Abstract
Objectives
To present a (molecular) definition of BCG failure which incorporates fluorescence in situ hybridization (FISH) testing to predict BCG failure before it becomes clinically evident.
This will help in trial designs for patients with non-muscle invasive bladder cancer (NMIBC) who fail BCG and thus lack an adequate control arm other than radical cystectomy.
Patients and Methods
We used data from 143 patients were followed prospectively for 2 years during intravesical BCG therapy during which time FISH assays were collected and correlated to clinical outcomes.
Results
Of the 95 patients with no evidence of tumor at 3-month cystoscopy, 23 developed tumor recurrence, and 17 developed disease progression by 2 years.
Patients with a positive FISH at both 6-weeks and 3-months were more likely to develop tumor recurrence (17/37, 46% and 16/28, 57%, respectively) compared to patients with a negative FISH (6/58, 10% and 3/39, 8%, respectively) (both: p<0.001).
Using hazard ratios for recurrence with positive 6-week and 3-month FISH results, we constructed clinical trial scenarios whereby patients with a negative 3-month cystoscopy and positive FISH result could be considered to have “molecular BCG failure” and enrolled in prospective, randomized clinical trials comparing BCG therapy (control) with an experimental intravesical therapy.
Conclusions
Patients with positive early FISH and negative 3-month cystoscopy results can be considered to have molecular BCG failure based on their high rates of recurrence and progression.
This is intended for use in designing clinical trials, thus potentially allowing continued use of BCG as an ethical comparator arm.
Keywords: BCG, BCG failure, bladder cancer, clinical trials, FISH
INTRODUCTION
Non-muscle-invasive bladder cancer (NMIBC) accounts for approximately 75% of newly diagnosed bladder cancer cases.1–2 As High-grade NMIBC is associated with high rates of recurrence3 and progression,4, most guidelines (i.e., the American Urological Association, European Association of Urology, International Consultation on Urological Diseases, and National Comprehensive Cancer Network) recommend intravesical bacillus Calmette-Guerin (BCG).1,5–7 While BCG has been shown to reduces rates of both recurrence and progression, BCG is also associated with failure rates as high as 40% and carries certain toxicity.8 Despite a need for improved therapy for NMIBC, few intravesical agents have been developed since BCG was approved by the Food and Drug Administration (FDA) in 1998, none of which are effective after BCG failure.
The lack of progress is in large part due to limitations with current clinical trial design. Most alternative intravesical agents have been tested in patients in whom BCG has already failed – traditionally, this is determined by cystoscopy and urine cytology, i.e. those with visible, clinical tumor recurrence. We reasoned that a different platform is needed to develop novel intravesical agents. The ability to identify patients at high risk for BCG failure before clinical endoscopic failure might make it possible to design clinical trials comparing novel intravesical agents to BCG for primary treatment and still allow timely incorporation of radical cystectomy if necessary.
We previously reported on the utility of fluorescence in situ hybridization (FISH) performed using the UroVysion® kit for monitoring response to BCG before recurrent disease is clinically evident.9 In that prospective clinical trial, we demonstrated the ability of FISH to predict response to BCG therapy. In this report, propose a new clinical trial paradigm by incorporating a FISH-based definition of BCG failure (we use expanded data from the aforementioned study) with the hope that it allow more trials to be conducted and further the to the development of new intravesical agents.
MATERIALS AND METHODS
Clinical Trial Design
The design of this prospective, institutional-review-board-approved clinical trial (National Clinical Trial #01007058) has been published previously.9 Briefly, between July 2005 and April 2011; patients with pathologically confirmed NMIBC within 6 weeks of enrollment, normal findings on upper tract imaging, and intermediate- or high-risk disease by European Organization for Research and Treatment of Cancer criteria were enrolled.10 Patients were excluded if they had a history of prior pelvic radiation, variant histological subtypes (i.e. squamous cell carcinoma, micropapillary, small cell, etc.). All patients with high grade Ta/T1 tumors underwent re-resection 4 to 6 weeks after initial diagnosis before initiation of BCG therapy. All participants were scheduled to receive intravesical BCG immunotherapy according to the SWOG trial 8507 protocol11.
Patients were monitored during the course of BCG therapy according to normal institutional practices that included conventional white light cystoscopy (blue light cystoscopy not available at the start of the study) and cytology at 3-month intervals for 2 years, and every 3–6 months thereafter. FISH assays were performed at baseline (before the first BCG instillation), 6 weeks (before the sixth induction BCG treatment), 3 months (before the first maintenance BCG treatment), and 6 months (before the second maintenance BCG treatment). Repeat TUR and other interventions were performed as deemed necessary during the course of surveillance. While patient management was not necessarily directed by the results of the FISH assays, results were provided to the treating physician to be acted on if desired.
