Abstract
Effective strategies to monitor pharmacotherapy adherence are necessary, and sensitive biological markers are lacking. This study examined a sub-therapeutic dose of quinine as a potential adherence tracer. Primary aims included examination of the plasma and urinary pharmacokinetic profile of once-daily quinine; secondary aims assessed pharmacokinetic/pharmacodynamic interactions with oxycodone (a CYP3A and CYP2D substrate). Healthy, non-dependent opioid users (n=9) were enrolled in this within-subject, double-blind, placebo-controlled, inpatient study. Participants received the following oral doses, Day 1: oxycodone (30 mg), Days 2-4: quinine (80 mg), Day 5: quinine and oxycodone (2 hrs post-quinine). Blood and 24-hr urine samples were collected throughout the study, and pharmacodynamic outcomes were assessed during experimental sessions (Days 1, 4, 5). Quinine displayed a plasma Tmax ∼2 hrs and t1/2 ∼10 hrs. Oxycodone and noroxycodone parameters (Tmax, Cmax, t1/2) were similar with or without quinine present, although drug exposure (AUC) was slightly greater when combined with quinine. No pharmacodynamic interactions were detected and doses were safely tolerated. During washout, quinine urinary concentrations steadily declined (elimination t1/2 ∼16 hrs), with a 94% decrease observed 72 hrs post-dose. Overall, low-dose quinine appears to be a good candidate for a medication additive to monitor adherence for detection of missed medication.
Keywords: Adherence, tracer, quinine, oxycodone, human, pharmacokinetic
Introduction
Medication adherence, (e.g., taking medication as directed in regard to both dose and frequency), is critical in determining pharmacotherapy efficacy in both clinical practice and research trials. Non-adherence (e.g., dosing omission, cessation) is common; it is estimated that chronically ill patients take ∼50-80% of their prescribed doses, with adherence inversely related to dose frequency (e.g., four doses/day = 50%; one dose/day = 80%).1 Non-adherence is associated with misdiagnosis, preventable medical problems, morbidity and mortality2,3 and costs the United States health care system $105 billion annually.4 Non-adherence is also problematic in clinical research trials, with an estimated 4% of participants never taking a single dose3 and an overall adherence rate of 75% (e.g., 25% missed dose rate).5 However, lower adherence rates (39%-42%) occur in alcohol or drug treatment trials,6,7 with up to 10% of participants never taking a single dose.8 Non-adherence in research trials can contribute to underestimation of medication side effects, inaccurate estimates of effective dose ranges, and inconclusive or misinterpreted results – particularly potentially incorrect conclusions that a putative pharmacotherapy is ineffective.3,9,10
Better strategies are needed to improve medication adherence monitoring and identify lapses as they occur. Indirect and direct monitoring approaches have been implemented (see reviews7,11); indirect measurements include monitoring medication quantity (e.g., pill counts, refill frequency), collecting self-report, and using electronic medication bottles/pill planners that record each time a medication container is opened. Clinicians may use patient assessments and biological outcomes (e.g., hemoglobin A1C levels in diabetic patients) to estimate medication adherence. However, these indirect methods have clear limitations: pills can be removed from containers without ingestion; self-reports often overestimate adherence;12 physiological markers are frequently influenced by factors other than adherence (e.g., poor medication response).3 Direct measurements typically provide more accurate information5 and include observed drug dosing and measurement of the prescribed medication in blood (e.g., drug/metabolite concentrations). However, these measures can be limited by logistical issues, such as frequent visits to the clinic and high costs of quantitative drug assays.11 Further, there is frequently no known quantifiable cut-off/range for most medications that determines adherence/non-adherence, medication concentrations are subject to high inter- and intra-individual variability, and placebo adherence, which appears to be an independent predictor of better treatment outcomes,13 cannot be measured with this model.3,9,10
A promising direct measurement approach is the inclusion of a medication additive (i.e., tracer) compounded with the prescribed medication (and placebo in clinical trials), which can be detected in blood, urine or saliva by qualitative or quantitative assays. An ideal tracer would be non-toxic, safe and pharmacologically inert at administered doses, display linear pharmacokinetic characteristics, an elimination half-life well matched to once or twice daily dosing, and reliably detected and easily quantifiable in urine or other biological matrices that do not require invasive collection procedures (e.g., saliva).9,10 If an appropriate tracer is identified, it could be combined with medications for which there is no established immunoassay, allowing researchers the ability to monitor adherence to a wide variety of pharmacotherapies (eliminating the need to develop expensive assays for every drug under clinical investigation).
Several tracer medications have been tested, including riboflavin, sodium bromide, phenazopyridine, digoxin, and low-dose phenobarbital.14,15 Riboflavin has been frequently used in clinical trials; however, several characteristics limit its utility: 1) narrow window of detection in urine after dosing (∼2-8 hrs),16-18 2) considerable inter-subject variability in absorption and elimination,18,19 3) interference from dietary sources/multivitamins,15,17 and 4) relatively high false positive rate (20-53%) for qualitative florescence.16,17 Because of these limitations, some have suggested exploring alternative adherence tracers.16
The current study examined a sub-therapeutic dose of quinine (80 mg) as a candidate tracer. Quinine is FDA-approved for the treatment of uncomplicated malaria (1944 mg/day; 3-7 days)20 and is also present in commercially available tonic water at a maximum concentration of 83 mg/L, per FDA regulations.21 Previous studies have indicated that quinine has a favorable profile: 1) it is readily absorbed following oral administration (∼88% bioavailability),22 2) maximal plasma concentrations (Tmax) occur within 2-3 hrs,23 3) plasma half-life (t1/2) is 9-11 hrs,23,24 and 4) ∼20% is excreted unchanged in the urine.25
The objectives of this study were to characterize the pharmacokinetic profile of a low dose of quinine in plasma and urine with once daily dosing. Secondary aims were to examine potential pharmacokinetic and pharmacodynamic interactions with oxycodone, a model medication that is a common drug of abuse, which may be encountered in trials examining pharmacotherapies for the treatment of drug dependence, and is hepatically metabolized similar to quinine (via cytochrome P450 (CYP) 3A and 2D).26,27
Methods
Human Subjects Protection
This study was approved by the University of Kentucky (UK) Institutional Review Board and conducted in accordance with the Helsinki guidelines for ethical research. All participants provided sober, written informed consent prior to study participation and were paid for their participation.
Participants
Participants were healthy adult recreational opioid users who were not physically dependent on opioids. In-person screening evaluations included medical history, physical exam, psychiatric assessments, blood chemistry (CBC, metabolic panel), urinalysis and ECG. Participants were literate, English-speaking adults ages 18-50 with adequate venous access; participants reported current occasional opioid abuse (∼1-2 times/week), confirmed by at least one observed opioid-positive urine sample. Participants were also required to provide an observed opioid-negative urine sample in the absence of opioid withdrawal signs and symptoms to exclude individuals with physiological opioid dependence. Additional exclusion criteria included current physiological drug dependence requiring medical care (e.g., benzodiazepine dependence), pregnancy, and serious medical or psychiatric problems (e.g., diabetes, suicidality).
Drugs
The study was conducted under an investigator-initiated Investigational Drug Application from the Food and Drug Administration (#69,214). Drug doses were prepared by the UK Investigational Pharmacy. Quinine sulfate capsules (324 mg/capsule, Qualaquin®, AR Scientific/URL Pharma, Philadelphia, PA) and oxycodone hydrochloride tablets (Mallinckrodt, Hazelwood, MO) were obtained. Lactose monohydrate powder (Medisca Pharmaceuticals, Plattsburgh, NY) served as the placebo. To formulate the active doses, 1) quinine capsules were emptied and the powder needed to produce a dose of 80 mg was measured (to account for excipients), and 2) oxycodone tablets (30 mg) were over-encapsulated. All doses were delivered in size 00 gelatin capsules (Health Care Logistics, Circleville, OH) loose-filled with lactose with two different capsule colors used.
