Abstract
Declarative memory and procedural memory are known to be two fundamentally different kinds of memory that are dissociable in their psychological characteristics and measurement (explicit versus implicit) and in the neural systems that subserve each kind of memory. Declarative memory abilities are known to improve from childhood through young adulthood, but the developmental maturation of procedural memory is largely unknown. We compared 10-year-old children and young adults on measures of declarative memory, working memory capacity, and four measures of procedural memory that have been strongly dissociated from declarative memory (mirror tracing, rotary pursuit, probabilistic classification, and artificial grammar). Children had lesser declarative memory ability and lesser working memory capacity than the adults, but exhibited learning equivalent to adults on all four measures of procedural memory. Declarative and procedural memory are, therefore, developmentally dissociable, with procedural memory being adult-like by age 10 and declarative memory continuing to mature into young adulthood.
Evidence has converged on a fundamental distinction between two forms of memory, declarative and procedural (Cohen & Squire, 1980). Declarative memory (“knowing that”) refers to conscious memory for events and facts, is assessed by explicit tests of recall and recognition, and depends upon medial temporal lobe and diencephalic brain structures. Procedural memory (“knowing how”) refers to unconscious memory, is assessed by experience-dependent learning of skilled performance, and depends on structures in the basal ganglia, cerebellum, and neocortex (Gabrieli, 1998). Declarative memory abilities improve across child and adolescent development (e.g., Kail, 1990), but surprisingly little is known about the development of procedural memory. Here we asked whether procedural memory continues to develop past middle childhood, as does declarative memory, or if instead procedural memory matures at an earlier age.
There is evidence that some forms of non-declarative memory mature earlier than declarative memory. Perceptual priming, based on stimulus form, appears to be adult-like early in development (Carroll, Byrne, & Kirsner, 1985; Drummey & Newcombe, 1995). Conceptual priming, based on stimulus meaning, develops more slowly (e.g., Billingsley, Smith, & McAndrews, 2002; Murphy, McKone, & Slee, 2003), perhaps because it relies on the growth of semantic knowledge through development.
Several studies have examined the development of sensorimotor sequence learning. Sequence learning of visuospatial locations appears to mature during infancy when measured by visual saccades (Amso & Davidow, 2012; Lum, Kidd, Davis, & Conti-Ramsden, 2010). Sequence learning for locations can also be measured by reaction times to button presses on the serial reaction time task. Developmental findings using this task have been mixed with findings of learning in children that is equal to adults (Meulemans, Van der Linden, & Perruchet, 1998; Thomas & Nelson, 2001), less than adults (Thomas, et al., 2004), or greater than adults (Janacsek, Fiser, & Nemeth, 2012). The inconsistent developmental findings may relate to factors that influence explicit awareness of the to-be-learned sequence, such as the nature of the sequences (Willingham & Goedert-Eschmann, 1999).
Here we examined age differences in learning on four diverse measures of procedural memory selected because they have been dissociated from declarative memory in studies of patients with global amnesia. Therefore, if children exhibit reduced procedural memory relative to adults on these tasks, it is unlikely to be a secondary consequence of immature declarative memory. Two tasks, mirror tracing (Milner, 1962) and rotary pursuit (Corkin, 1968), were the motor skill-learning tasks on which the amnesic patient H.M. and patients with impaired declarative memory due to Alzheimer’s disease have shown successful learning (Gabrieli, Corkin, Mickel, & Growdon, 1993; Heindel, Salmon, Shults, Walicke, & Butters, 1989). Despite their landmark status in memory research, neither of these tasks has been used to examine development.
We also examined two cognitive examples of procedural memory. One task was probabilistic classification, which has also revealed intact learning in amnesic patients (Knowlton, Squire, & Gluck, 1994). The other task was artificial grammar learning, the original example of implicit learning (Reber, 1967), and one that has also revealed intact learning in amnesia (Knowlton, Ramus, & Squire, 1992). Artificial grammar learning has been studied in children ages 9–11 years (Fischer, 1997) and 5–8 years (A. Witt & Vinter, 2012), but neither study compared learning between children and adults.
