Abstract
Objectives
To identify the clinical outcomes of mRCC patients with PM treated with either pazopanib or sunitinib and assess whether PM is an independent prognostic variable in the current therapeutic environment.
Patients and Methods
Retrospective review of mRCC patients in an outpatient clinic was done from January 2006 to November 2011. Patient characteristics including demographics, laboratory data, and outcomes were analyzed. Comparison of baseline characteristics was done using chi2 and t-test and Overall Survival (OS) and Cancer-Specific Survival (CSS) was estimated using Kaplan-Meier methods. Predictors of OS were analyzed using Cox regression.
Results
A total of 228 patients were reviewed of which 44 (19.3%) had metastases to the pancreas and 184 (81.7%) had metastasis to sites other than the pancreas. The distribution of baseline characteristics was equal in both groups with the exception of a higher incidence of prior nephrectomy, diabetes and number of metastatic sites in the pancreatic metastasis group. 4 patients had isolated metastases to the pancreas, however, the majority of patients (68%) with pancreatic metastases had at least three different organ sites of metastases, as compared to 29% in patients without pancreatic metastases (p<0.01). Distribution of organ sites of metastases was similar (p>0.05), excluding pancreas. Median OS was 39 months (95% confidence interval [CI], 24–57, HR=0.66, 95% CI = 0.42–0.94, p=0.02) for patients with pancreatic metastases, compared to 26 months (95% CI, 21–31) for patients without pancreatic metastases (p-value <0.01). CSS was 42 months (95% CI: 30–57 months) in the PM group and 27 months (95% CI: 22–33 months, p-value = 0.05) in the control group.
Conclusions
Despite a higher number of affected organ sites in the pancreatic metastasis cohort, mRCC behavior in this cohort appears to be more indolent, as demonstrated by a higher median OS. These findings suggest that host or tumor features associated with pancreatic metastases may represent a less aggressive tumor phenotype.
INTRODUCTION
The incidence of cancers arising from the kidney or the renal pelvis is approximately 65,000 new cases per year, accounting for 3 to 6% of all cancers in the United States, with renal cell carcinoma (RCC) being the most common subtype.1–3 The 5-year survival rate for patients with RCC confined to the kidney is approximately 95%. However, metastases are known to occur in approximately 30% of cases, which confers a 5-year survival rate ranging from 0% to 20% for patients with metastatic disease. 3,4
Validated algorithms that assess patient specific clinical and laboratory characteristics to define prognosis for patients with metastatic RCC (mRCC) are currently utilized in clinical practice. The Memorial Sloan Kettering Cancer Center (MSKCC) risk criteria and Heng criteria categorize patients into good, intermediate, and poor risk cohorts.5,6 Unfortunately, at this time there is limited insight into the molecular drivers of this clinical heterogeneity. Identifying unique disease subgroups with recognizable biological characteristics will provide an ideal starting point to perform comparative genomic, transcriptomic, and proteomic assessments of both the tumor and the host, and will provide valuable information on the molecular drivers of tumor prognosis.
In an effort to develop a biologically actionable categorization of patients with mRCC, we assessed patients with pancreatic metastases (PM) at our institution. Of the primary tumors that can metastasize to the pancreas, RCC is the most common, followed by lung and breast cancer. 7–9 PM are seen in a relatively small percentage of patients with mRCC, with published incidence ranging between 2% to 11%.4,10,11 Approximately 22% of the PM cases are identified at the time of their primary tumor diagnosis.8 Although metastasis to the pancreas is commonly associated with disseminated and advanced systemic disease for most cancers, nearly two-thirds of RCC metastases have been associated with an isolated spread to the pancreas.12,13 RCC can behave in a variable manner, with up to 20% of cases having periods of slow growth or stability lasting for many years. PM from RCC are frequently the only metastatic site and metastases have been reported to occur a long time after nephrectomy.14–16
In the past decade, multiple targeted therapies received US FDA regulatory approval for mRCC. This evolving treatment armamentarium has improved patient outcomes, with lengthened progression-free survival (PFS) and overall survival (OS). Currently, it is unknown how these agents have impacted the clinical outcome of patients with PM. A few case-series and retrospective studies, which were largely compiled in the pre-TKI era, have documented longer OS in patients with mRCC and PM. Herein, we sought to identify the clinical outcomes of patients with mRCC and PM who were treated with either pazopanib or sunitinib, assess whether PM is an independent prognostic variable in the current therapeutic environment, and determine whether they are appropriate candidates for detailed molecular characterization to identify tumor and host-specific determinants of disease indolence and therapeutic response.