Prior to study enrollment, in vitro dissolution studies were conducted (Murty Pharmaceuticals, Lexington, KY) with capsules containing quinine with no filler (control) or one of four excipients (lactose, povidone, methylcellulose, gelatin) to determine if any of these agents modified the dissolution profile of quinine. Methylcellulose slowed the release of quinine; all other agents performed similarly and did not differ from control. Lactose was selected for the current study because it is commonly used in the formulation of experimental, over-the-counter and prescription medications.
Study Design
The data presented here are from the second week of a 2.5-week within-subject, double-blind, placebo-controlled, inpatient study conducted at the UK Center for Clinical and Translational Science; this study consisted of two, 1-week segments that were nearly methodologically identical (Week 1 examined acetazolamide alone and in combination with oxycodone [results reported separately]). Week 2 examined the pharmacokinetic, subjective and physiological effects of quinine (80 mg), oxycodone (30 mg) and their combination (tested on the fourth consecutive day of quinine administration). Three experimental sessions (8.5 hrs) were conducted on Days 1, 4 and 5 (of Week 2) to capture the physiological, subjective and observer-rated effects of each drug alone and in combination.
General Methods
Participants were maintained on a diet free of caffeine and tonic water. A light standardized breakfast was provided 2 hrs prior to drug administration; no food was permitted until the end of session (or 4 hrs post-dose during non-session days). Smoking was not permitted 1 hr prior to drug administration and was allowed to resume after session completion (or 4 hrs after the last dose administered and after the blood draws were completed on non-session days). During each session, participants were provided one 16.9 fl oz bottle of water (Dasani®, Coca-Cola Company) 2 hrs prior to and 1 hr after placebo/opioid administration and were required to finish each bottle within 3 hrs (to ensure urine production).
Urine samples were tested each morning for drugs of abuse. Females were tested for pregnancy daily. Breath samples were tested for alcohol concentrations prior to each session.
Dosing Schedule
Dosing was as follows: Day 1: placebo + oxycodone (30 mg); Days 2 and 3: quinine (80 mg); Day 4: quinine (80 mg) + placebo; Day 5: quinine (80 mg) + oxycodone (30 mg) (Table 1).
Table 1.
Study dosing, blood sampling, urine collection, and experimental session schedule.
| Study Day, Doses | Plasma Collection | Plasma Analytes | 24 hr Urine | Session |
|---|---|---|---|---|
| Day 1: Placebo, Oxycodone | -2.5, 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12, 24 hrs post OXY | OXY, NOROXY | - - | Session 1 |
| Day 2: Quinine | -0.5 hrs (pre-QUIN baseline) | QUIN | Pre-dose; 6 hr intervals | - - |
| Day 3: Quinine | -0.5 hrs | QUIN | 6 hr intervals | - - |
| Day 4: Quinine | -0.5, 1, 2, 3, 6, 10, 14, 24 hrs post QUIN | QUIN | 3 hr intervals | Session 2 |
| Day 5: Quinine, Oxycodone | -0.5, 1, 2, 2.5, 3, 3.5, 4, 5, 6, 8, 10, 14, 24 hrs post QUIN* | QUIN, OXY, NOROXY | 3 hr intervals | Session 3 |
| Day 6 - 8: Washout | - - | - - | 6 hr intervals through morning of Day 8 | - - |
Study schema describing the doses administered, plasma and urine collection schedule, analytes for each plasma sample, and experimental session schedule. Urine samples were analyzed for quinine only.
The collection schedule for Day 5 is presented relative to quinine administration. Relative to oxycodone, samples occurred -2.5, 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12 and 24 post dose. Day 5 samples were analyzed for each analyte, except 1 and 2 hrs post quinine samples (only analyzed for quinine). Abbreviations: quinine (QUIN), oxycodone (OXY) and noroxycodone (NOROXY).
Quinine/matched placebo was administered 2 hrs prior to oxycodone/matched placebo (8am, 10 am respectively). On non-session days (Days 2, 3) a single oral quinine dose was administered; on session days (Days 1, 4, 5) two oral capsules were administered in order to maintain the double-blind and double-dummy dosing procedures for the behavioral sessions. Days 6-8 were washout days (no doses administered).
Blood and Urine Sampling Procedures
Peripheral blood was collected via an indwelling intravenous catheter or individual needle stick when needed. Catheters were regularly flushed with physiological saline and maintained for a maximum of 72 hr. Blood was collected in either a 5mL purple-top for a single assay or a 10 mL green-top sodium heparin vacutainer for multiple assays. Vacutainers were centrifuged at 1,300 g for 10 minutes at 4°C, and plasma aliquoted and frozen to -80°C.
Table 1 displays the plasma analytes evaluated (quinine, oxycodone, and noroxycodone) and the collection schedule, designed to capture ascending, peak and descending time-concentration curves for each analyte.
Twenty-four hour urine collection occurred throughout the study and samples were analyzed for quinine (Table 1). Each sample was mixed and volume (mL) and pH were measured; a 10-mL aliquot was frozen at -80° C. Urine collection ended on the morning of Day 7 for the first four participants enrolled; collection continued through the morning of Day 8 for the last five participants.
Physiological Measures and Safety Assessments
During sessions, heart rate, blood pressure and oxygen saturation (Dinamap Non-Invasive Patient Monitor, GE Medical Systems, Tampa, FL) were collected in 1-min intervals before and 6 hrs after opioid/placebo administration. Manual respiration rate and pupil diameter measurements (PLR-200, NeurOptics, Irvine, CA) were collected at baseline and at 15-min intervals for the first 2 hrs after opioid/placebo and at 30-min intervals for the remaining 4 hrs.
A baseline ECG was completed at study screening and after enrollment between the third and fourth day of quinine dosing. ECGs were compared and reviewed by a physician. Participants were queried regularly for adverse events.
Participant- and Observer-Rated Measures
Participant-rated measures included a locally-developed questionnaire to assess frequently reported non-specific (e.g., nausea) and quinine-specific (e.g., tinnitus) side effects; a six-item visual analog scale (VAS),28 collected at baseline, 90 minutes post-tracer administration and regularly after opioid/placebo administration (15-min intervals for the first 2 hrs, 30-min intervals for the remaining 4 hrs); the Participant-Rated Opioid Adjective Scale,29 presented at baseline, 90 minutes post-tracer administration and in 30-min intervals after opioid/placebo administration; and a street value estimate (e.g., rating the subjective dollar value of the drug) presented in 30-min intervals after opioid/placebo administration. Trained research assistants (blind to conditions) rated signs of opioid agonist effects on the Observer-Rated Opioid Adjective Scale29 at baseline, 90 minutes post-quinine dose and in 30-min intervals after opioid/placebo administration.
Sample Preparation and Analyses
All plasma samples were spiked with a quinine, oxycodone or noroxycodone internal standard (IS) solution (2H3-quinine, 2H6-oxycodone, 2H3-noroxycodone, respectively) and were extracted in the presence of NaCO3 using a EtOAc : tBME (3:1) solvent mix. Samples were then neutralized, dried under nitrogen and reconstituted in mobile phase. Urine samples were spiked with an IS but not extracted prior to LC/MS analyses.
