Abstract
Background
Gastric cancer is the second most common cancer in Korea. Fatigue is a common symptom among cancer survivors. The aim of this study was to identify factors associated with fatigue in gastric cancer survivors.
Methods
Data were analyzed from 199 gastric cancer survivors who visited a cancer survivor outpatient clinic from July 2013 to June 2014. Patients were surveyed using a questionnaire containing a fatigue severity scale (FSS) and questions regarding associated symptoms. Participants were divided into fatigue (FSS) and non-fatigue groups based on FSS scores (≥4 and <4, respectively). Age, sex, weight, body mass index, cancer stage, pathology, surgery type, chemotherapy, radiotherapy, comorbid disease, family history of cancer, smoking, alcohol consumption, exercise, and laboratory results were investigated.
Results
The fatigue and non-fatigue groups contained 42 and 157 survivors, respectively. Their mean age was 58 years, and the mean post-operative period was 6.58 years. Arthralgia (odds ratio [OR], 12.95; 95% confidence interval [CI], 3.21-52.34), dyspnea (OR, 10.54; 95% CI, 2.94-37.80), dyspepsia (OR, 8.26; 95% CI, 2.63-25.96), changed bowel habits (OR, 4.56; 95% CI, 1.09-19.11), anemia (OR, 3.18; 95% CI, 1.26-8.05), and regular exercise (OR, 0.31; 95% CI, 0.12-0.77) were significantly associated with fatigue in gastric cancer survivors, while weight, treatment, and depressive mood were not.
Conclusion
Arthralgia, dyspnea, dyspepsia, bowel habit change, anemia, and regular exercise are associated with fatigue in gastric cancer survivors.
Keywords: Stomach Neoplasms, Survivors, Fatigue
INTRODUCTION
Cancer-related fatigue is the most common symptom among cancer patients, reported in up to 60% to 90% of cases.1) Approximately 41% of cancer patients complain of fatigue during treatments such as chemotherapy, radiotherapy, and bone marrow transplantation. Fatigue persists in 30% to 40% of patients even after completing treatment.2) Previous studies have indicated that fatigue in cancer survivors can last for up to five years after completion of therapy3) and can be a persistent problem.4) Moreover, fatigue was associated with cancer recurrence and poor overall survival in a large longitudinal study of patients with breast cancer.5)
Gastric cancer is the second most common cancer in South Korea, accounting for 14.5% of all cancer diagnoses in 2011. The crude incidence rate of gastric cancer was 63.1 per 100,000 persons (85.1 in men and 41.1 in women). The five-year survival rate of gastric cancer has increased to 69.4%, mainly due to advances in screening tests and curative surgical treatment.6) As a consequence of increased numbers of gastric cancer survivors, attention has been focused on characterizing survivor fatigue, quality of life, and wellness.
Fatigue is a common problem associated with quality of life in gastric cancer survivors.7) In prospective studies of gastric cancer survivors, fatigue-related symptoms persisted even 12 months after surgery.8) Although previous studies have reported on factors associated with cancer-related fatigue, little information is available on long-term gastric cancer survivors and the factors associated with their fatigue. The aim of this study, therefore, was to identify factors associated with fatigue in gastric cancer survivors.
METHODS
1. Study Population
From July 2013 to June 2014, 322 gastric cancer survivors who visited cancer survivorship clinic in Samsung Medical Center were enrolled in this study. They did not show evidence of cancer recurrence during five years of follow-up after treatment. After excluding 123 subjects with missing questionnaire responses, 199 eligible patients were included in our analysis. This study was approved by the institutional review board of Samsung Medical Center (IRB no. SMC 2014-09-023-011). Because this study was a retrospective analysis of existing administrative and clinical data, the ethics committee waived the requirement for informed consent.
2. Data Collection
Patient age, sex, surgery type, cancer stage, pathology, and adjuvant chemotherapy or radiotherapy were investigated retrospectively based on medical records. Height and weight were measured and body mass index (BMI) calculated. We assessed the patients' medical history of physician-diagnosed hypertension, diabetes, and dyslipidemia. Comorbidities, family history of cancer, smoking, alcohol consumption, physical activity, current symptoms, and fatigue scale scores were surveyed using a self-administered questionnaire.
