Abstract
Objective
A double-blind, randomized controlled trial showed that low-dose glucocorticoid therapy in pediatric ARDS patients is feasible and may improve both ventilation and oxygenation indices in these patients. However, the molecular mechanisms underlying potential changes in outcomes remain unclear. Based on these clinical findings, this study was designed to examine the effects of intravenous methylprednisolone on circulating inflammatory biomarkers in pediatric ARDS patients.
Design
Double-blind, placebo-controlled randomized trial with blood collection on study entry and day 7.
Setting
Tertiary care children’s hospital.
Patients
Children (0–18 years) with ARDS undergoing mechanical ventilation.
Interventions
35 children were randomized within 72 hours of mechanical ventilation. The glucocorticoid group received methylprednisolone 2 mg/kg loading dose followed by 1 mg/kg/day continuous infusion from days 1–7. Both groups were ventilated following the ARDSnet recommendations. WBC and differential cell counts, plasma cytokines and CRP levels, and coagulation parameters were analyzed on days 0 and 7.
Results
At study entry, the placebo group had higher IL-15 and basophil levels. On day 7, in comparison to study entry, the placebo group had lower IL-1α, IFN-γ and IL-10 levels. The glucocorticoid group had lower INF-α, IL-6, IL-10, MCP-1, G-CSF and GM-CSF levels, and higher IL-17α levels on day 7 in comparison to study entry. Total and differential cell counts remained unchanged within the placebo group between days 0 and 7, whereas in the glucocorticoid group total WBC and platelets counts were increased on day 7. Pearson’s correlation studies within the placebo and glucocorticoid groups revealed positive and negative correlations between cytokine levels, cell counts, coagulation parameters and relevant clinical parameters of disease severity identified in our previous study. Multiple regression models identified several cytokines as predictors for alterations in clinical parameters of disease severity.
Conclusion
This pilot study shows the feasibility of simultaneously measuring multiple inflammatory cytokines, cell counts and coagulation parameters in pediatric ARDS patients. We report statistical models that may be useful for future, larger trials to predict ARDS severity and outcomes.
Keywords: ARDS, lung injury, cytokines, chemokines, mediators, pediatrics, steroids, glucocorticoids, inflammation
INTRODUCTION
The clinical constellation of acute hypoxia and bilateral chest X-ray infiltrates was first described by Ashbaugh in 1967[1] and was later defined as “Acute Respiratory Distress Syndrome” (ARDS) by the American-European Consensus Conference (AECC) in 1994[2]. Although mortality rates in both the adult[3] and pediatric populations[4] are declining, substantial morbidity persists[5], resulting in a steadily increasing burden on our health care budget.
Despite the clinical consequences and health care costs associated with ARDS, the development of new therapeutic strategies has faced multiple challenges over the years. Currently, oxygen supplementation and lung-protective ventilation strategies remain the cornerstones of ARDS treatment, although ultimately both therapies can exacerbate pre-existing lung damage and promote pro-inflammatory cytokine release[6]. Multiple other therapies including nitric oxide[7], surfactant[8], prostaglandins[9], fluid balance[10] and high frequency ventilation[11] have failed to improve survival rates. To ameliorate the exaggerated pulmonary and systemic proinflammatory response occurring in ARDS patients, intravenous glucocorticoid therapy has been studied in the adult population[12]. Early initiation of low-dose glucocorticoid therapy appears to provide particular therapeutic benefits in adults by reducing lung injury scores, ventilator days and mortality rates[13].
The scarcity of new therapeutic approaches for ARDS is partly related to our lack of understanding the underlying molecular mechanisms promoting this disease. Dysregulation of inflammatory mediator secretion both locally and systemically contributes to the development of ARDS. Plasma IL-1β, IL-6, IL-8, and IL-10 levels are elevated in adult ARDS patients[14], while TNF-α and IL-6 levels are increased in the BAL fluid[15]. Importantly, both serum and BAL cytokine levels correlate with increased mortality rates[15]. The large majority of these findings were obtained in adult studies and our knowledge about changes in inflammatory markers in pediatric ARDS patients is very limited[16]. As the pulmonary and immune systems of children are still in development, differences in their immune and inflammatory responses compared to adults are to be expected.
Not only are inflammatory mediator signaling networks incredibly complex but we also lack a clear understanding of their cellular sources. While macrophages, neutrophils and lymphocytes are known to produce a variety of pro- and anti-inflammatory mediators in the lung[17, 18], we have recently confirmed that alveolar epithelial cells also secrete substantial amounts of inflammatory cytokines[19–21]. Due to the complexity of cytokine signaling and cellular interactions, which ultimately determine the inflammatory microenvironment in the lungs of ARDS patients, employing a broad-spectrum anti-inflammatory approach by using intravenous glucocorticoids constitutes a reasonable clinical approach while we continue our search for more specific molecular targets.
The effects of intravenous glucocorticoid therapy in the pediatric population, including potentially adverse consequences for the developing child, are poorly defined. We recently reported the first randomized controlled pilot trial showing the feasibility of methylprednisolone therapy in children with ARDS and potential improvements in their oxygenation, ventilation and plateau pressures[22]. However, the molecular mechanisms underlying these clinical changes remained unknown. This study builds on the changes in 5 clinical parameters identified in our previous publication[22]. These included (1) P/F ratio on day 8, (2) plateau pressures (PP) on day 2, (3) pCO2 levels on day 2, (4) racemic epinephrine requirement following extubation, and (5) O2 requirement at PICU discharge. This is the first attempt to dissect the molecular mechanisms responsible for the observed alterations in these 5 clinical parameters by determining alterations in pro- and anti-inflammatory mediator concentrations in response to early, low-dose intravenous glucocorticoid therapy. In this follow-up study our main goal consisted in identifying potential ARDS biomarkers such as cytokines, cell counts, CRP levels and coagulation parameters and to determine potential relationships between these changes and predictors of disease severity.
MATERIALS AND METHODS
Study protocol
A total of 35 children (0–18 years) diagnosed with ARDS as defined by the Berlin definition[23] were initially randomized to placebo or glucocorticoid groups within 72 hours of mechanical ventilation as described in our previous study[22] (ClinicalTrials.gov number: NCT01274260). Briefly, exclusion criteria for study enrollment were exposure to glucocorticoids at the time of screening, terminal illness, hospice care, immunosuppressed status, extensive burns, adrenal insufficiency, vasculitis, diffuse alveolar hemorrhage, invasive fungal infection, chronic liver disease, gastrointestinal bleed within the past 1 month, or conditions with an estimated 6-month mortality of >50%. The steroid and placebo groups were similarly matched (p>0.05) in regards to the following patient characteristics: sex, race, PRISM III score, PIM-2 score, P/F ratio, PEEP, tidal volume, mean airway pressure, blood gas pH. PaCO2, and number of lobes affected on chest X ray. Furthermore, ARDS etiologies were similar between the two groups with pneumonia being the most common cause, followed in order of frequency by bronchiolitis, aspiration, trauma, TRALI, near drowning, hydrocarbon ingestion, preterm birth, and asthma. The duration of mechanical ventilation was 9.74±6.62 vs 9.59±5.21 days in the glucocorticoid and the placebo group (p=0.94), respectively. Two patients died in the placebo group, whereas all survived in the glucocorticoid group (p=0.15). No patients abandoned the study. Importantly, although we enrolled a total of 35 patients into our study, the number of patients across all study days was not a constant for either group since the numbers of patients in both groups decreased with increasing number of days. Not all cytokines were present in all subjects and by day 7 some patients from each study group had improved and were no longer on a ventilator. Therefore, each table contains the number of patients in which a certain cytokine or inflammatory parameter was detected or studied.
