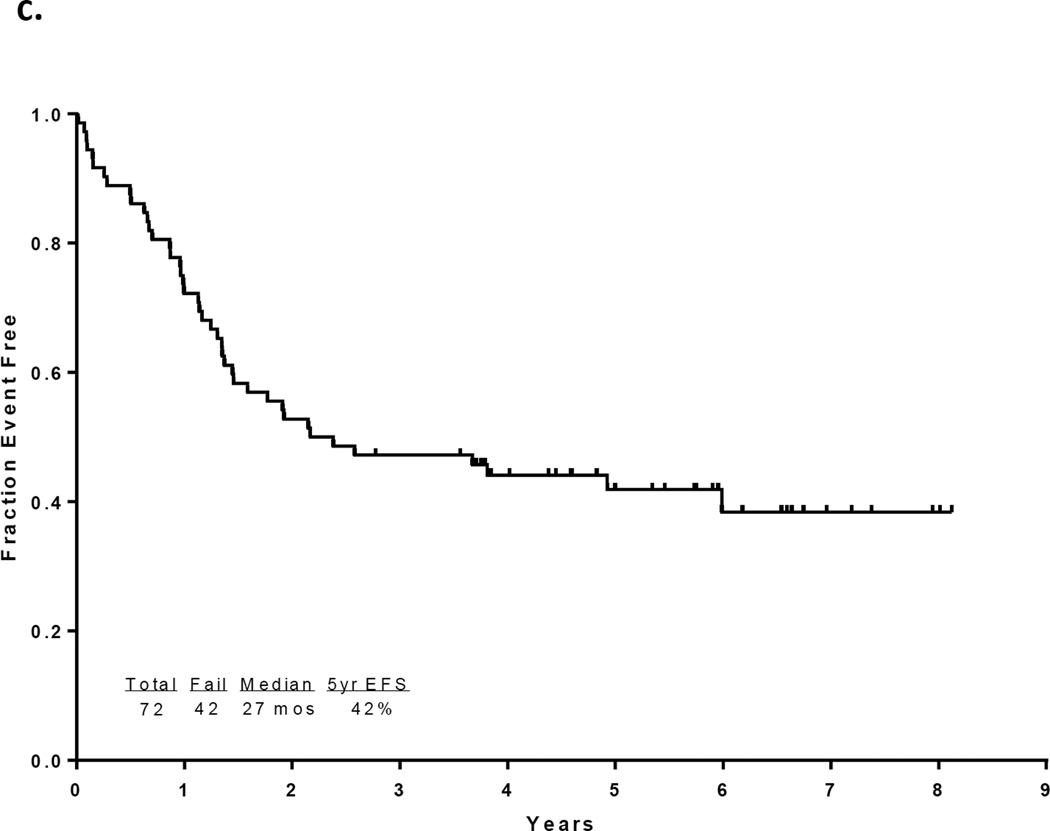
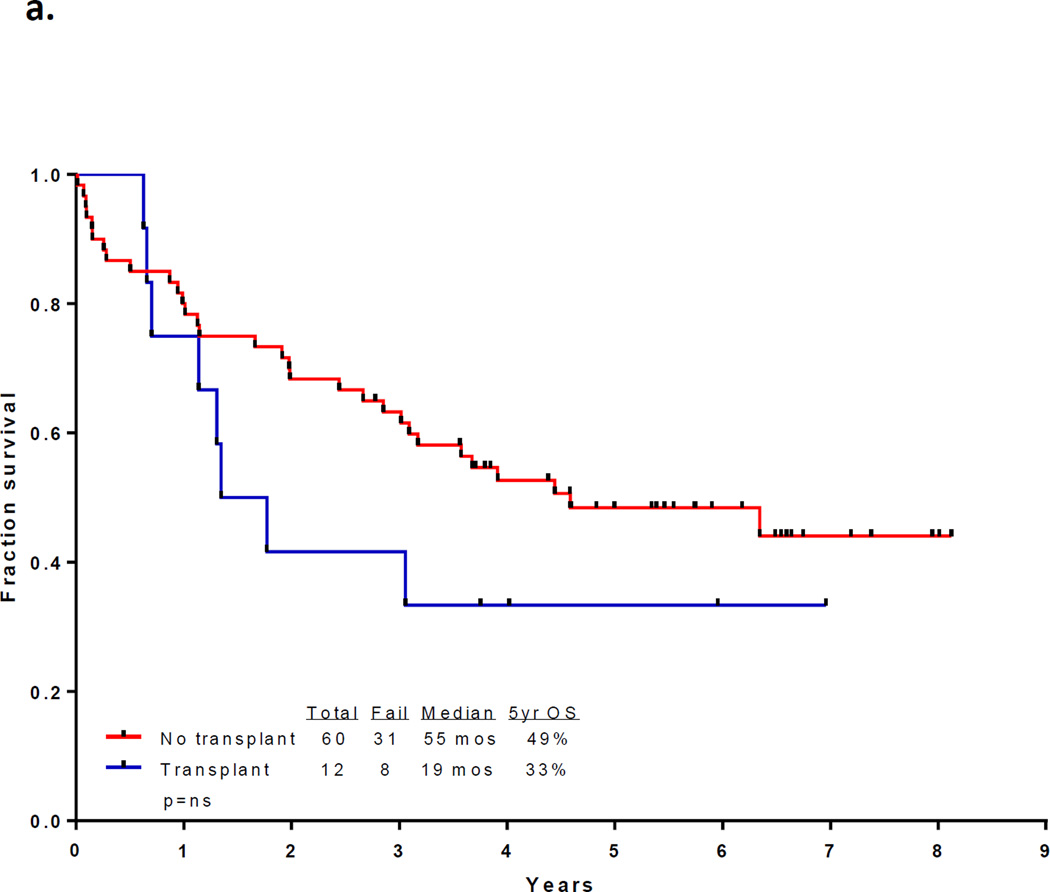
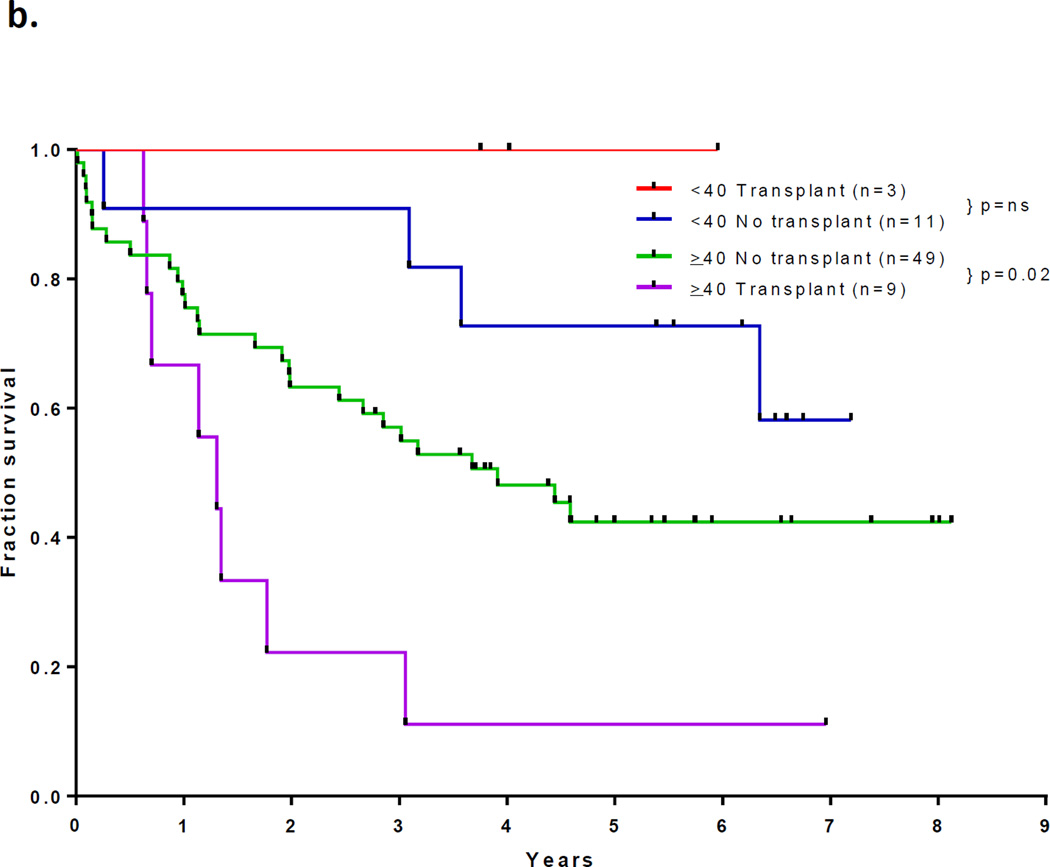
With a median follow up of 67 months in the surviving patients (range −33–97 months), 33 patients (46%) are alive and 30 (43%) remain in CR1. Twelve patients with a median age of 52.5 years (range, 23 – 63) underwent an allogeneic SCT in CR1 with a median of 5 induction/consolidation courses (range, 2–7) prior to transplant. Among the 60 patients who did not receive a transplant in CR1, 21 had no referral (including 13 who were older than 65 and deemed unfit, 4 who had early death, and 4 who declined). Among the other 39 patients referred for transplant, only 10 had a suitable sibling or matched unrelated donor (MUD) and only one received transplant in CR2. The preparative regimens for the 12 patients who underwent transplant in CR1 included busulfan plus melphalan in 4 (2 sibling donor, 2 MUD), clofarabine plus busulfan in 7 (5 sibling and 2 MUD) and fludarabine plus melphalan (cord blood