Fatigue was evaluated in all patients via the translated fatigue severity scale (FSS), which was validated in previous studies.9,10) The FSS, a self-administered questionnaire developed by Krupp et al.,9) is a useful fatigue questionnaire for chronic diseases.11) It is composed of nine items that measure how fatigue affects motivation; exercise; physical functioning; the ability to perform duties; and its effects on work, family, and social life. Respondents are directed to answer each question based on their subjective status during the previous week. Grading of each item ranges from 1 to 7, where 1 indicates strong disagreement and 7 indicates strong agreement, and the final score represents the mean value of the nine items. In the current study, the mean score of the nine categories were divided into two groups using a cut-off value as described previously12) (i.e., FSS ≥4 and FSS <4 for the fatigued and non-fatigued groups, respectively).
Each variable was categorized as follows. The type of surgery was categorized as total or subtotal gastrectomy, diagnostic stage was classified according to the American Joint Committee on Cancer TNM (tumor-node-metastasis) system. Early gastric cancer (EGC) was defined as a cancer invading no deeper than the submucosa, irrespective of lymph node metastasis (T1, any positive lymph nodes [N]), while advanced gastric cancer (AGC) was defined as a cancer invading deeper than the submucosa (>T1).
Pathology results were categorized as adenocarcinoma (the most common type of gastric cancer) or other pathology subgroups, including signet ring cell carcinoma. A questionnaire was used to investigate the presence of physician-diagnosed comorbidities including diabetes mellitus, hypertension, dyslipidemia, myocardial infarction, stroke, and osteoporosis. Family history of cancer was assessed for based on participant parents, siblings, and grandparents.
Smoking status was ascertained based on responses to the questions "have you ever smoked more than 20 packs of cigarettes during your lifetime?" and "do you currently smoke?" Alcohol drinking status was defined as having consumed more than one cup of alcohol during their lifetime. Regular exercise was defined as performing physical activity with sweating more than once per week.
Current symptoms were assessed using a self-administered questionnaire; respondents indicated "yes" or "no" to questions regarding anorexia (do you have loss of appetite?), xerostomia (do you feel dryness in your mouth?), dyspnea (do you feel shortness of breath with minimal exercise?), changed bowel habits (do you have changed bowel habits?), headache (do you feel headache?), chest pain (do you have tightness, compressive feeling, or pain in your anterior chest?), fever or chills (do you feel fevered or chilled?), dyspepsia (do you have digestion difficulties?), arthralgia (do you have joint swelling or pain?), sexual dysfunction (do you have difficulty in your sex life?), sleep disorder (is it hard to fall asleep or do you wake frequently?), depressive mood (do you feel depressed or hopeless?), and decreased interest (are you concerned about decreased interest or pleasure?). Serum aspartate aminotransferase, alanine aminotransferase, blood urea nitrogen, and serum creatinine levels were measured. Anemia was defined as hemoglobin levels <13 g/dL in men and <12 g/dL in women according to World Health Organization definitions.13)
3. Statistical Analysis
All statistical analyses were performed using IBM SPSS Statistics for Windows ver. 22.0 (IBM Co., Armonk, NY, USA). Mean values of the clinical characteristics were compared between groups (fatigued and non-fatigued). Continuous data were expressed as means and standard deviations, and Student t-tests were used to compare differences. Categorical data were reported as proportions, and chi-square or Fisher's exact tests were used for comparisons. Univariate and multivariate logistic regressions were used for association analyses. All variables with P-values less than 0.05 in the univariate analyses as well as clinically significant variables were included in the multivariate logistic regression. P-values less than 0.05 were considered statistically significant.