Pediatric ARDS was defined as (1) acute onset of disease (within 7 days) that could not be explained by acute left heart failure, (2) new, bilateral infiltrates on chest X ray consistent with parenchymal pulmonary disease, and (3) P/F ratio <300[23]. Both study groups were mechanically ventilated on the Servo i ventilators (Maquet, Inc.) in a patient-regulated volume control (PRVC) mode, with or without synchronized intermittent mandatory ventilation (SIMV), and with tidal volumes of 6–8 ml/kg (based on ideal body weight in obese children and actual body weight in non-obese children), as suggested by the ARDSnet recommendations modified for children[24].
Glucocorticoid group patients received methylprednisolone 2 mg/kg loading dose followed by 1 mg/kg/day continuous infusions from day 1 to day 7. The placebo group received equivalent saline infusions.
Blood sample collection
On days 0 and 7 of the study, we collected 1 ml of whole blood in a lavender-top, K2EDTA-containing tube. The samples were manually transferred on ice within 30 min from the blood draw to the hospital laboratory and centrifuged for 10 min at 1000 × g at 4°C. Plasma samples were stored at −80°C. Clinically required WBC and differential cell counts, C-reactive protein (CRP) levels and coagulation parameters (PT, PTT, fibrinogen) from days 0 and 7 were retrospectively analyzed when available.
Luminex assay
Plasma mediator concentrations were determined using the Millipore Human Cytokine panel (Millipore, Billerica, MA) following the manufacturer’s instructions. All samples were run in duplicates (or triplicates if plasma was available); 38 mediators were assayed and concentrations were expressed in [pg/mL]: EGF, eotaxin, FGF-2, Flt3L, fractalkine, G-CSF, GM-CSF, GRO, IFN-α2, IFN-γ, IL-1α, IL-1β, IL-1RA, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12(p40), IL-12(p70), IL-13, IL-15, IL-17α, IP-10, MCP-1, MCP-3, MDC, MIP-1α, MIP-1β, sCD40L, TGF-α, TNF-α, TNF-β and VEGF.
Statistical analyses
Univariate (descriptive statistics, frequency distributions), bivariate (Fisher’s test for variability, t-tests, Pearson’s correlations) and multivariate analyses (linear regressions using the least squares method) were used to evaluate the effects of glucocorticoids in pediatric ARDS. StatPlus (AnalystSoft, Inc.) was used for generating descriptive statistics and frequency distributions for all variables (cytokines, chemokines, and growth factors) at days 0 and 7 for the placebo and glucocorticoid groups. Prism 6 (GraphPad Software, Inc.) was used for bivariate analyses comparing within and between the glucocorticoid vs. placebo groups at days 0 and 7. Depending on the data distribution, we used either the unpaired t-test (parametric) or the Mann-Whitney U test (nonparametric) for group comparisons. We also generated a correlation matrix for pairwise associations of Pearson correlation coefficients with simultaneously run t-tests. These results were used for pathway analysis of the measured mediators.
Pairs of variables demonstrating strong correlation coefficients (R ≥0.7, p ≤0.01) were used for building multivariate regression models to predict the 5 relevant clinical outcomes reported in our previous study[22], which included PaO2/FiO2 (P/F) ratio on day 8, plateau pressure and PaCO2 on day 2, racemic epinephrine following extubation, and supplemental oxygen at PICU discharge.
Bivariate correlation matrices and bar graphs were prepared using only raw data without adjustment or imputation of missing data points. Rarely, imputation of missing values for immune mediators was necessary to build the multivariate regression models explaining or contributing to glucocorticoid treatment-associated clinical outcomes. Immune mediator values falling at or below the standard curve were assigned the lowest value on the generated standard curve. Immune factors falling at or above the standard curve were assigned the highest value on the generated standard curve. Since these were exploratory hypothesis-generating analyses we did not make corrections for multiple comparisons between groups.
The number of subjects (n) per group is listed in each table legend. We selected a 95% confidence interval.
RESULTS
1. Plasma cytokine levels, WBC counts, CRP levels, and coagulation tests
a. Between-group differences
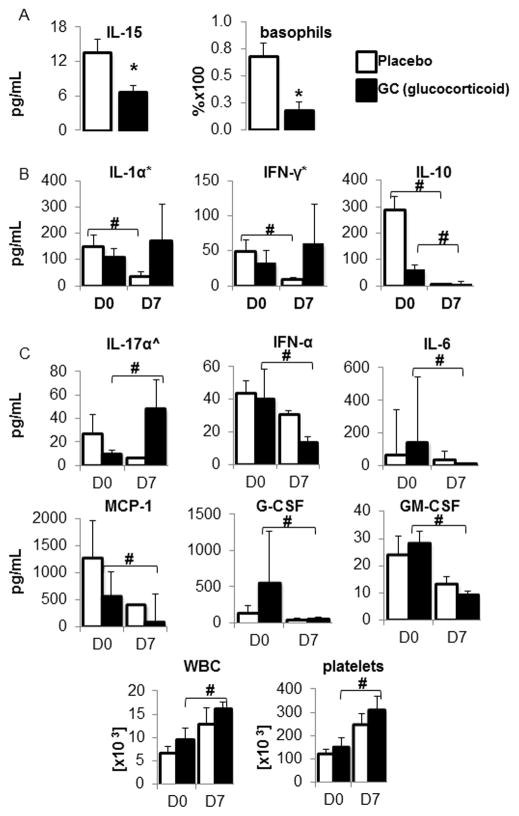
No baseline differences in any of the 38 cytokines occurred on day 0 between the placebo and GC groups, except for higher IL-15 levels in the placebo group (Table 1A and Figure 1A). We found no differences in total WBC counts (including neutrophils, monocytes, lymphocytes, eosinophils and basophils), platelet counts, CRP levels and coagulation parameters (including PT, PTT and fibrinogen levels) between the placebo and GC groups on day 0.
Table 1. Comparison of cytokine levels and cell counts between placebo and the glucocorticoid (GC) groups on days 0 and 7.
Table 1A shows an increased IL-15 concentration on day 0 and Table 1B an increased basophil count on day 7 in the placebo group; n=number of subjects. A p-value <0.05 was considered significant.
| A | ||
|---|---|---|
| Placebo n=8 |
GC n=11 |
P |
| Day 0 | ||
| IL - 15↑ | - | 0.012 |
| B | ||
|---|---|---|
| Placebo n=5 |
GC n=6 |
P |
| Day 7 | ||
| basophils ↑ | - | 0.008 |
Figure 1. Comparisons of inflammatory meditators.
Figure 1A shows comparisons of cytokine levels and cell counts between the placebo and the glucocorticoid (GC) groups on days 0 and 7. Using an unpaired 2-tailed t-test with a p-value of <0.05 as our level of significance (*), the only alteration in cytokine levels was an elevated IL-15 concentration [pg/mL] in the placebo group on day 0 and a higher basophil count in the placebo group on day 7; (mean±SEM).
Figure 1B shows decreased IL-1α, IFN-γ and IL-10 levels in the placebo group on day 7 (D7), and decreased IL-10 levels in the GC group on day 7. Cytokine concentrations are depicted in pg/mL (mean±SEM). A p-value of <0.05 was considered significant (#).
Figure 1C shows elevated IL-17α levels and decreased IFN-α, IL-6, MCP-1, G-CSF and GM-CSF levels on day 7 (D7) in the glucocorticoid (GC) group. Cytokine concentrations are depicted in pg/mL (mean±SEM). IL-17α levels were analyzed using an unpaired t-test (indicated by ^). The rest of the cytokine levels were analyzed using a 2-tailed Mann-Whitney test based on a Fisher test and data are depicted as median±SE. A p-value of <0.05 was considered significant (#).