PATIENTS AND METHODS
After Institutional Review Board approval, we conducted a retrospective evaluation of patients diagnosed with mRCC at the University Of Texas MD Anderson Cancer Center (MDACC) between January 2006 to November 2011. Patients 18 yr of age or older with clear cell mRCC, who received first line anti-angiogenic therapy with sunitinib or pazopanib, and available for adequate follow-up (at least one clinic visit at MDACC within 3 months of treatment initiation), were included. Patients were stratified according to the presence or absence of PM at the time of presentation or during the course of their disease. Institutional electronic medical records were used to extract patient information. A formal review of radiological scans and reports was conducted for the identification of metastasis to pancreas.
Statistical analysis
Patient characteristics were summarized using median and range for continuous variables and frequency and percentage for categorical variables. All statistical tests were two-sided and p-value less than 0.05 was considered statistically significant. To assess the difference between the metastasis groups, Wilcoxon-rank sum tests and Fisher’s exact tests were used for continuous and categorical variables, respectively. OS was calculated from systemic treatment initiation date to date of death. The cause of death was validated using our electronic medical record, which captures date and cause of death using social security death index and/or death certificate for most patients. OS was estimated by Kaplan-Meier method and comparison between the groups was performed by log-rank test. Cox proportional hazards regression model was used to assess the association between patient characteristics and OS, with goodness-of-fit assessed by the Grambsch-Therneau test and Martingale residual plots. Based on fitted univariable Cox models, variables with p-value<0.10 were included in the full multivariable Cox model. The final multivariable Cox model was derived using backward elimination method, where variables with p-value<0.05 were considered statistically significant. Using similar methodology, secondary analysis for cancer specific survival was further carried out comparing patients with metastasis to the pancreas (and/or other sites) versus metastasis to the sites other than the pancreas. Cancer specific survival (CSS) was defined as the period from date of diagnosis of mRCC until death due to progression of disease or causes directly related to progression of disease. Patients who died from causes other than the progression of disease were censored at their time of death. All computations were completed in STATA (version 11).
RESULTS
Among the 228 patients identified with mRCC, 44(19%) patients had PM along with metastases to other sites, and 184(81%) patients had metastases to sites other than the pancreas. Demographic and baseline characteristics were similar between the two groups, with the exception of a higher incidence of prior nephrectomy, diabetes, and number of metastatic sites in the PM group (Table 1). In the PM group 34(77%) patients had undergone nephrectomy, compared to 109(59%) patients in the group with no pancreatic metastases. 43% of our patients in the PM group developed metastases to the pancreas after 1 year of presentation. In this subgroup of patients, the median time to develop PM was 34 months (95% CI – 24–53 months). 30(68%) patients had 3 or more metastatic sites in the group with PM, while only 4(9%) patients had the pancreas as the only metastatic site. The most frequent metastatic sites in the PM cohort were lung (68%), bone (30%), adrenal glands (27%) and liver (27%). In patients without PM, lung (76%), bone (33%) and liver (21%) were the common sites of metastases. In the PM group, 20(45%) patients had pancreatic metastases at the time of presentation and only 6(14%) underwent pancreatic metastasectomy. According to MSKCC risk stratification, 9(20%) of the PM patients were low risk, 27 (62%) were intermediate risk, and 8(18%) were in the poor risk group, compared to 14 (8%) with low risk, 135 (73%) with intermediate risk and 35 (19%) with poor risk for patients without PM. Using Heng prognostic criteria, 32 (73%) patients with PM were intermediate risk and 12 (27%) patients were poor risk compared to 158 (86%) patients who were intermediate risk and 26 (14%) patients who were poor risk of patients without PM (p-value=0.04).
