Abstract
Obesity is one of the most pervasive and costly public-health problems. Clinicians need effective tools to address weight management in primary care, including evaluation and communication methods, guideline-based weight-management interventions, and safe and effective weight-loss medications and surgery. The objective of this Grand-Rounds presentation is to provide practicing clinicians with the latest information regarding effective ways to care for and communicate with patients about weight loss; evidence-based guidelines for selecting weight-management therapies; and safety, efficacy, and adverse effects of weight-loss medications and surgery.
Keywords: obesity, primary care, patient-centered communication, weight-loss drugs, weight-loss surgery
INTRODUCTION
Overweight, including obesity, is one of the most pervasive and costly public-health problems. Two in three adults in the United States (US) are overweight, including one in three who is obese.1,2 Physicians now care for patients with many weight-related problems that could be improved by a modicum of weight loss; however, physicians need guidance regarding how to provide high-quality, effective weight management in primary care. The objective of this Grand-Rounds presentation is to provide practicing clinicians with the latest information regarding effective ways to care for and communicate with patients about weight loss; evidence-based US guidelines for selecting weight-management therapies; and safety, efficacy, and adverse effects of weight-loss medications and surgery using evidence-based recommendations from the Guidelines (2013) for Managing Overweight and Obesity in Adults,3 recently released by the Obesity Society (TOS), American College of Cardiology (ACC), and American Heart Association (AHA); National Heart, Lung, and Blood Institute (NHLBI)-sponsored obesity guidelines4; and the 2008 Physical Activity Guidelines for Americans.5
Assessment
Identification
Identifying patients who are overweight is the first step in determining whether weight management is needed. Body mass index (BMI) is the recommended and most practical office-based tool to identify overweight and monitor patients over time.3 Individuals with a BMI of 25-<30 kg/m2 are considered overweight, and those with a BMI ≥ 30 kg/m2, obese. Most electronic medical records auto-calculate BMI, and many plot weight trajectory, which can be used to engage patients when communicating about weight management. An additional tool is waist circumference, which is measured at the level of the iliac crest and indicates increased cardiovascular-disease and health risks. Measuring waist circumference is particularly useful for patients who are overweight but otherwise “well.” A waist circumference ≥ 88 cm (35 inches) for women and ≥ 102 cm (40 in) for men reflects excessive abdominal fat and greater weight-related health risks than a patient with a normal waist circumference; weight management, therefore, may be particularly important for these high-risk patients.3,6,7
Obesity-Focused History and Evaluation
After identifying patients who are overweight, an obesity-focused history is critical for developing a tailored weight-management plan. Factors that impact weight-management treatment decisions include life circumstances contributing to weight gain/loss (for example, receiving supplemental nutrition assistance, working night shifts, pregnancy, or marital changes), physical and mental-health issues, prior weight-loss attempts, drug-induced weight gain, current dietary and activity habits, and readiness to make lifestyle changes.8 It is important to identify the presence and extent of alcohol or tobacco use and any disordered eating behaviors, such as binge eating, bulimia, or night eating.9 Identification of an alcohol addiction, active eating disorder, or significant mental health issue may warrant referral to a mental-health professional.10
To identify and communicate with patients regarding obesity-related health risks, clinicians can elicit an obesity-focused review of systems, physical exam, and laboratory evaluation. For example, a sleep history can help determine risk for sleep apnea, and particularly should be sought in patients who have a history of atrial fibrillation, thrombosis, pulmonary hypertension, or diastolic heart failure; an elevated blood-pressure reading or acanthosis nigricans can be used to communicate risk for hypertension and diabetes; and identifying and communicating with patients regarding early-stage diseases, such as pre-hypertension and prediabetes, can be used to partner with patients on preventing disease progression or medication initiation by using a weight-management intervention. Although there are no obesity-specific recommended laboratory screenings, current guidelines recommend universal lipid screening and screening for diabetes in overweight patients (and Asians with a BMI ≥ 23 kg/m2).11 Screening for fatty liver might also be considered, particularly when caring for male and Latino patients.12 Because obesity increases risk of post-menopausal breast and endometrial cancer, it is important to ensure women receive mammograms at recommended intervals and ask about post-menopausal vaginal bleeding, which might warrant referral to gynecology.13,14 Considerations regarding the conduct of and challenges inherent in the physical examination of patients with obesity previously have been published.15
Assessing Benefits of Weight Management
After identifying obesity-related health risks, clinicians and patients can determine potential benefits of weight management and develop non-weight-based goals for weight-management intervention—discussing improvement in weight-related conditions can aid realistic goal-setting that impacts health, particularly because patients may have larger goals for weight loss than are attainable. A 5–10% weight reduction can improve quality of life, reduce pain, and improve cardiovascular disease risk factors such as blood pressure, cholesterol, and blood sugar.16 Dietary changes, even without weight loss, can prevent progression from prediabetes to diabetes16; and normalizing glucose regulation reduces cardiovascular risk.17 A less-known weight-related complication that improves with weight loss is kidney disease. Being overweight or obese at the age of 17 is associated with an eight-fold increased risk of developing end-stage renal disease.18 A major cause is obesity-related glomerulopathy, which is independent of diabetes and hypertension, and improves with weight loss.19,20 A host of other conditions also improve with weight loss, including atrial fibrillation,21 fatty liver disease,22 polycystic ovarian syndrome,23 urinary incontinence,24 and erectile dysfunction.25 Thus, the benefits of weight loss underscore the critical importance of addressing weight management.
Treatment
Tools physicians can use to help patients lose weight include: patient-centered communication, tailoring medications that impact weight, behavior modification, weight-loss medications, and surgery.
Communicating with Patients about Weight Management
Physicians may be maximally effective in supporting weight management long-term by using patient-centered terms to describe excess weight (so that patients do not feel judged26) and sharing decision-making with patients. A patient-centered way to open a conversation about weight would be to frame the conversation around health, not size. For example, “I noted that your weight has been going up, and I’m concerned about the impact of this on your health.” Shared decision-making includes allowing patients to choose the behaviors, goals, and treatments that they consider important.27 Supporting patients in choosing and sustaining lifestyle changes is made easier by communication methods that facilitate conversations about change, including using patient-centered rather than doctor-centered communication (Table 1).27,28 Fundamental to patient-centered communication is spending time relationship-building, and there is evidence for its efficacy in weight management.29–31 In this style of communication, physicians elicit patients’ health needs, beliefs, and expectations, and engage patients in making decisions about their care.27
Table 1.
Differences Between Patient-Centered and Doctor-Centered Communication During Behavioral Weight-Loss Counseling
| Patient-Centered Communication | Doctor-Centered Communication |
|---|---|
|
| |
Physician:
|
Physician:
|
Motivational interviewing (MI) is one type of patient-centered communication that is used to address behavior change with an ambivalent patient.28 For effective use of MI, four skills are needed.17 The first skill is engaging the patient in non-medical conversation before eliciting their medical questions. The second is focusing the visit on a specific change. For example, after asking about the patient’s concerns, reflect back their concerns, and then ask permission to address weight changes: “I hear you’re concerned about knee pain, correct? Let’s talk about that; and, would it be okay to discuss your weight too? I noticed it’s been going up. I’d like to hear your thoughts about why that might be.” The third skill is evoking from the patient their own good reasons to change. The physician listens for evidence of some intent to change, then responds with reflective-listening statements to evoke further discussion of behavior change. The fourth skill is planning change. If the patient is not ready to make changes, then the plan is to follow-up at the next visit. If the patient is ready, one might ask, “If, as part of our plan to help your knee pain, you decide to work on getting to a healthier weight, what might be a first step?” If a patient replies that they do not know, one might respond, “May I offer some advice based on my experience? There are some options that you have. You could start tracking your diet and activity; try a weight-loss diet; or, if you already have tried these, there are weight-loss medicines or surgery. What makes the most sense to you?” In other words, give information that could be absorbed as advice, and let the patient choose. Patient-centered communication, including use of MI, may activate patients by helping them identify their weight status, risk/presence of related disease, and treatments that can promote a healthier future.
Medication Management
When communicating with patients regarding weight management, it is helpful to identify medications that cause weight gain, and switch patients to drugs that cause weight loss or are more weight neutral (Table 2). For example, in patients with migraines, whereas the migraine-prophylaxis drug propranolol (trade name Inderal) causes weight gain, topiramate (trade name Topamax) reduces migraine frequency and causes weight loss, even at dosages below those recommended for migraine treatment (for example 25–50 mg bid instead of 100–200 mg bid); however, topiramate is teratogenic (category X in pregnancy).32 For diabetes, whereas insulin, sulfonylureas, thiazolidinediones, and meglitinides cause weight gain, excellent alternative treatments are available that cause weight loss or are more weight neutral (Table 3). For patients who require insulin, consider using long-acting insulin, which is associated with less weight gain than rapid- or intermediate-acting insulins.33–34 Actively reviewing and managing medications that impact weight is an important part of weight management; however, off-label use of medications approved for other indications solely for the purpose of weight loss is not recommended.35
Table 2.