On day 7, the difference in IL-15 levels between the placebo and the GC groups was no longer present. Although total WBC and platelet counts remained unchanged between the two groups, basophil percentages were elevated on day 7 in the placebo group (Table 1B and Figure 1A). We had insufficient data points for statistical analysis of CRP levels, PT, PTT, and fibrinogen levels on day 7.
b. Within-group (time-dependent) changes
By day 7, compared to study entry, the placebo group (Table 2 and Figure 1B) had lower IL-1α, IFN-γ, and IL-10 levels but no changes occurred in total and differential WBC (neutrophils, monocytes, lymphocytes, eosinophils and basophils) counts, platelet counts, PT, PTT, fibrinogen or CRP levels.
Table 2.
Comparison of cytokine levels within the placebo group between days 0 (D0) and 7 (D7): ↓ arrows indicate a decrease in cytokine levels. (*) indicate data analyzed using an unpaired 2-tailed t-test, whereas IL-10 levels were analyzed using a 2-tailed Mann-Whitney test based on a Fisher test. A p-value of <0.05 was considered significant (#). n=number of subjects.
| Placebo Days 0 vs 7 | ||
|---|---|---|
| Cytokines: | p | n: day 0, day 7 |
| IL–1a* ↓ | 0.036 | 6,6 |
| INF–y*↓ | 0.037 | 6,6 |
| IL–10 ↓ | 0.002 | 7,6 |
By day 7, compared to study entry, the GC group (Table 3, Figures 1B and 1C) had increased IL-17α levels but lower IFN-α, IL-6, IL-10, MCP-1, G-CSF and GM-CSF levels. The GC group also showed increased platelet and total WBC counts, without any changes in differential WBC counts.
Table 3.
Comparison of cytokine levels within the glucocorticoid (GC) group between days 0 (D0) and 7 (D7): ↑ and ↓ arrows indicate an increase or decrease in cytokine levels, respectively. IL-17α levels were analyzed using an unpaired t-test (indicated by ^) and are depicted as mean±SEM. The rest of the cytokine levels were analyzed using a 2-tailed Mann-Whitney test based on a Fisher test. A p-value of <0.05 was considered significant (#). n=number of subjects.
| GC Days 0 vs 7 | ||
|---|---|---|
| Cytokines: | p | n: day 0, day 7 |
| IL-17a^ ↑ | 0.045 | 5,2 |
| INF-α ↓ | 0.026 | 6,6 |
| IL-6 ↓ | 0.003 | 7,6 |
| IL-10 ↓ | 0.035 | 7,6 |
| MCP-1 ↓ | 0.004 | 7,8 |
| G-CSF ↓ | 0.004 | 4,8 |
| GM-CSF ↓ | 0.026 | 7,7 |
| WBCs ↑ | 0.041 | 8,7 |
| platelets ↑ | 0.034 | 8,7 |
2. Pairwise correlations of serum cytokines and other parameters
Candidate biomarkers demonstrating differences between the placebo and GC groups, or between days 0 and 7 within these groups, were analyzed as Pearson correlations. Tables 4 and 5 depict positive and negative relationships between cytokines, cell counts, non-cellular inflammatory markers (PT, PTT, fibrinogen, CRP), and the 5 relevant clinical outcomes identified in our previous study[22], specifically: (1) P/F ratio on day 8, (2) plateau pressures (PP) on day 2, (3) PCO2 levels on day 2, (4) racemic epinephrine requirement following extubation, and (5) O2 requirement at PICU discharge.
Table 4.
Placebo group Pearson’s correlation table showing pairwise comparisons of cytokine levels, cell counts and clinical parameters of disease severity: r is the Pearson’s correlation coefficient. A p-value of <0.01 was considered significant and was derived from a paired, 2-tailed t-test. n=number of subjects (Day 0: cytokines n=8–18, cell counts n=18, clinical parameters of disease severity n=17. Day 7: cytokines n=3–7, cell counts n=7, clinical parameters of disease severity n=7).
| IL-15 | ||
|---|---|---|
| Placebo Day 0 | r | p |
| monocytes | −1.0 | 0.001 |
| Placebo Day 7 | r | p |
| MIP-1β | 1.0 | 0.007 |
| O2 at discharge | 1.0 | 0.001 |
| Racemic epi | 1.0 | 0.001 |
| IL-1α | ||
|---|---|---|
| Placebo Day 0 | r | p |
| eotaxin | 0.9 | 0.001 |
| GM-CSF | 1.0 | ≤0.000 |
| IL-10 | 1.0 | ≤0.000 |
| IL-7 | 1.0 | 0.002 |
| MIP-1α | 0.8 | 0.005 |
| MIP-1β | 0.9 | 0.001 |
| TNF-α | 0.9 | ≤0.000 |
| lymphocytes | 0.8 | 0.008 |
| O2 at discharge | 0.8 | 0.008 |
| IFN-y | ||
|---|---|---|
| Placebo Day 0 | r | p |
| Flt-3L | 1.0 | 0.002 |
| fractalkine | 0.7 | 0.002 |
| IL-17α | 0.9 | 0.002 |
| IL-1RA | 0.7 | 0.007 |
| IP-10 | 0.9 | ≤0.000 |
| Placebo Day 7 | r | p |
| racemic epi | 0.9 | 0.006 |
| IL-10 | ||
|---|---|---|
| Placebo Day 0 | r | p |
| eotaxin | 0.9 | ≤0.000 |
| Flt-3L | 1.0 | 0.003 |
| GM-CSF | 0.9 | ≤0.000 |
| IL-1RA | 0.7 | ≤0.000 |
| IL-1α | 1.0 | ≤0.000 |
| IL-6 | 0.8 | ≤0.000 |
| IL-7 | 1.0 | ≤0.000 |
| IP-10 | 0.8 | ≤0.000 |
| MCP-1 | 0.7 | 0.001 |
| MIP-1β | 0.9 | ≤0.000 |
| TNF-α | 0.9 | ≤0.000 |
| PT | 1.0 | 0.001 |
| PTT | 1.0 | ≤0.000 |
| O2 at transfer | 0.8 | ≤0.000 |
| Placebo Day 7 | r | p |
| FLt-3L | 1.0 | 0.008 |
| IL-6 | 1.0 | 0.003 |
| IL-8 | 1.0 | ≤0.000 |
| MCP-1 | 1.0 | 0.001 |
| MIP-1α | 1.0 | 0.007 |
| O2 at discharge | 1.0 | ≤0.000 |
Table 5.
Glucocorticoid (GC) group Pearson’s correlation table showing pairwise comparisons of cytokine levels, cell counts and clinical parameters of disease severity: r is the Pearson’s correlation coefficient. A p-value of <0.01 was considered significant and was derived from a paired, 2-tailed t-test. n=number of subjects (Day 0: cytokines n=8–16, cell counts n=16, clinical parameters of disease severity n=16. Day 7: cytokines n=6–8, cell counts n=8, clinical parameters of disease severity n=8).