FISH was performed using UroVysion® with at least 35 mL of freshly collected urine as previously reported. The assay was considered positive if 4 or more cells showed polysomy on at least 2 chromosomes (3, 7, or 17) and/or at least 12 cells showed a homozygous deletion for 9p21 (no signal).12 In the absence of polysomy on 2 of the chromosomes described or a homozygous deletion for 9p21, assays were considered negative. If results were indeterminate or if a processing error occurred, assays were repeated if adequate urine was available.
For the purpose of designing our virtual study, we limited our selected cohort to only include patients who had high-grade cTa tumors, carcinoma in situ (CIS), and/or T1 primary at entry and no evidence of early recurrence (visible tumor at 3-month cystoscopy). Since the cytopathologists were aware of the FISH results, and this has been known to affect their interpretation of cytology findings, we did not analyze cytology as an independent variable. Also, while follow up data is available well beyond 2 years among this cohort, as most experts believe that a tumor recurrence beyond two years is less clinically meaningful than an early recurrence and may not constitute a true BCG failure, we limited cut off to this time period.
Statistical Analysis and Definitions
Recurrence was defined as the presence of tumor of any stage or grade detected after the start of intravesical BCG therapy. Progression was defined as a clinically meaningful endpoint and includes patients who developed a tumor recurrence with muscularis propria invasion or who underwent radical cystectomy. In this prospective trial, a 2 year endpoint was selected in order to identify a clinically relevant endpoint as recurrences beyond two years are less clinically impactful. Negative cystoscopy was defined as no evidence of papillary tumor or findings concerning for CIS. Patient data were censored from the time of recurrence, progression, or conclusion of the 2-year study interval.
T-test (or Wilcoxon’s rank sum) and Pearson chi-square test (or Fisher’s exact test) were used to assess the clinical/demographic characteristics differences between the FISH results. Logistic regression models were used to identify any association with each of the variables and recurrence (or progression). Univariate and multivariate Cox proportional hazards regression models were used to model the association between FISH and recurrence-free or progression-free survival. The Kaplan-Meier product limit method (Kaplan & Meier, 1958) was used to estimate the median recurrence-free and progression-free survival. Statistical analysis was performed using IBM SPSS version 21 statistical software and Stata/SE version 12.1 statistical software (Stata Corp. LP, College Station, TX). Sample size calculations were performed using East 5, version: 5.4.2.0 using time to event modeling.
RESULTS
Patient and Tumor Characteristics
Of the 143 total patients, we selected data from 95 patients to serve as backbone of our trial design as follows: 119 patients presented with high-grade cTa tumors, carcinoma in situ (CIS), and/or T1 primary tumors; of these 19 were excluded because of early recurrence at the initial 3-month cystoscopy and 5 because of inadequate follow-up, leaving 95 patients with no visual evidence of tumor after the initial surveillance cystoscopy to form the basis of this virtual trial design. Of the presenting tumors, 38 (40%) were high grade Ta, 50 (53%) were T1, and 7 (7%) were pure CIS. CIS was detected concurrently in 48(51%) of the Ta/T1 tumors (Tables 1 and 2).
Table 1.
Patient and tumor characteristics for patients with negative findings at 3-month cystoscopy and available 6-week FISH results
| Total | Negative FISH Results | Positive FISH Results | p-value | |
|---|---|---|---|---|
| No. of patients | 95 | 58 | 37 | |
| Age: mean (±SD) | 66 ± 11.1 | 65 ± 12.2 | 66 ± 9.2 | 0.544 |
| Sex | ||||
| Female | 19 (20%) | 9 (16%) | 10 (27%) | 0.171 |
| Male | 76 (80%) | 49 (84%) | 27 (73%) | |
| Smoker (past or present) | 65 (68%) | 42 (72%) | 13 (35%) | 0.376 |
| Grade | ||||
| Low | 9 (10%) | 8 (14%) | 1 (3%) | 0.086 |
| High | 86 (90%) | 50 (86%) | 36 (97%) | |
| T Stage | ||||
| cTa | 38 (40%) | 24 (41%) | 14 (38%) | 0.948 |
| cT1 | 50 (53%) | 30 (52%) | 20 (54%) | |
| cTis | 7 (7%) | 4 (7%) | 3 (8%) | |
| Concurrent CIS | 48 (51%) | 21 (36%) | 27 (73%) | <0.001 |
| Prior Recurrence | ||||
| No | 16 (17%) | 10 (17%) | 6 (16%) | 0.914 |
| Single recurrence | 43 (45%) | 27 (47%) | 16 (43%) | |
| Multiple recurrence | 36 (38%) | 21 (36%) | 15 (41%) | |
| Recurrence | 23 (24%) | 6 (10%) | 17 (46%) | <0.001 |
| Progression | 17 (18%) | 5 (9%) | 12 (32%) | 0.005 |
| Radical cystectomy | 14 (15%) | 5 (9%) | 9 (24%) | 0.043 |
| Death | 6 (6%) | 3 (5%) | 3 (8%) | 0.677 |
| Cancer-specific death | 4 (4%) | 2 (3%) | 2 (5%) | 0.999 |
Of 100 patients in the entire cohort, 95 had 6-week FISH results sufficient for analysis; 2 had equivocal results.