Method
Participants
Thirty-two children (mean age 10.46 years, min = 10.04, max = 10.94; 16 female) and 29 adults (mean age 23.68 years; 16 female) participated. Twenty-six children and 28 adults completed all tasks detailed below; some participants were not able to complete all tasks for one or more reasons (ran out of time (children, n = 5; adults n = 0); a program crashed (children, n = 4; adults n = 2); data were overwritten (children, n = 1; adults n = 0); Appendix A). Both adults and children received Amazon gift cards for participation ($60) and gave written consent (along with parents of minors).
Apparatus, stimuli, and tasks
Participants were tested in a quiet room with one experimenter; they completed the tasks in the following order with opportunities to take breaks every 30 minutes: Probabilistic Classification, California Verbal Learning, Rotary Pursuit, Mirror Tracing, Count Span, Artificial Grammar Learning, and the Kaufman Brief Intelligence Test.
IQ
The Kaufman Brief Intelligence Test, Second Edition
This test consisted of three sections—1) Verbal Knowledge, 2) Riddles and 3) Matrices (Kaufman & Kaufman, 2004)—and was administered by an experimenter using a booklet. Reponses were scored and standardized in keeping with procedures recommended by the publisher.
Procedural Memory
Rotary Pursuit
A photoelectric pursuit rotor (Lafayette Instruments, Model 30014C*C) was used in which participants were asked to use a stylus to maintain contact with a photoelectric target that rotated in the shape of a rectangle with truncated corners. Participants first completed a 20-second practice trial to establish baseline speed (15, 30, 45, or 60 rotations per minute). The speed at which the participant’s time-on-target was closest to 5 seconds was selected as the baseline and used for all subsequent trials. Participants then completed four 20-second trials, took a break for 1 minute, and then completed four more 20-second trials. After 30 minutes of performing other tasks, participants completed eight more 20-second trials, taking a 1-minute break after the first four trials as before. The dependent measure was time-on-target per trial.
Mirror Tracing
Participants traced the outline of a six-sided star while watching their hands in a mirror (Gabrieli, et al., 1993; Milner, 1962) using a Lafayette Instruments Auto-scoring mirror tracer (Model 58024A*C). In this device, the stylus is metal and the test plate is metal except for the star pattern. When the stylus goes off the star and touches the metal plate, it completes an electrical circuit and an error is recorded. Participants were instructed to stay inside the outline of the star and to trace as quickly and accurately as possible. Participants first completed a practice trial, then traced four times. After 30 minutes of performing other tasks, participants traced five more times. The dependent measures were completion time and number of errors per trial.
Probabilistic Classification Task
This task was modeled after prior weather prediction tasks (Knowlton, et al., 1994; Shohamy, et al., 2004) (Supplementary Table 1; Supplementary Figure 1a). Participants viewed a series of cards on a computer and were asked whether a particular combination of cards indicated sun or rain. At first, participants did not know whether a set of cards indicated sun or rain; after deciding, they were given feedback in the form of a smiling or frowning face. Participants completed 100 trials. The dependent measure was the percent of chosen optimal outcomes (which was more probable given prior feedback).
Artificial Grammar Learning
In the initial study phase, participants viewed a series of letter strings on a computer screen and were instructed to write these down. There was no time limit. After copying each string, participants were asked to cover their response before moving on to the next string. Twenty-three study strings were generated from a Markov chain grammar (Supplementary Figure S1b) and presented twice each in two sets, with 23 strings presented in random order once and then the same 23 presented in random order again (46 training trials). Next, in the test phase,, participants were asked unexpectedly to decide if new strings were grammatical: 16 were and 16 were not, and half of each kind were high- and half were low-chunk strength (high = frequent letter pairings during the study phase). The dependent measure was the proportion of items endorsed correctly as grammatical.
Declarative Memory
California Verbal Learning Test (CVLT-II)
Both adult and child versions of this standardized test of declarative memory (Delis, Kramer, Kaplan, & Ober, 2000) were used. These versions are the same in terms of procedure, but differ in the words that are to be remembered and the list lengths (16 for adults and 15 for children). The long-delay measure, in which participants were asked to remember words from a study list after a 20-minute delay, was the dependent measure. Standardized scores were used to compare the two groups relative to their age peers; a raw percentage correct score was used to compare the two groups directly.