Table 1.
Baseline patient characteristics
| Variable | PM(44) | No PM(184) | P = |
|---|---|---|---|
| Age (yrs, range) | 63(44–79) | 61(23–82) | 0.35 |
| Male | 30(68%) | 139(76%) | 0.32 |
| BMI > 30 | 15(34%) | 77(42%) | 0.39 |
| Caucasian | 35(80%) | 133(72%) | 0.57 |
| Prior nephrectomy | 34(77%) | 109(59%) | 0.03 |
| Diabetes | 27(61%) | 55(36%) | 0.001 |
| Hypertension | 24(55%) | 85(46%) | 0.32 |
| Number of metastases | 3 (1–5) | 3 (1–4) | 0.001 |
| 1 | 4(9%) | 57(31%) | |
| 2 | 10(23%) | 74(40%) | |
| ≥3 | 30(68%) | 53(29%) | |
| Sites of Metastases | |||
| Lung | 30(68%) | 139(76%) | 0.34 |
| Bone | 13(30%) | 60(33%) | 0.52 |
| Liver | 12(27%) | 39(21%) | 0.39 |
| Brain | 5(11%) | 25(14%) | 0.69 |
| Adrenal | 12(27%) | 26(14%) | 0.38 |
| MSKCC group | 0.04 | ||
| Good | 9(20%) | 14(8%) | |
| Intermediate | 27(62%) | 135(73%) | |
| Poor | 8(18%) | 35(19%) | |
| Heng* | 0.04 | ||
| Intermediate | 32(73%) | 158(86%) | |
| Poor | 12(27%) | 26(14%) | |
| ECOG | 0.71 | ||
| 0 | 13(30%) | 46(25%) | |
| 1 | 23(52%) | 107(58%) | |
| ≥2 | 8(18%) | 31(17%) | |
| Hb (< LLN) | 31(70%) | 143(78%) | 0.32 |
| Corrected Calcium (> LLN) | 8(18.2%) | 33(18%) | 0.56 |
| Platelets (> ULN) | 25(57%) | 98(53%) | 0.50 |
| LDH (> 1.5 ULN) | 18(41%) | 80(44%) | 0.6 |
| ANC (> ULN) | 11(25%) | 55(30%) | 0.7 |
| Diagnosis to treatment < 1 year | 21(48%) | 120(65%) | 0.05 |
No patients in Heng – Good prognostic cohort
Table 2 shows the variables that were predictive for overall survival in univariable Cox proportional hazards model. Based on the final fitted multivariable Cox model (Table 3) for OS, nephrectomy (HR=0.73, 95% CI= 0.52–0.92, p=0.02), surgically no evidence of disease (NED) (HR=0.52, 95% CI= 0.35–0.95, p=0.04) and PM (HR=0.66, 95% CI = 0.42–0.94, p=0.02) were associated with an increased OS; whereas higher tumor burden (HR=1.27, 95% CI=1.19–2.5, p=0.03), intermediate (HR=2.79, 95% CI=1.29–6.06, p=0.009) and poor risk (HR=5.29, 95% CI=2.20–12.74, p<0.000) stratification by MSKCC criteria, Heng poor risk (HR=1.49, 95% CI=1.02–2.43, p=0.01) category, and lung metastases (HR=1.45, 95% CI=1.00–2.34, p=0.001) were associated with decreased OS.
Table 2.