Table 2.
Patient and tumor characteristics for patients with negative findings at 3-month cystoscopy and available 3-month FISH results
| Total | Negative FISH Results | Positive FISH Results | p-value | |
|---|---|---|---|---|
| No. of patients | 67 | 39 | 28 | |
| Age: mean (±SD) | 66 ± 11.3 | 66 ± 12.2 | 65 ± 10.2 | 0.688 |
| Sex | ||||
| Female | 13 (19%) | 6 (15%) | 7 (25%) | 0.326 |
| Male | 54 (81%) | 33 (85%) | 21 (75%) | |
| Smoker (past or present) | 38 (57%) | 21 (54%) | 17 (61%) | 0.629 |
| Grade | ||||
| Low | 6 (9%) | 4 (10%) | 2 (7%) | 0.999 |
| High | 61 (91%) | 35 (90%) | 26 (93%) | |
| cT Stage | ||||
| cTa | 28 (42%) | 17 (44%) | 11 (39%) | 0.303 |
| cT1 | 37 (55%) | 22 (56%) | 15 (54%) | |
| cTis | 2 (3%) | 0 (0%) | 2 (7%) | |
| Concurrent CIS | 39 (58%) | 21 (54%) | 18 (64%) | 0.393 |
| Prior Recurrence | ||||
| No | 4 (6%) | 2 (5%) | 2 (7%) | 0.868 |
| Single recurrence | 32 (48%) | 18 (46%) | 14 (50%) | |
| Multiple recurrence | 31 (46%) | 19 (49%) | 12 (43%) | |
| Recurrence | 19 (28%) | 3 (8%) | 16 (57%) | <0.001 |
| Progression | 15 (22%) | 1 (3%) | 14 (50%) | <0.001 |
| Radical cystectomy | 13 (19%) | 1 (3%) | 12 (43%) | <0.001 |
| Death | 5 (7%) | 2 (5%) | 3 (11%) | 0.643 |
| Cancer-specific death | 3 (4%) | 0 (0%) | 3 (11%) | 0.100 |
Of 100 patients in the entire cohort who had negative findings at 3-month cystoscopy, 67 had 3-month FISH results sufficient for analysis.
Virtual Study Design using Recurrence and Progression Rates after a Positive FISH
Since the purpose of our manuscript was to provide a definition that could be used in designing clinical trials for patients who are likely to fail BCG therapy, we studied the results of various FISH permutations. The results that we found potentially most useful were results at 6 weeks and 3 months. Positive 6-week FISH results dichotomized patients as follows: 46% of patients with a positive FISH result developed tumor recurrence within 2 years versus 10% with a negative FISH result (p<0.001), while 32% of patients developed tumor progression with a positive 6-week FISH result versus 9% with a negative FISH result (p=0.003) (Table 1). Using time to event analysis, this translates to a 2-year recurrence free survival (RFS) of 52% and a 2-year progression free survival (PFS) of 83%. Positive 3-month FISH results similarly dichotomized patients: 57% of patients with a positive 3-month FISH result developed tumor recurrence within 2 years versus 8% with a negative FISH result (p<0.001), while 50% developed tumor progression within 2 years with a positive FISH result versus 3% with a negative 3-month FISH result, (p<0.001) (Table 2). With time to event analysis, this corresponds to a 2-year RFS of 43% and a 2-year PFS of 71%.
On the basis of these data, we propose that patients with positive FISH results at or before 3-month cystoscopy in the absence of visible tumor at 3-month cystoscopy can be considered to have a “molecular BCG failure”. In other words, we propose a definition of BCG failure based on the ability of FISH to predict response to BCG therapy in order to suggest a new paradigm for clinical trial enrollment (see Discussion section for more details).