Complex Working Memory
Count Span
This task was modeled after previous work (Case, Kurland, & Goldberg, 1982; Cowan et al., 2005). Participants viewed an array on a computer with blue circles, blue triangles, and red circles and were instructed to count only the blue circles (targets), after which they were instructed to press the spacebar to move forward. If they did not press within 5 seconds, the screen would forward automatically. After anywhere from one to six consecutive arrays were presented, participants were prompted to enter the number of targets per array in the order that they were presented. The dependent measure was calculated by determining the highest load (from one to six) at which two of three trials were answered correctly, plus 0.5 if one of three trials at the next highest load was answered correctly.
Results
IQ, Declarative, and Working Memory
Adult and child groups did not differ significantly on standardized measures of IQ, including the composite score, t(57) = .51 p = .62, d = −.136 (Adults=119; Children=117.2), verbal subscale, t(57) = .46, p = .644, d = −.124 (Adults=117.97; Children=116.03), and non-verbal subscale, t(57) = .18, p = .86, d = −.046 (Adults=114.72; Children=114.17). Adults had superior declarative memory performance (California Verbal Learning Test: t(59)= 2.80, p = .007, d = −.719; Adults=.84; Children=.72) and working memory performance (Count Span: t(55) = 5.56, p < 0.001, d = −1.47; (Adults=5.41; Children=3.57). When CVLT-II scores were age-normed, there was no significant difference between groups, t(59) = .37, p = .71, d = −0.10. See Supplementary Table 2 for means and standard deviations.
Procedural Memory
Rotary Pursuit
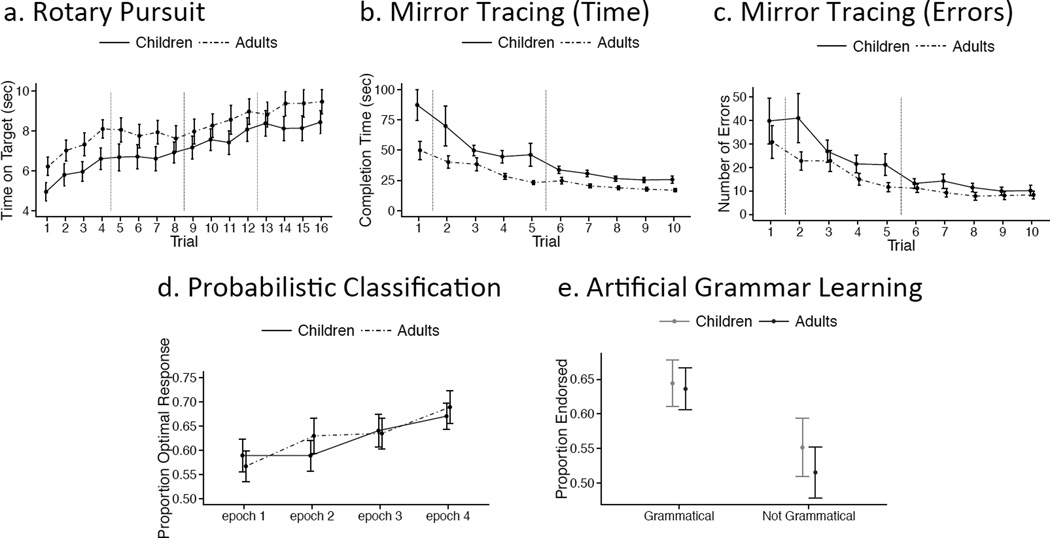
A repeated measures analysis of variance ANOVA with time-on-target as the dependent measure revealed a main effect of trial number, F(15,885) = 23.50, p < .001, np2 = .285 (Figure 1a), but no main effect of age, F(2,59) = 2.1, p = .153, np2 = .034, and no trial number by age group interaction, F(15,885) = .570, p = .899, np2 = .01. Adults performed the task at a faster initial speed (mean of 34.66 rotations per minute) than children (mean of 26.25 rotations per minute); independent samples t-test: t(56) = 2.96 p = .004, d = .762.