donor). Thirty-nine patients have died including 3 at induction, 19 after relapse, 7 post stem cell transplant performed in CR1, and 10 in CR (6 from infections, 1 from unrelated cardiac event, 1 from unrelated cancer, and 2 from an unknown cause). The 7 deaths occurring after transplant in CR1 were due to pneumonia (viral in 1, bacterial in 1, aspergillus in 1 and unknown pathogen in 1), graft-versus host disease in 1, myocardial infarction in 1, and unknown cause in 1. The relatively high transplant-related mortality is likely related to the relatively advanced age of the cohort. The median disease free survival is 31 months (range, 0.3 to 97 months) and the median overall survival is 47 months (range, 0.2 to 97 months). (Figures 1a and 1b) The median event-free survival is 27 months (range, 0.2 to 97) (Figure 1c) Figure 2 compares the outcomes of patients who did or did not undergo SCT in CR1. When comparing the outcomes of the patients above or below the age 40, it appears that patients younger than 40 years, but not those over 40 benefit from SCT inCR1, although the numbers are very small (p values NS and 0.02, respectively; Figure 2b).
Figure 1.
a) Disease-free survival for all patients, b) Overall survival for all patients c) Event-free survival for all patients
Figure 2.
(a) Survival by whether or not received allogeneic stem cell transplant in CR1; (b) Survival by whether or not received transplant in CR1 and age < and ≥ 40 years
Analysis of the outcomes based on minimal residual disease (MRD) assessment at CR and at 3 months is limited due to small number of patients. At initial CR, 21 patients remained positive for BCR-ABL fusion transcripts by polymerase chain reaction (PCR) analysis; 4 patients underwent transplant in CR1. Overall, 7 patients relapsed and 11 died with 5-year survival of 52% and no significant difference between those who did or did not receive transplant. At 3 months, 6 patients remained positive for MRD; none were transplanted, 2 relapsed, and 3 are still alive with a 5-year survival of 50%.