Impact of CIS
It is well recognized among experts that carcinoma in situ remains a poorly understood variable when developing clinical trials. We attempted to provide some insight on this sub group by analyzing the cohort with CIS present in the primary specimen, recognizing the limitations of such subset analysis. While the presence of CIS did not predict recurrence or progression on time to event analysis, it was associated with higher rates of recurrence in general (OR = 2.86; 95% CI: 1.05–7.79; p=0.040). Of the 56 tumors with primary or concurrent CIS at baseline, 17 (30%) recurred within 2 years. In patients with CIS, positive baseline FISH results were unable to predict response to BCG (p=0.464) (71% positive after TUR alone). However, the 6-week FISH results, were associated with response to BCG: 14 of 30 patients (47%) with positive FISH results had recurrence, compared to only 3 of 25 patients (12%) with negative FISH results (p=0.006). As expected with CIS and BCG, many patients converted to a negative FISH result over the course of BCG therapy, including 9 patients with a positive 6-week FISH (1 recurrence) and 7 patients with a positive 3-month FISH (1 recurrence).
Other Variables Affecting Recurrence and Progression
As stated previously, the purpose of this manuscript is to provide a definition that can be used in designing clinical trials for patients who are likely to fail BCG therapy; for this we used the FISH results at 6 weeks and 3 months. For completeness however, we also report, herein results of univariate and multivariate analyses.
Over the 2-year period, 23 patients developed tumor recurrence. Of the clinicodemographic features listed in Table 1, on univariate analysis only the following variables predicted tumor recurrence: positive 6-week FISH result (HR = 5.45; 95% CI: 2.14–13.86; p<0.001), and positive 3-month FISH result (HR = 9.61; 95% CI, 2.789–33.11; p<0.001) and prior bladder tumor recurrence (HR 1.98, 95% CI, 1.034–3.772; p=0.039), Neither the initial stage, grade, nor presences of CIS (HR 2.27, 95% CI, 0.93–5.52; p=0.070) predicted tumor recurrence (likely because of the homogeneous nature of the cohort). In a multivariate model only the 3-month FISH (p=0.002) predicted tumor recurrence.
Over the 2-year period, 17 patients developed clinical evidence of progression (10 with muscle invasion). On univariate analysis, only the following variables predicted tumor progression: positive 6-week FISH result (HR = 4.25; 95% CI: 1.496–12.075; p=0.007), and positive 3-month FISH result (HR 24.52; 95% CI: 3.22–186.84; p=0.002) and prior bladder tumor recurrence (HR 2.19, 95% CI, 1.01–4.78; p=0.039); tumor size approached significance (HR 0.55, 95% CI, 0.298–1.031; p<0.062). In a multivariate model only the 3-month FISH (p=0.003) predicted tumor progression.
DISCUSSION
Our results show how the FISH status of a patient at 6-weeks or 3-months after the start of induction BCG therapy correlates with increased rates of recurrence and progression and hence can be used as a surrogate for clinical recurrence. On the basis of these data, we propose that positive FISH results at or before 3-month cystoscopy in the absence of visible tumor at 3-month cystoscopy be considered “molecular BCG failure”. This definition of BCG failure based on the ability of FISH to predict response to BCG therapy lends itself to a new paradigm for clinical trial design and enrollment. The use of this new definition of BCG failure would permit clinical trials to test new intravesical agents or technologies in a head-to-head comparison with BCG therapy based on early identification of patients most likely to experience BCG failure.
The proposed design of such trials is outlined in Figure 2. Patients with a positive6-week FISH (i.e., at the sixth instillation of BCG) would be counseled that they have an almost 50% chance of tumor recurrence if they continue with BCG therapy. These patients could then be offered participation in a clinical trial early, provided no disease is clinically evident at 3-month cystoscopy. We propose using 6-week FISH results for recruitment to facilitate early discussion and the logistics of registration. Eligible Patients would then be randomly assigned to an experimental treatment or control (i.e., continue BCG) and followed prospectively. Assuming a 2-year RFS of 52% for patients with positive 6-week FISH results, this head-to-head clinical trial would need approximately 106 patients with positive FISH results (53 in each arm) to detect a 20% absolute improvement in RFS (i.e. 2-year RFS of 72%) between intravesical BCG and an experimental agent (2 sided α=0.05 with 80% power).
Figure 2.