| IL-17α | ||
|---|---|---|
| GC Day 0 | r | p |
| IL-2 | 1.0 | 0.008 |
| IFN-α | ||
|---|---|---|
| GC Day 0 | r | p |
| IL-1RA | 0.8 | 0.002 |
| IL-7 | 0.8 | 0.008 |
| IL-8 | 0.9 | ≤0.000 |
| MCP-1 | 0.8 | 0.001 |
| monocytes | 0.7 | 0.007 |
| GC Day 7 | r | p |
| monocytes | 0.9 | 0.008 |
| IL-10 | ||
|---|---|---|
| Steroid Day 0 | r | p |
| EGF | 0.7 | 0.007 |
| fractalkine | 0.8 | 0.002 |
| INF-α | 0.7 | 0.006 |
| GRO | 0.7 | 0.003 |
| IL-1RA | 0.7 | 0.004 |
| IL-8 | 0.7 | 0.008 |
| MCP-1 | 0.7 | 0.003 |
| MIP-1α | 0.7 | 0.006 |
| GC Day 7 | r | p |
| IL-15 | 1.0 | 0.009 |
| PP Day 2 | 0.9 | 0.004 |
| MCP-1 | ||
|---|---|---|
| GC Day 0 | R | p |
| EGF | 0.8 | 0.002 |
| FGF-2 | 0.8 | 0.001 |
| eotaxin | 0.9 | ≤0.000 |
| GM-CSF | 0.9 | ≤0.000 |
| fractalkine | 0.8 | ≤0.000 |
| IFN-α | 0.8 | 0.001 |
| GRO | 0.9 | ≤0.000 |
| IL-10 | 0.7 | 0.003 |
| IL-1RA | 1.0 | ≤0.000 |
| IL-8 | 0.7 | 0.001 |
| MIP-1α | 0.8 | 0.008 |
| MIP-1β | 0.7 | 0.006 |
| basophils | 0.9 | ≤0.000 |
| GC Day 7 | r | p |
| EGF | 1.0 | 0.002 |
| FGF-2 | 1.0 | 0.002 |
| TGF-α | 1.0 | 0.008 |
| IL-8 | 1.0 | ≤0.000 |
| VEGF | 0.9 | 0.003 |
| PTT-Day 0 | 0.9 | 0.005 |
| G-CSF | ||
|---|---|---|
| GC Day 7 | r | p |
| neutrophils | −0.9 | 0.008 |
| eosinophils | 0.9 | 0.005 |
| GM-CSF | ||
|---|---|---|
| GC Day 0 | r | p |
| eotaxin | 0.7 | 0.007 |
| GRO | 0.8 | ≤0.000 |
| IL-8 | 0.8 | ≤0.000 |
| MCP-1 | 0.9 | ≤0.000 |
| EGF | 0.9 | 0.001 |
| FGF-2 | 0.8 | ≤0.000 |
| IFN-α | 0.9 | ≤0.000 |
| IL-1RA | 0.9 | ≤0.000 |
| IL-10 | 0.7 | 0.0074 |
| GC Day 7 | r | p |
| neutrophils | −0.9 | 0.008 |
| eosinophils | 0.9 | 0.006 |
| IL-6 | ||
|---|---|---|
| GC Day 0 | r | p |
| EGF | 0.9 | 0.002 |
| IL-8 | 0.8 | 0.002 |
| monocytes | 0.7 | 0.004 |
a. In the Placebo Group
Although only IL-15 levels were increased on day 0 in the placebo group compared to the GC group, IL-15 positively correlated with MIP-1β levels on day 7 and negatively with monocyte counts on day 0 (Table 4). O2 requirement at ICU discharge and racemic epinephrine after extubation were also positively correlated with IL-15 levels on day 7.
Other cytokines of particular interest were IL-1α, IFN-γ and IL-10 since they were decreased on day 7 (Table 2). IL-1α levels in the placebo group on day 0 positively correlated with eotaxin, GM-CSF, IL-10, IL-7, MIP-1α, MIP-1β and TNF-α levels. Interestingly, IL-1α levels positively correlated with the anti-inflammatory cytokine IL-10 but not with the WBC counts, CRP levels or coagulation parameters (PT, PTT, fibrinogen). O2 requirement at ICU discharge also positively correlated with IL-1 α levels on day 0.
IFN-γ levels on day 0 positively correlated with Flt-3L, fractalkine, IL-17α, IL-1RA and IP-10 levels. On day 7, IFN-γ levels positively correlated with racemic epinephrine requirement after extubation.
IL-10 levels on day 0 positively correlated with eotaxin, Flt-3L, GM-CSF, IL-1RA, IL-1α, IL-6, IL-7, IP-10, MCP-1, MIP-1β and TNF-α levels, as well as with PT and PTT levels. On day 7, IL-10 levels in the placebo group positively correlated with Flt-3L, IL-6, IL-8, MCP-1 and MIP-1α levels. Interestingly, IL-10 levels on both days 0 and 7 positively correlated with O2 requirement at ICU discharge in the placebo group.
b. In the GC Group
Cytokines of particular interest were IL-17α, IFN-α, IL-10, IL-6, MCP-1, G-CSF and GM-CSF since their levels were altered on day 7 compared to day 0 (Table 3).
IL-17α positively correlated with IL-2 levels on day 0 (Table 5), but not with any other cytokines, cell counts, CRP levels, coagulation parameters, or clinical outcomes.
On day 0, IFN-α levels positively correlated with IL-1RA, IL-7, IL-8, MCP-1 levels and monocyte counts (Table 5). This positive correlation between IFN-α levels and monocyte counts persisted on day 7.
IL-10 levels positively correlated on day 0 with EGF, fractalkine, IFN-α, GRO, IL1RA, IL-8, MCP-1, and MIP-1α, whereas on day 7, IL-10 levels positively correlated with IL-15 levels (Table 5). Interestingly, IL-10 levels on day 7 positively correlated with PPs on day 2.
IL-6 levels positively correlated with EGF, IL-8 and monocyte counts on day 0 (Table 5), but not with any clinical outcomes on days 0 or 7.
MCP-1 levels positively correlated on day 0 with EGF, FGF-2, eotaxin, GM-CSF, fractalkine, IFN-α, GRO, IL-10, IL-1RA, IL-8, MIP-1α, MIP-1β and basophil levels (Table 5). On day 7, the positive correlation between MCP-1 and EGF, FGF-2 and IL-8 persisted but MCP-1 also positively correlated with TGF-α and VEGF levels. MCP-1 levels on day 7 also correlated with PTT levels on day 0.
On day 7, we found a negative correlation between G-CSF levels and neutrophil counts but a positive correlation between G-CSF levels and eosinophil counts (Table 5).
GM-CSF levels positively correlated on day 0 with several chemokines (eotaxin, GRO, IL-8, MCP-1), cytokines (IFN-α, IL-1RA and IL-10) and growth factors (EGF, FGF-2) in the GC group (Table 5). Similar to G-CSF, on day 7 GM-SCF levels inversely correlated with neutrophil counts but positively correlated with eosinophil counts. None of the clinical parameters of interest correlated with GM-CSF levels on days 0 or 7.
3. Cytokines, cell counts, CRP levels and coagulation parameters as predictors for clinical outcomes
Based on the alterations in 5 clinical outcomes identified in our previous study[22], (P/F ratio on day 8, plateau pressures (PP) on day 2, PCO2 levels on day 2, racemic epinephrine requirement following extubation, and O2 requirement at PICU discharge), we set these clinical outcomes as dependent variables and used pairwise correlations from Tables 4 and 5 as predictors for these outcomes (Tables 6A–C).
Table 6.
Tables 6A and B. Multiple regression models based on clinical parameters of disease severity identified in our previous study[22] using the least squared method.
Table 6C reports the Pearson’s r, adjusted r2 and p-ANOVA values for the data in Tables 6A and B.