All plasma samples were separated on a 5 μm, 4.6 × 100 mm, C18 HPLC column with an isocratic mobile phase (90 ACN :10 H2O :0.05 HCOOH; 5mM NH4HCOO). Quantification was conducted via selective-ion monitoring using atmospheric pressure chemical ionization and positive ionization by multiple reaction monitoring modes, recording the following m/z ratios: 325-307 (quinine), 328-175 (quinine IS), 316-298 (oxycodone), 322-304 (oxycodone IS), 302-284 (noroxycodone), 305-287 (noroxycodone IS). The range of the standard curve for quinine in plasma and urine was 20 to 8000 ng/mL with a lower limit of quantitation (LLOQ) of 20 ng/mL. The range of the standard curves for both oxycodone and noroxycodone in plasma was 0.2 to 200 ng/mL, with an LLOQ of 0.2 ng/mL.
Statistical Analyses
Non-compartmental pharmacokinetic analyses of plasma and urine data were conducted (Phoenix® WinNonlin®, Pharsight®, Certara, L.P.) and included all available data within each dose condition; Shapiro-Wilk normality tests were conducted to confirm that the curves were normally distributed and appropriate for parametric testing and t-tests were conducted to determine dose condition effects. Trough plasma concentrations were analyzed with a one-factor model (time); behavioral and physiological data were initially analyzed as raw time course data using a two-factor repeated measures model (drug condition, time) with AR(1) covariance structure. Physiological measures, collected minute-by-minute, were averaged across 15-30 min intervals corresponding to subjective reporting intervals. Peak/trough scores were analyzed using a one-factor model (drug condition). Time-to-peak effect (e.g., Tmin or Tmax) was calculated for individual participants and dose conditions and was analyzed in a one-factor model (drug condition). Tukey's post-hoc tests were performed to examine the time course of drug effects, effects of individual doses and differences between comparator dose conditions. All models were analyzed with Proc Mixed in SAS 9.3 (Cary, NC) with significance at p<.05.
Results
Participants
Thirty-nine participants signed screening consent and 14 signed main study consent; 12 participants met qualification criteria and were enrolled. Data from 3 enrolled participants were not included in the analyses: 2 due to early discharge (behavioral issues, need for daily allergy medications) and 1 due to a pharmacy dose error. In total, data from 9 participants (2 Caucasian women, 1 mixed race male (African American/Caucasian), 6 Caucasian males) were analyzed. They were 31.5 (±1.6) years old and reported current illicit short-acting opioid use of 11.2 (±1.9) days out of the 30 days prior to enrollment. Seven participants were daily smokers (14.4 (±1.7) cigarettes/day), 2 were non-smokers. Other past month drug use included alcohol (2.9 [±1.4] days; n=5); benzodiazepines (1.8 [±0.4] days; n=5); cocaine (1.5 [±0.2] days; n=4); amphetamines (6 days; n=1); and marijuana (17.1 [±4.1] days; n=7).
Pharmacokinetic Outcomes
Quinine Concentrations in Plasma
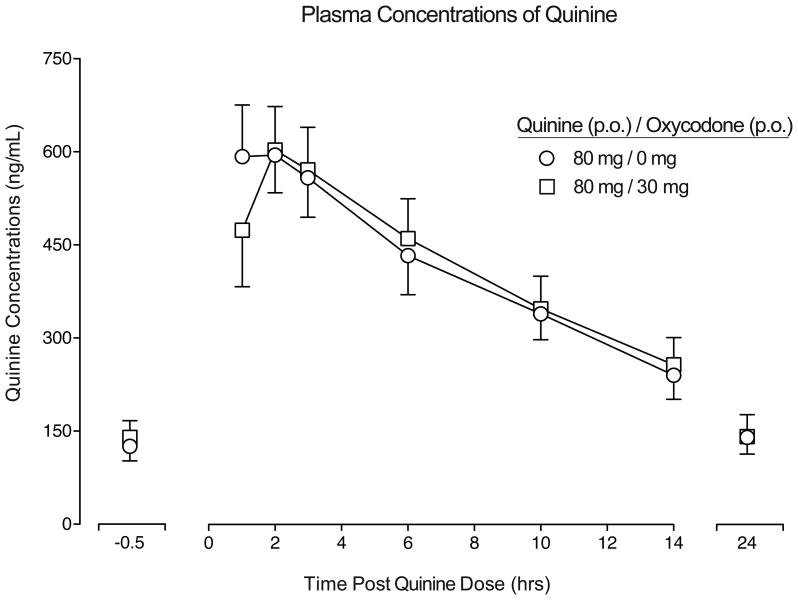
Figure 1 presents the time course of quinine plasma concentrations when quinine was administered alone (Day 4) and in combination with oxycodone (Day 5). The time concentration curves were similar across conditions and peak quinine concentrations (Tmax) occurred ∼2 hrs post-dose with a half-life of ∼10 hrs (Table 2). AUC, Tmax, Cmax, t1/2 were similar across the two conditions (p>.05; Table 2).
Figure 1.
Mean quinine plasma concentrations displayed as a function of dose condition and time following quinine administration (n=9, ±1 SEM; circles = quinine alone, squares = quinine + oxycodone). Pharmacokinetic parameters for each dose condition are displayed in Table 2; no differences were detected as a function of dose condition (p > .05).
Table 2.
Pharmacokinetic parameters for quinine, oxycodone and noroxycodone in plasma (ng/mL, ±SEM).
| Plasma Quinine Concentrations | |||
|---|---|---|---|
| Quinine | Quinine + Oxycodone | p value | |
|
| |||
| AUC (ng/ml_)*h | 7524 (±972) | 7597 (±1141) | 0.79 |
| Cmax (ng/mL) | 680 (±79) | 664 (±78) | 0.73 |
| Tmax (h) | 2.0 (±0.2) | 2.1 (±0.2) | 0.78 |
| t1/2 (h) | 10.5 (±0.9) | 10.3 (±1.1) | 0.78 |
| Plasma Oxycodone Concentrations | |||
| Oxycodone | Quinine + Oxycodone | p value | |
|
| |||
| AUC (ng/ml_)*h | 324 (±43) | 347 (±41) | 0.02* |
| Cmax (ng/mL) | 66 (±7) | 68 (±6) | 0.79 |
| Tmax (h) | 1.1 (±0.1) | 1.1 (±0.1) | 0.78 |
| t1/2 (h) | 4.3 (±0.3) | 4.4 (±0.3) | 0.31 |
| Plasma Noroxycodone Concentrations | |||
| Oxycodone | Quinine + Oxycodone | p value | |
|
| |||
| AUC (ng/mL)*h | 221 (±24) | 279 (±24) | <.001* |
| Cmax (ng/mL) | 30 (±3) | 35 (±3) | 0.06 |
| Tmax (h) | 1.2 (±0.1) | 1.2 (±0.1) | 0.73 |
| t1/2 (h) | 6.7 (±0.3) | 6.8 (±0.4) | 0.73 |
All parameters, as listed above, were calculated using non-compartmental pharmacokinetic analyses (Phoenix® WinNonlin®). Significant effects between comparator drug conditions (p <.05) are indicated with a bolded p value and an asterisk in the final column.
Mean 24-hr trough plasma concentrations of quinine increased 50% across the four days of dosing (i.e., 94; 125; 139; 140 ng/mL). However, concentrations appeared stable after the second dose (with a modest, non-significant 11% increase from second to third dose) and nearly identical values for the last two doses, suggesting that plasma concentrations approached steady-state rapidly.