Univariate Cox proportional hazards model for Overall Survival
| Variable | HR | 95% CI | P = |
|---|---|---|---|
| Nephrectomy | 0.54 | 0.42–0.88 | 0.01 |
| Metastasis ≥2 vs 1 | 1.22 | 1.1–2.8 | 0.001 |
| Pancreatic metastasis | 0.67 | 0.42–0.95 | 0.01 |
| Lung metastasis | 1.55 | 1.08–2.22 | 0.01 |
| MSKCC Inter vs Good | 2.6 | 1.36–5.00 | 0.001 |
| MSKCC Poor vs Good | 4.9 | 2.52–9.93 | <0.001 |
| Heng (Poor vs Inter) | 1.58 | 1.01–2.22 | 0.003 |
| Surgically NED | 0.49 | 0.25–0.88 | 0.03 |
| Radiation treatment | 0.84 | 0.60–1.18 | 0.33 |
| Liver | 1.31 | 0.91–1.88 | 0.14 |
| Bone | 1.25 | 0.90–1.72 | 0.18 |
| ECOG (1 vs 0) | 1.54 | 1.05–2.27 | 0.03 |
| ECOG (≥2 vs 0) | 1.86 | 1.17–2.99 | 0.009 |
| Hemoglobin (< LLN) | 1.93 | 1.38–2.89 | 0.0005 |
| Corrected calcium (> ULN) | 2.11 | 1.52–3.10 | 0.0002 |
| Platelet (>ULN) | 1.49 | 1.32–1.75 | 0.0002 |
| LDH (> 1.5 ULN) | 5.67 | 4.31–10.4 | 0.0001 |
| ANC (> ULN) | 1.40 | 1.30–1.58 | 0.0000 |
| Diagnosis to treatment < 1 year | 1.58 | 1.14–2.18 | 0.004 |
Table 3.
Multivariable Cox proportional hazards model for Overall Survival
| Variable | HR | 95% CI | P = |
|---|---|---|---|
| Pancreatic metastasis | 0.66 | 0.42–0.94 | 0.02 |
| Nephrectomy | 0.73 | 0.52–0.92 | 0.02 |
| Metastasis ≥2 vs 1 | 1.27 | 1.19–2.5 | 0.03 |
| Lung metastasis | 1.45 | 1.00–2.34 | 0.001 |
| MSKCC Inter vs Good | 2.79 | 1.29–6.06 | 0.009 |
| MSKCC Poor vs Good | 5.29 | 2.20–12.74 | <0.000 |
| Heng (Poor vs Inter) | 1.49 | 1.02–2.43 | 0.01 |
| Surgically NED | 0.52 | 0.35–0.95 | 0.04 |
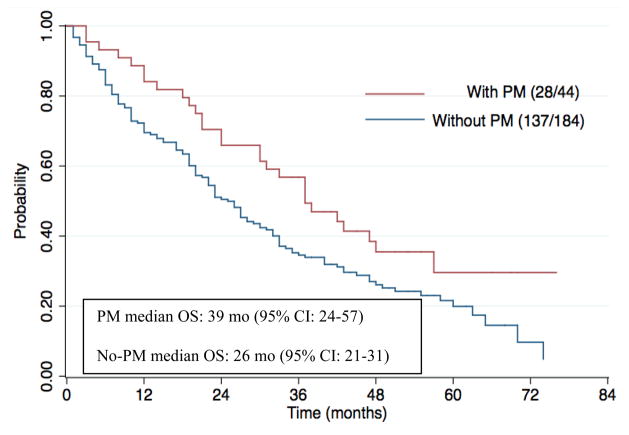
After a median follow-up time of 27 months (95% CI: 23–33 months), the median OS was 39 months (95% CI: 24–57 months) in the PM group and 26 months (95% CI: 21–31 months, p-value < 0.01) in the control group. Out of the 44 patients in the PM group, there were 28 deaths whereas in the control group, out of the 184 patients, there were 137 deaths. (Figure 1)
Figure 1.