| Tables 6A | |||
|---|---|---|---|
| P/F ratio day 8 | |||
| vs placebo D0, n=7 | Coefficients | SE | p |
| Intercept | 227 | 14.7 | ≤0.000 |
| FGF-2 | −2.480 | 0.266 | 0.001 |
| IL-7 | 11.4 | 1.910 | 0.004 |
| vs placebo D7, n=5 | Coefficients | SE | P |
| none | |||
| vs GC, n=9 | Coefficients | SE | p |
|---|---|---|---|
| Intercept | 119.044 | 41.933 | 0.025 |
| WBC | 5.386 | 5.956 | 0.396 |
| Neutrophils | 1.855 | 1.283 | 0.192 |
| vs GC D7, n=7 | Coefficients | SE | p |
| Intercept | 456.169 | 23.987 | ≤0.000 |
| fractalkine | −1.256 | 0.360 | 0.025 |
| lymphocytes | −2.419 | 0.646 | 0.020 |
| PP day 2 | |||
|---|---|---|---|
| vs placebo D0, n=18 | Coefficients | SE | P |
| none | |||
| vs placebo D7, n=17 | Coefficients | SE | P |
| Intercept | 7.743 | 0.497 | 0.001 |
| IL-10 | 0.115 | 0.006 | ≤0.000 |
| IL-12P70 | −0.005 | 0.001 | 0.002 |
| lymphocytes | 0.071 | 0.004 | ≤0.000 |
| vs GC D0, n=15 | Coefficients | SE | p |
|---|---|---|---|
| none | |||
| vs GC D7, n=8 | Coefficients | SE | p |
| none |
| PaCO2 day 2 | |||
|---|---|---|---|
| vs placebo D0, n=18 | Coefficients | SE | P |
| none | |||
| vs placebo D7, n=17 | Coefficients | SE | P |
| none | |||
| vs GC D0, n=15 | Coefficients | SE | P |
|---|---|---|---|
| none | |||
| vs GC D7, n=8 | Coefficients | SE | P |
| none |
| Tables 6B | |||
|---|---|---|---|
| Racemic epinephrine | |||
| vs placebo D0, n=17 | Coefficients | SE | P |
| Intercept | 0.755 | 0.216 | 0.004 |
| MCP-1 | ≤0.000 | ≤0.000 | 0.016 |
| lymphocytes | 0.015 | 0.005 | 0.012 |
| vs placebo D7, n=7 | Coefficients | SE | P |
| Intercept | 0.175 | 0.154 | 0.318 |
| IFN-y | 0.083 | 0.021 | 0.017 |
| lymphocytes | 0.017 | 0.006 | 0.039 |
| vs GC D0, n=16 | Coefficients | SE | p |
|---|---|---|---|
| none |
| vs GC D7, n=8 | Coefficients | SE | p |
|---|---|---|---|
| none |
| O2 at discharge | |||
|---|---|---|---|
| vs placebo D0, n=17 | Coefficients | SE | P |
| Intercept | 2.017 | 0.008 | ≤0.000 |
| Flt-3L | −0.002 | ≤0.000 | 0.001 |
| IL-10 | ≤0.000 | ≤0.000 | 0.001 |
| IL-1RA | ≤0.000 | ≤0.000 | ≤0.000 |
| IL-6 | ≤0.000 | ≤0.000 | 0.004 |
| MCP-1 | ≤0.000 | ≤0.000 | 0.013 |
| MIP-1β | 0.001 | ≤0.000 | ≤0.000 |
| lymphocytes | ≤0.000 | ≤0.000 | 0.007 |
| vs placebo D7, n=7 | Coefficients | SE | P |
| Intercept | 1.933 | 0.004 | ≤0.000 |
| eotaxin | ≤0.000 | ≤0.000 | 0.010 |
| Fit-3L | ≤0.000 | ≤0.000 | 0.003 |
| IL-10 | −0.002 | ≤0.000 | 0.008 |
| IL-15 | 0.027 | 0.001 | 0.001 |
| vs GC D0, n=16 | Coefficients | SE | P |
|---|---|---|---|
| none |
| vs GC D7, n=6 | Coefficients | SE | P |
|---|---|---|---|
| Intercept | 3.711 | 0.157 | ≤0.000 |
| PTT | −0.009 | 0.005 | 0.168 |
| Table 6C | |||
|---|---|---|---|
| P/F ratio day 8 | r | Adjusted r2 | p-ANOVA |
| vs placebo day 0 | 0.097 | 0.935 | 0.002 |
| vs GC day 0 | 0.833 | 0.694 | 0.016 |
| vs placebo day 7 | 0.978 | 0.932 | 0.002 |
| vs GC day 7 | 0.978 | 0.932 | 0.002 |
| PP day 2 | |||
| vs placebo day 0 | none | - | - |
| vs GC day 0 | none | - | - |
| vs placebo day 7 | 1 | 1 | ≤0.000 |
| vs GC day 7 | none | - | - |
| PaCO2 day 2 | |||
| vs placebo day 0 | none | - | - |
| vs GC day 0 | none | - | - |
| vs placebo day 7 | none | - | - |
| vs GC day 7 | none | - | - |
| Racemic epinephrine | |||
| vs placebo day 0 | 0.809 | 0.604 | 0.001 |
| vs GC day 0 | none | - | - |
| vs placebo day 7 | 0.978 | 0.936 | 0.002 |
| vs GC day 7 | none | - | - |
| O2 at transfer | |||
| vs placebo day 0 | 1 | 1 | ≤0.000 |
| vs GC day 0 | none | - | - |
| vs placebo day 7 | 1 | 1 | ≤0.000 |
| vs GC day 7 | 0.991 | 0.970 | 0.002 |
In the placebo group, FGF levels on day 0 were negative predictors whereas IL-7 levels were positive predictors for improved P/F ratios on day 8 (Table 6A). In the GC group, WBC and neutrophil counts on day 0 positively predicted improved P/F ratios on day 8, whereas fractalkine levels and lymphocyte counts on day 7 were negative predictors.
No variables from day 0 were predictive of plateau pressures in either the placebo or the GC group (Table 6A). On day 7, IL-10 levels and lymphocyte counts were positive predictors for plateau pressures in the placebo group, whereas IL-12(p70) levels were a negative predictor.
Neither cytokines, nor cell counts, coagulation parameters, nor CRP levels were predictive of PaCO2 levels on day 2 in the placebo or GC group (Table 6A).
In the placebo group, MCP-1 levels on day 0 and IFN-γ levels on day 7 were positive predictors for racemic epinephrine requirement as were lymphocyte counts on both days 0 and 7 (Table 6B).
In the placebo group, several cytokines (IL-10, IL-1RA, IL-6, MCP-1, MIP-1β) and lymphocyte counts from day 0 were positive predictors for supplemental O2 requirement at ICU discharge, whereas Flt-3L and IL-17α levels were negative predictors (Table 6B). On day 7, eotaxin, Flt-3L and IL-15 levels were positive predictors of O2 requirement at ICU discharge, whereas IL-10 levels were a negative predictor. In the GC group, no variables were predictive of O2 requirement at ICU discharge on day 0, but PTT levels were a negative predictor on day 7.
Table 6C demonstrates the r, adjusted r2 and p-ANOVA values for the described alterations in clinical parameters and the different groups and study days.
DISCUSSION
Based on our recent pilot trial describing the effects of low-dose glucocorticoid (GC) therapy in early pediatric ARDS[22], we identified 5 clinical parameters of interest, namely P/F ratio on day 8, plateau pressures (PP) on day 2, PCO2 levels on day 2, racemic epinephrine requirement following extubation, and O2 requirement at PICU discharge. We now designed this study to analyze inflammatory mediators, WBC and differential cell counts, CRP levels and coagulation factors between placebo- and GC-treated children on study days 0 and 7, and to determine if these parameters could explain the alterations in 5 clinical outcomes reported in our previous study[22]. Despite following all patients for up to 28 days, the number of samples collected past day 7 was too small for statistical analysis. However, since in adults with ARDS the benefits of GC treatment occurred as far out as 32 days[25], future trials should determine the progression or resolution of inflammatory processes past the first week of treatment.
The pathophysiology of ARDS is not limited to the lung but is associated with a systemic inflammatory response that provides a rationale for systemic GC therapy[26]. Interestingly, non-resolving ARDS has been linked to GC resistance[27], while prolonged low-dose GC therapy downregulated systemic inflammation[26]. Of note, all our measurements were performed in plasma and not BAL samples, since BAL is not routinely performed in pediatric ARDS patients and often has no diagnostic or therapeutic value. In addition, due to the heterogeneous pattern of lung disease in ARDS, the technical difficulty of performing BAL in small children and the high dilution factor of BAL fluid in a small pediatric lung, this procedure is not standard practice in acutely ill children with ARDS.