Figure 1. Procedural Memory Performance.
Performance for adults (dotted line) and children (solid line) plotted across trials for Rotary Pursuit (a), Mirror Tracing (b, c), Probabilistic Classification (d), and Artificial Grammar Learning (e). For Probabilistic Classification, proportion of optimal responses is plotted by epoch (binning all 100 trials into 4 25-trial epochs). For Artificial Grammar Learning, adults (black) and children (grey) endorsed more grammatical than non-grammatical items as grammatical. In this and all other graphs, error bars reflect standard error of the mean. Dashed vertical lines reflect a task break.
Mirror Tracing
A repeated measures ANOVA with completion time as the dependent measure revealed a main effect of trial number (trials 2–10 as the first trial was practice: F(8,464) = 16.23, p < .001, np2 = .219; Bonferroni adjusted α = .025; Figure 1b), a main effect of age, F(1,58) = 7.31, p = .009, np2 = .112; Bonferroni adjusted α = .025, and no trial number by age group interaction, F(8,464) = 1.86, p = .065, np2 = .031; Bonferroni adjusted α = .025. The trend toward an interaction reflected greater learning in children, which may have resulted from the children’s slower initial performance. When baseline speed was taken into consideration (the average of trials two and three minus the average of trials 9 and 10 divided by the sum of those trials), there was no trend toward a group difference, t(58) = .725, p = .472, d = .188; Bonferroni adjusted α = .025.
The same analysis using number of errors as the dependent measure yielded a main effect of trial number (trials 2–10: F(8,464) = 16.78, p < .001, np2 = .224; Bonferroni adjusted α = .025; Figure 1c), no main effect of age, F(1,58) = 2.46, p = .122, np2 = .042; Bonferroni adjusted α = .025, and no trial by age group interaction, F(8,464) = 1.84, p = .067, np2 = .037; Bonferroni adjusted α = .025, with a trend for greater learning in the children. When baseline errors were taken into consideration (same calculation as in completion time), there was no trend toward a group difference, t(58) = .911, p = .366, d = .236; Bonferroni adjusted α = .025.
Probabilistic Classification Task
A repeated measures ANOVA in which performance was binned into 4 epochs (25 trials each) revealed a main effect of epoch, F(3,168) = 6.40, p < .001, np2 = .103 (Figure 1d), no main effect of age group, F(1,56) = .01, p = .95, np2 = .001, and no epoch by group interaction, F(3,168) = .84, p = .48, np2 = .015. Further, children and adults did not differ in their learning of any of the 14 card combinations (which ranged in their association with an outcome; see Supplementary Materials).
Artificial Grammar Learning
A repeated measures ANOVA with grammaticality (grammatical or not) and chunk strength (low or high) as factors revealed a main effect of grammaticality, F(1,52) = 13.81, p < .001, np2 = .210, a main effect of chunk strength, F(1,52) = 38.99, p < .001, np2 = .428, and no main effect of age group, F(1,52) = .28 p = .596, np2 = .005. There was no interaction between grammaticality and age, F(1,52) = 3.08, p = .085, np2 = .056, or grammaticality, chunk strength, and age, F(1,52) = .98, p = .326, np2 = .019, but there was a marginal interaction between chunk strength and age, F(1,52) = 3.96, p = .052, np2 = .071, such that children were more likely to incorrectly endorse low-chunk strength items. Both groups learned the artificial grammar as shown by selecting grammatical strings significantly more often than non-grammatical strings (paired t-test, t(53) = 4.18, p < .001, d = .578; Figure 1e), and did so to a similar extent.