Kaplan-Meier estimates of Overall Survival stratified by the presence of pancreatic metastasis. There were 28 deaths out of the 44 patients in the PM group and 137 deaths out of the 184 patients in the group without PM. Number at risk at 2 years and 5 years in the PM group were 31 and 5, respectively, whereas in the non-PM group, it was 89 and 13, respectively.
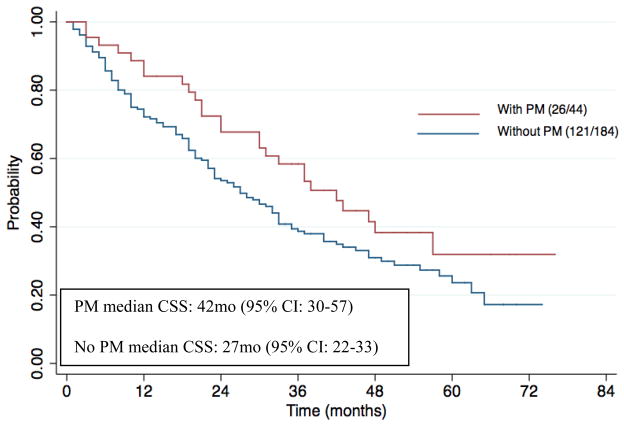
In our secondary analysis looking at CSS, the median follow-up time was 29 months (95% CI: 24–35 months), the median CSS was 42 months (95% CI: 30–57 months) in the PM group and 27 months (95% CI: 22–33 months, p-value = 0.05) in the control group. Out of the 44 patients in the PM group, there were 26 deaths whereas in the control group, out of the 184 patients, there were 121 deaths. (Figure 2)
Figure 2.
Kaplan-Meier estimates of Cancer Specific Survival stratified by the presence of pancreatic metastasis. There were 26 deaths out of the 44 patients in the PM group and 121 deaths out of the 184 patients in the group without PM. Number at risk at 2 years and 5 years in the PM group were 31 and 5, respectively, whereas in the non-PM group, it was 89 and 13, respectively.
DISCUSSION
There have been major advances in the management of patients with mRCC due to the introduction of various targeted therapy agents. Despite the advent of these new therapies, prognosis for this patient group remains quite poor. As evident in large cohorts of patients with mRCC, clinical outcome is quite heterogeneous. This is illustrated by the MSKCC and Heng criteria, relatively simple algorithms using clinical and laboratory features which are capable of stratifying patients into dramatically different prognostic groups. Currently, we have a limited understanding of the underlying drivers for this clinical heterogeneity. The identification of subsets of patients with mRCC who have uniquely favorable clinical features and potentially informative biology can form the basis of a more extensive molecular characterization of that group, which will inform us regarding the drivers of this unique clinical phenotype.
Although PM are only seen in a small fraction of the patient population with mRCC, in our study patients in the PM cohort had a significantly longer median OS when compared to patients with no PM. The PM cohort also demonstrated a statistically significant increase in favorable risk categorization by MSKCC and Heng prognostic nomograms, although the PM group had a higher median number of affected organ sites than the non-PM group. Exploratory analyses comparing the CSS between the two groups yielded results consistent with the OS. Although the PM cohort and the non-PM cohort had a fairly even distribution of patients with lung, liver and bone metastases, the PM-cohort was still associated with improved survival outcomes than the non-PM cohort in both univariable and multivariable analyses. These clinical clues further suggest that a unique biological state exists in patients with PM.