RESULTS
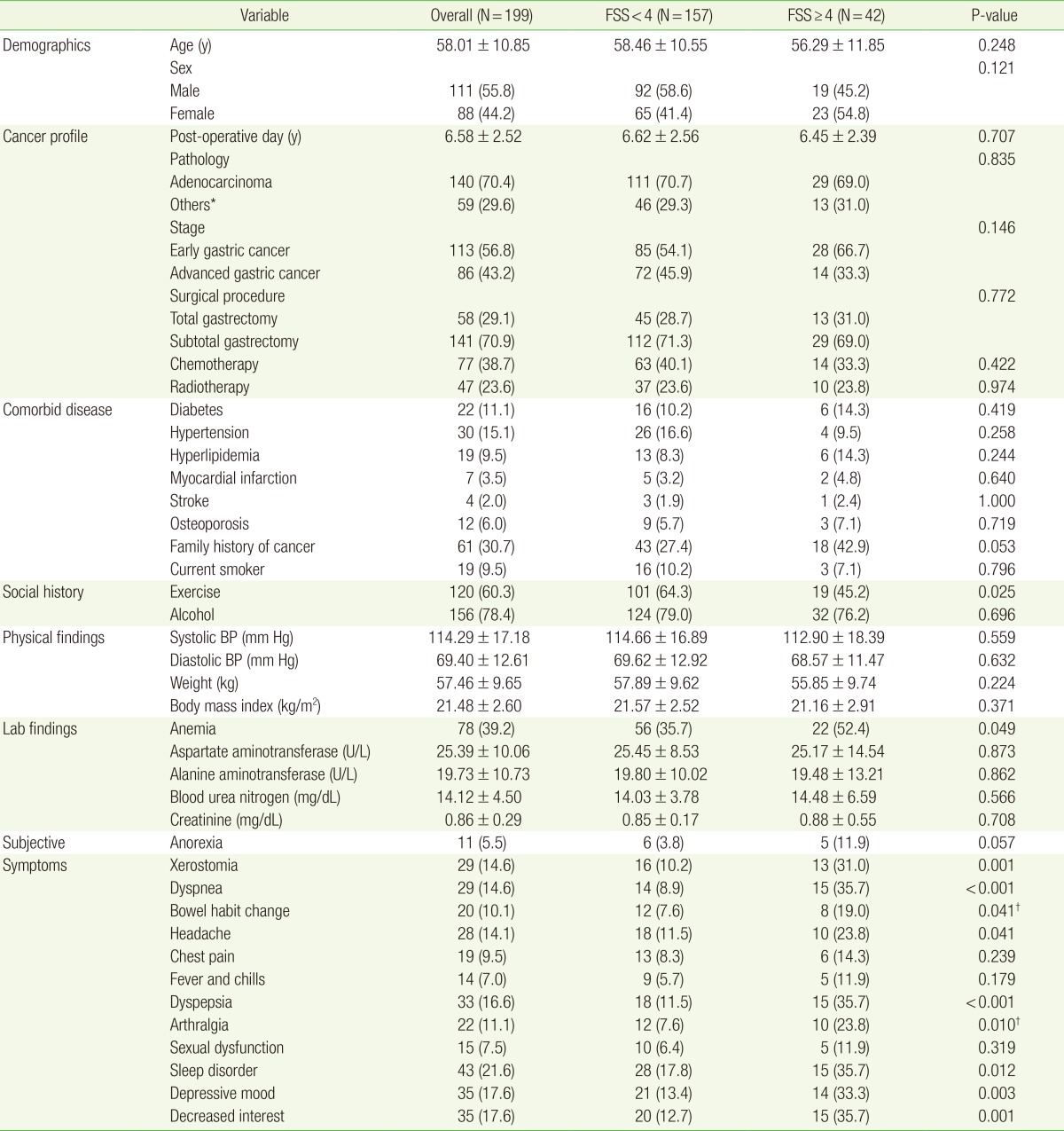
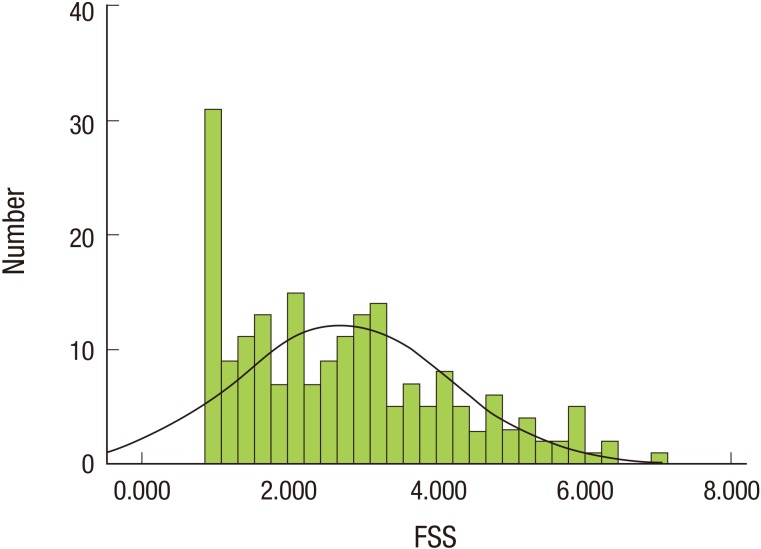
The baseline characteristics of the men (n=111) and women (n=88) enrolled in this study are shown in Table 1. Their mean age was 58.1 years; and 55.8% were men. The mean postoperative follow-up period was 6.58 years. The mean of FSS score for all participants was 2.728±1.441; the distribution of FSS score is shown in Figure 1. Among 199 subjects, 42 were placed in the fatigued group (21.11%). There was no significant difference between the two groups in BMI, weight, pathology, stage, surgical type, chemotherapy, or radiotherapy. The number of patients who regularly exercised was higher in the non-fatigued group than the fatigued group. The incidence of anemia in the fatigued group was significantly higher than in the non-fatigued group.