Relapse Characteristics and Outcomes
Twenty-two patients have relapsed including 8 patients who only had CNS relapse (Table 2). The median time to relapse was 17 months (range, 6–72 months) and patients had received a median of 8 cycles of induction/consolidation (range, 2–8) prior to relapse. The majority of relapses occurred during maintenance cycles while receiving dasatinib (n=16) while 3 occurring after receiving alternative TKI (imatinib in 2 and nilotinib in 1 patient), 1 during consolidation, 1 after allogeneic SCT and 1 off any therapy. Thirteen relapsed patients had mutational analysis and 7 had ABL mutations (4 T315I, 1 F359V, and 2 V299L). They were treated with a variety of regimens including single agent TKI in 2 (9%), combination of hyperCVAD and another TKI in 8 (36%), single agent monoclonal antibody in 2 (9%) and other in 2 (9%). The 8 patients with CNS only relapse received a combination of IT chemotherapy as well as radiation, systemic chemotherapy and alternative TKIs. Overall, 16 patients received a TKI for salvage including 4 ponatinib (1 with T315I, 1 V299L, 2 no mutation), 2 nilotinib (1 with F359V), 1 imatinib (V299L) AND 9 dasatinib (1 with T315I, 8 no mutation); 6 patients did not receive TKI during salvage. A second CR was achieved in all 8 patients with CNS relapse (8 of 8 or 100%) and 9 of 14 patients (64%) with systemic relapse; 5 (36%) had no response or died during salvage. Ten patients underwent an allogeneic SCT during CR2. Outcome of the 17 patients who achieved CR2 is summarized in Table 3.
Table 2.
Outcomes after relapse – treatment and outcome
| Regimen | N (%) |
|---|---|
| HCVAD + Another TKI | 8 (36) |
| Single Agent TKI | 2 (9) |
| Single Agent Monoclonal Antibody | 2 (9) |
| Other | 2 (9) |
| Intrathecal chemo/RT/TKI (CNS relapse) | 8 (36) |
| Salvage Outcome | |
| Complete Remission (CR) | 7/14 (50) |
| CRi | 2/14 (14) |
| Overall Response | 9/14 (64) |
| No response/Died | 5/14 (36) |
| Response in CNS only relapse | 8/8 |
| OS after Salvage 1 (months) | 11.7 (0.6 – 52.8) |
Table 3.
Outcome of relapsed patients who achieved CR2
| Patients in CR2 after salvage | Received SCT in CR2 (N=10) |
No SCT in CR2 (N=7) |
|---|---|---|
| 3-year survival | 20% | 0% |
| Median OS from salvage (range) in months | 14.5 (3.2 – 52.8) | 16.5 (5.8 _ 27.5) |
| Patients alive | 2 | 1 |
Toxicity
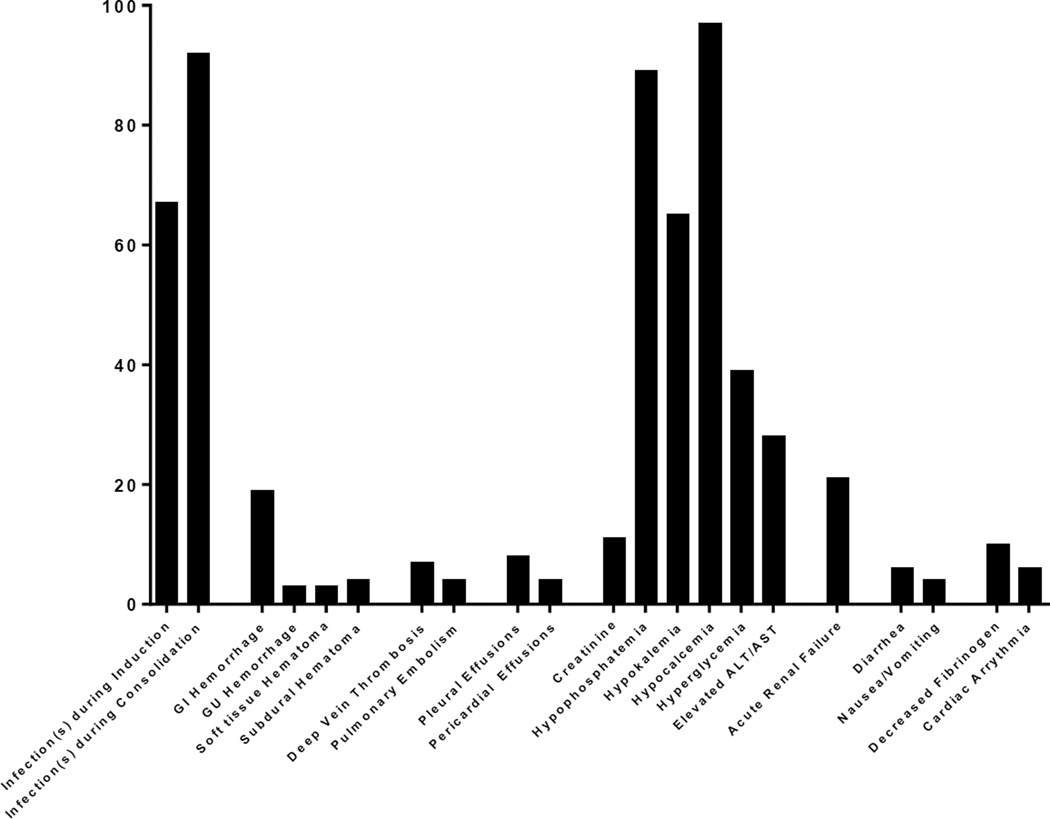
The median time to neutrophil and platelet recovery for cycle 1 is 18 and 22 days and for subsequent cycles is 15 and 20 days. Grade 3 and 4 adverse events have included bleeding (GI, GU, soft tissue and subdural hematomas), pleural effusions, pericardial effusions, reversible rise in creatinine, deep vein thromboses, pulmonary emboli, as well as diarrhea, infections, hypophosphatemia, hypokalemia, hypocalcemia, hyperglycemia, and elevated transaminases. Figure 3 is a summary of the grade 3 and 4 toxicities encountered by the enrolled patients. Dasatinib was discontinued in 12 patients and an alternative TKI was started including imatinib in 8 patients, bosutinib in 1patient, nilotinib in 1 patient, and ponatinib in 1patient; 1patient did not receive further TKI therapy. Toxicites that led to the discontinuation of dasatinib were pleural effusions in 6 patients, pulmonary artery hypertension in 2 patients, gastrointestinal bleeding in 2 patients, skin cancer in 1 patient, and subdural bleed in 1 patient.