Our results are comparable to previously published case series and studies assessing the impact of PM on prognosis in patients with mRCC. In a similar retrospective study of mRCC patients treated with surgery or targeted therapies, median OS was 39 mo in patients with PM vs. 23 mo in patients without PM (P = 0.0004).17 In comparison, the PM cohort had similar greater metastatic sites of disease, yet favorable prognostic variables as compared to the control cohort. In another study looking at the outcomes of mRCC patients treated with pazopanib after disease progression on other targeted therapies, pancreatic metastases were associated with increased OS (HR=0.38, 95% CI = 0.17–0.86, p = 0.019).18 Tanis et al conducted a systematic literature search for 311 surgically and 73 non-surgically treated patients with pancreatic RCC metastasis. They observed that disease free survival rate was 57 percent and overall survival was 72.6 percent. In the unresected patients, the overall survival at 5 years was only 14 percent.19 In a similar study by Schwarz et al, 62 surgically treated patients were followed up for assessing their survival outcomes. 3 year, 5 year and 10 year overall survival rates were 72, 63 and 32 percent. The corresponding disease-free survival rates were 54, 35 and 27 percent, respectively.20 These findings suggest that host or tumor features associated with pancreatic metastases may induce a less aggressive tumor phenotype.
The next steps in our understanding of this unique subgroup of patients with mRCC will be to perform comprehensive analyses of both tumor and host biology. There are several caveats to this analysis. First, it appears that developing PM is associated with a favorable outcome regardless of the number of additional metastatic sites. Indeed, our PM patient cohort had a higher mean number of involved organs compared to the control group, so the differences in prognosis are not due to a lower probability of disease dissemination. These observations lead to the hypothesis that prognosis of patients with PM may be driven by host determinants, which include unique host tissue polymorphisms that affect tumor growth or the composition of the cellular tumor microenvironment. A second possibility is that there is a unique endocrine dependence of RCC from patients who develop PM, which enhances the likelihood of growing in the pancreas, but allows survival in distant organs provided this endocrine environment is sufficiently maintained in those distant organs. Both of these scenarios are testable using appropriate tools.
Our results must be carefully scrutinized for inherent biases and limitations. Limitations of our analysis include their retrospective nature; accuracy and availability of documentation, no randomization or blinding, and difficulty establishing cause and effect. Selection bias is also of great concern; further analysis is underway to limit such bias.
In conclusion, we show that PM in patients with mRCC are associated with a statistically significant higher OS. This unique patient group can form the basis of detailed molecular studies that elucidate key drivers of RCC tumor behavior, and determinants of tumor-host interaction.
Footnotes
DISCLOSURES
Authors SK, BJA, MRM, SFM, CGW, PT, KS, PR, PGC have no financial disclosures. JAK reports personal fees from Pfizer. NMT received grants from UT MD Anderson Cancer Center; has Research Support and is on Advisory Board for Pfizer, Novartis, GlaxoSmithKline, and is on Advisory Board for Aveo. EJ reports grants from Pfizer, GSK, Novartis, Onyx and Exelixis and Personal fees from Pfizer, GSK and Novartis.
References
- 1.Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Motzer RJ, Jonasch E, Agarwal N, et al. Kidney cancer, version 2.2014. J Natl Compr Canc Netw. 2014;12(2):175–182. doi: 10.6004/jnccn.2014.0018. [DOI] [PubMed] [Google Scholar]