Table 1. Comparison of baseline characteristics by fatigue group (±one standard deviation).
Values are presented as mean±standard deviation or number (%).
FSS, fatigue severity scale; BP, blood pressure.
*Other subgroup of pathology was included signet ring cell carcinoma and lymphoepithelioma-like carcinoma. †Obtained by Student t-test and chi-square tests or Fisher's exact test between the fatigue and non-fatigue groups.
Figure 1. Distribution of FSS scores in gastric cancer survivors. The normal curve for FSS scores among all participants is represented by the solid line. FSS, fatigue severity scale.
Among current symptoms, anorexia, xerostomia, dyspnea, changed bowel habits, headache, dyspepsia, arthralgia, sleep disorders, depressive mood, and decreased interest were significantly more prevalent in the fatigue group. Under univariate logistic regression analysis, dyspnea, dyspepsia, xerostomia, decreased interest, arthralgia, anorexia, depressive mood, change in bowel habits, and headache were associated with fatigue in gastric cancer survivors. Conversely, regular exercise was associated with less fatigue.
All covariates with P-values less than 0.05 in the univariate analyses as well as clinically significant variables were included in the multivariate logistic regression model (Table 2). Arthralgia (adjusted OR, 12.95; 95% CI, 3.21-52.34), dyspnea (adjusted OR, 10.54; 95% CI, 2.94-37.80), dyspepsia (adjusted OR, 8.25; 95% CI, 2.63-25.96), changed bowel habits (adjusted OR, 4.56; 95% CI, 1.09-19.11), and anemia (adjusted OR, 3.18; 95% CI, 1.26-8.05) were associated with fatigue in gastric cancer survivors. Regular exercise (adjusted OR, 0.31; 95% CI, 0.12-0.77) was associated with a low fatigue score. Advanced gastric cancer (adjusted OR, 0.34; 95% CI, 0.13-0.89) was associated with a lower risk of fatigue.
Table 2. Logistic regression of the ORs (95% CI) for fatigue and associated factors.