Oxycodone and Noroxycodone in Plasma
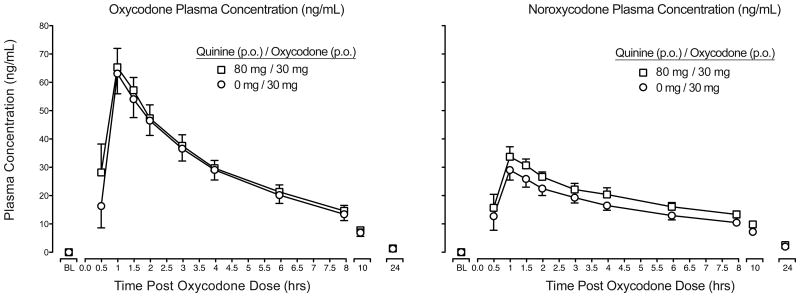
Figure 2 (left panel) displays the time course profile of plasma concentrations (ng/mL) of oxycodone when administered alone (Day 1) and with quinine (Day 5). Peak plasma concentrations occurred ∼1 hr post-dose with a half-life of ∼4 hrs and minimal concentrations (≤5 ng/mL) detected at 24 hrs. No differences were observed in Cmax, Tmax, or t1/2 as a function of quinine administration (Table 2); however, oxycodone AUC was slightly elevated (e.g., 7% increase) in the quinine condition (p<.05).
Figure 2.
Mean oxycodone (left panel) and noroxycodone (right panel) plasma concentrations displayed as a function of dose condition and time following oxycodone administration (n=9, ±1 SEM; circles = oxycodone alone; squares = quinine + oxycodone). Pharmacokinetic parameters for each dose condition are displayed in Table 2; no differences were detected in Cmax, Tmax or t1/2 as a function of dose condition (p > .05); AUC values for oxycodone and noroxycodone were greater in the quinine condition (p < .05; Table 2).
Figure 2 (right panel) presents the time course of noroxycodone across the two dosing conditions (Day 1, Day 5). Peak noroxycodone concentrations occurred ∼1 hr post-dose with a half-life of ∼7 hrs (Table 2). There was a moderate upward shift (∼26%) in the noroxycodone curve when quinine was co-administered, producing a significant increase in AUC values (p<.05); however, all other pharmacokinetic estimates were similar across conditions (Table 2).
Pharmacodynamic Outcomes
Physiological, Subject- and Observer Rated Measures
Oxycodone produced prototypical μ-opioid agonist effects on an array of physiological, subjective, and observer-rated measures. Specifically, oxycodone increased subjective ratings of positive drug effect (e.g., drug liking), increased observer ratings of opioid agonist effects, and produced pupillary miosis (p<.05). These effects were consistent across time course, AUC, and peak effect analyses (Table 3).
Table 3.
Mean peak and trough values of measures for which a significant drug effect was detected.
| Outcome Measure | F(2,16) | QUIN | OXY | QUIN + OXY |
|---|---|---|---|---|
| Physiological Effects | ||||
| Pupil Diameter | 105.2 | 4.8 (0.2) | 2.5 (0.1) | 2.4 (0.1) |
| Subjective Effects | ||||
| Visual Analog Scales | ||||
| Drug Effect | 19.3 | 1.4 (1.0) | 37.2 (7.0) | 40.2 (7.2) |
| High | 19.9 | 1.3 (0.9) | 38.1 (7.1) | 41.1 (7.2) |
| Good Drug Effect | 19.8 | 1.1 (0.8) | 37.3 (7.1) | 40.7 (7.3) |
| Like Drug Effect | 19.9 | 1.1 (0.8) | 37.7 (7.1) | 40.9 (7.3) |
| Street Value Estimate | 22.4 | 1.1 (0.7) | 20.0 (2.9) | 26.1 (5.2) |
| Participant Adjectives | ||||
| Agonist Sub-Scale | 11.7 | 7.0 (1.6) | 12.8 (1.6) | 13.2 (1.3) |
| Itchy Skin | 24.4 | 0.0 (0.0) | 1.4 (0.2) | 1.6 (0.2) |
| Nodding | 4.2 | 0.1 (0.1) | 0.6 (0.2) | 0.9 (0.3) |
| Relaxed | 12.8 | 1.0 (0.4) | 2.0 (0.3) | 1.6 (0.2) |
| Talkative | 7.4 | 0.6 (0.2) | 1.1 (0.4) | 1.3 (0.4) |
| Heavy or Sluggish | 4.0 | 0.0 (0.0) | 0.3 (0.2) | 0.3 (0.2) |
| Dry Mouth | 5.1 | 0.0 (0.0) | 0.4 (0.2) | 0.7 (0.2) |
| Good Mood | 6.9 | 1.9 (0.4) | 2.4 (0.4) | 2.4 (0.3) |
| Friendly | 7.0 | 1.9 (0.4) | 2.3 (0.3) | 2.4 (0.3) |
| Observer-Rated Effect | ||||
| Observer Adjectives | ||||
| Agonist Sub-Scale | 29.9 | 4.6 (0.6) | 7.6 (0.6) | 7.4 (0.5) |
| Itchy Skin | 18.2 | 0.0 (0.0) | 1.1 (0.3) | 1.7 (0.4) |
| Talkative | 8.9 | 1.1 (0.3) | 2.0 (0.2) | 1.9 (0.2) |
| Good Mood | 12.3 | 1.0 (0.2) | 1.9 (0.2) | 1.6 (0.2) |
All measures were analyzed as peak maximum score, with the exception of pupil diameter (which is presented as trough or minimum scores). Values are mean peak scores and standard error of the mean for quinine (QUIN), oxycodone (OXY), and the combination of the oxycodone and quinine (QUIN + OXY). Bolded values indicate the mean is significantly different from quinine alone (p < 0.05, Tukey post-hoc). None of the values were significantly different between oxycodone alone compared to quinine combined with oxycodone (p > 0.05, Tukey post-hoc).
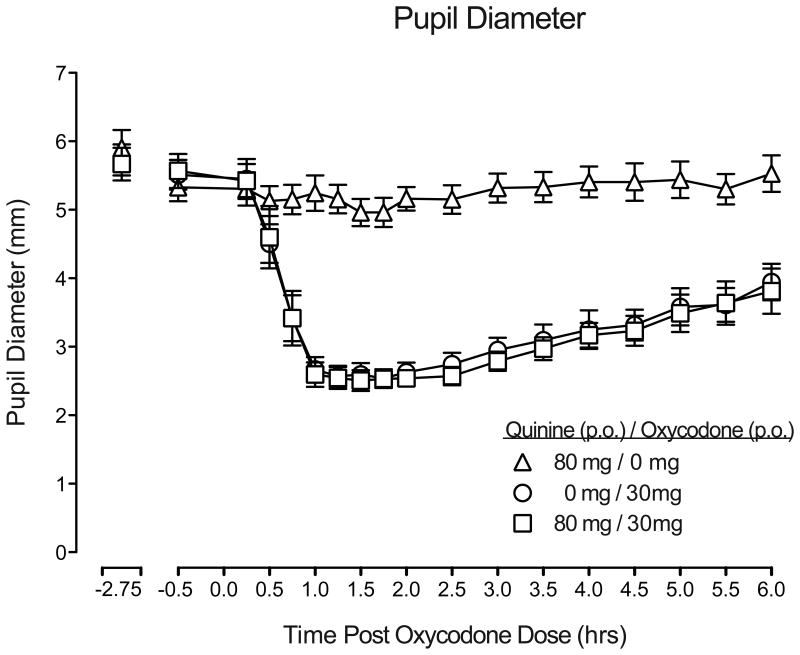
Quinine alone did not produce any psychoactive effects, increase subjective or observer-rated measures (Table 3), or produce any changes from baseline physiological outcomes. In addition, quinine did not modify the time course, AUC, or peak magnitude of any of the pharmacodynamic effects of oxycodone (p>.05; Table 3). Figure 3 presents the time course effects of quinine alone, oxycodone alone and the dose combination on pupil diameter. Oxycodone alone produced miosis, with peak effects occurring at ∼1-2 hrs, and effects lasting through the end of session. Quinine alone did not alter pupil diameter (compared to baseline) and did not modify the time course or magnitude of oxycodone effects (Table 3). In addition, none of the dose conditions tested modified heart rate, blood pressure, respiratory rate, or oxygen saturation (p>.05).