Figure 3.
Grade 3 and 4 toxicities
DISCUSSION
The introduction of TKIs has been associated with a significant improvement in the outcome of patients with Ph+ ALL.16 Several studies have demonstrated a significant benefit from the addition of imatinib to conventional chemotherapy in both pediatric and adult patients.6,17,18 The introduction of second and third generation TKIs able to overcome resistance to imatinib and to induce responses in patients who have failed imatinib based therapy, has raised the possibility of improving the outcome of these patients even further.4,19 Preclinical data suggest that combining TKIs with chemotherapy is likely to be beneficial and prevent development of mutations responsible for resistance.9 This has been further corroborated by early data from trials where TKIs were administered with minimal chemotherapy.8 In the study by Foa et al, all patients received dasatinib in addition to steroids and IT chemotherapy and although the achievement of CR was universal, almost half of patients relapsed within 2 years.8 Relapses were more common in patients with limited post-remission therapy. Mutations in the ABL kinase domain occurred in 13 of 17 relapsed patients who had the analysis and included T315I in 12.8
In this report, we have demonstrated that durable remissions and long-term survival can be achieved with the combination of chemotherapy and dasatinib in patients with Ph+ ALL, in many without the use of allogeneic SCT in first remission. Twelve patients underwent an allogeneic SCT in CR1 and 7 died from transplant-related complications. Furthermore, 3 patients died during induction and 10 later in CR1 mainly from infectious causes. This can be partly attributed to the advanced age of the cohort with a median age of 55 years, a population less able to tolerate the intensive chemotherapy regimen.
Overall 22 patients relapsed including 8 (11%) patients with CNS relapse alone. This is comparable with the incidence of CNS only relapse among the patients treated with our prior regimen of hyperCVAD plus imatinib (5 of 54 patient, 9%)(unpublished data). Seven of these patients had 6 to 8 IT chemotherapy prophylaxis (4 had 8 and 3 had 6) and the eighth patient presented with CNS disease and had a total of 12 IT treatments. Only one had a non-TBI-based allogeneic SCT in CR1. This relatively high incidence of CNS only relapse suggests that the number of prophylactic IT treatments may not be sufficient and may be a function of more patients living longer thereby being at a higher risk of CNS relapse. We have instituted this practice in our current regimens for Ph+ ALL increasing the number of IT therapy to 12. It is also important to note that all of the patients with CNS only relapse and 9 of 14 (64%) of patients with systemic relapse were able to achieve CR2 and 10 underwent an allogeneic SCT in CR2.