Drugs that Cause Weight Gain and More Weight-Neutral Alternatives
| Drugs that Cause Weight Gain | More Weight-Neutral Alternatives | |
|---|---|---|
|
| ||
| Migraine drugs: |
|
|
|
| ||
| Psychiatric disorders | ||
|
| ||
| Tri-cyclic:a |
|
|
|
| ||
| SSRI: |
|
|
|
| ||
| Other/atypical |
|
|
| Antipsychotics |
|
|
| Atypical: |
|
|
| Conventional: |
|
|
|
| ||
| Lithium: |
|
|
|
| ||
| Anticonvulsants |
|
|
|
| ||
| Beta Blockers: |
|
|
Abbreviations: CR = continuous release, ER = extended release, ACE = angiotensin-converting enzyme inhibitor, ARB = angiotensin-receptor blocker
Tertiary tricyclic antidepressants (such as amitriptyline, imipramine, and doxepin) may cause greater weight gain than secondary tricyclics (such as nortriptyline and desipramine).72
Table 3.
Diabetes Drugs that Cause Weight Gain and More Weight-Neutral Alternatives
| Diabetes Drugs that Cause Weight Gain | More Weight-Neutral Alternatives | ||
|---|---|---|---|
| Insulin |
|
Biguanides |
|
| Sulfonylureas |
|
SGLT2 inhibitors |
|
| Thiazolidinediones |
|
Incretins/GLP-1 agonists |
|
| Meglitinides |
|
Alpha-glucosidase inhibitor |
|
| DPP4 inhibitors |
|
||
Abbreviations: SGLT2 = sodium-glucose cotransporter 2, GLP-1 = glucagon-like peptide-1, DPP4 = dipeptidyl peptidase 4
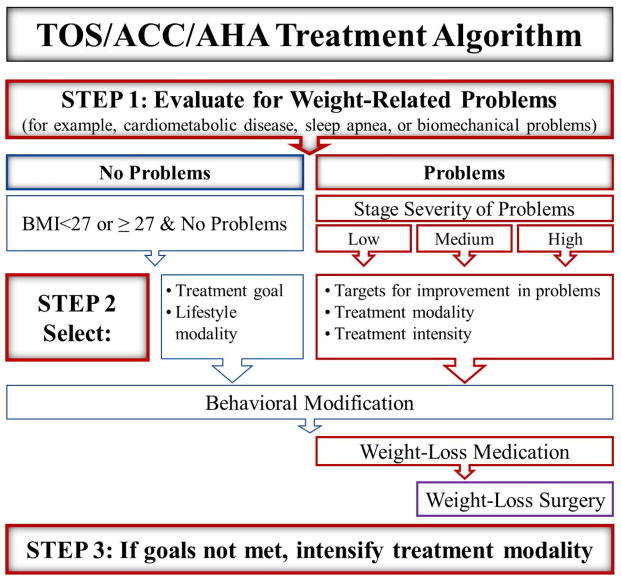
The 2013 Guidelines for the Management of Overweight and Obesity in Adults outlines when to use behavior modification, weight-loss medications, and surgery (Figure 1).3 The algorithm consists of three steps:
Figure 1.
-
Evidence-based weight-management guidelines, released by TOS, ACC, and AHA, include guidance regarding appropriate use of behavior modification, weight-loss medications, and surgery, based upon the patient’s severity of overweight and presence of weight-related comorbidities.Abbreviations: TOS = The Obesity Society, ACC = American College of Cardiology AHA = American Heart Association, BMI = body mass index
Step 1—determine the patient’s BMI, presence of any cardiometabolic or biomechanical problems, and their severity. For patients who are overweight—including those with a BMI <27 kg/m2 or BMI ≥ 27–<30 kg/m2 without weight-related problems, only behavior modification is indicated.
-
Step 2—treatment goals, modality, and intensity are selected based on BMI and severity of weight-related problems:
For all patients, tailor medications that could be contributing to weight gain (Tables 2 and 3), and address behavior modification.
For patients with a BMI ≥ 27 kg/m2 with weight-related problems, in addition to behavioral changes, FDA-approved weight-loss medications should be considered, as should surgery, for patients who have a BMI ≥ 35 kg/m2 with weight-related problems or ≥ 40 kg/m2.
Select weight-loss treatment intensity based on severity of weight-related problems; and
Step 3—reevaluate whether goals for weight-loss and comorbidity improvement have been met, and intensify lifestyle, medication, or surgical weight-loss treatments as needed.
Behavior modification
The cornerstone of weight management in the Guidelines (2013) for Managing Overweight and Obesity in Adults is comprehensive behavior modification with frequent follow-up. The goal of behavior modification is to modify diet and lifestyle behaviors to create negative energy balance. Regarding diet, reducing caloric intake is recommended using one of three approaches: 1) 1,200–1,500 kcal/day for women and 1,500–1,800 kcal/day for men, adjusting as needed; 2) prescribe a 500–750 kcal/day energy deficit; or 3) prescribe an evidence-based diet that restricts certain types of foods (for example, high-carbohydrate, high-fat, or low-fiber foods) to create an energy deficit. Recommendations for physical activity include working up to a goal of moderate-to-vigorous physical activity (for example, brisk walking) 30 minutes/day most days of the week (>150 minutes/week).5 Exercise, particularly when performed at higher-intensity levels and combined with dietary changes, can promote weight loss; even when no weight is lost, exercise improves cardiovascular disease risk factors.36 Recommended behavioral techniques include self-monitoring weight, diet, and activity; removing highly palatable high-calorie foods and drinks from one’s environment; and addressing triggers to unhealthy eating behaviors, such as sleep deprivation, time constraints, and stress.3 Although not discussed in the Guidelines, practitioners (or ancillary staff) might help patients who have smart phones download and use mobile applications to track diet, activity, sleep, and goals. The follow-up recommended in the Guidelines is in-person meetings more than twice a month in the first six months (≥14 meetings in the first six months) delivered by a trained interventionist. In places that lack access to clinics specializing in obesity treatment or that cannot offer this frequency of follow-up, second-line alternatives include commercial and remotely-delivered (for example, by telephone or internet) programs.
Safety, Efficacy, and Adverse Effects of Weight-Loss Medications
Weight-loss drugs that are FDA-approved for adults as adjuncts to a reduced-calorie diet and increased physical activity include orlistat (Xenical, Alli), lorcaserin (Belviq), phentermine/topiramate extended release (Qsymia), naltrexone/bupropion sustained release (Contrave), liraglutide (Saxenda), and the sympathomimetic amines, diethylpropion (Tenuate, Tenuate Dospan), phentermine (Adipex-P, Suprenza), benzphetamine (Didrex), and phendimetrazine (Bontril, Bontril extended release).3,35,37–46 Lisdexamfetamine (Vyvanse) is approved for binge-eating disorder, not weight loss, and will not be reviewed here.47 To be eligible for a weight-loss medication, one must have failed to achieve weight-loss goals by lifestyle alone, have a BMI of ≥ 30 kg/m2, or ≥ 27 kg/m2 with at least one weight-related disease, and not be pregnant, seeking pregnancy, or nursing.3 Measure success by seeing weight loss of one or more pounds per week in the first month, loss of >5% body weight by 3–6 months, and improvement in baseline risk factors.