Overall Pattern of Learning and Memory
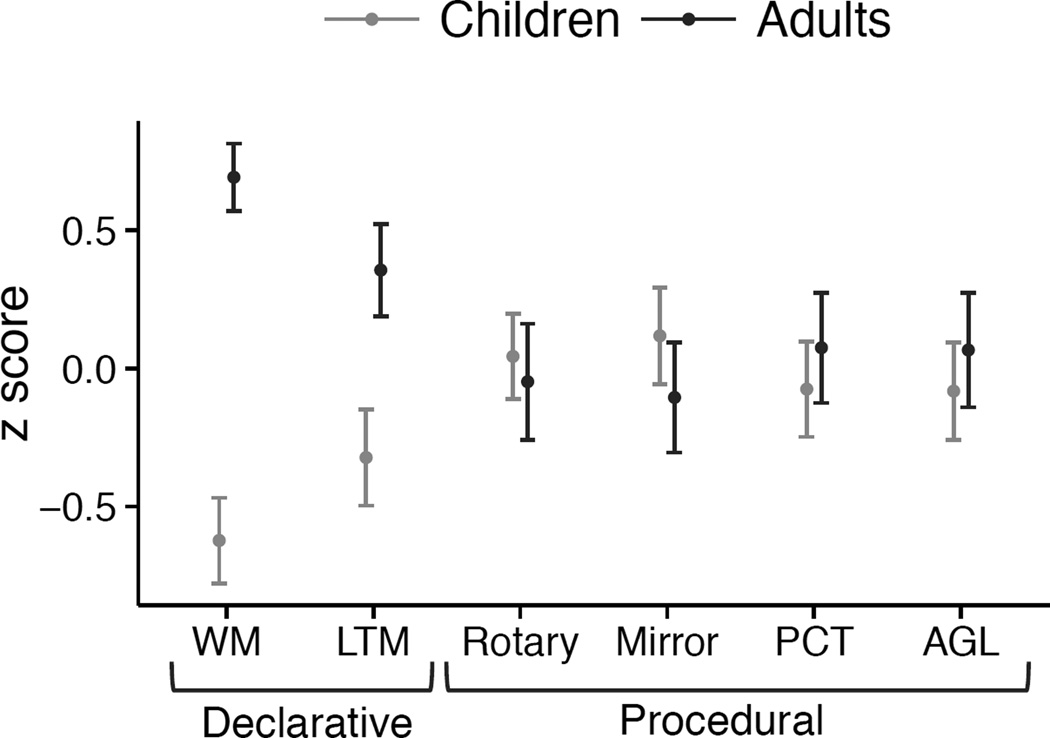
In order to provide an overview of age-related differences and similarities in learning and memory, z scores were calculated for the six main dependent measures (percent errors for mirror tracing) (Figure 2). The children had worse scores on the declarative-memory and working-memory tasks, but had scores similar to adults on the skill learning tests of procedural memory.
Figure 2. Summary of Performance on Declarative and Procedural Memory Tasks.
Z-scores of performance across tasks for adults (black) and children (grey) show an adult advantage for declarative (LTM) and working memory (WM) measures, but not for procedural measures (PCT = procedural classification task; AGL = artificial grammar learning). To plot learning of procedural memory, z-scores were taken of the difference in performance in later as compared to earlier in learning. For Rotary Pursuit this was time on target during trial 16 minus trial 1; for Mirror Tracing this was the percent change scores for errors as described in the results; for PCT this was proportion of optimal responses during block 4 minus block 1; for AGL, this was overall accuracy (regardless of stimulus-type).
Discussion
There was a clear dissociation between the age groups’ declarative memory ability, which was lesser in the children, and procedural memory, which was mature or adult-like in the children. Although the procedural tasks varied in their nature, children exhibited adult-like skill learning on all the tasks. The comparison between these children and adults was valid because both groups scored similarly relative to their age-group peers on standardized tests of IQ and declarative memory (Supplementary Table 2).
The findings that children had lesser declarative memory ability and lesser working memory capacity are consistent with many studies reporting age-related growth of declarative memory ability (e.g., Kail, 1990) and working memory capacity (e.g., Gathercole, Pickering, Ambridge, & Wearing, 2004) into young adulthood. In sharp contrast, the children exhibited adult-like rates of learning on all four skill-learning tests of procedural memory. The skill learning tasks varied on several dimensions. Two were perceptual-motor and two were cognitive. The nature of feedback also varied across the tasks, from none at all, to observed motor-based, to explicit. In three tasks, learning was measured continuously, but not in artificial grammar for which there was a final test phase. Despite this variation, children exhibited adult-like learning on all the tests of procedural memory.