Figure 3.
Mean pupil diameter, displayed as a function of time following drug administration, from baseline through the end of the session (n=9; ±1 SEM). Time course analyses detected a significant effect of dose on pupil diameter (F(2,16) = 58.27, p<.0001). Tukey post-hoc tests indicated that oxycodone alone and oxycodone in combination with quinine significantly decreased pupil diameter relative to quinine alone (p<.01).
Quinine Concentrations in Urine
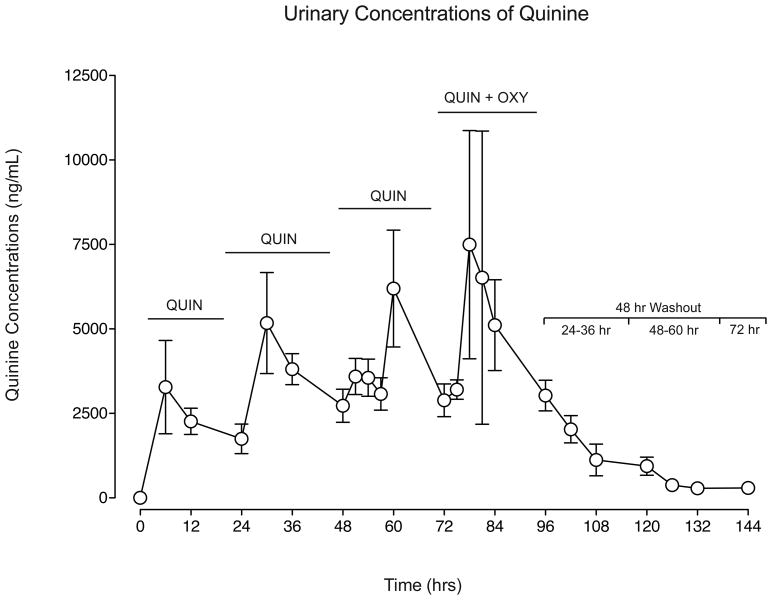
Figure 4 displays mean urinary concentrations of quinine (without creatinine normalization), expressed in ng/mL (±SEM), across quinine dosing: 1) prior to first dose on Day 2 (0 hrs), 2) across Days 2-5 (6-84 hrs) of dosing, and 3) during the washout (Days 6-8; 96-144 hrs). Across the four days of active quinine dosing, urine concentrations increased, with mean and peak concentrations (Cmax) increasing 98% and 141%, respectively, while trough concentrations (Cmin) displayed a 20% increase, suggesting moderate urinary accumulation during this early period of daily dosing (Table 4). Quinine urine concentrations displayed substantial variability on Day 5 (Figure 4) when quinine was administered in combination with oxycodone; however, trough quinine concentration values were similar to those observed on Day 4 (quinine alone) (p>.05), indicating that oxycodone did not alter the profile of quinine in urine.
Figure 4.
Mean urinary concentrations of quinine (ng/mL) displayed across each hour of quinine dosing: prior to the first dose on Day 2 (0 hrs), during the four days of daily quinine administration (Days 2-5: 6-84 hrs), and through the 48-hr washout period (Days 6-8: 96-144 hrs; values below the bracket represent the time relative to last quinine dose). On Days 2-4, quinine was administered alone (QUIN); on Day 5, quinine was administered in combination with oxycodone (QUIN + OXY). Samples were not creatinine normalized. Means (±1 SEM) from 0-96 hrs include n=9; means from 102-144 hrs include n=5.
Table 4.
Concentrations (ng/mL) and elimination rate (μg/h) of quinine in urine.
| Urinary Quinine Concentrations (ng/mL, ± SEM) | ||||
|---|---|---|---|---|
| Observed Range | Mean | Cmax | Cmin | |
|
| ||||
| Acute Dosing (hrs post first dose) | ||||
| Day 2 (0 -12hrs) | 159 – 13,400 | 2,772 (± 668) | 3,974 (±1215) | 1,569 (±239) |
| Day 3 (24-36 hrs) | 771 – 16,300 | 3,577 (± 563) | 5,880 (±1326) | 2,308 (±308) |
| Day 4 (48-60 hrs) | 860 – 17,300 | 3,830 (±406) | 7,518 (±1445) | 1,898 (±252) |
| Day 5 (72-96 hrs) | 993 – 36,800 | 5,011 (±1045) | 9,863 (±3430) | 1,956 (±257) |
| Washout (hrs post last dose) | ||||
| Day 6 (24-36 hrs) | 309 – 5,130 | 2,061 (± 277) | -- | |
| Day 7 (48-60 hrs) | 126 – 2,820 | 618 (±138) | -- | |
| Day 8 (72 hrs) | 240 – 327 | 296 (±16) | --- | --- |
| Urinary Elimination Rate of Quinine (μg/h, ± SEM) | ||||
| Observed Range | Mean | |||
|
|
||||
| Time post last dose | ||||
| Day 5 (0-12 hrs) | 1034 – 92 | 403 (±30) | ||
| Day 6 (24-36 hrs) | 539 – 40 | 167 (±24) | ||
| Day 7 (48-60 hrs) | 175 – 14 | 53 (±9) | ||
| Day 8 (72 hrs) | 48 – 12 | 31 (±6) | ||
Observed ranges and means are displayed above for quinine urine concentrations (ng/mL) and elimination rate (μg/h). Peak (Cmax) and trough (Cmin) values that occurred during active dosing are also displayed. Calculations were conducted on raw, non-modeled data that was not normalized with respect to creatinine concentrations.
During the 48-hr washout phase, urinary concentrations steadily decreased (Figure 4, Table 4), with a urinary half-life of ∼16 hrs. In the first 24 hrs after dosing cessation (Day 6), mean concentrations decreased 59% from those observed on Day 5. At 48-60 hrs post-dose (Day 7), an 88% reduction occurred, while a 94% decrease was observed 72 hrs post-dose (Day 8).
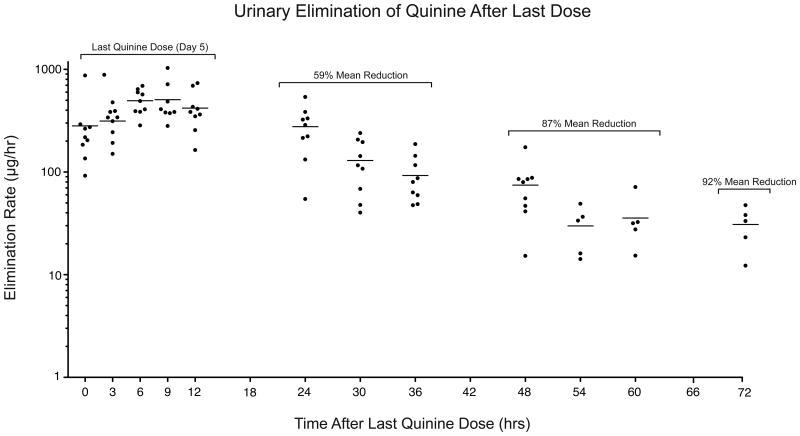
Quinine Elimination Rate
Figure 5 displays urinary elimination of quinine (μg/h) prior to and 72 hrs following the last dose of quinine (Days 5-8); Table 4 presents the observed ranges and mean elimination rates during this period. On the last day of dosing (Day 5, labeled 0-12 hrs; Figure 5), the mean elimination rate was 403 (±30) μg/h. On Day 6 (24-36 hrs), a 59% reduction from the Day 5 elimination rate was observed. This rate continued to decrease, with an 87% reduction occurring the following day (e.g., Day 7, 48-60 hrs post-dose) and, by 72 hrs post-dose, a 92% decrease was observed (mean = 31 (±6) μg/h).