Orlistat, by inhibiting lipase, causes fecal-fat excretion and malabsorption of 25–30% of calories from fat.37,39–40 Benefits include: FDA-approval as safe for long-term use39; placebo-adjusted weight loss of 3% of body weight (7–8% total) after up to four years of use37; and risk-factor improvement, including reducing the conversion of impaired-glucose tolerance to diabetes by 40%, reducing LDL cholesterol 5–10%, and improving blood pressure and hemoglobin A1C.40 Despite its efficacy and safety, however, orlistat’s limitations include delayed time to effect, side effects, and cost: modest weight loss occurs over an extended period of time, and patients seeking weight loss want rapid results; up to a third of people report borborygmi, cramps, flatus, or anal discharge/incontinence; orlistat causes malabsorption of some drugs and fat-soluble vitamins—patients taking orlistat should be advised to take a multivitamin, and patients on warfarin need closer INR monitoring; and the drug is expensive, costing $45–175 per month. Orlistat should not be used in patients with a history of malabsorption, cholecystectomy, or who are taking cyclosporine or amiodarone (due to impaired drug absorption).39
Lorcaserin, like orlistat, is approved for long-term use, and lorcaserin and orlistat’s efficacy are comparable. Lorcaserin increases satiety through selective serotonin 2C-receptor agonism.41 Benefits include placebo-adjusted weight loss of 3–4 kg at 1 year; those on drug (vs. placebo) are twice as likely to lose 5–10% of their body weight (25% lose ≥ 10% and 50% lose ≥ 5%); and risk-factor improvement (in blood pressure, heart rate, total and LDL cholesterol, C-reactive protein, fasting blood glucose, and insulin).41 Disadvantages of lorcaserin are: side effects, though typically mild, include headache (18% drug vs. 11% placebo) and nausea42; and expense—a usual dose, 10 mg twice daily, costs $200 per month without insurance coverage, although patients may be able to get a reduced price ($70–75/month in 2015) through manufacturer discount programs. Contraindications include use of drugs that alter serotonin or dopamine metabolism (SSRIs, bupropion, lithium, antipsychotics, tricyclic antidepressants, MAOIs, tramadol, triptans) or CYP 2D6-substrate drugs, valvular heart disease, and a glomerular filtration rate (GFR) <30.42
Phentermine/topiramate extended release (ER), trade name Qsymia, is a combination of the anorectic agent phentermine and the seizure medication topiramate in an extended-release formulation—separately, neither drug has an extended-release form, and must be taken at a considerably higher dose to be effective for weight loss (typical weight-loss dosing for phentermine is 37.5 mg, and for topiramate, 50–100 mg twice a day).37 Available dosage combinations of phentermine and topiramate are 7.5/26 mg and 15/92 mg, respectively.43 Benefits include: placebo-adjusted weight loss of approximately 10% of initial body weight, equal to 8–9 kg or 20 lb. at one year44; and risk-factor improvement, including an almost 80% reduction in conversion from impaired glucose tolerance to diabetes, increased HDL, and reductions in hemoglobin A1C, triglycerides, LDL-cholesterol, and blood pressure (however, it can lead to an increase in heart rate of about one beat per minute).45 Disadvantages of phentermine/topiramate extended release are: side effects (although less than for either drug alone because of lower doses and the extended-release formulation), including both those related to the sympathomimetic phentermine (e.g., an increase in heart rate) and topiramate’s dose-related impact on cognition (e.g., word-finding problems, mental dulling) and depression43; and expense—a prescription costs $160 per month out-of-pocket, although manufacturer discounts may be available. Caution is recommended if a patient has insulin-requiring diabetes or hypertension (the phentermine component may worsen hypertension or cause hypoglycemia). Contraindications include pregnancy—topiramate is teratogenic (and, as part of a Risk Evaluation and Mitigation Strategy, the FDA requires prescribing physicians to complete a healthcare provider training program and the drug can only be dispensed through certified pharmacies)—glaucoma, hyperthyroidism, cardiovascular disease, uncontrolled hypertension, and use of MAOIs or stimulants.43
Naltrexone/bupropion sustained release (SR), trade name Contrave, is a combination of the opioid antagonist naltrexone and the dopamine-reuptake inhibitor bupropion in a sustained-release pill.37 Benefits of a 32 mg/360 mg dose combination include: placebo-adjusted weight loss of up to 6% of body weight and 6 kg or 13 lb. at one year, >60% on drug lose ≥ 5%, and more than one-third lose ≥ 10%46,48–50; and improved ability to control eating behavior and response to food cravings. Disadvantages of naltrexone/bupropion SR include side effects, such as nausea, headache, dizziness, constipation, and dry mouth; possible elevation in blood pressure or heart rate, especially during the first three months of use (patients with hypertension should be monitored closely)46,48–50; and expense—a prescription costs $160 per month without insurance, although manufacturer discount programs ($70–75/month in 2015) may be available. The drug is not approved for treatment of major depressive or other psychiatric disorders. Caution is recommended if a patient has a history of suicidality, because antidepressants like bupropion increase the risk of suicidal thoughts in children, adolescents, and young adults. Contraindications include uncontrolled hypertension, seizures disorder or history of seizures, use of other bupropion-containing products, bulimia (which may increase seizure risk), long-term opioid or opiate agonists, and use of MAOIs.46,48–50
Liraglutide is an agonist of the gut hormone glucagon-like peptide-1 (GLP-1) that suppresses food intake and appetite, particularly at the 3 mg dose approved for the treatment of obesity.51 GLP-1 receptor agonists initially were developed to treat type 2 diabetes, because they enhance endogenous meal-induced insulin secretion while inhibiting glucagon secretion to improve glucose homeostasis. Benefits of the 3 mg dose in patients without diabetes include placebo-adjusted weight loss of 4–6 kg at 6–12 months (~3 kg in those with diabetes); 75% on drug vs. ~30% on placebo lost ≥ 5% of initial body weight and ~30% lost ≥ 10% (vs. 10% on placebo).52 Marked improvements were noted in hemoglobin A1C, insulin, C-reactive protein, systolic blood pressure and the urine protein/creatinine ratio; improvements in lipids were modest and heart rate slightly increased.53 Disadvantages of liraglutide include gastrointestinal side effects (nausea, diarrhea, and vomiting), increased lipase, headache, fatigue, and hypoglycemia (of note, the risk of hypoglycemia markedly increased in patients who were taking sulfonylureas, despite halving of the sulfonylurea dose53; therefore, consider stopping sulfonylureas prior to liraglutide use). Although pricing information for Saxenda (3 mg/0.5 mL pens) has not been released, a one-month supply of 18 mg/3 mL liraglutide pens costs ~$650 without insurance; reduced pricing ($25–$150/month) is available for eligible patients. Contraindications include pregnancy, confirmed pancreatitis, or personal or family histories of medullary thyroid carcinoma or Multiple Endocrine Neoplasia type 2. Clinicians should counsel patients regarding possible increased risk of medullary thyroid carcinoma and symptoms of thyroid tumors. The effects of Saxenda on cardiovascular endpoints have not been established (although a cardiovascular outcomes trial for lower-dose liraglutide is underway), it should not be used with insulin, and caution is recommended in patients with a history of gallbladder disease.
The sympathomimetic amines, benzphetamine, diethylpropion, phendimetrazine, and phentermine, blunt appetite by eliciting noradrenaline release.37–38 Although these drugs only are approved for short-term use (up to 12 weeks), phentermine currently is the most widely prescribed weight-loss medication and many physicians prescribe it off-label beyond 12 weeks. Recommendations regarding long-term prescribing of phentermine are published elsewhere.35 Two meta-analyses report that the placebo-subtracted weight loss associated with phentermine is ~4 kg (~9 lbs.) after an average duration of 13 weeks.38,54 Another benefit is relative low-cost—a typical prescription costs only $10–50 per month. Adverse effects of the sympathomimetic amines include: sympathetic side effects (increased heart rate, blood pressure, insomnia, dry mouth, constipation, and nervousness), which partly can be mitigated by starting the drugs at low dosages and increasing after 3–7 days on each dosage increase; potential for addiction (most are Schedule IV drugs, although clinically addiction is not seen); rare psychosis; and possible worsening of hypertension and hypoglycemia in patients with hypertension and insulin-requiring diabetes, respectively. The drugs are contraindicated in patients with renal insufficiency, valvular heart disease, coronary artery disease, hyperthyroidism, or taking MAOIs or stimulants.
Importantly, although many weight-loss drugs are now approved for long-term use, drug responsiveness needs to be determined.37 In general, if a patient has lost <5% of their initial body weight after three months, consider discontinuing the medicine and trying an alternative. The official recommendation for determining drug responsiveness on phentermine/topiramate ER, however, is that, at three months, if weight loss is <3% of initial weight, either advance to a full dose or discontinue the medication; and at six months, if weight loss is <5% of initial weight, discontinue the medication by tapering the drug in patients taking the 15/92 mg formulation to every other day for one week.
Weight-loss drugs should be chosen based on risk and side-effect profiles, and may be needed indefinitely—as is needed for other chronic diseases, including hypertension, hyperlipidemia, and diabetes. For patients who are pregnant or seeking pregnancy, only behavior modification is recommended. For patients with suboptimally-controlled hypertension, cardiovascular disease, or anxiety disorders consider using orlistat or lorcaserin instead of a sympathomimetic amine (such as phentermine or phentermine/topiramate) or naltrexone/bupropion. For patients with depression on an SSRI, although lorcaserin is contraindicated, drug options include orlistat, sympathomimetic amines, and liraglutide. In patients with a history of bulimia or seizures, although naltrexone/bupropion and sympathomimetic amines (including phentermine/topiramate) are contraindicated, orlistat, lorcaserin, or liraglutide could be considered.