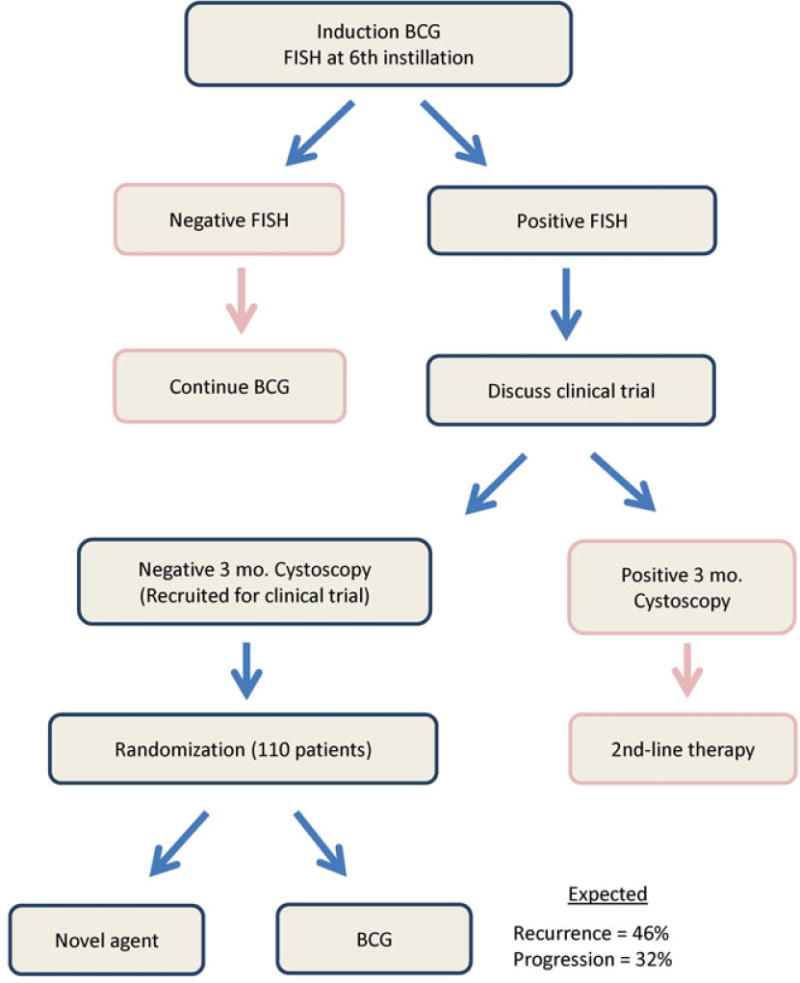
Proposed clinical trial design using FISH at 6 weeks.
While the National Comprehensive Cancer Network guidelines do presently accommodate urinary urothelial tumor markers (category 2B),7 the guidelines do not call for routine use of such markers before 3 months. Thus, alternatively, since 6 week FISH is not currently standard, a 3-month FISH result (performed as standard of care at cystoscopy) could also be used for enrollment. Assuming a 2-year RFS of 43% for patients with a positive 3-month FISH, a similarly designed clinical trial would need 122 patients (61 for each arm) to detect a 20% absolute improvement in 2-year RFS (Figure 3). Additional permutations using FISH might be possible to improve the predictive value of FISH and thus enhance recruitment. For example, if only patients with both a positive 6 week and 3 month FISH were enrolled, the 2-year RFS becomes 33%. However, due to the added complexities introduced by such a design, we would prefer to restrict our proposed definition to a single time point.
Figure 3.