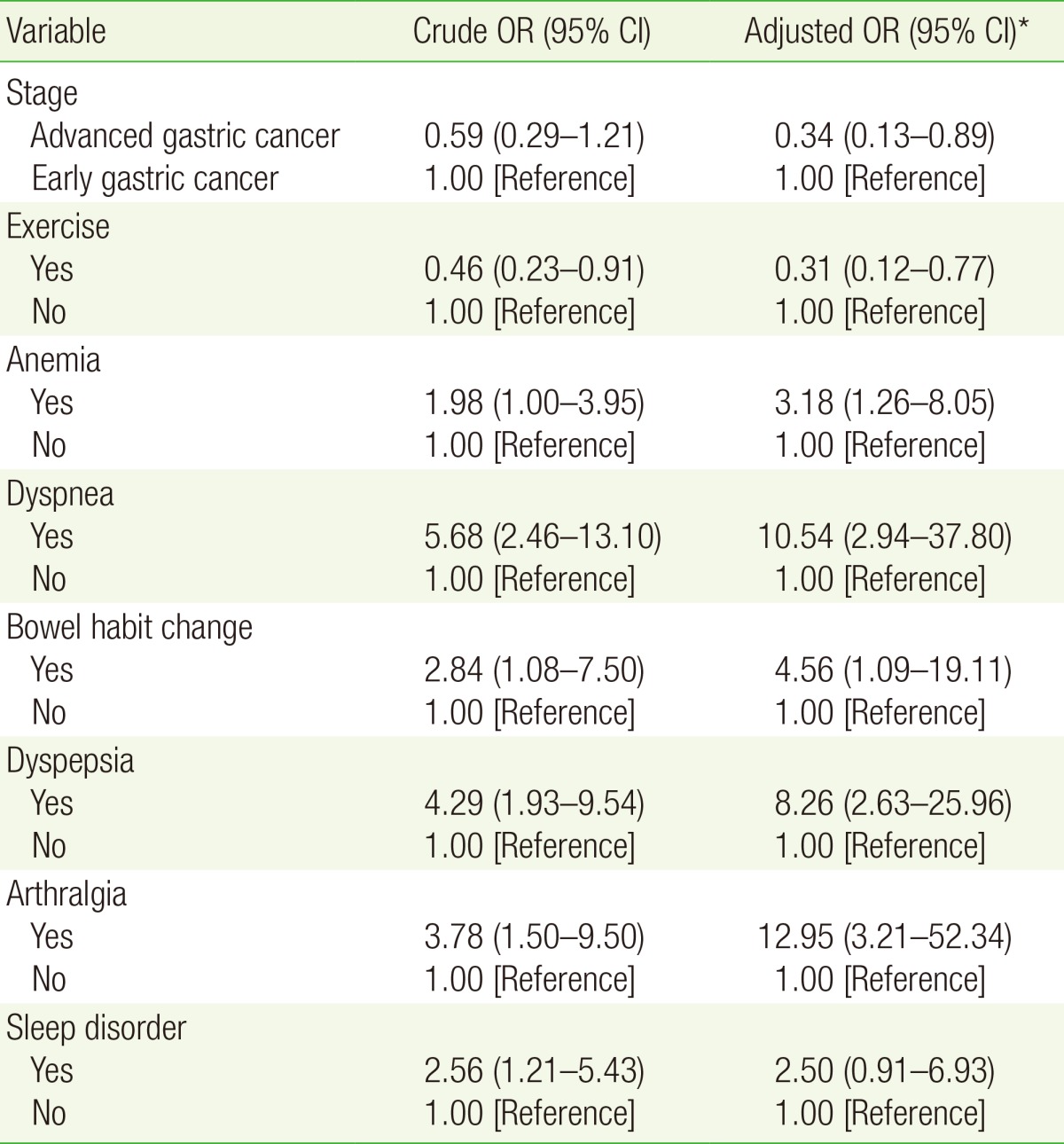
Fatigue is divided into fatigue (FSS ≥4) and non-fatigued (FSS <4) groups.
OR, odds ratio; CI, confidence interval; FSS, fatigue severity scale.
*Covariates with P-values less than 0.05 in univariate analysis and clinically significant variables including sex, age, body mass index, systolic blood pressure, diastolic blood pressure, pathology, stage, surgery, chemotherapy, radiotherapy, family history of cancer, smoking, exercise, alcohol, anemia, and subjective symptoms were included in the multivariate analysis. Other variables, not shown in this table, were removed using optimized multivariate regression analysis.
DISCUSSION
The results of the present study show that subjective symptoms including arthralgia, dyspnea, dyspepsia, and changes in bowel habits are associated with fatigue in gastric cancer survivors. Anemia, a known factor linked with fatigue, was also associated with fatigue in gastric cancer survivors. Regular exercise was associated with lower fatigue scores in gastric cancer survivors. However, weight, surgery type, chemotherapy, radiotherapy, comorbid disease, smoking habit, and depressive mood were not associated with fatigue in these long-term survivors.