In summary, FDA-approved weight-loss drugs provide clinicians with effective weight-management tools that, for the first time, are recommended as part of a comprehensive primary-care weight-management plan. Drugs should be adjuncts to, not substitutes for, necessary changes in lifestyle—one can “eat through” the drugs’ effects on appetite and satiety. Also, weight regain is the rule upon drug cessation—thus, if patients meet drug-responsiveness criteria for body-weight loss at 3–6 months, drugs approved for long-term use should be continued.
Choosing Weight-Loss Surgery Wisely
What primary-care providers need to know about bariatric surgery includes indications for and available weight-loss surgeries, relative risks and benefits of each procedure, and how to manage patients in the post-operative period and long-term. Emerging surgical weight-loss techniques will not be reviewed. Eligibility criteria for bariatric surgery include, BMI ≥ 40 kg/m2 or ≥ 35 kg/m2 with at least one weight-related health condition (standard conditions include diabetes, hypertension, and obstructive sleep apnea, although what insurers accept as weight-related conditions may vary) and failure at non-surgical weight loss attempts; no active drug or alcohol abuse or uncontrolled psychiatric illness; comprehension of the risks, benefits, anticipated outcomes, and lifestyle changes required after surgery; and patient commitment, which can be assessed by the patient’s ability to track food and activity (using a log or mobile application), and reviewing whether they keep their appointments and adhere to medications and treatment plans.3,55 Available weight-loss procedures include roux-en-y gastric bypass (RYGB), both open and laparoscopic, laparoscopic gastric sleeve (LGS), and laparoscopic adjustable gastric band (LAGB, also called “lap-band,” although this also is a trade name for a specific band).55 Biliopancreatic diversion (BPD) and BPD with duodenal switch are two additional procedures that account for <2% of bariatric procedures in the US, are reserved for patients with a BMI ≥ 50 kg/m2, and have adverse effects that are equal to or greater than those following RYGB; given the infrequency of these operations, they will not be reviewed here. Bariatric surgery’s efficacy appears to be due to physiologic alterations in gut hormones and adipose-tissue mass, and to anatomical changes, which classically have been divided into three categories—restrictive, malabsorptive, or both.55
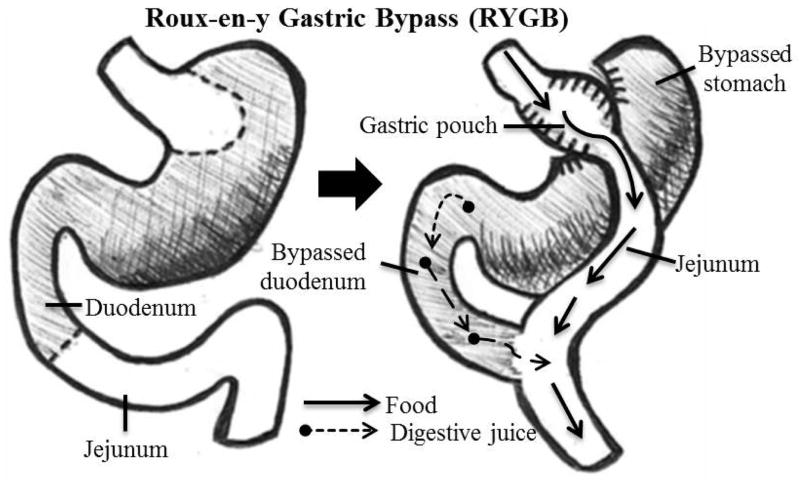
In the RYGB procedure, a surgeon uses a surgical stapler to cut a small pouch at the top of the stomach, then ~two feet from the stomach, the small bowel is divided, and one end is attached to the new stomach pouch (Fig. 2). The other end, which remains connected to the bypassed stomach, is reconnected to the intestinal tract. Food bypasses most of the stomach and the first part of the small intestine. The procedure is both restrictive, because of the small stomach pouch, and malabsorptive, because it bypasses part of the small intestine causing a reduction in nutrient absorption.55 Advantages of the RYGB include: increased weight loss due to gastric restriction plus malabsorption; and improvements in weight-related conditions, particularly diabetes. Disadvantages include: greater risks than other procedures for bleeding, dumping syndrome, vitamin deficiencies, and death; and the post-procedure diet is very restrictive.56
Figure 2.
Roux-en-Y Gastric Bypass
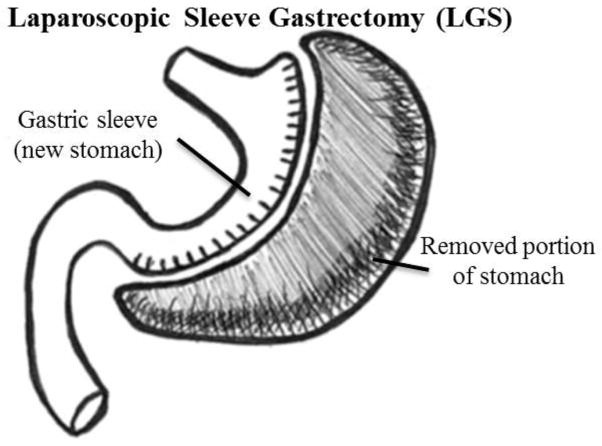
Gastric sleeve surgery, also called “sleeve gastrectomy,” is a restrictive weight-loss procedure that reduces the size of the stomach up to 85%, by surgical removal of a large portion of the greater curvature of the stomach—this results in a sleeve or tube-like structure.55 Advantages of LGS are: a less-restrictive post-surgical diet compared to post-RYGB; and for patients in whom a RYGB would be too risky, the procedure may precede RYGB, which can be performed after improvement in weight and comorbidities. Disadvantages of LGS include stretching of the gastric pouch with time, possible leakage at the staple site, and weight loss is slightly less than following RYGB.57
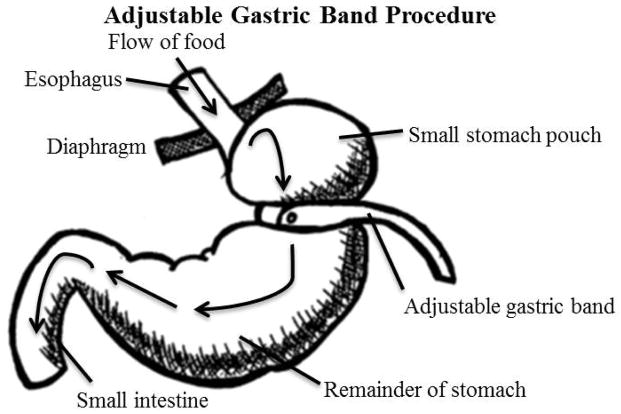
In the adjustable gastric-band procedure, an adjustable belt is placed around the upper portion of the stomach to restrict the amount of food that can be held in the stomach. It also slows the rate of passage of food into the intestines.55 Advantages include: shorter operation, hospitalization, and recovery times than other procedures; it is considered reversible (although not inconsequential, because it requires surgery); and there is less risk of dumping syndrome or vitamin deficiencies, compared to other procedures. Disadvantages include: once placed, the band is not tightened for up to six weeks leading to a delay in weight loss; follow-up procedures are needed to reposition, replace, or remove the band; the band may slip, leak saline, erode into the stomach, and cause infection or rejections; and weight loss is slower and of lower magnitude compared to other procedures.55,57
In a meta-analysis of surgery-related weight and comorbidity improvement, those undergoing RYGB had a net (after subtracting the effect of active lifestyle control) one-year loss of 22% body weight (%BW) (Table 4).55 LGS led to a net one-year weight loss of 15%. Data for %BW after five years are shown only for RYGB and LGS, but are similar to the one-year results. In terms of comorbidity improvement, diabetes improved the greatest after RYGB (any improvement—not complete resolution), hypertension improved the most following RYGB and LAGB (though not LGS, the reason for which is unclear and inconsistent with other studies54), and dyslipidemia improved in all procedures.
Table 4.
Meta-analysis of the impact of weight-loss surgical procedure on weight loss and comorbidity improvement55
| Characteristic | Control | RYGB | LGS | LAGB |
|---|---|---|---|---|
| Percent body weight lost year 1a,b | 10% | 32% | 25% | 17% |
| Percent body weight lost at year 5c | — | 32% | 28% | — |
| Diabetes improvement,c % | 18% | 95% | — | 74% |
| Hypertension improvement,d % | 49% | 81% | 54%c | 80% |
| Dyslipidemia improvement,c % | 5% | 63% | 83% | 61% |
Abbreviations: RYGB = Roux-en-Y Gastric Bypass, LGS = laparoscopic sleeve gastrectomy, LABG = laparoscopic gastric bypass
Numbers adjusted from excess body weight to reflect percent change in total body weight.