The tests of procedural memory share the property that they do not depend on the integrity of medial temporal-lobe structures, but they are heterogeneous in regards to what neural systems are necessary for learning. Procedural memory for both rotary pursuit (Gabrieli, Stebbins, Singh, Willingham, & Goetz, 1997) and probabilistic classification (Knowlton, Mangels, & Squire, 1996) depends upon the integrity of the basal ganglia. Lesions to the basal ganglia, however, do not impair skill learning for mirror tracing (Gabrieli et al., 1997); instead lesions to the cerebellum impair such learning (Sanes, Dimitrov, & Hallett, 1990). The necessary brain regions for artificial grammar learning are less well known, but they may be neocortical. Patients with basal ganglia degeneration, due to Parkinson’s disease, or cerebellar degeneration have shown intact artificial grammar learning (K. Witt, Nuhsman, & Deuschl, 2002), and neuroimaging studies have suggested that neocortices in left occipital and parietal regions mediate such learning (e.g., Thiel, Shanks, Henson, & Dolan, 2003). Thus, mature procedural memory in children may reflect not so much the maturation of a particular neural circuit, but rather the shared properties of multiple procedural learning mechanisms that are independent of declarative memory. Thus, it is unknown as to whether or not the same mechanisms support the various forms of adult-like procedural memory in 10-year-old children.
There are several important limitations to the present study. First, further research with a broader age of younger children is needed to determine at what age these forms of procedural learning become adult-like, and whether the different kinds of procedural memory become adult-like at similar ages. Second, the different kinds of learning involved different kinds of measurement, with the declarative memory task having been measured by a standardized test. In patient studies these concerns have been mitigated by double dissociations between declarative and procedural memory, but this is not possible in the study of typical development. Third, the critical findings of mature procedural learning in 10 year olds is based on the absence of a learning difference, which could reflect limited measurement power. This concern is mitigated by the fact that the children exhibited somewhat better learning than adults on two procedural learning measures.
The differential development of procedural and declarative memory may have implications for learning at various ages. For example, there is a suggestion that some aspects of language (e.g., grammar), for which there is a critical or sensitive period, may depend on procedural memory (Ullman, 2001). Indeed, adults, who have greater declarative and working memory ability, outperform children for learning words and their meanings, but struggle to learn grammar as well as children (Snow & Hoefnagel-Höhle, 1978). Although more research is needed to understand the consequences of having a relatively more developed procedural than declarative memory system, this developmental imbalance in childhood could have beneficial implications for learning some aspects of one’s native language (Ramscar & Gitcho, 2007; Thompson-Schill, Ramscar, & Chrysikou, 2009). In terms of their memory systems, the more rapid maturation of procedural relative to declarative memory may promote particular kinds of learning in children.
Supplementary Material
Research Highlights.
Procedural memory is mature by age 10 while declarative memory is not.
The maturity of procedural memory extends across cognitive and motor modalities and different types of feedback.
This developmental dissociation corresponds with landmark dissociations in the neuropsychology literature.
The developmental imbalance of these core mnemonic systems can help understand age-related differences in learning outcomes, especially for language.
Acknowledgements
This research was funded by the National Institutes of Health (R01MH08344 to J.D.E.G. and 1F32MH095354-01 to A.S.F.). We thank Jennifer Minas, John Salvatore, Brian Chan, Caitlin Tan, Will Levinson, and Kelly Halverson for their help. We thank the participants and their parents for their participation.