There are several potential ways that the outcomes can be improved further using this regimen. Clearly the intensity of chemotherapy can be tailored to the patient tolerability and age. This is likely to be more achievable with the introduction of more potent TKIs, where the reliance on the cytotoxic agents can be mitigated using a more powerful BCR-ABL inhibitor able to suppress mutant clones such as T315I, such as ponatinib.20 The potential cardiovascular toxicity of ponatinib should be weighed against its potential benefit in this setting. Another potential strategy is to tailor therapy based upon the eradication or persistence of markers of minimal residual disease (MRD), including multi-parameter flow cytometry as well as RT-qPCR for BCR-ABL transcripts and for patient specific immunoglobulin heavy chain gene (IGH) rearrangements.11,21 We have previously reported that persistence of MRD beyond three months of therapy with the hyperCVAD plus TKI based regimens can be associated with a lower likelihood of long-term success.11 Younger patients with an available matched donor should still be considered for an allogeneic SCT in CR1, particularly if they have persistent MRD. However, post-transplant strategies including resumption of TKI treatment should be studied further.22,23
Acknowledgments
This study was supported by a grant from Bristol Myers Squibb (BMS). FR, JC, and HK have received research funding from BMS. This research is supported in part by the MD Anderson Cancer Center Support Grant CA016672
REFERENCES
- 1.Kantarjian H, O'Brien S, Jabbour E, et al. Improved survival in chronic myeloid leukemia since the introduction of imatinib therapy: a single-institution historical experience. Blood. 2012;119(9):1981–1987. doi: 10.1182/blood-2011-08-358135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoelzer D. Advances in the management of Ph-positive ALL. Clin Adv Hematol Oncol. 2006;4(11):804–805. [PubMed] [Google Scholar]
- 3.Ottmann OG, Druker BJ, Sawyers CL, et al. A phase 2 study of imatinib in patients with relapsed or refractory Philadelphia chromosome-positive acute lymphoid leukemias. Blood. 2002;100(6):1965–1971. doi: 10.1182/blood-2001-12-0181. [DOI] [PubMed] [Google Scholar]
- 4.Ottmann O, Dombret H, Martinelli G, et al. Dasatinib induces rapid hematologic and cytogenetic responses in adult patients with Philadelphia chromosome positive acute lymphoblastic leukemia with resistance or intolerance to imatinib: interim results of a phase 2 study. Blood. 2007;110(7):2309–2315. doi: 10.1182/blood-2007-02-073528. [DOI] [PubMed] [Google Scholar]
- 5.Thomas DA, Faderl S, Cortes J, et al. Treatment of Philadelphia chromosome-positive acute lymphocytic leukemia with hyper-CVAD and imatinib mesylate. Blood. 2004;103(12):4396–4407. doi: 10.1182/blood-2003-08-2958. [DOI] [PubMed] [Google Scholar]
- 6.Yanada M, Takeuchi J, Sugiura I, et al. High complete remission rate and promising outcome by combination of imatinib and chemotherapy for newly diagnosed BCR-ABL-positive acute lymphoblastic leukemia: a phase II study by the Japan Adult Leukemia Study Group. J Clin Oncol. 2006;24(3):460–466. doi: 10.1200/JCO.2005.03.2177. [DOI] [PubMed] [Google Scholar]
- 7.Tanguy-Schmidt A, Rousselot P, Chalandon Y, et al. Long-term follow-up of the imatinib GRAAPH-2003 study in newly diagnosed patients with de novo Philadelphia chromosome-positive acute lymphoblastic leukemia: a GRAALL study. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2013;19(1):150–155. doi: 10.1016/j.bbmt.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 8.Foa R, Vitale A, Vignetti M, et al. Dasatinib as first-line treatment for adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 2011;118(25):6521–6528. doi: 10.1182/blood-2011-05-351403. [DOI] [PubMed] [Google Scholar]