Observational study data
RCT, active lifestyle control
Fewer trials of LGS compared to LAGB were included, and other sources report greater improvement in hypertension following LGS compared to LAGB55
Risks of bariatric surgery include a small risk of mortality and complications, both immediately post-operation and months to years later.55–56 The proportion of patients who die within 30 days of a procedure is 0% for LAGB, 0.2% for laparoscopic RYGB, and 2% for open RYGB (patients who undergo open RYGB tend to have higher BMIs and surgical risk).56 The majority of post-operative deaths are due to pulmonary embolism, myocardial infection, congestive heart failure, or respiratory failure.55–56
Other early complications include bleeding at anastomoses or staple sites, infections, abscesses, post-operative pneumonia, leaks—which are fairly common after revision surgeries and are associated with a 15% mortality risk—thrombosis, and respiratory failure (Table 5).55–59 An overview of the presentation, evaluation, and treatment of each is provided.
Table 5.
Early post-operative risks of weight-loss surgery: complications, evaluation, and management55
| Complication | Presentation | Evaluation/Treatment |
|---|---|---|
| Bleeding at anastomoses or staple sites |
|
|
| Infection at wound site (e.g., abscess, pneumonia) |
|
|
| Leaks: 2–6% initial RYGB, 35% revisions, 15% mortality |
|
|
| Thromboses: PE/VTE/MI |
|
|
| Respiratory compromise or failure |
|
|
Abbreviations: RYGB = Roux-en-Y Gastric Bypass, Upper GI = upper gastrointestinal series, CT = computed tomography, PE = pulmonary embolism, VTE = venous thromboembolism, MI = myocardial infarction, EKG = electrocardiogram, CHF = congestive heart failure, OSA = obstructive sleep apnea
Late complications of RYGB are shown in the Tables 6–7.55–60 Common complications include dumping syndrome, which presents months or years after surgery. When it occurs early, symptoms are usually caused by eating simple carbohydrates (for example, rice, bread, or candy), are rapid in onset—within 15 minutes of eating. The hyperosmolality of simple carbohydrate causes rapid fluid shifts, resulting in hypotension and a sympathetic-nervous-system response. Late dumping results from a rapid rise in glucose, from food dumping into the small bowel, causing a robust insulin response followed by relative hypoglycemia. Other late complications, not listed, include iron deficiency, metabolic bone disease, gastrointestinal problems, kidney problems, short-bowel syndrome, elevated risk of suicide (4.1/10,000 person-years within 10 years after bariatric surgery vs., in the general population, 0.7/10,000 for females and 2.4/10,000 for males61), and need for reoperation (ranging in frequency from 6.7%–24% of time, according to a meta-analysis62).55–60
Table 6.
Late complications of RYGB surgery: evaluation and management59
| Complication | Timing | Risk Factors | Presentation | Evaluation/Treatment |
|---|---|---|---|---|
| Dumping syndrome: ~50% prevalence |
|
|
|
|
| Gallstones: ~40% prevalence |
|
|
|
|
| Stomal stenosis: 6–20% prevalence |
|
|
|
|
| Marginal ulcers: 2–16% prevalence |
|
|
|
|
| Hernias (ventral, internal): 0–5% prevalence for laparoscopic, up to 25% for open |
|
|
|
|
| Gastric-remnant distension/rupture |
|
|
|
|
| Failure to lose weight and weight regain |
|
|
|
|
Abbreviations: M/ERCP = magnetic/endoscopic resonance cholangiopancreatography, NSAID = non-steroidal anti-inflammatory drugs, G-G = gastrogastric, G-J = gastro-jejunal, H. pylori = helicobacter pylori, PPI = proton-pump inhibitor, KUB = abdominal x-ray
Table 7.
Nutrient deficiencies associated with weight-loss surgery60
| Deficiency | Timing | Risk Factors | Presentation | Evaluation/Treatment |
|---|---|---|---|---|
| Thiamine deficiency |
|
|
|
|
| Folate deficiency |
|
|
|
|
| B12 deficiency |
|
|
|
|
| Copper deficiency |
|
|
|
|
| Zinc deficiency: 6–40% post-gastrectomy |
|
|
|
|
Abbreviations: TKA = transketolase, CBC = complete blood count, MCV = mean corpuscular volume, RBC = red blood cell, MMA = methylmalonic acid, IDA = iron deficiency anemia
For gastric banding, early complications that are unique to LAGB include acute stomal obstruction, band infection, gastric perforation, and delayed gastric emptying.55,58 Late complications that are unique to gastric banding include band erosion (>1 year post-band, <10% prevalence), slippage (2–14% prevalence), or prolapse, port malfunction, esophagitis, esophageal dilation (pseudoachalasia), and hiatal-hernia formation.55,60 Most complications can be identified by upper GI series. Treatment may include band deflation and dietary modification, band repositioning, or band removal, with or without conversion to another procedure, such as RYGB. High reoperation rates (~41%) for band removal were cited in a meta-analysis.62
For sleeve gastrectomy, the most common early complication is bleeding; sub-acute to late complications include narrowing or stenosis of the stoma, and leaks.55,59–60 Whereas stomal stenosis can be treated by endoscopic dilation, leaks are some of the most serious complications, and early diagnosis, drainage, and gastric decompression are crucial.59–60
Long-Term Care of Patients Following Bariatric Surgery
In the period immediately following bariatric surgery, providers should be familiar with post-operative dietary advancement and vitamin/mineral-supplementation requirements (Table 8). Following RYGB/LGS, patients are advanced from clear to full liquids, then soft, moist protein-rich foods (for example, an egg), soft fruits and vegetables, and as more volume is tolerated, solid foods. Patients are counseled to drink fluids to prevent dehydration, 30 minutes apart from consuming solids. Lifelong vitamin/mineral supplements are required, although specific supplements are tailored using regular bloodwork.60
Table 8.
Standard postoperative vitamin and mineral supplements for RYGB/LSG patients60
| Supplement | Dosagea |
|---|---|
| Multivitamin |
|
| Calcium citrate (PPI recommended after bariatric surgery) with vitamin Da |
|
| Elemental iron (menstruating females only)a |
|
| Vitamin B12a |
|
Patients with deficiency states need treatment beyond these recommendations.59
For LAGB, following band placement, patients resume a normal diet, and the first band tightening occurs 4–6 weeks after placement. Band tightening occurs gradually, at 4–6-week intervals over 1–2 years. As patients begin to feel satiety, they are encouraged to eat smaller meals and chew food more thoroughly.
Follow-up visits in the immediate post-operative period are particularly important for patients receiving RYGB and LSG who take medications for diabetes and high blood pressure. Patients who require only oral diabetes medications usually are able to stop all agents following bariatric surgery. In patients with insulin-requiring diabetes, consider checking a c-peptide level to determine how much endogenous insulin the patient makes. Those with undetectable c-peptide levels will most likely require exogenous insulin administration despite weight loss. Typically, at the time of surgery, basal insulin is decreased by half. Because dietary intake and insulin requirements vary depending on the patient’s post-operative course, basal-bolus insulin regimens (for example, insulin 70/30) are untenable for most patients, and typically are replaced by use of regular or rapid-acting insulin with meals. Patients eat very slowly following bariatric surgery, which means that regular insulin can be taken at meal initiation instead of 20–30 minutes before. Rapid-acting insulin 15–20 minutes after meal initiation should be used, however, in patients who have a lot of nausea; because they may anticipate eating a meal only to find they are too nauseous to eat. In patients with fluctuating insulin needs, consider having patients email or fax glucose logs weekly for the first three months post-operatively. Further guidance regarding caring for patients with diabetes following bariatric surgery has been published.63–64 For patients with hypertension, the first medications to reduce or stop in the setting of weight loss (regardless of whether through diet, medication, or surgery-induced weight loss) are diuretics—as the liver releases glycogen stores, because glycogen is heavily complexed with water, diuresis ensues, which can lead to dehydration in the setting of diuretic use.
Long-term, after all surgical weight-loss procedures, lifestyle recommendations mirror those for patients who have not undergone weight-loss surgery, with adjustments for food aversions/intolerances that may develop following RYGB (for example, red meat).60 Focus shifts to weight-loss maintenance, including regular exercise, sleep, minimal sedentary activity, and promoting optimal mental health, by addressing any issues not addressed or revealed prior to weight-loss surgery.