Appendix A. Sample size by group and task
| Task | Group | Sample Size |
|---|---|---|
| KBIT | Children | 31 |
| Adults | 29 | |
| CVLT | Children | 32 |
| Adults | 29 | |
| Count Span | Children | 30 |
| Adults | 27 | |
| PCT | Children | 29 |
| Adults | 29 | |
| Rotary | Children | 32 |
| Adults | 29 | |
| Mirror | Children | 31 |
| Adults | 29 | |
| AGL | Children | 26 |
| Adults | 28 |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amso D, Davidow J. The development of implicit learning from infancy to adulthood: item frequencies, relations, and cognitive flexibility. Developmental Psychobiology. 2012;54:664–673. doi: 10.1002/dev.20587. [DOI] [PubMed] [Google Scholar]
- Billingsley RL, Smith ML, McAndrews MP. Developmental patterns in priming and familiarity in explicit recollection. Journal of Experimental Child Psychology. 2002;82:251–277. doi: 10.1016/s0022-0965(02)00007-3. [DOI] [PubMed] [Google Scholar]
- Carroll M, Byrne B, Kirsner K. Autobiographical memory and perceptual learning: a developmental study using picture recognition, naming latency, and perceptual identification. Memory & Cognition. 1985;13:273–279. doi: 10.3758/bf03197690. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Squire LR. Preserved learning and retention of pattern-analyzing skill in amnesia: dissociation of knowing how and knowing that. Science. 1980;210:207–210. doi: 10.1126/science.7414331. [DOI] [PubMed] [Google Scholar]
- Corkin S. Acquisition of motor skill after bilateral medial temporal-lobe excision. Neuropsychologia. 1968;6:255–265. [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test® - Second Edition (CVLT® -II) Pearson. 2000 [Google Scholar]
- Drummey AB, Newcombe N. Remembering versus knowing the past: children's explicit and implicit memories for pictures. Journal of Experimental Child Psychology. 1995;59:549–565. doi: 10.1006/jecp.1995.1025. [DOI] [PubMed] [Google Scholar]
- Fischer JP. L'apprentissage d'une grammaire artificielle par des enfants de 9 à 11 ans. L'année psychologique. 1997:207–236. [Google Scholar]
- Gabrieli JD. Cognitive neuroscience of human memory. Annual Review of Psychology. 1998;49:87–115. doi: 10.1146/annurev.psych.49.1.87. [DOI] [PubMed] [Google Scholar]
- Gabrieli JD, Corkin S, Mickel SF, Growdon JH. Intact acquisition and long-term retention of mirror-tracing skill in Alzheimer's disease and in global amnesia. Behavioral Neuroscience. 1993;107:899–910. doi: 10.1037//0735-7044.107.6.899. [DOI] [PubMed] [Google Scholar]
- Gabrieli JD, Stebbins GT, Singh J, Willingham DB, Goetz CG. Intact mirror-tracing and impaired rotary-pursuit skill learning in patients with Huntington's disease: Evidence for dissociable memory systems in skill learning. Neuropsychology. 1997;11:272–281. doi: 10.1037//0894-4105.11.2.272. [DOI] [PubMed] [Google Scholar]
- Gathercole SE, Pickering SJ, Ambridge B, Wearing H. The Structure of Working Memory From 4 to 15 Years of Age. Developmental Psychology. 2004;40:177–190. doi: 10.1037/0012-1649.40.2.177. [DOI] [PubMed] [Google Scholar]
- Heindel WC, Salmon DP, Shults CW, Walicke PA, Butters N. Neuropsychological evidence for multiple implicit memory systems: a comparison of Alzheimer's, Huntington's, and Parkinson's disease patients. Journal of Neuroscience. 1989;9:582–587. doi: 10.1523/JNEUROSCI.09-02-00582.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janacsek K, Fiser J, Nemeth D. The best time to acquire new skills: agerelated differences in implicit sequence learning across the human lifespan. Developmental Science. 2012;15:496–505. doi: 10.1111/j.1467-7687.2012.01150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kail RV. The development of memory in children. 3rd ed. New York: Freeman; 1990. [Google Scholar]
- Kaufman AS, Kaufman NL. Kaufman Brief Intelligence Test, Second Edition (KBIT-2) San Antonio, TX: Pearson; 2004. [Google Scholar]
- Knowlton BJ, Mangels JA, Squire LR. A neostriatal habit learning system in humans. Science. 1996;273:1399–1402. doi: 10.1126/science.273.5280.1399. [DOI] [PubMed] [Google Scholar]