- 3.Mekhail TM, Abou-Jawde RM, Boumerhi G, et al. Validation and extension of the Memorial Sloan-Kettering prognostic factors model for survival in patients with previously untreated metastatic renal cell carcinoma. J Clin Oncol. 2005;23(4):832–841. doi: 10.1200/JCO.2005.05.179. [DOI] [PubMed] [Google Scholar]
- 4.Ballarin R, Spaggiari M, Cautero N, et al. Pancreatic metastases from renal cell carcinoma: the state of the art. World J Gastroenterol. 2011;17(43):4747–4756. doi: 10.3748/wjg.v17.i43.4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27(22):3584–3590. doi: 10.1200/JCO.2008.20.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heng DYC, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol. 2009;27(34):5794–5799. doi: 10.1200/JCO.2008.21.4809. [DOI] [PubMed] [Google Scholar]
- 7.Lavu Harish, Yeo CJ. Metastatic Renal Cell Carcinoma to the Pancreas. Gastroenterology & Hepatology. 2011;7(10):699. [PMC free article] [PubMed] [Google Scholar]
- 8.Minni F, Casadei R, Perenze B, et al. Pancreatic metastases: Observations of three cases and review of the literature. Pancreatology. 2004;4(6):509–520. doi: 10.1159/000080248. [DOI] [PubMed] [Google Scholar]
- 9.Moussa A, Mitry E, Hammel P, et al. Pancreatic metastases: a multicentric study of 22 patients. Gastroenterol Clin Biol. 2004;28(10 Pt 1):872–876. doi: 10.1016/s0399-8320(04)95151-2. [DOI] [PubMed] [Google Scholar]
- 10.Faure JP, Tuech JJ, Richer JP, et al. Pancreatic metastasis of renal cell carcinoma: presentation, treatment and survival. J Urol. 2001;165:20–2. doi: 10.1097/00005392-200101000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Janzen NK, Kim HL, Figlin RA, et al. Surveillance after radical or partial nephrectomy for localized renal cell carcinoma and management of recurrent disease. Urol Clin North Am. 2003;30(4):843–852. doi: 10.1016/s0094-0143(03)00056-9. [DOI] [PubMed] [Google Scholar]
- 12.Medioni J, Choueiri TK, Zinzindohoué F, et al. Response of renal cell carcinoma pancreatic metastasis to sunitinib treatment: a retrospective analysis. J Urol. 2009;181(6):2470–5. doi: 10.1016/j.juro.2009.02.020. discussion 2475. [DOI] [PubMed] [Google Scholar]
- 13.Law C, Wei AC, Hanna SS, et al. Pancreatic resection for metastatic renal cell carcinoma: presentation, treatment, and outcome. Ann Surg Oncol. 2003;10:922. doi: 10.1245/aso.2003.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Thompson LD, Heffess CS. Renal cell carcinoma to the pancreas in surgical pathology material. Cancer. 2000;89(5):1076–1088. doi: 10.1002/1097-0142(20000901)89:5<1076::aid-cncr17>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 15.Sellner F, Tykalsky N, De Santis M, et al. Solitary and multiple isolated metastases of clear cell renal carcinoma to the pancreas: an indication for pancreatic surgery. Ann Surg Oncol. 2006;13(1):75–85. doi: 10.1245/ASO.2006.03.064. [DOI] [PubMed] [Google Scholar]
- 16.Bassi C, Butturini G, Falconi M, et al. High recurrence rate after atypical resection for pancreatic metastases from renal cell carcinoma. Br J Surg. 2003;90(5):555–559. doi: 10.1002/bjs.4072. [DOI] [PubMed] [Google Scholar]
- 17.Grassi P, Verzoni E, Mariani L, et al. Prognostic role of pancreatic metastases from renal cell carcinoma: results from an Italian center. Clin Genitourin Cancer. 2013;11(4):484–488. doi: 10.1016/j.clgc.2013.04.022. [DOI] [PubMed] [Google Scholar]
- 18.Matrana MR, Duran C, Shetty A, et al. Outcomes of patients with metastatic clear-cell renal cell carcinoma treated with pazopanib after disease progression with other targeted therapies. Eur J Cancer. 2013;49(15):3169–75. doi: 10.1016/j.ejca.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanis PJ, van der Gaag NA, Busch ORC, van Gulik TM, Gouma DJ. Systematic review of pancreatic surgery for metastatic renal cell carcinoma. Br J Surg. 2009;96(6):579–592. doi: 10.1002/bjs.6606. [DOI] [PubMed] [Google Scholar]
- 20.Schwarz L, Sauvanet A, Regenet N, et al. Long-term Survival After Pancreatic Resection for Renal Cell Carcinoma Metastasis. Ann Surg Oncol. 2014 doi: 10.1245/s10434-014-3821-4. [DOI] [PubMed] [Google Scholar]