Long-term monitoring of bariatric-surgery patients includes regular follow-up visits and laboratory assessments (Table 9).60,65 During follow-up visits, review patients’ diet and lifestyle habits, medications and supplements, and reinforce the importance of medication adherence, daily exercise, smoking cessation, and not taking NSAIDs (because both smoking and NSAIDs increase the likelihood of marginal ulcers). Also, conduct a thorough assessment of depression/suicidality, alcohol intake, sexual activity, and dysfunctional eating. Following RYGB: there are increased risks of incident alcoholism (incidence increases 50% two years postoperatively compared to pre-surgical rates; prevalence increases from 7.6% to ~10%65) and eating disorders (prevalence estimates vary67; for example, in one study in which more than 60% of a post-operative sample reported vomiting, 12% admitted to vomiting for weight loss68).65,67 Further resources regarding psychiatric aspects of bariatric surgery have been published.69 Women who seek to become pregnant should be advised to delay pregnancy for 12–18 months following surgery (to optimize weight loss and avert adverse effects of surgery-related nutritional deficiencies).70
Table 9.
Monitoring of patients following bariatric surgery60
| Clinical Practice | RYGB | LSG | LAGB |
|---|---|---|---|
| Visit interval | |||
| Immediately post-procedure (first three months) | Monthly | Monthly | Monthly |
| During active weight-loss (up to 12–18 months) | 3 months | 3–6 months | 3–6 months |
| Once weight stable | 6–12 months | 12 months | 12 months |
|
| |||
| Consider gout and gallstone prophylaxis | 0–12 months | 0–6 months | 1–6 months |
|
| |||
| Recommended at 3 months, 6 months, then annually thereafter: | |||
| Complete blood count | x | x | x |
| Electrolytes, AST/ALT, alk phos, bilirubin, and albumin | x | x | x |
| Glucose and hemoglobin A1C | x | x | x |
| Iron studies, ferritin (in males, check only until stable) | x | x | x |
| Vitamin B12, folate, and thiamine | x | x | x |
| Lipid profile | x | x | x |
| 25-hydroxyvitamin D, parathyroid hormone (intact PTH) | x | x | x |
| Zinc, copper | x | x | – |
|
| |||
| Bone densitometry every 1–2 years | x | x | x |
Abbreviations: RYGB = Roux-en-Y Gastric Bypass, LSG = laparoscopic sleeve gastrectomy, LABG = laparoscopic adjustable gastric band, AST = aspartate aminotransferase, ALT = alanine aminotransferase, alk phos = alkaline phosphatase, PTH = parathyroid hormone
SUMMARY
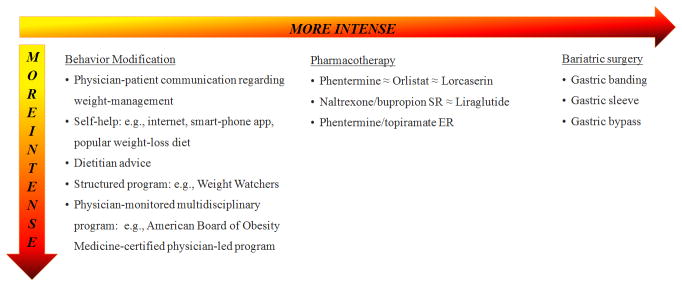
A summary of available weight-management interventions, by mode and intensity, is shown in Fig. 5. Behavior modification ranges from weight-loss advice to a physician-monitored multidisciplinary program. Now that there is an obesity medicine certification exam, the American Board of Obesity Medicine or ABOM, primary-care providers may consider partnering with these obesity specialists who also are knowledgeable about obesity medications and surgeries. For pharmacotherapy, orlistat, lorcaserin, and naltrexone/bupropion SR are the least effective, providing 3–6% net weight loss at a year and phentermine/topiramate ER is the most effective, providing 8–10% net weight loss at a year. For surgery, gastric banding is the least effective, at 7–10% net weight loss at a year; and gastric bypass is the most effective, providing 20–30% net weight loss.
Figure 5.
Available weight-management interventions, by intensity
Future Directions
The field of primary-care weight management is moving toward personalization, early intervention, and expansion of drug options. Factors amenable to treatment tailoring include comorbid illnesses, specific behavioral patterns, and genotypes—for example, treating prediabetes/diabetes with drugs that promote weight loss and targeting problems with hunger and satiety with anorexic agents. Early intervention is a hot topic, and pediatricians are being urged to identify and treat obesity in children and families earlier, more seriously, and more aggressively. In the future, new therapeutic targets may be identified through high-throughput drug-testing platforms (looking to repurpose drugs in use, and for new experimental targets), and drug engineering, harnessing new knowledge about the neuro-hormonal bases for changes in metabolism and appetite following bariatric surgery, or induced by cold temperatures and high altitude.71–72
CONCLUSIONS
Right now, physicians care for overweight patients in their practices, and to maximize the effectiveness of this opportunity to help overweight patients manage their weight, can apply evidence-based clinical practices, including patient-centered communication, partnered decision-making regarding weight-management goals, weight-loss medications, and bariatric surgery. The first maxim we are taught in medicine is, “First do no harm.” We know that obesity causes harm—the outcome is going to be bad unless something is done; therefore, not addressing weight management with overweight and obese patients is causing harm. The health costs of obesity and benefits of weight loss demand action. Although patient progress in weight management can be slow and fraught with setbacks, we must remember the ancient Chinese proverb, “A journey of a thousand miles begins with a single step.” Supporting patients in weight management, including activating their intrinsic motivation by providing tools and working towards feasible goals, could stimulate lifelong, self-sustained successful weight management.
Figure 3.
Adjustable Gastric Band Procedure
Figure 4.
Laparoscopic Sleeve Gastrostomy
Acknowledgments
Funding Source: Supported in part by Award # K23HL118152-01A1 from the National Heart, Lung, and Blood Institute (NHLBI; to Dr. Turer).
Footnotes
Financial disclosure: The author has no financial disclosures.
Conflict of interest: The author has no conflicts of interest to disclose.
Prior presentation: Presented in part at University of Texas Southwestern Medical Center (UTSW) Division of General Internal Medicine Grand Rounds on March 6, 2014 and UTSW Department of Internal Medicine Grand Rounds on October 3, 2014 in Dallas, TX.
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity among adults: United States, 2011–2012. NCHS Data Brief. 2013;131:1–8. [PubMed] [Google Scholar]
- 2.Finkelstein EA, Trogdon JG, Cohen JW, et al. Annual medical spending attributable to obesity: payer and service-specific estimates. Health Aff. 2009;28(5):w822–31. doi: 10.1377/hlthaff.28.5.w822. [DOI] [PubMed] [Google Scholar]
- 3.Expert Panel Members. Jensen MD, Ryan DH, Donato KA, et al. Executive summary: Guidelines (2013) for the management of overweight and obesity in adults. Obesity. 2013;22:S5–S39. doi: 10.1002/oby.20821. [DOI] [PubMed] [Google Scholar]
- 4.National Heart, Lung, and Blood Institute. [Accessed March 21, 2015];Managing overweight and obesity in adults: Systematic evidence review from the Obesity Expert Panel. 2013 http://www.nhlbi.nih.gov/guidelines/obesity/ser/index.htm.
- 5.US Department of Health and Human Services. [Access March 21, 2015];2008 physical activity guidelines for Americans. http://www.health.gov/paguidelines.