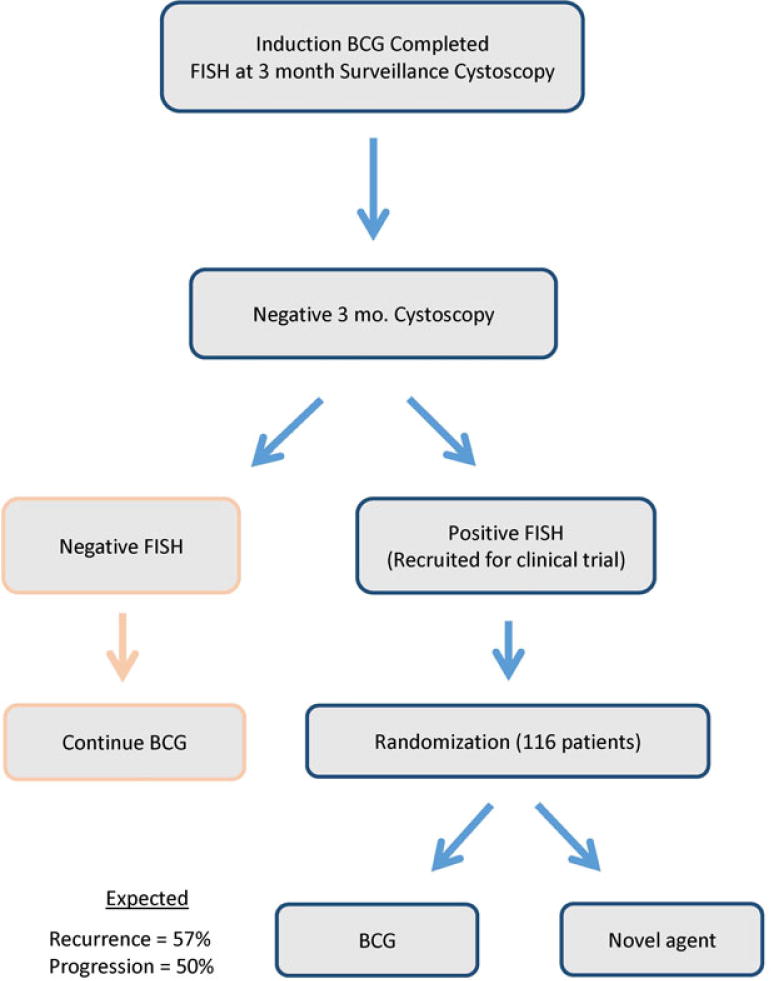
Proposed clinical trial design using FISH at 3 month cystoscopy.
There are many problems with the existing paradigm of clinical trial designs that define BCG failure after the endoscopic detection of tumor, all of which have historically led to poor recruitment. First, there is no ethical control arm short of radical cystectomy, making comparison and randomization impractical. Thus, most trials performed after traditional BCG failure are single arm studies making the interpretation of results difficult. Furthermore, only patients who accept the risks of tumor progression and forgo radical cystectomy can be enrolled, leaving only patients who adamantly refuse surgery or who are too ill to tolerate radical cystectomy. This issue is clinically relevant because patients who experience progression to muscle-invasive bladder cancer after BCG failure have worse survival than patients with de novo muscle-invasive bladder cancer. Survival rates as low as 35% among patients with progression to muscle-invasive disease after BCG therapy have been reported in multiple forums,13 including systematic reviews,14 case-control studies,15 and even in the recently published European Organization for Research and Treatment of Cancer randomized trial of different doses and durations of BCG therapy (cancer-specific mortality rate of 38.6%).16,17
The difficulties with current clinical trial designs has been recently highlighted by attempts made to study Urocidin™, a novel mycobacterial cell wall-DNA complex that has been described as having both immunologic activity and direct anticancer activity with a less toxicity than BCG. After encouraging preliminary results from Phase III trials were presented at the 2011 AUA annual meeting, Endo Pharmaceuticals and Bioniche Life Sciences were forced to abandon their planned large scale phase III trial with Urocidin™ as a result of poor recruitment and difficulties with their clinical trial design.
While our proposed clinical trial design is not without limitations, it overcomes the aforementioned obstacles because it is provides an ethical trial design offering treatment options that maintain clinical equipoise and allows randomization to BCG as a valid control arm. Most importantly, our proposed clinical trial design adheres to sound oncologic principles as patients who fail either BCG or an experimental agent still have the opportunity for radical cystectomy within an accepted time frame.
The purpose of this report is not to put forth a new clinical definition of BCG failure to supplant current conventional terminology. Rather, it is to generate a predictive definition of BCG failure in order to enhance clinical trial designs for intravesical therapy for NMIBC. We considered various modifications to improve the predictive power of FISH. First, we considered incorporating cytology results, which are readily available. However, cytology is highly subjective, further confounded by results such as “atypical” or “suspicious”, and recent publications question its reliability in bladder cancer monitoring.18 Furthermore, when cytopathologists are aware of the FISH results, it can affect their interpretation of cytology findings. The FISH assay is objective and allows consistent interpretation of positive and negative assays across institutions and hence more suited for incorporation into clinical trial design. Second, we considered expanding our analysis to include different FISH probes. However, we limited the analysis to the FDA approved UroVysion® assay to increase the likelihood of acceptance by most clinical practices and institutional review boards.