The only baseline difference in cytokine levels between the two groups was a higher IL-15 level in the placebo group (Table 1A and Figure 1A). IL-15 orchestrates T-cell responses during viral infections[28] and promotes T cell differentiation[29]. Interestingly, in our previous study[22], pneumonia and bronchiolitis were the two most common etiologies for ARDS in both study groups. Although IL-15 establishes homeostasis of NK and CD8+ T cells, emerging literature also links IL-15 to anti-viral T-cell responses in acute infections. In fact, IL-15 KO mice showed lower mortality following influenza infection despite no changes in viral loads[30] and the combination of IL-15 with hydrocortisone was a particularly powerful activator of NK cells[31]. In our study, lymphocyte counts were similar at baseline and at day 7, whereas total WBC and platelet counts were elevated on day 7 in the GC group (Table 3 and Figure 1C). Systemic GC treatment may plausibly cause increased bone marrow release of WBCs, decreased vascular emargination and decreased lung infiltration[32]. To evaluate this hypothesis, plasma, interstitial and BAL cell counts would need to be collected simultaneously, which may be very challenging in small children. Of note, in adults, methylprednisolone reduced BAL neutrophilia and albumin levels[33]. Our Pearson’s correlations revealed an inverse relationship between IL-15 and monocytes on day 0, whereas IL-15 positively correlated with MIP-1β on day 7 (Table 4).
Furthermore, IL-15 levels positively correlated with racemic epinephrine use after extubation and supplemental O2 at ICU discharge (Table 4), two parameters of interest identified in our previous study[22]. It is possible that although total lymphocyte counts were unchanged, more lymphocytes were activated by the elevated IL-15 levels in the placebo group, resulting in increased O2 and racemic epinephrine requirements. This hypothesis may be supported by the fact that IL-1α and IFN-γ, other T-cell cytokines, also showed a positive correlation with O2 at ICU discharge and racemic epinephrine requirements in the placebo group (Table 4). The multiple regression model (Tables 6A–C) revealed that in the placebo group on day 7, IL-15 levels positively correlated with O2 requirement at ICU discharge, but not with other clinical parameters of disease severity.
The increased IL-17α levels in the GC group on day 7 also merit further discussion (Table 3 and Figure 1C). IL-17α is closely linked to IL-22 as both cytokines coordinate aspects of innate lung immunity[34]. A major source for these cytokines during acute infections are γδ-T cells and NK cells, whereas CD4+ T helper (Th17) cells contribute more to vaccine-induced immunity[34]. With the discovery of Th17 cells, a role for IL-17α in ARDS was proposed[35]. In the lung epithelium, the primary target for IL-17α, this cytokine stimulates the production of antimicrobial proteins, neutrophil chemoattractants and macrophage differentiation, ultimately promoting pulmonary fibrosis[36].
Some investigators found that early activation of the IL-1β/IL-17α axis resulted in a proinflammatory effect and increased pulmonary fibrosis [37], whereas others showed that a lack of γδT cell-derived IL-17α actually increased lung fibrosis[38]. In a rodent sepsis model, IL-17α neutralization improved survival by decreasing neutrophil infiltration, IL-6 and TNF-α levels[36]. Therefore, with our current knowledge a final pro- or anti-inflammatory role cannot yet be assigned to IL-17α and the timing of IL-17α peaks may determine its function in a particular environment. Interestingly, IL-15, the only cytokine difference between the study groups, can decrease IL-17α levels and IL-17α-mediated lung injury[39].
In a rodent model of LPS-induced ARDS, methylprednisolone reduced IL-17α levels and ameliorated lung injury[40]. Although IL-17α was unchanged between the study groups, its levels were increased in the GC group on day 7 (Table 3 and Figure 1C). We speculate that an increase in IL-17α in the GC group could be related to the clinical improvements reported in our previous study[22]. Interestingly, total WBC but not WBC differential counts were elevated on day 7 in the GC group (Table 3 and Figure 1C). It is conceivable that the increased IL-17α levels were caused by increased activation of lymphocytes without changes in differential lymphocyte counts. Although we attempted to rescue lymphocytes from frozen buffy coat samples to study the Th17 population, we were unable to obtain adequate cell numbers. However, fresh lymphocytes for Th17 subtyping should be collected in future studies.
We also found a positive correlation of IL-17α with IL-2 levels in the GC group on day 0 (Table 5). IL-2, another T-cell cytokine, regulates T-cell proliferation, including Tregs, which in turn regulate Th17 cells[41]. In our multiple regression model (Table 6), IL-17α was a positive predictor for an increased O2 requirement at ICU discharge in the placebo group, potentially indicating a proinflammatory role for IL-17α.
The alterations in IL-10 levels also appeared intriguing. IL-10 is generally considered an anti-inflammatory cytokine and its role in ARDS is well recognized. We found no differences in IL-10 levels between the study groups despite some improvements in clinical disease parameters (P/F ratio, PPs, racemic epinephrine requirement, O2 requirement at ICU discharge) as shown in our previous study[22]. Nevertheless, we found a decrease in IL-10 levels within both groups on day 7 (Tables 2 and 3, Fig 1B), potentially coupled with a simultaneous downregulation of several proinflammatory cytokines, such as IL-1α and IFN-γ in the placebo group, and IL-6 and neutrophil chemoattractants (MCP-1, G-CSF and GM-CSF) in the GC group. Despite lower neutrophil chemoattractant levels in the GC group, differential neutrophil counts were unchanged on day 7. Elevated total WBC and platelet counts may again be more related to a GC-induced release of hematopoietic cells and decreased tissue emargination than chemotaxis. Nevertheless, a potential relationship between neutrophils and IL-10 is possible, since in the placebo group several chemoattractants, including eotaxin, GM-CSF and MCP-1 on day 0, and IL-8 and MCP-1 on day 7 positively correlated with IL-10 levels (Table 4). In the GC group, IL-10 levels positively correlated with neutrophil chemoattractants (GM-CSF, GRO, IL-8 and MCP-1) before (day 0) but not after GC therapy (day 7).
Interestingly, a positive correlation between IL-10 levels and O2 requirement at ICU discharge, one of the clinical parameters of interest identified in our previous study[22], occurred in the placebo group on both days 0 and 7 (Table 4) and our multiple regression model identified IL-10 as a positive predictor for O2 requirement at ICU discharge on both days 0 and 7 (Table 6), supporting a beneficial role of IL-10 in the resolution of ARDS.
While IL-10 levels positively correlated with plateau pressures in the GC group on day 7 (Table 5), in our multiple regression model (Table 6), IL-10 levels on day 7 predicted improved plateau pressures in the placebo but not the GC group. Thus, it remains unclear how IL-10 relates to the GC-mediated improvement in plateau pressures observed in our previous study[22].
Clear limitations of this pilot study consist in its small sample size and the lack of functional assays uncovering the mechanistic and functional consequences of the mediators measured. While our original study[22] was designed to show feasibility of patient recruitment, randomization and sample collection, in this follow-up study our main goal consisted in identifying potential ARDS biomarkers such as cytokines, cell counts, CRP levels and coagulation parameters. Due to these limitations, we caution the reader to not draw any major conclusions on the effects of GC therapy on clinical outcomes in pediatric ARDS patients while the specific molecular mechanisms underlying potential GC effects in pediatric ARDS remain to be unraveled. Nevertheless, this study unveiled that inflammatory mediators can be successfully measured in pediatric patients with commercially available techniques. Our correlation and regression models can aid future studies to focus on a more concise number of molecular targets and encourage the critical mind to speculate on new potential targets for ARDS therapies.
Highlights.
A recent RCT in pediatric ARDS patients showed that low-dose glucocorticoid therapy in pediatric ARDS patients is feasible and may improve both ventilation and oxygenation indices.
The molecular mechanisms underlying potential changes in these outcomes remained unclear.
This study was designed to examine the effects of intravenous methylprednisolone on circulating inflammatory biomarkers in pediatric ARDS patients.
We show the feasibility of simultaneously measuring multiple inflammatory cytokines, cell counts and coagulation parameters in pediatric ARDS patients.