- 6.Wormser D, Kaptoge S, De Angelantonio E, et al. Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: collaborative analysis of 58 prospective studies. Lancet. 2011;377:1085–1095. doi: 10.1016/S0140-6736(11)60105-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neeland IJ, Ayers CR, Rogatgi AK, Turer AT, Berry JD, Das SR, Vega GL, Khera A, McGuire DK, Grundy SM, de Lemos JA. Associations of visceral and abdominal subcutaneous adipose tissue with markers of cardiac and metabolic risk in obese adults. Obesity. 2013;21:E439–E447. doi: 10.1002/oby.20135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ogden J, Stavrinaki M, Stubbs J. Understanding the role of life events in weight loss and weight gain. Psychol Health Med. 2009;14(2):239–249. doi: 10.1080/13548500802512302. [DOI] [PubMed] [Google Scholar]
- 9.Mechanick JI, Youdim A, Jones DB, et al. Clinical practice guidelines for the perioperative nutritional, metabolic, and non-surgical support of the bariatric surgery patient—2013 update: co-sponsored by American Association of Clinical Endocrinologists, the Obesity Society, and American Society for Metabolic and Bariatric Surgery. Surg Obes Relat Dis. 2013;9(2):159–91. doi: 10.1016/j.soard.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 10.King WC, Chen JY, Mitchell JE, et al. Prevalence of alcohol use disorders before and after bariatric surgery. JAMA. 2012;307(23):2516–25. doi: 10.1001/jama.2012.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Romeo GR, Abrahamson MJ. The 2015 standards for diabetes care: maintaining a patient-centered approach. Ann Intern Med. 2015 Mar 24; doi: 10.7326/M15-0385. (e-pub ahead of print) [DOI] [PubMed] [Google Scholar]
- 12.Pan J, Fallon MB. Gender and racial differences in nonalcoholic fatty liver disease. World J Hepatol. 2014;6(5):274–283. doi: 10.4254/wjh.v6.i5.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahn J, Schatzkin A, Lacey JV, Jr, et al. Adiposity, adult weight change, and postmenopausal breast cancer risk. Arch Intern Med. 2007;167(19):2091–102. doi: 10.1001/archinte.167.19.2091. [DOI] [PubMed] [Google Scholar]
- 14.Esposito K, Chiodini P, Capuano A, Bellastella G, Maiorino MI, Giugliano D. Metabolic syndrome and endometrial cancer: a meta-analysis. Endocrine. 2014;45(1):28–36. doi: 10.1007/s12020-013-9973-3. [DOI] [PubMed] [Google Scholar]
- 15.Silk AW, McTigue KM. Reexamining the physical examination for obese patients. JAMA. 2011;305(2):193–194. doi: 10.1001/jama.2010.1950. [DOI] [PubMed] [Google Scholar]
- 16.Wing RR, Lang W, Wadden TA, et al. Look AHEAD Research Group. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34(7):1481–86. doi: 10.2337/dc10-2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perreault L, Temprosa M, Mather KJ, et al. Diabetes Prevention Program Research Group. Regression from prediabetes to normal glucose regulation is associated with reduction in cardiovascular risk: results from the Diabetes Prevention Program Outcomes Study. Diabetes Care. 2014;37:2622–31. doi: 10.2337/dc14-0656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vivante A, Golan E, Tzur D, et al. Body mass index in 1. 2 million adolescents and risk for end-stage renal disease. Arch Intern Med. 2012;172(21):1644–50. doi: 10.1001/2013.jamainternmed.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fowler SM, Kon V, Ma L, Richards WO, Fogo AB, Hunley TE. Obesity-related focal and segmental glomerulosclerosis: normalization of proteinuria in an adolescent after bariatric surgery. Pediatr Nephrol. 2009;24:851–55. doi: 10.1007/s00467-008-1024-6. [DOI] [PubMed] [Google Scholar]
- 20.Kambham N, Markowitz GS, Valeri AM, Lin J, D’Agati VD. Obesity-related glomerulopathy: an emerging epidemic. Kidney Int. 2001;59:1498–1509. doi: 10.1046/j.1523-1755.2001.0590041498.x. [DOI] [PubMed] [Google Scholar]
- 21.Abed HS, Wittert GA, Leong DP, et al. Effect of weight reduction and cardiometabolic risk factor management on symptom burden and severity in patient with atrial fibrillation. JAMA. 2013;310(19):2050–2060. doi: 10.1001/jama.2013.280521. [DOI] [PubMed] [Google Scholar]
- 22.Patel AA, Torres DM, Harrison SA. Effect of weight loss on nonalcoholic fatty liver disease. J Clin Gastroenterol. 2009;43(10):970–974. doi: 10.1097/MCG.0b013e3181b57475. [DOI] [PubMed] [Google Scholar]
- 23.Thomson RL, Buckley JD, Noakes M, Clifton PM, Normal RJ, Brinkworth GD. The effect of a hypocaloric diet with and without exercise training on body composition, cardiometabolic risk profile, and reproductive function in overweight and obese women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93(9):3373–3380. doi: 10.1210/jc.2008-0751. [DOI] [PubMed] [Google Scholar]
- 24.Subak LL, Wing R, West DS, et al. Weight loss to treat urinary incontinence in overweight and obese women. N Engl J Med. 2009;20(5):481–490. doi: 10.1056/NEJMoa0806375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Esposito K, Giugliano F, Di Palo C, et al. Effect of lifestyle changes on erectile dysfunction in obese men: a randomized controlled trial. JAMA. 2004;291(24):2978–2984. doi: 10.1001/jama.291.24.2978. [DOI] [PubMed] [Google Scholar]
- 26.Volger S, Vetter ML, Dougherty M, et al. Patients’ preferred terms for describing their excess weight: discussing obesity in clinical practice. Obesity (Silver Spring) 2012;20(1):147–50. doi: 10.1038/oby.2011.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roter D. Patient-centered communication. Br Med J. 2004;328(7453):E303–4. doi: 10.1136/bmj.328.7453.E303. [DOI] [PubMed] [Google Scholar]
- 28.Rollnick S, Butler CC, Kinnersley P, et al. Motivational interviewing. Br Med J. 2010;340:c1900. doi: 10.1136/bmj.c1900. [DOI] [PubMed] [Google Scholar]
- 29.Williams GC, Grow VM, Freedman ZR, Ryan RM, Deci EL. Motivational predictors of weight loss and weight-loss maintenance. J Pers Soc Psychol. 1996;70:115–26. doi: 10.1037//0022-3514.70.1.115. [DOI] [PubMed] [Google Scholar]
- 30.Rutten GM, Meis J, Hendriks M, Hamers F, Veenhof C, Kremers S. The contribution of lifestyle coaching of overweight patients in primary care to more autonomous motivation for physical activity and healthy dietary behavior: results of a longitudinal study. Int J Behav Nutr Phys Act. 2014;11(1):86. doi: 10.1186/s12966-014-0086-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pollak KI, Alexander SC, Coffman CJ, et al. Physician communication techniques and weight loss in adults: Project CHAT. Am J Prev Med. 2010;39(4):321–328. doi: 10.1016/j.amepre.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silberstein SD, Holland S, Freitag F, et al. Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Evidence-based guideline update: pharmacologic treatment of episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78(17):1337–1345. doi: 10.1212/WNL.0b013e3182535d20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raccah D, Lin J, Wang E, et al. Once-daily prandial lixisenatide versus once-daily rapid-acting insulin in patients with type 2 diabetes mellitus insufficiently controlled with basal insulin: analysis of data from five randomized, controlled trials. J Diabetes Complications. 2014;28(1):40–44. doi: 10.1016/j.jdiacomp.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 34.Fajardo Montañana C, Hernández Herrero C, Rivas Fernández M. Less weight gain and hypoglycemia with once-daily insulin detemir than NPH insulin in intensification of insulin therapy in overweight type 2 diabetes patients: the PREDICTIVE BMI clinical trial. Diabet Med. 2008;25(8):916–923. doi: 10.1111/j.1464-5491.2008.02483.x. [DOI] [PubMed] [Google Scholar]
- 35.Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2015;100(2):342–362. doi: 10.1210/jc.2014-3415. [DOI] [PubMed] [Google Scholar]
- 36.Shaw KA, Gennat HC, O’Rourke P, Del Mar C. Exercise for overweight and obesity. Cochrane Database of Systematic Reviews. 2006;(4):Art. No.: CD003817. doi: 10.1002/14651858.CD003817.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yanovski SZ, Yanovski JA. Long-term drug treatment for obesity: a systematic and clinical review. JAMA. 2014;311:74–86. doi: 10.1001/jama.2013.281361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Z, Maglione M, Tu W, et al. Meta-analysis: pharmacologic treatment of obesity. Ann Intern Med. 2005;142:532–546. doi: 10.7326/0003-4819-142-7-200504050-00012. [DOI] [PubMed] [Google Scholar]
- 39. [Accessed December 6, 2014];FDA Highlights of Prescribing Information: XENICAL (orlistat) capsules. http://www.fda.gov/Safety/MedWatch/SafetyInformation/ucm215504.htm.
- 40.Davidson MH, Hauptman J, DiGirolamo M, et al. Weight control and risk factor reduction in obese subjects treated for 2 years with orlistat: a randomized controlled trial. JAMA. 1999;281(3):235–242. doi: 10.1001/jama.281.3.235. [DOI] [PubMed] [Google Scholar]
- 41.Chan EW, He Y, Chui CS, Wong AY, Lau WC, Wong IC. Efficacy and safety of lorcaserin in obese adults: a meta-analysis of 1-year randomized controlled trials (RCTs) and narrative review on short-term RCTs. Obes Rev. 2013;14(5):383–392. doi: 10.1111/obr.12015. [DOI] [PubMed] [Google Scholar]
- 42. [Accessed December 8, 2014];FDA Highlights of Prescribing Information: BELVIQ (lorcaserin hydrochloride) tablets, for oral use. http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/022529lbl.pdf.
- 43. [Accessed December 8, 2014];FDA Highlights of Prescribing Information: QSYMIA (phentermine and topiramate extended-release) capsules. http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/022580s000lbl.pdf.