Another potential drawback of our clinical trial design is that approximately half of the enrolled patients would have been expected to respond to BCG therapy, but may instead be randomized to the experimental arm. However, since half the patients would correspondingly not respond to BCG, we submit that this might be acceptable for a disease where survival after progression is poor. Furthermore, we believe that ethical investigators will only study agents with robust pre-clinical activity and phase 2 data in such a study design. Note however, that we did not power for progression events, due to the large number of patients that would be required for such comparisons, even though a recent multicenter retrospective analysis did show that FISH positivity was an independent risk factor for progression and time to recurrence (but not likelihood of recurrence).19
While we initially considered that the presence of CIS might be a confounding factor, our analysis showed that patients with concomitant CIS can also be included in this study definition. For discussion, however, we admit that the pattern of response of CIS to BCG is different from that of papillary tumors. This is underscored by several studies showing that CIS may not be eradicated until after induction plus maintenance BCG, and that evaluating for response after induction BCG alone is premature for CIS. However, these data predate the use of FISH and, in our prospective study, there was no significant difference in the rates of prediction for FISH between lesions with and without CIS.
CONCLUSION
Our data supports a study design for patients with NMIBC that incorporates FISH as a marker of response to BCG therapy. Patients with positive 6-week or 3-month FISH results but negative findings at 3-month surveillance cystoscopy can be considered to have “molecular BCG failure.” Based on the high likelihood of tumor recurrence and progression among patients with positive FISH results, such patients can be ethically enrolled in clinical trials to compare new intravesical therapies yet maintaining the option of continued BCG therapy as an ethical control arm.
Figure 1.
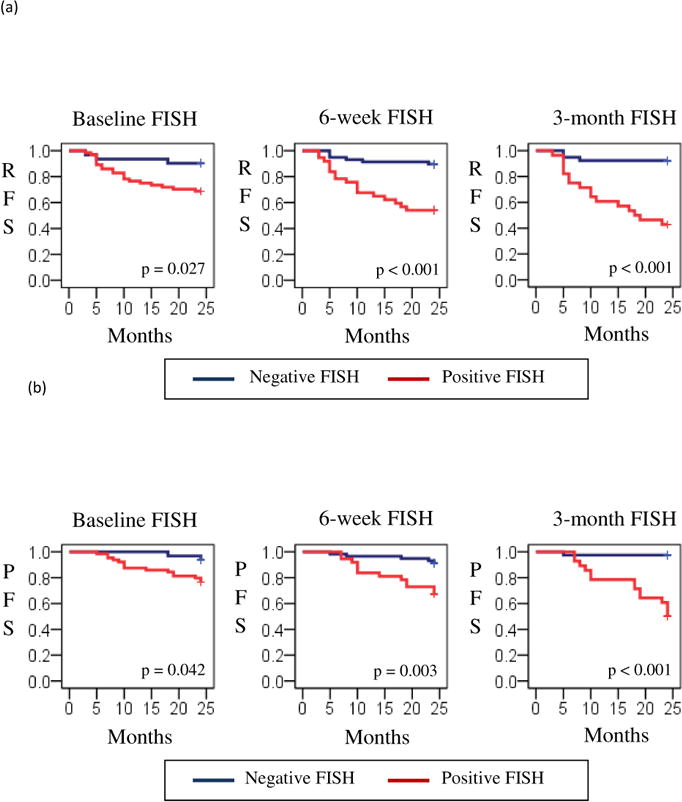
Recurrence-free survival (RFS) (a) and progression-free survival (PFS) (b) by results on early FISH assays.
Acknowledgments
This research was supported by a grant to AMK from Flight Attendant Medical Research Institute (FAMRI). The University of Texas MD Anderson Cancer Center is supported in part by a Cancer Center Support Grant (CA016672) from the National Institutes of Health.
Dr. Kamat reports grants from Flight Attendant Medical Research Institute, during the conduct of the study; and honoraria from Abbott Molecular, Sanofi Pasteur, Merck, Photocure, grants from FKD, outside the submitted work.
Dr. Grossman reports personal fees from Abbott Molecular, Heat Biologics, Nucleix, Telormedix, outside the submitted work.
Dr. Katz reports grants from The University of Texas, MD Anderson Cancer Center, during the conduct of the study.
Footnotes
Conflicts of interest
Dr. Anderson has nothing to disclose.