We report statistical models that may be useful for future, larger trials to predict ARDS severity and outcomes.
Acknowledgments
This study was funded by the NIH grants HL118118-01A1, R01AI090059, R01ES015050, P42ES013648 and the American Lung Association grant RG-260120-N.
Abbreviations
- ARDS
Acute Respiratory Distress Syndrome
- GC
glucocorticoid
- EGF
epidermal growth factor
- FGF-2
fibroblast growth factor-2
- Flt3L
fms-like tyrosine kinase 3 ligand
- G-CSF
granulocyte-colony stimulating factor
- GM-CSF
granulocyte monocyte-colony stimulating factor
- GRO
growth related cytokine
- INF-α2
interferon-α2
- IFN-γ
interferon-γ
- IL-1α
interleukin1α
- IL-1β
interleukin-1β
- IL-1ra
interleukin-1 receptor antagonist
- IL-2
interleukin-2
- IL-3
interleukin-3
- IL-4
interleukin-4
- IL-5
interleukin-5
- IL-6
interleukin-6
- IL-7
interleukin-7
- IL-8
interleukin-8
- IL-9
interleukin-9
- IL-10
interleukin-10
- IL-12(p40)
interleukin-12(p40)
- IL-12(p70)
interleukin-12(p70)
- IL-13
interleukin-13
- IL-15
interleukin-15
- IL-17α
interleukin-17α
- IP-10
interferon-inducible protein-10
- MCP-1
monocyte chemotactic protein-1
- MCP-3
monocyte chemotactic protein-3
- MDC
macrophage-derived chemokine
- MIP-1α
macrophage inhibitory protein-1α
- MIP-1β
macrophage inhibitory protein-1β
- sCD40L
soluble CD40 ligand
- TGF-α
transforming growth factor-α
- TNF-α
tumor necrosis factor-α
- TNF-β
tumor necrosis factor-β
- VEGF
vascular endothelial growth factor
- WBC
white blood cells
- PT
prothrombin time
- PTT
partial thromboplastin time
- CRP
C-reactive protein
- ICU
intensive care unit
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Andreas Schwingshackl, Assistant Professor, Department of Pediatrics, Mattel Children’s Hospital at UCLA, Los Angeles, CA
Dai Kimura, Assistant Professor, Department of Pediatrics, University of Tennessee Health Science Center, Memphis, TN
Cynthia R. Rovnaghi, Research Associate and Lab Manager, Pain Neurobiology Laboratory, University of Tennessee Health Science Center, Memphis, TN
Jordy S. Saravia, Postdoctoral Fellow, Department of Pediatrics, University of Tennessee Health Science Center, Memphis, TN.
Stephania A Cormier, Professor of Pediatrics, Department of Pediatrics, University of Tennessee Health Science Center, Memphis, TN.
Bin Teng, Research Associate, Department of Physiology, University of Tennessee Health Science Center, Memphis, TN
Alina N. West, Research Coordinator, Department of Pediatrics, University of Tennessee Health Science Center, Memphis, TN.
Umberto G. Meduri, Professor of Medicine and Physiology, Department of Internal Medicine, University of Tennessee Health Science Center, Memphis, TN
Kanwaljeet J. S. Anand, Professor of Pediatrics, Anesthesiology, Anatomy & Neurobiology, Department of Pediatrics, Stanford University, Stanford, CA 94305
References
- 1.Ashbaugh DG, Bigelow DB, Petty TL, Levine BE. Acute respiratory distress in adults. Lancet. 1967;2(7511):319–323. doi: 10.1016/s0140-6736(67)90168-7. [DOI] [PubMed] [Google Scholar]
- 2.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. American journal of respiratory and critical care medicine. 1994;149(3 Pt 1):818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 3.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. The New England journal of medicine. 2005;353(16):1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 4.Zimmerman JJ, Akhtar SR, Caldwell E, Rubenfeld GD. Incidence and outcomes of pediatric acute lung injury. Pediatrics. 2009;124(1):87–95. doi: 10.1542/peds.2007-2462. [DOI] [PubMed] [Google Scholar]
- 5.Zambon M, Vincent JL. Mortality rates for patients with acute lung injury/ARDS have decreased over time. Chest. 2008;133(5):1120–1127. doi: 10.1378/chest.07-2134. [DOI] [PubMed] [Google Scholar]
- 6.Slutsky AS, Ranieri VM. Ventilator-induced lung injury. The New England journal of medicine. 2014;370(10):980. doi: 10.1056/NEJMc1400293. [DOI] [PubMed] [Google Scholar]
- 7.Afshari A, Brok J, Moller AM, Wetterslev J. Inhaled nitric oxide for acute respiratory distress syndrome (ARDS) and acute lung injury in children and adults. The Cochrane database of systematic reviews. 2010;(7):CD002787. doi: 10.1002/14651858.CD002787.pub2. [DOI] [PubMed] [Google Scholar]
- 8.Willson DF, Thomas NJ, Tamburro R, Truemper E, Truwit J, Conaway M, Traul C, Egan EE. Pediatric calfactant in acute respiratory distress syndrome trial. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2013;14(7):657–665. doi: 10.1097/PCC.0b013e3182917b68. [DOI] [PubMed] [Google Scholar]
- 9.Sawheny E, Ellis AL, Kinasewitz GT. Iloprost improves gas exchange in patients with pulmonary hypertension and ARDS. Chest. 2013;144(1):55–62. doi: 10.1378/chest.12-2296. [DOI] [PubMed] [Google Scholar]
- 10.Silva PL, Pelosi P, Rocco PR. Fluids in acute respiratory distress syndrome: pros and cons. Curr Opin Crit Care. 2014;20(1):104–112. doi: 10.1097/MCC.0000000000000049. [DOI] [PubMed] [Google Scholar]
- 11.Sud S, Sud M, Friedrich JO, Wunsch H, Meade MO, Ferguson ND, Adhikari NK. High-frequency ventilation versus conventional ventilation for treatment of acute lung injury and acute respiratory distress syndrome. The Cochrane database of systematic reviews. 2013;2:CD004085. doi: 10.1002/14651858.CD004085.pub3. [DOI] [PubMed] [Google Scholar]
- 12.Meduri GU, Golden E, Freire AX, Taylor E, Zaman M, Carson SJ, Gibson M, Umberger R. Methylprednisolone infusion in early severe ARDS results of a randomized controlled trial. 2007. Chest. 2009;136(5 Suppl):e30. doi: 10.1378/chest.06-2100. [DOI] [PubMed] [Google Scholar]
- 13.Meduri GU, Marik PE, Chrousos GP, Pastores SM, Arlt W, Beishuizen A, Bokhari F, Zaloga G, Annane D. Steroid treatment in ARDS: a critical appraisal of the ARDS network trial and the recent literature. Intensive Care Med. 2008;34(1):61–69. doi: 10.1007/s00134-007-0933-3. [DOI] [PubMed] [Google Scholar]
- 14.Lee YL, Chen W, Chen LY, Chen CH, Lin YC, Liang SJ, Shih CM. Systemic and bronchoalveolar cytokines as predictors of in-hospital mortality in severe community-acquired pneumonia. Journal of critical care. 2010;25(1):176 e177–113. doi: 10.1016/j.jcrc.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Meduri GU, Kohler G, Headley S, Tolley E, Stentz F, Postlethwaite A. Inflammatory cytokines in the BAL of patients with ARDS. Persistent elevation over time predicts poor outcome. Chest. 1995;108(5):1303–1314. doi: 10.1378/chest.108.5.1303. [DOI] [PubMed] [Google Scholar]
- 16.Smith LS, Zimmerman JJ, Martin TR. Mechanisms of acute respiratory distress syndrome in children and adults: a review and suggestions for future research. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2013;14(6):631–643. doi: 10.1097/PCC.0b013e318291753f. [DOI] [PubMed] [Google Scholar]
- 17.Mokart D, Guery BP, Bouabdallah R, Martin C, Blache JL, Arnoulet C, Mege JL. Deactivation of alveolar macrophages in septic neutropenic ARDS. Chest. 2003;124(2):644–652. doi: 10.1378/chest.124.2.644. [DOI] [PubMed] [Google Scholar]