Figure 5.
Urinary elimination rate, expressed as μg/h, as a function of time after the last dose of quinine was administered: Day 5 (0-12 hrs), Day 6 (24-36 hrs), Day 7 (48-60 hrs) and Day 8 (72 hrs). The small filled circles indicate individual subject data and the nested horizontal line represents the mean elimination rate (n=9 for samples occurring 0-48 hrs; n=5 for 54-72 hrs).
Safety Outcome Measures
Quinine did not increase ratings of side effects (e.g., tinnitus, vision/hearing disturbances; p>.05). Baseline and post-dose ECGs did not display any differences (e.g., QT interval) as a function of quinine exposure.
Discussion
This study examined the pharmacokinetic and pharmacodynamic effects of a sub-therapeutic dose of once daily oral quinine to determine its potential as a medication additive for monitoring medication adherence; secondary aims included examination of potential interaction effects of quinine and oral oxycodone, a model medication that shares similar metabolic pathways with quinine.
Quinine was readily detected in plasma by HPLC even with the low dose employed in this study. Quinine displayed a favorable plasma profile, with a half-life of ∼10 hrs, Tmax of ∼2 hrs, and Cmax = 680 ng/mL, consistent with previous reports.30 Trough plasma concentrations increased ∼50% across the first 3 days of dosing, but stabilized thereafter. Similar findings were observed in an extended dosing study (e.g., 80 mg/day, 3 weeks) during which trough serum quinine concentrations were measured every 3-4 days during dosing and no evidence of accumulation was observed.25 Together, these data suggest that steady-state may be reached after 2-3 daily 80 mg doses.
Quinine plasma concentrations were also examined after co-administration of oxycodone and quinine plasma AUC, Cmax and Tmax and t1/2 were unaltered in combination with oxycodone (Figure 1, Table 2). These findings are supported by previous research indicating that potent CYP3A inhibitors (ketoconazole, troleandomycin), but neither moderate inhibitors (e.g., grapefruit juice) nor CYP3A substrates (e.g., oral contraceptives) interfere with quinine metabolism.27,31-32 Correspondingly, the pharmacokinetic profiles (Cmax, Tmax, t1/2) of oxycodone and its CYP3A metabolite, noroxycodone, were similar across conditions (Figure 2, Table 2); however, greater oxycodone and noroxycodone AUC values (+7%, +26%, respectively) were observed in the quinine condition. It is not completely clear why these increases occurred, as it does not appear to be due to quinine inhibition of CYP3A, which would likely cause decreased metabolite concentrations (not increases, as seen here). No clinical data are available on the effects of low-dose quinine on CYP3A substrates/metabolite concentrations and should be further investigated to exclude interaction effects. The observed AUC increases were also not likely due to inhibition of CYP2D, as others have demonstrated that 80 mg of quinine does not alter CYP2D substrate metabolism in healthy humans.33 Although the source of this interaction does not appear to be mediated by hepatic enzyme inhibition, quinine should be investigated further to determine if it could impact metabolism of other test agents. Nonetheless, the AUC increases observed here occurred in the absence of changes in other pharmacokinetic parameters (i.e., no changes in Cmax, Tmax, t1/2), suggesting that quinine did not greatly impact the overall pharmacokinetic profile of oxycodone, which is corroborated by the pharmacodynamic data. Specifically, oxycodone produced several prototypic mu-opioid agonist effects, including increases in ratings of positive drug effects, observer-rated opioid agonist effects and miosis, as previously demonstrated.28 Quinine did not modify any of these effects and did not produce any psychoactive effects when administered alone (Table 3).
Quinine was detected in urine and was present in each post-quinine urine sample (e.g., beginning 6 hrs post-dose). During the first two days of acute dosing, trough urine concentrations (Cmin) displayed a 20% increase (Table 4) but were generally stable over the remainder of active dosing days, with <5% change occurring between the last two days (Table 2). Similarly, others have reported consistent urine concentrations measured every 3-4 days during repeated dosing (80 mg/day, 3 weeks) (e.g., 12-13 mg quinine excreted in 24-hr samples).25 In the current study, mean and Cmax urine concentrations increased ∼30% from Day 4 (quinine alone) to Day 5 (dose combination) and greater variability was observed around Day 5 mean concentrations (Figure 4). Because trough concentrations were similar after reaching steady-state and across repeated dosing observed elsewhere,25 these changes may have been due to the lack of precision from non-creatinine normalized samples or the variability present in a relatively small number of participants (potential limitations for the clinical applicability of these results); however, these data do not suggest that oxycodone substantially modified quinine excretion in urine, although this should be explored in future studies.
During washout, quinine urinary concentrations (ng/mL) steadily decreased, with a 94% decrease occurring 72 hrs after the final dose (Day 8), indicating fairly rapid excretion in urine. Steep decreases occurred within each 24-hr washout period as follows: 70% from Day 6 (24-36 hrs) to Day 7 (48-60 hrs), and 52% from Day 7 to Day 8 (72 hrs). Elimination rates (μg/h) followed the same pattern, with an overall decrease of 92% (Day 5 to Day 8), with 68% and 42% decreases in the 24-hr washout periods. Although quinine is excreted rather quickly, this study did not observe urine concentrations reaching the LLOQ (20 ng/mL). One exploratory study (n=2) examined urinary excretion of quinine (35 mg) for 24 hrs (n=1) and up to 190 hr (n=1).34 At 24 hrs post-dose, quinine concentrations decreased 68%-80% relative to Cmax. While significant declines were observed at ∼72 hrs and 100 hrs post dose (n=1) (decreasing 90% and 99%), low concentrations were still detected 190 hrs post dose. While additional research is needed to establish the full window of detection in urine, these data indicate that within 3-4 days urinary quinine concentrations decrease substantially (92-99%) and suggest that standardized cut-off values can be developed to determine recent quinine administration and omission. Although this study cannot determine those cut-offs, concentrations of 240-327 ng/mL were observed 72 hrs post-dose (n=5), and White and colleagues34 observed 146-410 ng/mL at 70-75 hrs after a 35 mg dose (n=1), indicating that concentrations below ∼500 ng/mL could indicate 1-2 missed daily doses. However, additional studies should be conducted to determine validated cut-off values for potential assay development of point-of-care immuno- or fluorescence-assays to monitor adherence with quinine.
An additional objective was to determine the safety of quinine under repeated dosing conditions and when combined with oxycodone. Quinine, available in 324 mg capsules and typically administered at ∼1950 mg/day for malaria, can produce serious health risks, including idiopathic thrombocytopenic purpura (ITP), temporary loss of vision or hearing, and hypoglycemia,35 prompting the FDA to warn against off-label prescribing for leg cramps and suggest removal of over-the-counter products containing quinine from the U.S. market. However, the FDA has permitted low doses of quinine (≤ 83 mg/L) to be added to tonic water and bitter lemon drinks, due to the minimal risk of lower doses. Here, repeated doses of 80 mg of quinine did not produce any signal of quinine-specific complaints (e.g., tinnitus, changes in color vision or hearing/vision acuity) or physiological changes (e.g., heart rate, blood pressure, ECG activity). Further, quinine did not alter the safety profile of oxycodone (e.g., no changes in respiration rate). Although some have reported transient nystagmus after daily quinine (105 mg/day, 2 weeks),36 others have reported no detectable cardiovascular or auditory/ophthalmologic effects of 100-120 mg daily doses.25,37 Overall, the current data, along with other safety reports, indicate that low doses of quinine (≤80 mg) are safe and well-tolerated in healthy populations.