- 9.Boulos N, Mulder HL, Calabrese CR, et al. Chemotherapeutic agents circumvent emergence of dasatinib-resistant BCR-ABL kinase mutations in a precise mouse model of Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 2011;117(13):3585–3595. doi: 10.1182/blood-2010-08-301267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ravandi F, O'Brien S, Thomas D, et al. First report of phase 2 study of dasatinib with hyper-CVAD for the frontline treatment of patients with Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia. Blood. 2010;116(12):2070–2077. doi: 10.1182/blood-2009-12-261586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ravandi F, Jorgensen JL, Thomas DA, et al. Detection of MRD may predict the outcome of patients with Philadelphia chromosome-positive ALL treated with tyrosine kinase inhibitors plus chemotherapy. Blood. 2013;122(7):1214–1221. doi: 10.1182/blood-2012-11-466482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lilly MB, Ottmann OG, Shah NP, et al. Dasatinib 140 mg once daily versus 70 mg twice daily in patients with Ph-positive acute lymphoblastic leukemia who failed imatinib: Results from a phase 3 study. American journal of hematology. 2010;85(3):164–170. doi: 10.1002/ajh.21615. [DOI] [PubMed] [Google Scholar]
- 13.Cortes J, O'Brien SM, Pierce S, Keating MJ, Freireich EJ, Kantarjian HM. The value of high-dose systemic chemotherapy and intrathecal therapy for central nervous system prophylaxis in different risk groups of adult acute lymphoblastic leukemia. Blood. 1995;86(6):2091–2097. [PubMed] [Google Scholar]
- 14.Kantarjian H, Thomas D, O'Brien S, et al. Long-term follow-up results of hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (Hyper-CVAD), a dose-intensive regimen, in adult acute lymphocytic leukemia. Cancer. 2004;101(12):2788–2801. doi: 10.1002/cncr.20668. [DOI] [PubMed] [Google Scholar]
- 15.Alvarnas JC, Brown PA, Aoun P, et al. Acute lymphoblastic leukemia. Journal of the National Comprehensive Cancer Network : JNCCN. 2012;10(7):858–914. doi: 10.6004/jnccn.2012.0089. [DOI] [PubMed] [Google Scholar]
- 16.Fielding AK. Current treatment of Philadelphia chromosome-positive acute lymphoblastic leukemia. Hematology / the Education Program of the American Society of Hematology. American Society of Hematology. 2011;2011:231–237. doi: 10.1182/asheducation-2011.1.231. [DOI] [PubMed] [Google Scholar]
- 17.Schultz KR, Bowman WP, Aledo A, et al. Improved early event-free survival with imatinib in Philadelphia chromosome-positive acute lymphoblastic leukemia: a children's oncology group study. J Clin Oncol. 2009;27(31):5175–5181. doi: 10.1200/JCO.2008.21.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fielding AK, Rowe JM, Buck G, et al. UKALLXII/ECOG2993: addition of imatinib to a standard treatment regimen enhances long-term outcomes in Philadelphia positive acute lymphoblastic leukemia. Blood. 2014;123(6):843–850. doi: 10.1182/blood-2013-09-529008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cortes JE, Kim DW, Pinilla-Ibarz J, et al. A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias. N Engl J Med. 2013;369(19):1783–1796. doi: 10.1056/NEJMoa1306494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cortes JE, Kantarjian H, Shah NP, et al. Ponatinib in refractory Philadelphia chromosome-positive leukemias. N Engl J Med. 2012;367(22):2075–2088. doi: 10.1056/NEJMoa1205127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bassan R, Spinelli O, Oldani E, et al. Improved risk classification for risk-specific therapy based on the molecular study of minimal residual disease (MRD) in adult acute lymphoblastic leukemia (ALL) Blood. 2009;113(18):4153–4162. doi: 10.1182/blood-2008-11-185132. [DOI] [PubMed] [Google Scholar]
- 22.Fielding AK. Philadelphia-positive acute lymphoblastic leukemia--is bone marrow transplant still necessary? Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2011;17(1 Suppl):S84–S88. doi: 10.1016/j.bbmt.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 23.Pfeifer H, Wassmann B, Bethge W, et al. Randomized comparison of prophylactic and minimal residual disease-triggered imatinib after allogeneic stem cell transplantation for BCR-ABL1-positive acute lymphoblastic leukemia. Leukemia. 2013;27(6):1254–1262. doi: 10.1038/leu.2012.352. [DOI] [PubMed] [Google Scholar]