Physical activity has been linked with cancer-related fatigue. Fatigue severity in long-term breast cancer survivors was associated with physical inactivity.14) Prospective longitudinal studies have shown that low levels of physical activity after completing treatment is a predictor of fatigue in breast cancer survivors.15,16) In a cross-sectional study of 200 endometrial cancer survivors, high levels of physical activity were associated with relief of fatigue.17) Based on the results of a previous meta-analysis on exercise intervention in cancer survivors, regular exercise may be a beneficial intervention for cancer-related fatigue by improving muscle metabolism and mental health.18)
The anemia associated with gastric cancer could occur as a complication of the cancer itself or as a consequence of the treatment. Iron deficiency anemia or megaloblastic anemia often results from gastrectomy. Adjuvant chemotherapy and radiotherapy are also reported causes of anemia in gastric cancer patients.19) According to National Comprehensive Cancer Network guidelines, anemia that is a consequence of chemotherapy or radiotherapy should be addressed if patients with hemoglobin levels <11 g/dL complain of severe fatigue, dyspnea, tachycardia, chest pain, or lightheadedness. The guidelines also recommend management for hemoglobin levels <10 g/dL, even without these symptoms.20) Moreover, quality of life in cancer survivors may be related to correction of their anemia.21) Consequently, based on the association between anemia and fatigue in the present study, screening for anemia may improve of quality of life and reduce fatigue.
In this study, AGC survivors had lower fatigue levels than EGC survivors (adjusted OR, 0.34; 95% CI, 0.13-0.89; P=0.028). However, little is known about the association between cancer stage and fatigue. Although AGC patients who achieve complete remission could be more vulnerable to fatigue due to aggressive surgical interventions and adjuvant therapy, they unexpectedly showed a lower risk of fatigue. One reason for this unusual finding may be that the patients had good prognoses primarily enrolled from the outpatient clinic, except those subjects with poor outcome. Those individuals may seek a higher level of health care.
In a longitudinal study of breast cancer survivors, BMI was a predictor of fatigue during the 42 months following the end of adjuvant treatment.15,22) However, we did not find an association between BMI and fatigue in this study. Compared to other cancers, weight loss after treatment in a gastric cancer patient is a serious complaint and has multifactorial causes. In our study, therefore, weight at the time of outpatient clinic visit may not be related with patient fatigue. Serial measurements of weight before and after the surgery may be more representative for evaluation of fatigue. Serial measurements of weight during follow-up are required to further investigate the association between fatigue and weight or BMI change.
Depression is strongly correlated with fatigue in cancer patients and is a risk factor for cancer-related fatigue.23) Several studies suggested that depression and anxiety were significant predictors of cancer-related fatigue, regardless of treatment period.24) In previous studies, anxiety and depression were associated with the severity of fatigue among 267 survivors of Hodgkin's lymphoma.25) Current depression is one of the strongest risk factors for clinically relevant fatigue in disease-free gastric cancer survivors.7) In our study, depressive moods were more frequent in the fatigued group. However, it was not significantly associated with fatigue in multivariate analysis.
Both chemotherapy and radiotherapy could aggravate fatigue by increasing levels of inflammatory markers.26) Previous studies have shown that greater post-treatment fatigue is associated with increased levels of C-reactive protein (CRP) in breast cancer survivors,27) CRP and interleukin-1 in testicular cancer survivors,28) and interleukin-6 in ovarian cancer survivors.29) In our study, chemotherapy and radiotherapy were not associated with fatigue. We believe that chemotherapy and radiotherapy may have a trivial effect on fatigue in long-term survivors. Further evaluation with inflammatory markers in gastric cancer survivors may be required to understand the lack of association of chemotherapy or radiotherapy with fatigue.
A longitudinal cohort study reported that gastric cancer patients experienced fatigue, diarrhea, dysphagia, and eating restrictions after gastrectomy, and suggested the necessity to monitor these symptoms for improvement of quality of life in gastric cancer patients.30) In the present study, several subjective symptoms were associated with fatigue in gastric cancer survivors. However, the questions used in the questionnaire were not standardized, and further development of an assessment tool for evaluation of each symptom is necessary.
This study has several limitations. First, because this study was a cross-sectional analysis, it could not be used to establish the exact pathophysiologic link between fatigue and subjective symptoms. Second, several subjects were removed from analysis due to missing data. Because this population was enrolled at a clinic for long-term survivors, an enrollment bias toward relatively healthy subjects with good prognosis was possible. Therefore, selection bias was not fully considered in these analyses. Finally, socioeconomic factors including monthly income, socioeconomic status, and residential area, which are known to influence fatigue incidence in previous studies,7) were not included in the analysis.