Another consideration for choice of an adherence marker is the prevalence of outside sources of the drug (e.g., dietary sources) and their potential interference with testing. Although the maximum quinine concentration permitted in liquids in the United States is 83 mg/L, commercially available tonic water often contains less than this maximum: 59-68 mg/L34,38 or 1.75 - 2.0 mg/fl oz. Because relatively small volumes of tonic water are consumed in cocktails (e.g., 2-4 oz.), total quinine exposure is minimal (e.g., 4-8 mg/cocktail). However, if immunoassays are developed to detect quinine in urine for adherence testing, urinary cut-off concentrations should account for dietary exposure so that low concentrations do not produce a false positive result (e.g., a result that suggests medication adherence after dietary quinine exposure only). Exposure could also occur through illicit drug use, as quinine has been reported as an additive/cutting agent in street heroin;39 however, the risks of intravenous administration of quinine-laced heroin include serious cardiac complications40 and ITP.41 Therefore, quinine would not be an appropriate adherence marker for individuals who heavily consume tonic water (e.g., those who self-treat leg cramps with quinine), those who abuse heroin, or those at risk of injecting the medication for which adherence is being monitored.
Taken together, these data indicate that low-dose quinine should be explored further as a medication adherence tracer, as it 1) is safe, non-toxic and seemingly inert at low oral doses, 2) has high oral bioavailability, 3) is easily detected in plasma and urine using standard laboratory techniques, 4) displays a plasma half-life of ∼10 hrs, suitable to detect adherence for a variety of medication regimens, 5) displays generally linear pharmacokinetics, with steady-state plasma concentrations occurring with three consecutive doses, 6) does not produce any pharmacodynamic interactions when co-administered with a model hepatically-metabolized drug, 7) produces consistent, steady-state urine concentrations within the first 2-3 days of dosing, 8) is rapidly excreted in urine, with a ∼94% reduction in urine concentrations within 72 hrs following dosing cessation, and 9) exhibits a urinary elimination half-life of ∼16 hrs. However, additional research should be conducted to explore potential pharmacokinetic interactions between quinine and other model drugs to exclude clinically significant effects. Thus, overall, quinine appears to be a good candidate for further development as a tracer drug and should be explored as a potential tool to monitor drug adherence and detect several missed doses of medication in both research and clinical settings.
Acknowledgments
Grants from the National Institute on Drug Abuse (R01DA016718-08S1 [SLW]) and the National Center for Research Resources and National Center for Advancing of Translational Sciences (UL1TR000117-04 [UK CTSA]; KL2TR000116-04 [SB]) provided support for this research. We thank the staff at the University of Kentucky (UK) Center on Drug and Alcohol Research for research support: Stacy Conley, RN, Victoria Vessels, Tasia York, and John Beninato; Leta Hommel, Ken Westberry of the UK CCTS Laboratory for assistance with specimens; the UK Investigational Pharmacy for preparing study medication, UK CCTS Inpatient Unit nursing staff for patient care, especially Lisa Chamblin, RN for exceptional phlebotomy; and Dr. Samy-Claude Elayi (UK Department of Cardiology, Gill Heart Institute) for patient support.
We also thank staff at the University of California San Francisco Drug Studies Unit for assay development and sample analyses: Dr. Young Huang, Winnie Gee, Shirley Yee and Karen Kuncze; and Dr. Nora Chiang and Dr. Philip Krieter at the National Institute on Drug Abuse, Division of Pharmacotherapies and Medical Consequences of Drug Abuse, Chemistry and Pharmaceuticals Branch, for pharmacokinetic expertise and support.
Footnotes
Author Contributions: Shanna Babalonis, Aidan Hampson and Sharon Walsh were responsible for the study concept and design. Shanna Babalonis directly supervised the conduct of the study, interviewed and consented the participants, directed the statistical analyses and wrote the manuscript. Michelle Lofwall conducted medical interviews and physical examinations, reviewed laboratory results, provided medical coverage and edited the manuscript. Paul Nuzzo trained the staff, provided technical support services, and supervised daily operations. Paul Nuzzo and Aidan Hampson conducted data analyses. Sharon Walsh supervised the conduct of the study, statistical analyses and interpretation of the data. Sharon Walsh, Aidan Hampson, Michelle Lofwall and Paul Nuzzo provided critical revision of the manuscript for important intellectual content. All authors critically reviewed content and approved the final version for publication.
References
- 1.Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001;23(8):1296–1310. doi: 10.1016/s0149-2918(01)80109-0. [DOI] [PubMed] [Google Scholar]
- 2.Lieber SR, Helcer J, Shemesh E. Monitoring drug adherence. Transplant Rev (Orlando) 2014 doi: 10.1016/j.trre.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blaschke TF, Osterberg L, Vrijens B, Urquhart J. Adherence to medications: insights arising from studies on the unreliable link between prescribed and actual drug dosing histories. Annu Rev Pharmacol Toxicol. 2012;52:275–301. doi: 10.1146/annurev-pharmtox-011711-113247. [DOI] [PubMed] [Google Scholar]
- 4.IMS Health. Avoidable Costs in U.S. Health Care: The $200 Billion Opportunity from Using Medicines More Responsibly. IMS Insitute for Healthcare Informatics. 2013:1–62. [Google Scholar]
- 5.DiMatteo MR. Variations in patients' adherence to medical recommendations: a quantitative review of 50 years of research. Med Care. 2004;42(3):200–209. doi: 10.1097/01.mlr.0000114908.90348.f9. [DOI] [PubMed] [Google Scholar]
- 6.Swift R, Oslin DW, Alexander M, Forman R. Adherence monitoring in naltrexone pharmacotherapy trials: a systematic review. J Stud Alcohol Drugs. 2011;72(6):1012–1018. doi: 10.15288/jsad.2011.72.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiss RD. Adherence to pharmacotherapy in patients with alcohol and opioid dependence. Addiction. 2004;99(11):1382–1392. doi: 10.1111/j.1360-0443.2004.00884.x. [DOI] [PubMed] [Google Scholar]
- 8.Anderson AL, Li SH, Biswas K, et al. Modafinil for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2012;120(1-3):135–141. doi: 10.1016/j.drugalcdep.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Insull W., Jr Evaluation and recommendations for adherence marker development by working groups of the workshop. Control Clin Trials. 1984;5(4 Suppl):579–583. doi: 10.1016/0197-2456(84)90022-9. [DOI] [PubMed] [Google Scholar]
- 10.Insull W., Jr Statement of the problem and pharmacological and clinical requirements for the ideal marker. Control Clin Trials. 1984;5(4 Suppl):459–462. doi: 10.1016/0197-2456(84)90003-5. [DOI] [PubMed] [Google Scholar]