- 44.Allison DB, Gadde KM, Garvey WT, et al. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP) Obesity (Silver Spring) 2012;20(2):330–342. doi: 10.1038/oby.2011.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gadde KM, Allison DB, Ryan DH, et al. Effects of low-dose controlled-release phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomized, placebo-controlled, phase 3 trial. Lancet. 2011;377(9774):1341–1352. doi: 10.1016/S0140-6736(11)60205-5. [DOI] [PubMed] [Google Scholar]
- 46.Greenway FL, Fujioka K, Plodkowski RA, et al. COR-I Study Group. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicenter, randomized, double-blind, placebo-controlled, phase 3 trial. Lancet. 2010;376(9741):595–605. doi: 10.1016/S0140-6736(10)60888-4. [DOI] [PubMed] [Google Scholar]
- 47.McElroy SL, Hudson JI, Mitchell JE, et al. Efficacy and safety of lisdexamfetamine for treatment of adults with moderate to severe binge-eating disorder: a randomized clinical trial. JAMA Psychiatry. 2015 Jan 14; doi: 10.1001/jamapsychiatry.2014.2162. (e-pub ahead of print) [DOI] [PubMed] [Google Scholar]
- 48.Apovian CM, Aronne L, Rubino D, et al. COR-II Study Group. A randomized, phase 3 trial of naltrexone SR/bupropion SR on weight and obesity-related risk factors (COR-II) Obesity (Silver Spring) 2013;21(5):935–943. doi: 10.1002/oby.20309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wadden TA, Foreyt JP, Foster GD, et al. Weight loss with naltrexone SR/bupropion SR combination therapy as an adjunct to behavior modification: the COR-BMOD trial. Obesity (Silver Spring) 2011;19(1):935–943. doi: 10.1038/oby.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gadde KM ACOR-II Study Group. A randomized, phase 3 trial of naltrexone SR/bupropion SR on weight and obesity-related risk factors (COR-II) Obesity. 2013;21(5):935–43. doi: 10.1002/oby.20309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Visboll T, Christensen M, Junker AE, Knop FK, Gluud LL. Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomized controlled trials. BMJ. 2012;344:d7771–8882. doi: 10.1136/bmj.d7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Astrup A, Rossner S, Van Gaal L, et al. Effects of liraglutide in the treatment of obesity: a randomized, double-blind, placebo-controlled study. Lancet. 2009;374:1601–1616. doi: 10.1016/S0140-6736(09)61375-1. [DOI] [PubMed] [Google Scholar]
- 53.FDA Briefing Document. NDA 206321. Liraglutide Injection, 3 mg. Sponsor: Novo Nordisk. Endocrinologic and Metabolic Drugs Advisory Committee Meeting; Sept 11, 2014; [Accessed March 25, 2015]. http://www.fda.gov/downloads/advisorycommittees/committeesmeetingmaterials/drugs/endocrinologicandmetabolicdrugsadvisorycommittee/ucm413317.pdf. [Google Scholar]
- 54.Haddock CK, Poston WSC, Dill PL, Foreyt JP, Ericsson M. Pharmacotherapy for obesity: a quantitative analysis of four decades of published randomized clinical trials. Int J Obes. 2002;26:262–273. doi: 10.1038/sj.ijo.0801889. [DOI] [PubMed] [Google Scholar]
- 55.Chang SH, Stoll CR, Song J, Varela JE, Eagon CJ, Colditz GA. The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003–2012. JAMA Surg. 2014;149(3):275–87. doi: 10.1001/jamasurg.2013.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lancaster RT, Hutter MM. Bands and bypasses: 30-day morbidity and mortality of bariatric surgical procedures as assessed by prospective, multi-center, risk-adjusted ACS-NSQIP data. Surg Endosc. 2008;22(12):2554–63. doi: 10.1007/s00464-008-0074-y. [DOI] [PubMed] [Google Scholar]
- 57.Hutter MM, Schirmer BD, Jones DB, Ko CY, Cohen ME, Merkow RP, Nguyen NT. First report from the American College of Surgeons Bariatric Surgery Center Network: laparoscopic sleeve gastrectomy has morbidity and effectiveness positioned between the band and the bypass. Ann Surg. 2011;254(3):410–20. doi: 10.1097/SLA.0b013e31822c9dac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stenberg E, Szabo E, Agren G, et al. for the Scandinavian Obesity Surgery Registry Study Group. . Early complications after laparoscopic gastric bypass surgery: results from the Scandinavian Obesity Surgery Registry. Ann Surg. 2013;11:1–8. doi: 10.1097/SLA.0000000000000431. [DOI] [PubMed] [Google Scholar]
- 59.Longitudinal Assessment of Bariatric Surgery (LABS) Consortium. Flum DR, Belle SH, King WC, et al. Perioperative safety in the longitudinal assessment of bariatric surgery. N Engl J Med. 2009;361(5):445–54. doi: 10.1056/NEJMoa0901836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mechanick JI, Youdim A, Jones DB, et al. Clinical practice guidelines for the perioperative nutritional, metabolic, and non-surgical support of the bariatric surgery patient—2013 update: co-sponsored by American Association of Clinical Endocrinologists, the Obesity Society, and American Society for Metabolic and Bariatric Surgery. Surg Obes Relat Dis. 2013;9(2):159–91. doi: 10.1016/j.soard.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 61.Peterhänsel C, Petroff D, Klinitzke G, Kersting A, Wagner B. Risk of completed suicide after bariatric surgery: a systematic review. Obes Rev. 2013;14(5):369–82. doi: 10.1111/obr.12014. [DOI] [PubMed] [Google Scholar]
- 62.Colquitt JL, Pickett K, Loveman E, Frampton GK. Surgery for weight loss in adults. Cochrane Database of Systematic Reviews. 2014;8:Art. No.: CD003641. doi: 10.1002/14651858.CD003641.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fujioka K. Follow-up of nutritional and metabolic problems after bariatric surgery. Diabetes Care. 2005;28(2):481–484. doi: 10.2337/diacare.28.2.481. [DOI] [PubMed] [Google Scholar]
- 64.Ardestani A, Rhoads D, Tavakkoli A. Insulin cessation and diabetes remission after bariatric surgery in adults with insulin-treated type 2 diabetes. Diabetes Care. 2015;38:659–664. doi: 10.2337/dc14-1751. [DOI] [PubMed] [Google Scholar]
- 65.Sarwer DB, Dilks RJ, West-Smith L. Dietary intake and eating behavior after bariatric surgery: threats to weight loss maintenance and strategies for success. Surg Obes Relat Dis. 2011;7(5):644–51. doi: 10.1016/j.soard.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 66.King WC, Chen JY, Mitchell JE, et al. Prevalence of alcohol use disorders before and after bariatric surgery. JAMA. 2012;307(23):2516–25. doi: 10.1001/jama.2012.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marino JM, Ertelt TW, Lancaster K, et al. The emergence of eating pathology after bariatric surgery: a rare outcome with important clinical implications. Int J Eat Disord. 2012;45(2):179–84. doi: 10.1002/eat.20891. [DOI] [PubMed] [Google Scholar]
- 68.De Zwaan M, Hilbert A, Swan-Kremeier L, et al. Comprehensive interview assessment of eating behavior 18–35 months after gastric bypass surgery for morbid obesity. Surg Obes Relat Dis. 2010;6:79–87. doi: 10.1016/j.soard.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 69.Yen Y, Huang C, Tai C. Psychiatric aspects of bariatric surgery. Curr Opin Psychiatry. 2014;27(5):374–379. doi: 10.1097/YCO.0000000000000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Beard JH, Bell RL, Duffy AJ. Reproductive considerations and pregnancy after bariatric surgery: current evidence and recommendations. Obes Surg. 2008;18(8):1023–27. doi: 10.1007/s11695-007-9389-3. [DOI] [PubMed] [Google Scholar]
- 71.Lichtenbelt Wv, Kingma B, van der Lans A, Schellen L. Cold exposure—an approach to increasing energy expenditure in humans. Trends Endocrinol Metab. 2014;25(4):165–67. doi: 10.1016/j.tem.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 72.Boyer SJ, Blume FD. Weight loss and changes in body composition at high altitude. J Appl Physiol Respir Exerc Physiol. 1984;57(5):1580–85. doi: 10.1152/jappl.1984.57.5.1580. [DOI] [PubMed] [Google Scholar]
- 73.Fernstrom MH, Kupfer DJ. Antidepressant-induced weight gain: a comparison study of four medications. Psychiatry Res. 1988;26:265–271. doi: 10.1016/0165-1781(88)90120-5. [DOI] [PubMed] [Google Scholar]