Despite these limitations, we believe that the present study facilitates better understanding of fatigue in gastric cancer survivors. Anemia, regular exercise, subjective symptoms including dyspnea, dyspepsia, and bowel habit change are associated with fatigue in gastric cancer survivors. These findings may help in establishing comprehensive management of long-term gastric cancer survivors.
ACKNOWLEDGMENTS
This study was based on a questionnaire provided by the Cancer Survivor Clinic in the Department of Family Medicine of Samsung Medical Center.
Footnotes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Silver JK. After cancer treatment: heal faster, better, stronger. Baltimore (MD): Johns Hopkins University Press; 2006. [Google Scholar]
- 2.Berger AM, Bruera E, Cimprich B. Recognition and treatment of the symptom of cancer-related fatigue. Alexandria (VA): American Society of Clinical Oncology; 2010. [Google Scholar]
- 3.Minton O, Stone P. How common is fatigue in disease-free breast cancer survivors?: a systematic review of the literature. Breast Cancer Res Treat. 2008;112:5–13. doi: 10.1007/s10549-007-9831-1. [DOI] [PubMed] [Google Scholar]
- 4.Bower JE, Ganz PA, Desmond KA, Bernaards C, Rowland JH, Meyerowitz BE, et al. Fatigue in long-term breast carcinoma survivors: a longitudinal investigation. Cancer. 2006;106:751–758. doi: 10.1002/cncr.21671. [DOI] [PubMed] [Google Scholar]
- 5.Groenvold M, Petersen MA, Idler E, Bjorner JB, Fayers PM, Mouridsen HT. Psychological distress and fatigue predicted recurrence and survival in primary breast cancer patients. Breast Cancer Res Treat. 2007;105:209–219. doi: 10.1007/s10549-006-9447-x. [DOI] [PubMed] [Google Scholar]
- 6.Ministry of Health and Welfare; The Korea Central Cancer Registry; National Cancer Center. Annual report of cancer statistics in Korea in 2011. Goyang: National Cancer Center; 2013. [Google Scholar]
- 7.Hwang IC, Yun YH, Kim YW, Ryu KW, Kim YA, Kim S, et al. Factors related to clinically relevant fatigue in disease-free stomach cancer survivors and expectation-outcome consistency. Support Care Cancer. 2014;22:1453–1460. doi: 10.1007/s00520-013-2110-2. [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi D, Kodera Y, Fujiwara M, Koike M, Nakayama G, Nakao A. Assessment of quality of life after gastrectomy using EORTC QLQ-C30 and STO22. World J Surg. 2011;35:357–364. doi: 10.1007/s00268-010-0860-2. [DOI] [PubMed] [Google Scholar]
- 9.Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale: application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46:1121–1123. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- 10.Kleinman L, Zodet MW, Hakim Z, Aledort J, Barker C, Chan K, et al. Psychometric evaluation of the fatigue severity scale for use in chronic hepatitis C. Qual Life Res. 2000;9:499–508. doi: 10.1023/a:1008960710415. [DOI] [PubMed] [Google Scholar]
- 11.Hjollund NH, Andersen JH, Bech P. Assessment of fatigue in chronic disease: a bibliographic study of fatigue measurement scales. Health Qual Life Outcomes. 2007;5:12. doi: 10.1186/1477-7525-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valko PO, Bassetti CL, Bloch KE, Held U, Baumann CR. Validation of the fatigue severity scale in a Swiss cohort. Sleep. 2008;31:1601–1607. doi: 10.1093/sleep/31.11.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nutritional anaemias: report of a WHO scientific group. World Health Organ Tech Rep Ser. 1968;405:5–37. [PubMed] [Google Scholar]
- 14.Winters-Stone KM, Bennett JA, Nail L, Schwartz A. Strength, physical activity, and age predict fatigue in older breast cancer survivors. Oncol Nurs Forum. 2008;35:815–821. doi: 10.1188/08.ONF.815-821. [DOI] [PubMed] [Google Scholar]