- 11.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 12.McDonald HP, Garg AX, Haynes RB. Interventions to enhance patient adherence to medication prescriptions: scientific review. JAMA. 2002;288(22):2868–2879. doi: 10.1001/jama.288.22.2868. [DOI] [PubMed] [Google Scholar]
- 13.Granger BB, Swedberg K, Ekman I, et al. Adherence to candesartan and placebo and outcomes in chronic heart failure in the CHAEM prgramme: double-blind, randomized, controlled clinical trial. Lancet. 2005;366(9502):2005–11. doi: 10.1016/S0140-6736(05)67760-4. [DOI] [PubMed] [Google Scholar]
- 14.Wolff K, Hay AA, Raistrick D, Feely M. Use of ‘very low-dose phenobarbital’ to investigate compliance in patients on reducing doses of methadone (detoxification) J Subst Abuse Treat. 1993;10(5):453–458. doi: 10.1016/0740-5472(93)90006-n. [DOI] [PubMed] [Google Scholar]
- 15.Insull W., Jr Appendix: List of Actual and potential adherence markers and devices. Control Clin Trials. 1984;5:584–587. [Google Scholar]
- 16.Babiker IE, Cooke PR, Gillett MG. How useful is riboflavin as a tracer of medication compliance? J Behav Med. 1989;12(1):25–38. doi: 10.1007/BF00844747. [DOI] [PubMed] [Google Scholar]
- 17.Herron AJ, Mariani JJ, Pavlicova M, et al. Assessment of riboflavin as a tracer substance: comparison of a qualitative to a quantitative method of riboflavin measurement. Drug Alcohol Depend. 2013;128(1-2):77–82. doi: 10.1016/j.drugalcdep.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramanujam VM, Anderson KE, Grady JJ, Nayeem F, Lu LJ. Riboflavin as an oral tracer for monitoring compliance in clinical research. Open Biomark J. 2011;2011(4):1–7. doi: 10.2174/1875318301104010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ryu YW, Kim ES, Song CS. Riboflavin and thiamine absorption. Yonsei Med J. 1968;9(1):11–13. doi: 10.3349/ymj.1968.9.1.11. [DOI] [PubMed] [Google Scholar]
- 20.Qualaquin® Package Insert. AR Scientific Inc./URL Pharma; Philadelphia, PA: 2009. [Google Scholar]
- 21.U.S. Department of Health and Human Services, U.S. Food and Drug Administration. Code of Federal Regulations, 21 CFR 172.575. 2014 [Google Scholar]
- 22.Salako LA, Sowunmi A. Disposition of quinine in plasma, red blood cells and saliva after oral and intravenous administration to healthy adult Africans. Eur J Clin Pharmacol. 1992;42(2):171–174. doi: 10.1007/BF00278479. [DOI] [PubMed] [Google Scholar]
- 23.Paintaud G, Alvan G, Ericsson O. The reproducibility of quinine bioavailability. Br J Clin Pharmacol. 1993;35(3):305–307. doi: 10.1111/j.1365-2125.1993.tb05698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wanwimolruk S, Kaewvichit S, Tanthayaphinant O, Suwannarach C, Oranratnachai A. Lack of effect of oral contraceptive use on the pharmacokinetics of quinine. Br J Clin Pharmacol. 1991;31(2):179–181. doi: 10.1111/j.1365-2125.1991.tb05509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drewitt PN, Butterworth KR, Springall CD, Walters DG, Raglan EM. Toxicity threshold of quinine hydrochloride following low-level repeated dosing in healthy volunteers. Food Chem Toxicol. 1993;31(4):235–245. doi: 10.1016/0278-6915(93)90073-8. [DOI] [PubMed] [Google Scholar]
- 26.Steiner E, Dumont E, Spina E, Dahlqvist R. Inhibition of desipramine 2-hydroxylation by quinidine and quinine. Clin Pharmacol Ther. 1988;43(5):577–581. doi: 10.1038/clpt.1988.76. [DOI] [PubMed] [Google Scholar]
- 27.Mirghani RA, Ericsson O, Tybring G, Gustafsson LL, Bertilsson L. Quinine 3-hydroxylation as a biomarker reaction for the activity of CYP3A4 in man. Eur J Clin Pharmacol. 2003;59(1):23–28. doi: 10.1007/s00228-003-0575-5. [DOI] [PubMed] [Google Scholar]
- 28.Walsh SL, Nuzzo PA, Lofwall MR, Holtman JR., Jr The relative abuse liability of oral oxycodone, hydrocodone and hydromorphone assessed in prescription opioid abusers. Drug Alcohol Depend. 2008;98(3):191–202. doi: 10.1016/j.drugalcdep.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fraser HF, Van Horn GD, Martin WR, Wolbach AB, Isbell H. Methods for evaluating addiction liability. (A) “Attitude” of opiate addicts toward opiate-like drugs. (B) a short-term “direct” addiction test. J Pharmacol Exp Ther. 1961;133:371–387. [PubMed] [Google Scholar]
- 30.Sowunmi A, Salako LA. Effect of dose size on the pharmacokinetics of orally administered quinine. Eur J Clin Pharmacol. 1996;49(5):383–386. doi: 10.1007/BF00203782. [DOI] [PubMed] [Google Scholar]
- 31.Ho PC, Chalcroft SC, Coville PF, Wanwimolruk S. Grapefruit juice has no effect on quinine pharmacokinetics. Eur J Clin Pharmacol. 1999;55(5):393–398. doi: 10.1007/s002280050646. [DOI] [PubMed] [Google Scholar]
- 32.Wanwimolruk S, Paine MF, Pusek SN, Watkins PB. Is quinine a suitable probe to assess the hepatic drug-metabolizing enzyme CYP3A4? Br J Clin Pharmacol. 2002;54(6):643–651. doi: 10.1046/j.1365-2125.2002.01687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Donovan JL, DeVane CL, Boulton D, Dodd S, Markowitz JS. Dietary levels of quinine in tonic water do not inhibit CYP2D6 in vivo. Food Chem Toxicol. 2003;41:1199–1201. doi: 10.1016/s0278-6915(03)00112-1. [DOI] [PubMed] [Google Scholar]
- 34.White VL, Canfield DV, Hordinsky JR. Elimination of quinine in two subjects after ingestion of tonic water: an exploratory study. U S Department of Transportation, Federal Aviation Administration, Commissioned Report. 1994 [Google Scholar]
- 35.Taylor WR, White NJ. Antimalarial drug toxicity: a review. Drug Safe. 2004;27(1):25–61. doi: 10.2165/00002018-200427010-00003. [DOI] [PubMed] [Google Scholar]
- 36.Zajtchuk JT, Mihail R, Jewell JS, Dunne MJ, Chadwick SG. Electronystagmographic findings in long-term low-dose quinine ingestion. A preliminary report. Arch Otolaryngol. 1984;110(12):788–791. doi: 10.1001/archotol.1984.00800380018006. [DOI] [PubMed] [Google Scholar]
- 37.Worden AN, Frape DL, Shephard NW. Consumption of quinine hydrochloride in tonic water. Lancet. 1987;1(8527):271–272. doi: 10.1016/s0140-6736(87)90087-0. [DOI] [PubMed] [Google Scholar]
- 38.Samanidou VF, Evaggelopoulou EN, Papadoyannis IN. Simple and rapid HPLC method for determination of quinine in soft drinks using fluorescence detection. J Liq Chromatogr Relat Technol. 2004;27(15):2397–2406. [Google Scholar]
- 39.Swift RM, Griffiths W, Cammera P. False positive urine drug screens from quinine in tonic water. Addict Behav. 1989;14(2):213–215. doi: 10.1016/0306-4603(89)90051-8. [DOI] [PubMed] [Google Scholar]
- 40.Phillips KA, Hirsch GA, Epstein DH, Preston KL. Cardiac complications of unwitting co-injection of quinine/quinidine with heroin in an intravenous drug user. J Gen Intern Med. 2012;27(12):1722–1725. doi: 10.1007/s11606-012-2089-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Christie DJ, Walker RH, Kolins MD, Wilner FM, Aster RH. Quinine-induced thrombocytopenia following intravenous use of heroin. Arch Intern Med. 1983;143(6):1174–1175. [PubMed] [Google Scholar]