- Knowlton BJ, Ramus SJ, Squire LR. Intact Artificial Grammar Learning in Amnesia: Dissociation of Classification Learning and Explicit Memory for Specific Instances. Psychological Science. 1992;3:172–179. [Google Scholar]
- Knowlton BJ, Squire LR, Gluck MA. Probabilistic classification learning in amnesia. Learning & Memory. 1994;1:106–120. [PubMed] [Google Scholar]
- Lum J, Kidd E, Davis S, Conti-Ramsden G. Longitudinal study of declarative and procedural memory in primary school-aged children. Australian Journal of Psychology. 2010;62:139–148. [Google Scholar]
- Meulemans T, Van der Linden M, Perruchet P. Implicit Sequence Learning in Children. Journal of Experimental Child Psychology. 1998;69:199–221. doi: 10.1006/jecp.1998.2442. [DOI] [PubMed] [Google Scholar]
- Milner B. Les troubles de la memoire accompagnant des lesions hippocampiques bilaterales [Memory problems with bilateral hippocampal lesions] Paris: Centre National de la Recherche Scientiflque; 1962. [Google Scholar]
- Murphy K, McKone E, Slee J. Dissociations between implicit and explicit memory in children: the role of strategic processing and the knowledge base. Journal of Experimental Child Psychology. 2003;84:124–165. doi: 10.1016/s0022-0965(03)00002-x. [DOI] [PubMed] [Google Scholar]
- Ramscar M, Gitcho N. Developmental change and the nature of learning in childhood. Trends in Cognitive Sciences. 2007;11:274–279. doi: 10.1016/j.tics.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Reber AS. Implicit learning of artifical grammars. Journal of verbal learning and verbal behavior. 1967;6:317–327. [Google Scholar]
- Sanes JN, Dimitrov B, Hallett M. Motor learning in patients with cerebellar dysfunction. Brain. 1990;113(Pt 1):103–120. doi: 10.1093/brain/113.1.103. [DOI] [PubMed] [Google Scholar]
- Shohamy D, Myers CE, Grossman S, Sage J, Gluck MA, Poldrack RA. Cortico-striatal contributions to feedback-based learning: converging data from neuroimaging and neuropsychology. Brain. 2004;127:851–859. doi: 10.1093/brain/awh100. [DOI] [PubMed] [Google Scholar]
- Snow CE, Hoefnagel-Höhle M. The critical period for language acquisition: Evidence from second language learning. Child Development. 1978;49:1114–1128. [Google Scholar]
- Thiel CM, Shanks DR, Henson RN, Dolan RJ. Neuronal correlates of familiarity-driven decisions in artificial grammar learning. Neuroreport. 2003;14:131–136. doi: 10.1097/00001756-200301200-00024. [DOI] [PubMed] [Google Scholar]
- Thomas KM, Hunt RH, Vizueta N, Sommer T, Durston S, Yang Y, Worden MS. Evidence of Developmental Differences in Implicit Sequence Learning: An fMRI Study of Children and Adults. Journal of Cognitive Neuroscience. 2004;16:1339–1351. doi: 10.1162/0898929042304688. [DOI] [PubMed] [Google Scholar]
- Thomas KM, Nelson CA. Serial Reaction Time Learning in Preschooland School-Age Children. Journal of experimental child psychology. 2001;79:364–387. doi: 10.1006/jecp.2000.2613. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, Ramscar M, Chrysikou EG. Cognition Without Control: When a Little Frontal Lobe Goes a Long Way. Current Directions in Psychological Science. 2009;18:259–263. doi: 10.1111/j.1467-8721.2009.01648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullman MT. A neurocognitive perspective on language: The declarative/procedural model. Nature Reviews Neuroscience. 2001;2:717–726. doi: 10.1038/35094573. [DOI] [PubMed] [Google Scholar]
- Willingham DB, Goedert-Eschmann K. The Relation Between Implicit and Explicit Learning: Evidence for Parallel Development. Psychological Science. 1999;10:531–534. [Google Scholar]
- Witt A, Vinter A. Artificial grammar learning in children: abstraction of rules or sensitivity to perceptual features? Psychological Research. 2012;76:97–110. doi: 10.1007/s00426-011-0328-5. [DOI] [PubMed] [Google Scholar]
- Witt K, Nuhsman A, Deuschl G. Intact artificial grammar learning in patients with cerebellar degeneration and advanced Parkinson's disease. Neuropsychologia. 2002;40:1534–1540. doi: 10.1016/s0028-3932(02)00027-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.