- 15.Donovan KA, Small BJ, Andrykowski MA, Munster P, Jacobsen PB. Utility of a cognitive-behavioral model to predict fatigue following breast cancer treatment. Health Psychol. 2007;26:464–472. doi: 10.1037/0278-6133.26.4.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alfano CM, Smith AW, Irwin ML, Bowen DJ, Sorensen B, Reeve BB, et al. Physical activity, long-term symptoms, and physical health-related quality of life among breast cancer survivors: a prospective analysis. J Cancer Surviv. 2007;1:116–128. doi: 10.1007/s11764-007-0014-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Basen-Engquist K, Scruggs S, Jhingran A, Bodurka DC, Lu K, Ramondetta L, et al. Physical activity and obesity in endometrial cancer survivors: associations with pain, fatigue, and physical functioning. Am J Obstet Gynecol. 2009;200:288.e1–288.e8. doi: 10.1016/j.ajog.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown JC, Huedo-Medina TB, Pescatello LS, Pescatello SM, Ferrer RA, Johnson BT. Efficacy of exercise interventions in modulating cancer-related fatigue among adult cancer survivors: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2011;20:123–133. doi: 10.1158/1055-9965.EPI-10-0988. [DOI] [PubMed] [Google Scholar]
- 19.Sohn T. Nutritional treatment after gastrectomy. J Korean Med Assoc. 2010;53:1124–1127. [Google Scholar]
- 20.Rodgers GM, 3rd, Becker PS, Blinder M, Cella D, Chanan-Khan A, Cleeland C, et al. Cancer- and chemotherapy-induced anemia. J Natl Compr Canc Netw. 2012;10:628–653. doi: 10.6004/jnccn.2012.0064. [DOI] [PubMed] [Google Scholar]
- 21.Cella D, Zagari MJ, Vandoros C, Gagnon DD, Hurtz HJ, Nortier JW. Epoetin alfa treatment results in clinically significant improvements in quality of life in anemic cancer patients when referenced to the general population. J Clin Oncol. 2003;21:366–373. doi: 10.1200/JCO.2003.02.136. [DOI] [PubMed] [Google Scholar]
- 22.Andrykowski MA, Donovan KA, Laronga C, Jacobsen PB. Prevalence, predictors, and characteristics of off-treatment fatigue in breast cancer survivors. Cancer. 2010;116:5740–5748. doi: 10.1002/cncr.25294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacobsen PB, Donovan KA, Weitzner MA. Distinguishing fatigue and depression in patients with cancer. Semin Clin Neuropsychiatry. 2003;8:229–240. [PubMed] [Google Scholar]
- 24.Reinertsen KV, Cvancarova M, Loge JH, Edvardsen H, Wist E, Fossa SD. Predictors and course of chronic fatigue in long-term breast cancer survivors. J Cancer Surviv. 2010;4:405–414. doi: 10.1007/s11764-010-0145-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daniels LA, Oerlemans S, Krol AD, Creutzberg CL, van de Poll-Franse LV. Chronic fatigue in Hodgkin lymphoma survivors and associations with anxiety, depression and comorbidity. Br J Cancer. 2014;110:868–874. doi: 10.1038/bjc.2013.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bower JE. Cancer-related fatigue: mechanisms, risk factors, and treatments. Nat Rev Clin Oncol. 2014;11:597–609. doi: 10.1038/nrclinonc.2014.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alfano CM, Imayama I, Neuhouser ML, Kiecolt-Glaser JK, Smith AW, Meeske K, et al. Fatigue, inflammation, and ω-3 and ω-6 fatty acid intake among breast cancer survivors. J Clin Oncol. 2012;30:1280–1287. doi: 10.1200/JCO.2011.36.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orre IJ, Murison R, Dahl AA, Ueland T, Aukrust P, Fossa SD. Levels of circulating interleukin-1 receptor antagonist and C-reactive protein in long-term survivors of testicular cancer with chronic cancer-related fatigue. Brain Behav Immun. 2009;23:868–874. doi: 10.1016/j.bbi.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 29.Schrepf A, Clevenger L, Christensen D, DeGeest K, Bender D, Ahmed A, et al. Cortisol and inflammatory processes in ovarian cancer patients following primary treatment: relationships with depression, fatigue, and disability. Brain Behav Immun. 2013;30(Suppl):S126–S134. doi: 10.1016/j.bbi.2012.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim AR, Cho J, Hsu YJ, Choi MG, Noh JH, Sohn TS, et al. Changes of quality of life in gastric cancer patients after curative resection: a longitudinal cohort study in Korea. Ann Surg. 2012;256:1008–1013. doi: 10.1097/SLA.0b013e31827661c9. [DOI] [PubMed] [Google Scholar]