References
- 1.Babjuk M, Oosterlinck W, Sylvester R, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder, the 2011 update. Eur Urol. 2011;59:997. doi: 10.1016/j.eururo.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 2.Brausi M, Witjes JA, Lamm D, et al. A review of current guidelines and best practice recommendations for the management of nonmuscle invasive bladder cancer by the International Bladder Cancer Group. J Urol. 2011;186:2158. doi: 10.1016/j.juro.2011.07.076. [DOI] [PubMed] [Google Scholar]
- 3.Allard P, Bernard P, Fradet Y, et al. The early clinical course of primary Ta and T1 bladder cancer: a proposed prognostic index. Br J Urol. 1998;81:692. doi: 10.1046/j.1464-410x.1998.00628.x. [DOI] [PubMed] [Google Scholar]
- 4.Kurth KH, Denis L, Bouffioux C, et al. Factors affecting recurrence and progression in superficial bladder tumours. Eur J Cancer. 1995;31A:1840. doi: 10.1016/0959-8049(95)00287-s. [DOI] [PubMed] [Google Scholar]
- 5.Hall MC, Chang SS, Dalbagni G, et al. Guideline for the management of nonmuscle invasive bladder cancer (stages Ta, T1, and Tis): 2007 update. J Urol. 2007;178:2314. doi: 10.1016/j.juro.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Burger M, Oosterlink W, Konety B, et al. ICUD-EAU International Consultation on Bladder Cancer 2012: Non-muscle-invasive urothelial carcinoma of the bladder. Eur Urol. 2013;63:36. doi: 10.1016/j.eururo.2012.08.061. [DOI] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network. NCCN Guidelines for Bladder Cancer Version 2.2012. Accessed March 1, 2013. http://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf.
- 8.Sylvester RJ, van der MA, Lamm DL. Intravesical bacillus Calmette-Guerin reduces the risk of progression in patients with superficial bladder cancer: a meta-analysis of the published results of randomized clinical trials. J Urol. 2002;168:1964. doi: 10.1016/S0022-5347(05)64273-5. [DOI] [PubMed] [Google Scholar]
- 9.Kamat AM, Dickstein RJ, Messetti F, et al. Use of fluorescence in situ hybridization to predict response to bacillus Calmette-Guerin therapy for bladder cancer: results of a prospective trial. J Urol. 2012;187:862. doi: 10.1016/j.juro.2011.10.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sylvester RJ, van der Meijden AP, Oosterlinck W, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol. 2006;49:466. doi: 10.1016/j.eururo.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 11.Lamm DL, Blumenstein BA, Crissman JD, et al. Maintenance bacillus Calmette-Guerinimmunotherapy for recurrent TA, T1 and carcinoma in situ transitional cell carcinoma of the bladder: a randomized Southwest Oncology Group Study. J Urol. 2000;163:1124. [PubMed] [Google Scholar]
- 12.Caraway NP, Khanna A, Fernandez RL, et al. Fluorescence in situ hybridization for detecting urothelial carcinoma: a clinicopathologic study. Cancer Cytopathol. 2010;118:259. doi: 10.1002/cncy.20099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herr HW, Sogani PC. Does early cystectomy improve the survival of patients with high risk superficial bladder tumors? J Urol. 2001;166:1296. [PubMed] [Google Scholar]
- 14.van den Bosch S, Alfred Witjes J. Long-term cancer-specific survival in patients with high-risk, non-muscle-invasive bladder cancer and tumour progression: a systematic review. Eur Urol. 2011;60:493. doi: 10.1016/j.eururo.2011.05.045. [DOI] [PubMed] [Google Scholar]
- 15.Schrier BP, Hollander MP, van Rhijn BW, et al. Prognosis of muscle-invasive bladder cancer: difference between primary and progressive tumours and implications for therapy. Eur Urol. 2004;45:292. doi: 10.1016/j.eururo.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 16.Witjes JA. Maintenance bacillus Calmette-Guerin for non-muscle-invasive bladder cancer: do we finally know the best schedule? Eur Urol. 2013;63:473. doi: 10.1016/j.eururo.2012.11.023. [DOI] [PubMed] [Google Scholar]
- 17.Oddens J, Brausi M, Sylvester R, et al. Final Results of an EORTC-GU Cancers Group Randomized Study of Maintenance Bacillus Calmette-Guerin in Intermediate- and High-risk Ta, T1 Papillary Carcinoma of the Urinary Bladder: One-third Dose Versus Full Dose and 1 Year Versus 3 Years of Maintenance. Eur Urol. 2013;63:462. doi: 10.1016/j.eururo.2012.10.039. [DOI] [PubMed] [Google Scholar]
- 18.Reid MD, Osunkoya AO, Siddiqui MT, et al. Accuracy of grading of urothelial carcinoma on urine cytology: an analysis of interobserver and intraobserver agreement. Int J Clin Exp Pathol. 2012;5:882. [PMC free article] [PubMed] [Google Scholar]
- 19.Seideman C, Canter D, Kim P, et al. Multicenter evaluation of the role of UroVysion FISH assay in surveillance of patients with bladder cancer: does FISH positivity anticipate recurrence? World J Urol. 2014 Nov 25; doi: 10.1007/s00345-014-1452-9. [DOI] [PubMed] [Google Scholar]