- 18.Williams AE, Chambers RC. The mercurial nature of neutrophils: still an enigma in ARDS? Am J Physiol Lung Cell Mol Physiol. 2014;306(3):L217–230. doi: 10.1152/ajplung.00311.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwingshackl A, Teng B, Ghosh M, West AN, Makena P, Gorantla V, Sinclair SE, Waters CM. Regulation and function of the two-pore-domain (K2P) potassium channel Trek-1 in alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2012;302(1):L93–L102. doi: 10.1152/ajplung.00078.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwingshackl A, Teng B, Ghosh MC, Lim KG, Tigyi GJ, Narayanan D, Jaggar JH, Waters CM. Regulation of Interleukin-6 Secretion by the Two-Pore-Domain Potassium (K2P) Channel Trek-1 in Alveolar Epithelial Cells. Am J Physiol Lung Cell Mol Physiol. 2012 doi: 10.1152/ajplung.00299.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwingshackl A, Teng B, Ghosh M, Waters CM. Regulation of Monocyte Chemotactic Protein-1 secretion by the Two-Pore-Domain Potassium (K2P) channel TREK-1 in human alveolar epithelial cells. American journal of translational research. 2013;5(5):530–542. [PMC free article] [PubMed] [Google Scholar]
- 22.Drago BB, Kimura D, Rovnaghi CR, Schwingshackl A, Rayburn M, Meduri GU, Anand KJ. Double-blind, placebo-controlled pilot randomized trial of methylprednisolone infusion in pediatric acute respiratory distress syndrome. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2015;16(3):e74–81. doi: 10.1097/PCC.0000000000000349. [DOI] [PubMed] [Google Scholar]
- 23.Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin Definition. JAMA : the journal of the American Medical Association. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 24.Pediatric acute respiratory distress syndrome: consensus recommendations from the Pediatric Acute Lung Injury Consensus Conference. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2015;16(5):428–439. doi: 10.1097/PCC.0000000000000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meduri GU, Headley AS, Golden E, Carson SJ, Umberger RA, Kelso T, Tolley EA. Effect of prolonged methylprednisolone therapy in unresolving acute respiratory distress syndrome: a randomized controlled trial. JAMA : the journal of the American Medical Association. 1998;280(2):159–165. doi: 10.1001/jama.280.2.159. [DOI] [PubMed] [Google Scholar]
- 26.Meduri GU, Annane D, Chrousos GP, Marik PE, Sinclair SE. Activation and regulation of systemic inflammation in ARDS: rationale for prolonged glucocorticoid therapy. Chest. 2009;136(6):1631–1643. doi: 10.1378/chest.08-2408. [DOI] [PubMed] [Google Scholar]
- 27.Meduri GU, Rocco PR, Annane D, Sinclair SE. Prolonged glucocorticoid treatment and secondary prevention in acute respiratory distress syndrome. Expert Rev Respir Med. 2010;4(2):201–210. doi: 10.1586/ers.10.2. [DOI] [PubMed] [Google Scholar]
- 28.Verbist KC, Rose DL, Cole CJ, Field MB, Klonowski KD. IL-15 participates in the respiratory innate immune response to influenza virus infection. PLoS One. 2012;7(5):e37539. doi: 10.1371/journal.pone.0037539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huntington ND, Legrand N, Alves NL, Jaron B, Weijer K, Plet A, Corcuff E, Mortier E, Jacques Y, Spits H, et al. IL-15 trans-presentation promotes human NK cell development and differentiation in vivo. The Journal of experimental medicine. 2009;206(1):25–34. doi: 10.1084/jem.20082013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakamura R, Maeda N, Shibata K, Yamada H, Kase T, Yoshikai Y. Interleukin-15 is critical in the pathogenesis of influenza a virus-induced acute lung injury. Journal of virology. 2010;84(11):5574–5582. doi: 10.1128/JVI.02030-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salagianni M, Lekka E, Moustaki A, Iliopoulou EG, Baxevanis CN, Papamichail M, Perez SA. NK cell adoptive transfer combined with Ontak-mediated regulatory T cell elimination induces effective adaptive antitumor immune responses. J Immunol. 2011;186(6):3327–3335. doi: 10.4049/jimmunol.1000652. [DOI] [PubMed] [Google Scholar]
- 32.Fehr J, Grossmann HC. Disparity between circulating and marginated neutrophils: evidence from studies on the granulocyte alkaline phosphatase, a marker of cell maturity. American journal of hematology. 1979;7(4):369–379. doi: 10.1002/ajh.2830070409. [DOI] [PubMed] [Google Scholar]
- 33.Meduri GU, Headley S, Tolley E, Shelby M, Stentz F, Postlethwaite A. Plasma and BAL cytokine response to corticosteroid rescue treatment in late ARDS. Chest. 1995;108(5):1315–1325. doi: 10.1378/chest.108.5.1315. [DOI] [PubMed] [Google Scholar]
- 34.McAleer JP, Kolls JK. Directing traffic: IL-17 and IL-22 coordinate pulmonary immune defense. Immunological reviews. 2014;260(1):129–144. doi: 10.1111/imr.12183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharma AK, LaPar DJ, Zhao Y, Li L, Lau CL, Kron IL, Iwakura Y, Okusa MD, Laubach VE. Natural killer T cell-derived IL-17 mediates lung ischemia-reperfusion injury. American journal of respiratory and critical care medicine. 2011;183(11):1539–1549. doi: 10.1164/rccm.201007-1173OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li J, Zhang Y, Lou J, Zhu J, He M, Deng X, Cai Z. Neutralisation of peritoneal IL-17A markedly improves the prognosis of severe septic mice by decreasing neutrophil infiltration and proinflammatory cytokines. PLoS One. 2012;7(10):e46506. doi: 10.1371/journal.pone.0046506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gasse P, Riteau N, Vacher R, Michel ML, Fautrel A, di Padova F, Fick L, Charron S, Lagente V, Eberl G, et al. IL-1 and IL-23 mediate early IL-17A production in pulmonary inflammation leading to late fibrosis. PLoS One. 2011;6(8):e23185. doi: 10.1371/journal.pone.0023185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Braun RK, Ferrick C, Neubauer P, Sjoding M, Sterner-Kock A, Kock M, Putney L, Ferrick DA, Hyde DM, Love RB. IL-17 producing gammadelta T cells are required for a controlled inflammatory response after bleomycin-induced lung injury. Inflammation. 2008;31(3):167–179. doi: 10.1007/s10753-008-9062-6. [DOI] [PubMed] [Google Scholar]
- 39.Pandiyan P, Yang XP, Saravanamuthu SS, Zheng L, Ishihara S, O’Shea JJ, Lenardo MJ. The role of IL-15 in activating STAT5 and fine-tuning IL-17A production in CD4 T lymphocytes. J Immunol. 2012;189(9):4237–4246. doi: 10.4049/jimmunol.1201476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.You QH, Zhang D, Niu CC, Zhu ZM, Wang N, Yue Y, Sun GY. Expression of IL-17A and IL-17F in lipopolysaccharide-induced acute lung injury and the counteraction of anisodamine or methylprednisolone. Cytokine. 2014;66(1):78–86. doi: 10.1016/j.cyto.2013.12.019. [DOI] [PubMed] [Google Scholar]
- 41.Attridge K, Walker LS. Homeostasis and function of regulatory T cells (Tregs) in vivo: lessons from TCR-transgenic Tregs. Immunological reviews. 2014;259(1):23–39. doi: 10.1111/imr.12165. [DOI] [PMC free article] [PubMed] [Google Scholar]